Lignin for Bioeconomy: The Present and Future Role of Technical Lignin
Abstract
1. Introduction
Classification of Technical Lignin
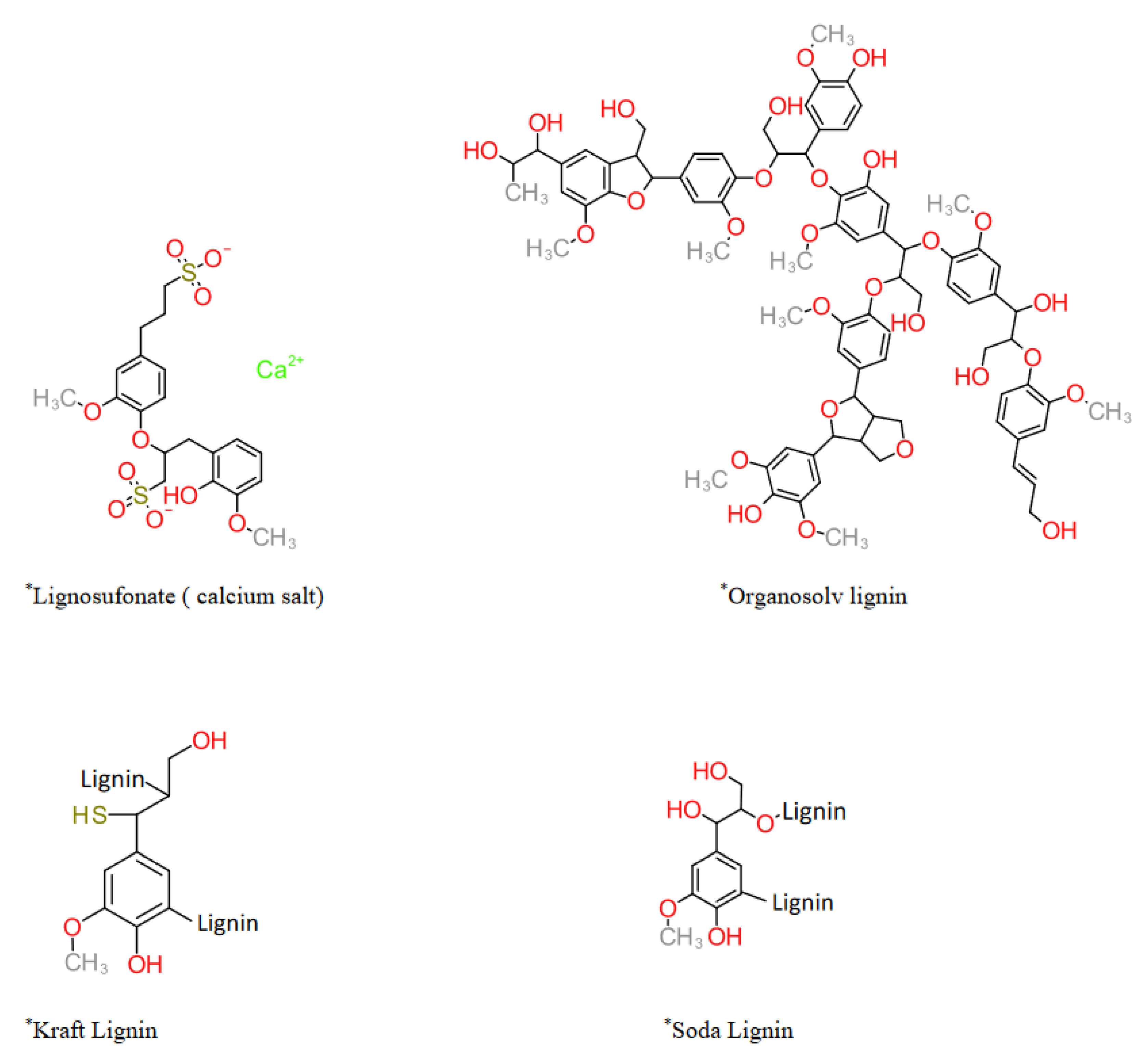
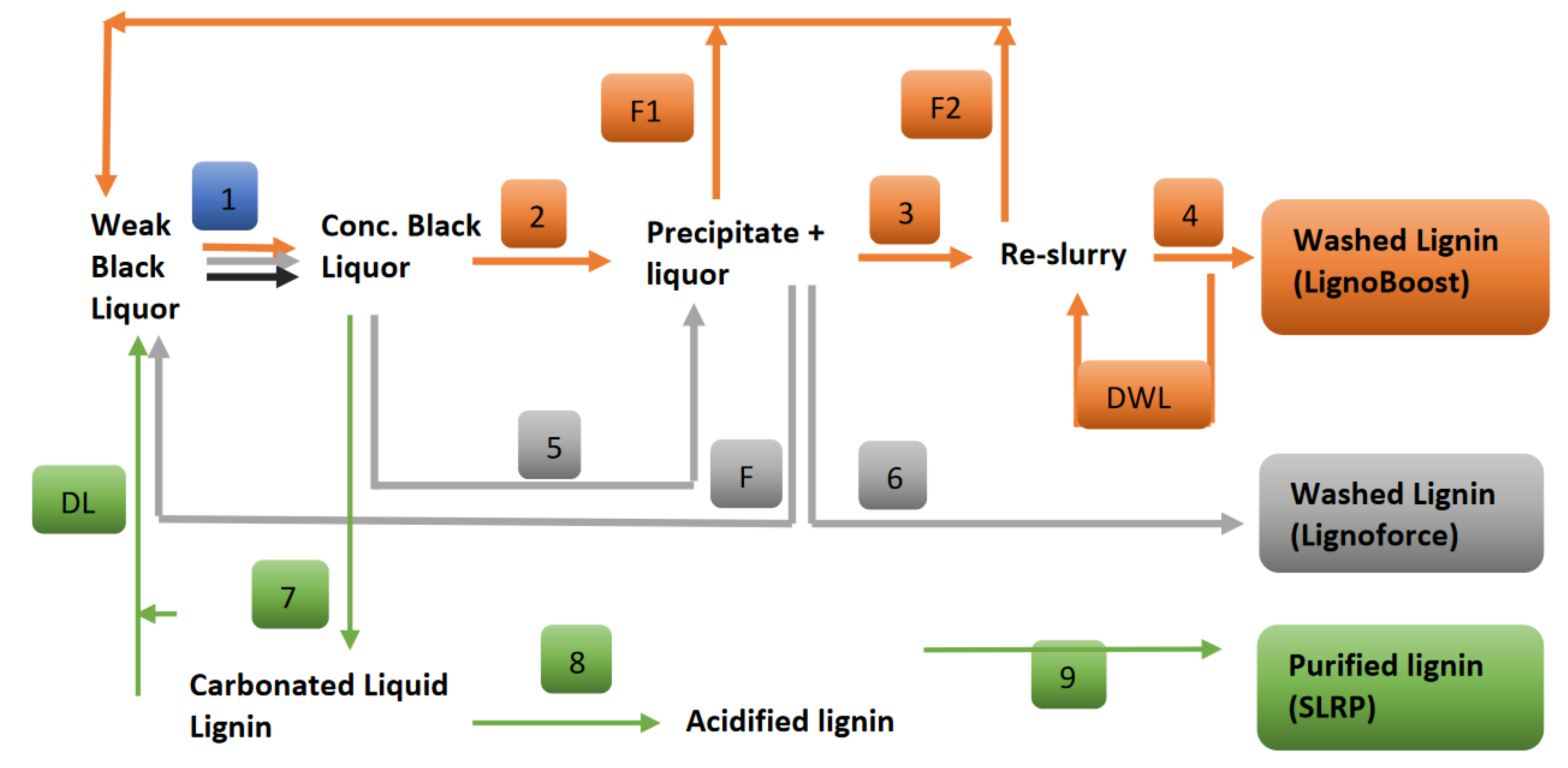
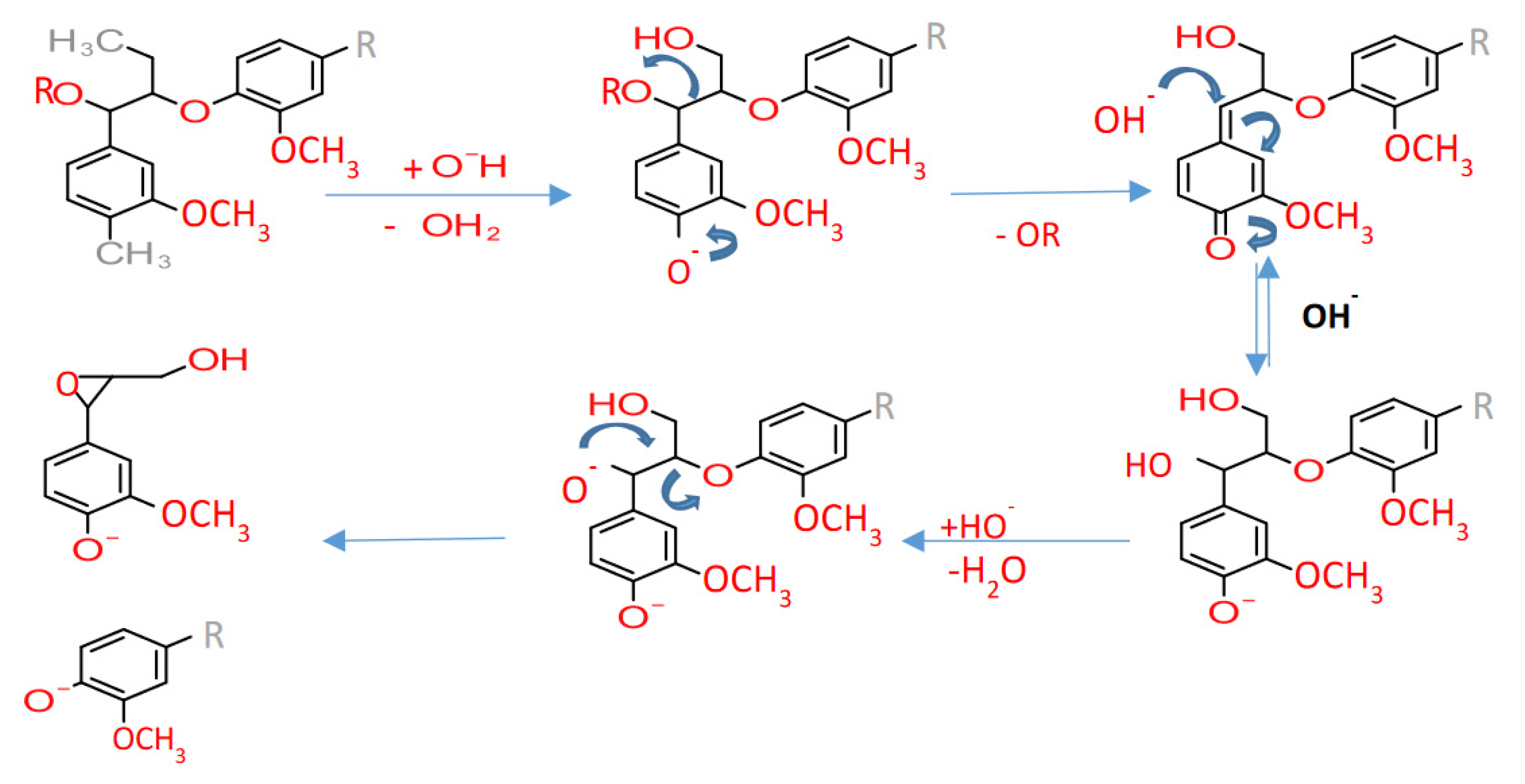
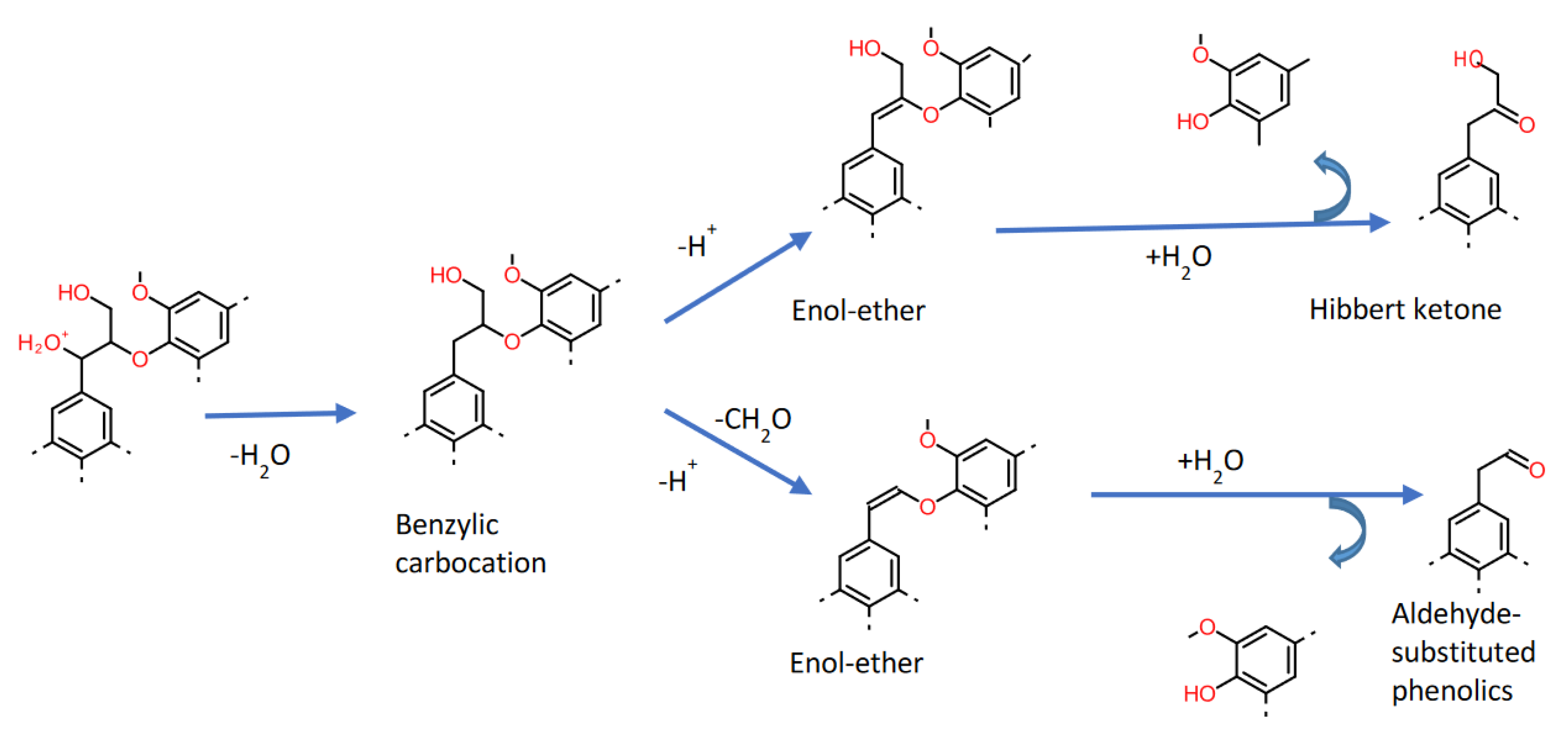
2. Kraft Lignin
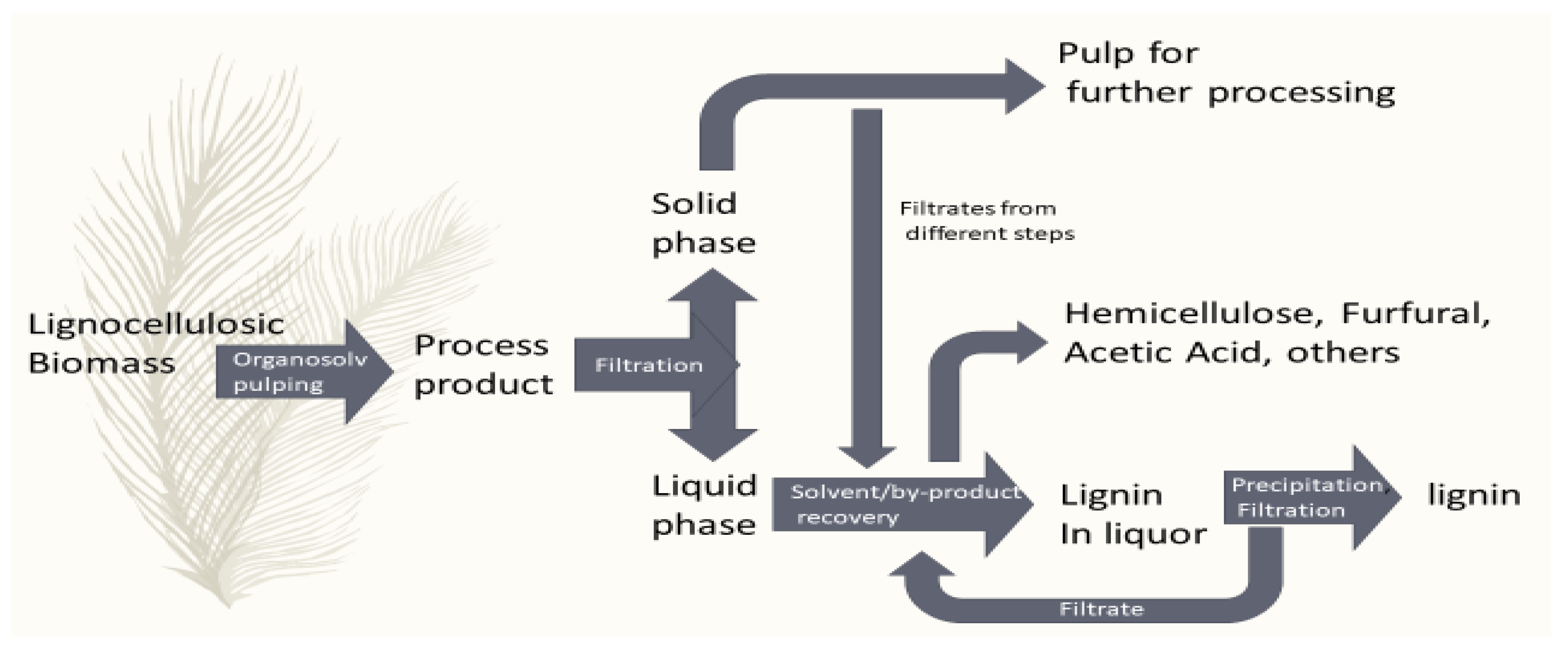
3. Organosolv Lignin
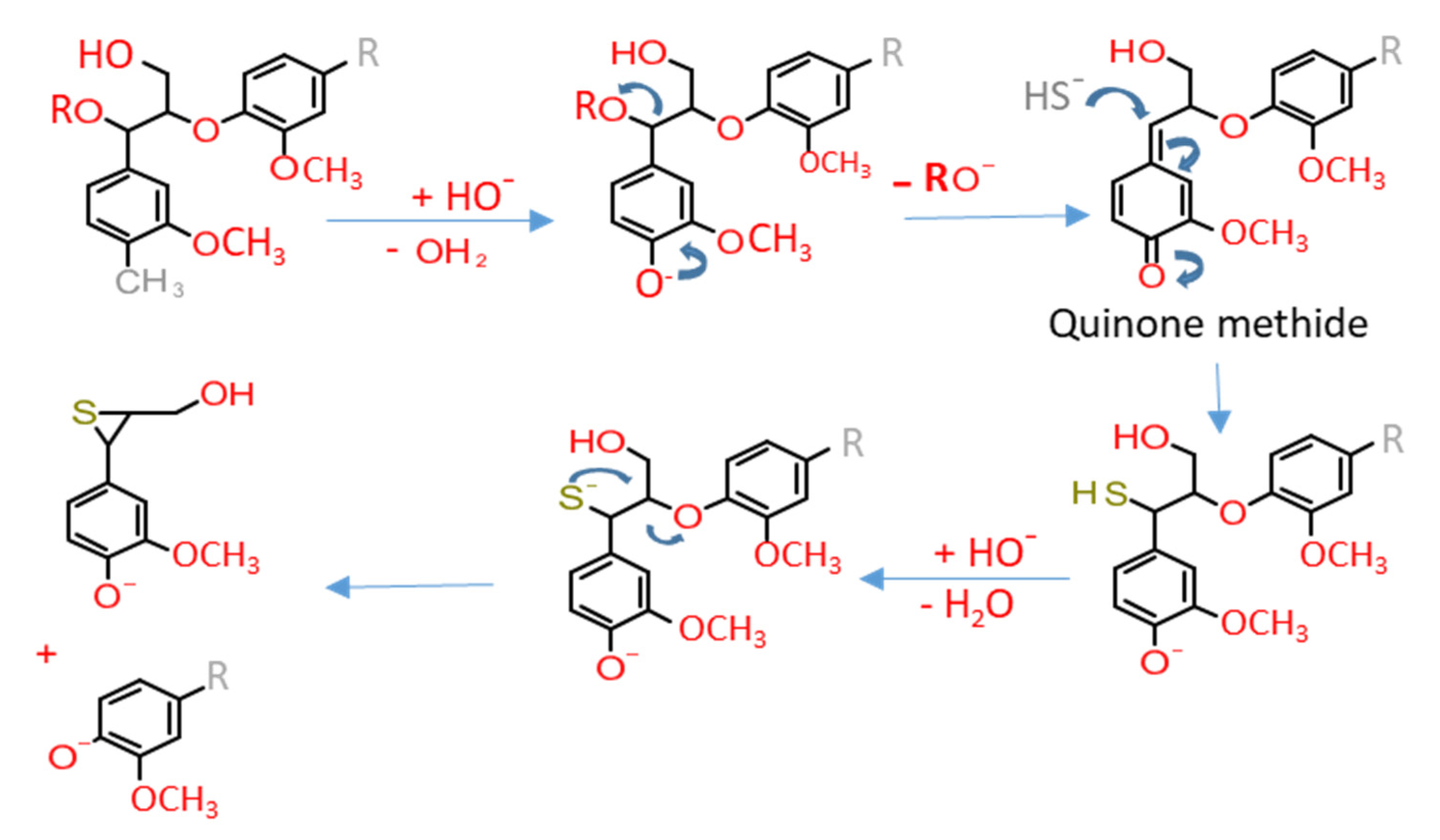
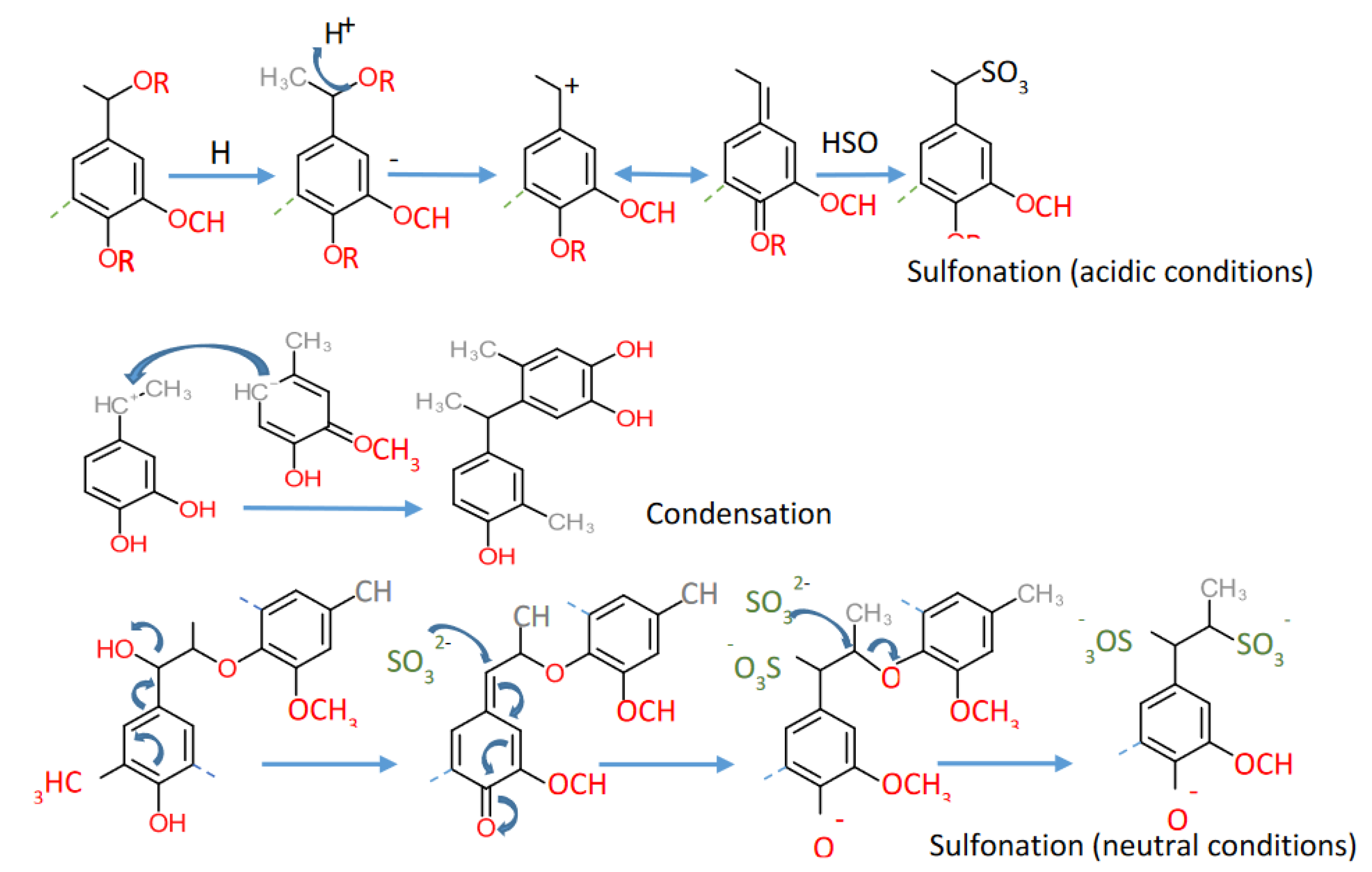
4. Lignosulfonates
5. Soda Lignin
6. Hydrolytic Lignin
Technical Lignin Based Nanoparticle Synthesis: Potential and Practicability
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Achyuthan, K.E.; Achyuthan, A.M.; Adams, P.D.; Dirk, S.M.; Harper, J.C.; Simmons, B.A.; Singh, A.K. Supramolecular Self-Assembled Chaos: Polyphenolic Lignin’s Barrier to Cost-Effective Lignocellulosic Biofuels. Molecules 2010, 15, 8641–8688. [Google Scholar] [CrossRef]
- Kopsahelis, N.; Agouridis, N.; Bekatorou, A.; Kanellaki, M. Comparative Study of Spent Grains and Delignified Spent Grains as Yeast Supports for Alcohol Production from Molasses. Bioresour. Technol. 2007, 98, 1440–1447. [Google Scholar] [CrossRef]
- Ashori, A. Nonwood Fibers—A Potential Source of Raw Material in Papermaking. Polym. Plast. Technol. Eng. 2006, 45, 1133–1136. [Google Scholar] [CrossRef]
- Argyropoulos, D.S. Quantitative Phosphorus-31 NMR Analysis of Six Soluble Lignins. J. Wood Chem. Technol. 1994, 14, 65–82. [Google Scholar] [CrossRef]
- Balakshin, M.Y.; Berlin, A.; DelliColli, H.T.; Grunert, C.A.N.J.; Gutman, V.M.; Ortiz, D.; Pye, E.K. Derivatives of Native Lignin. U.S. Patent 8,445,562, 21 May 2013. [Google Scholar]
- Dawy, M.; Shabaka, A.A.; Nada, A.M.A. Molecular Structure and Dielectric Properties of Some Treated Lignins. Polym. Degrad. Stab. 1998, 62, 455–462. [Google Scholar] [CrossRef]
- Mansfield, S.D. Solutions for Dissolution—Engineering Cell Walls for Deconstruction. Curr. Opin. Biotechnol. 2009, 20, 286–294. [Google Scholar] [CrossRef]
- Ahvazi, B.; Cloutier, É.; Wojciechowicz, O.; Ngo, T.-D. Lignin Profiling: A Guide for Selecting Appropriate Lignins as Precursors in Biomaterials Development. ACS Sustain. Chem. Eng. 2016, 4, 5090–5105. [Google Scholar] [CrossRef]
- Siddiqui, L.; Bag, J.; Mittal, D.; Leekha, A.; Mishra, H.; Mishra, M.; Verma, A.K.; Mishra, P.K.; Ekielski, A.; Iqbal, Z. Assessing the Potential of Lignin Nanoparticles as Drug Carrier: Synthesis, Cytotoxicity and Genotoxicity Studies. Int. J. Biol. Macromol. 2020, 152, 786–802. [Google Scholar] [CrossRef]
- Siddiqui, L.; Mishra, H.; Mishra, P.K.; Iqbal, Z.; Talegaonkar, S. Novel 4-in-1 Strategy to Combat Colon Cancer, Drug Resistance and Cancer Relapse Utilizing Functionalized Bioinspiring Lignin Nanoparticle. Med. Hypotheses 2018, 121, 10–14. [Google Scholar] [CrossRef]
- Mishra, P.K.; Ekielski, A. A Simple Method to Synthesize Lignin Nanoparticles. Colloids Interfaces 2019, 3, 52. [Google Scholar] [CrossRef]
- Poletto, M.; Zattera, A.J. Materials Produced from Plant Biomass: Part III: Degradation Kinetics and Hydrogen Bonding in Lignin. Mater. Res. 2013, 16, 1065–1070. [Google Scholar] [CrossRef]
- Santos, R.B.; Capanema, E.A.; Balakshin, M.Y.; Chang, H.; Jameel, H. Lignin Structural Variation in Hardwood Species. J. Agric. Food Chem. 2012, 60, 4923–4930. [Google Scholar] [CrossRef]
- Negro, M.; Manzanares, P.; Oliva, J.; Ballesteros, I.; Ballesteros, M. Changes in Various Physical/Chemical Parameters of Pinus Pinaster Wood after Steam Explosion Pretreatment. Biomass Bioenergy 2003, 25, 301–308. [Google Scholar] [CrossRef]
- Poulomi, S.; Dong Ho, K.; Seokwon, J.; Arthur, R. Pseudo-Lignin and Pretreatment Chemistry. Energy Environ. Sci. 2011, 4, 1306–1310. [Google Scholar]
- Hu, F.; Jung, S.; Ragauskas, A. Pseudo-Lignin Formation and Its Impact on Enzymatic Hydrolysis. Bioresour. Technol. 2012, 117, 7–12. [Google Scholar] [CrossRef]
- Kumar, R.; Hu, F.; Sannigrahi, P.; Jung, S.; Ragauskas, A.J.; Wyman, C.E. Carbohydrate Derived-pseudo-lignin Can Retard Cellulose Biological Conversion. Biotechnol. Bioeng. 2013, 110, 737–753. [Google Scholar] [CrossRef]
- Rinaldi, R.; Jastrzebski, R.; Clough, M.T.; Ralph, J.; Kennema, M.; Bruijnincx, P.C.; Weckhuysen, B.M. Paving the Way for Lignin Valorisation: Recent Advances in Bioengineering, Biorefining and Catalysis. Angew. Chem. Int. Ed. 2016, 55, 8164–8215. [Google Scholar] [CrossRef]
- Bouxin, F.P.; McVeigh, A.; Tran, F.; Westwood, N.J.; Jarvis, M.C.; Jackson, S.D. Catalytic Depolymerisation of Isolated Lignins to Fine Chemicals Using a Pt/Alumina Catalyst: Part 1—Impact of the Lignin Structure. Green Chem. 2015, 17, 1235–1242. [Google Scholar] [CrossRef]
- Hubbe, M.A.; Alén, R.; Paleologou, M.; Kannangara, M.; Kihlman, J. Lignin Recovery from Spent Alkaline Pulping Liquors Using Acidification, Membrane Separation, and Related Processing Steps: A Review. Bioresources 2019, 14, 2300–2351. [Google Scholar] [CrossRef]
- Norgren, M.; Edlund, H. Lignin: Recent Advances and Emerging Applications. Curr. Opin. Colloid Interface Sci. 2014, 19, 409–416. [Google Scholar] [CrossRef]
- Thakur, V.K.; Thakur, M.K.; Raghavan, P.; Kessler, M.R. Progress in Green Polymer Composites from Lignin for Multifunctional Applications: A Review. ACS Sustain. Chem. Eng. 2014, 2, 1072–1092. [Google Scholar] [CrossRef]
- Figueiredo, P.; Lintinen, K.; Hirvonen, J.T.; Kostiainen, M.A.; Santos, H.A. Properties and Chemical Modifications of Lignin: Towards Lignin-Based Nanomaterials for Biomedical Applications. Prog. Mater. Sci. 2018, 93, 233–269. [Google Scholar] [CrossRef]
- Mishra, P.K.; Ekielski, A. The Self-Assembly of Lignin and Its Application in Nanoparticle Synthesis: A Short Review. Nanomaterials 2019, 9, 243. [Google Scholar] [CrossRef]
- Kai, D.; Tan, M.J.; Chee, P.L.; Chua, Y.K.; Yap, Y.L.; Loh, X.J. Towards Lignin-Based Functional Materials in a Sustainable World. Green Chem. 2016, 18, 1175–1200. [Google Scholar] [CrossRef]
- Gael Febdinand Dahl. U.S. Patent 296,935, 15 April 1884.
- Zhang, Y.-H.P. Reviving the Carbohydrate Economy via Multi-Product Lignocellulose Biorefineries. J. Ind. Microbiol. Biotechnol. 2008, 35, 367–375. [Google Scholar] [CrossRef]
- Ramirez, F.; González, V.; Crespo, M.; Meier, D.; Faix, O.; Zúñiga, V. Ammoxidized Kraft Lignin as a Slow-Release Fertilizer Tested on Sorghum Vulgare. Bioresour. Technol. 1997, 61, 43–46. [Google Scholar] [CrossRef]
- Kadla, J.; Kubo, S.; Venditti, R.; Gilbert, R.; Compere, A.; Griffith, W. Lignin-Based Carbon Fibers for Composite Fiber Applications. Carbon 2002, 40, 2913–2920. [Google Scholar] [CrossRef]
- Gosselink, R.; de Jong, E.; Abächerli, A.; Guran, B. Activities and Results of the Thematic Network Eurolignin. In Proceedings of the 7th ILI Forum, Barcelona, Spain, 27–28 April 2005; pp. 25–30. [Google Scholar]
- Tejado, A.; Peña, C.; Labidi, J.; Echeverria, J.M.; Mondragon, I. Physico-Chemical Characterization of Lignins from Different Sources for Use in Phenol–Formaldehyde Resin Synthesis. Bioresour. Technol. 2007, 98, 1655–1663. [Google Scholar] [CrossRef]
- Zoumpoulakis, L.; Simitzis, J. Ion Exchange Resins from Phenol/Formaldehyde Resin-modified Lignin. Polym. Int. 2001, 50, 277–283. [Google Scholar] [CrossRef]
- Carrott, P.; Carrott, M.R. Lignin–from Natural Adsorbent to Activated Carbon: A Review. Bioresour. Technol. 2007, 98, 2301–2312. [Google Scholar]
- Vishtal, A.G.; Kraslawski, A. Challenges in industrial applications of technical lignins. Bioresources 2011, 6, 3547–3568. [Google Scholar]
- Brauns, F.E.; Brauns, D.A. The Chemistry of Lignin: Covering the Literature for the Years 1949–1958; Elsevier: Amsterdam, The Netherlands, 2013; ISBN 1-4832-7595-7. [Google Scholar]
- ÖHMAN, F.; Theliander, H.; Norgren, M.; Tomani, P.; Axegård, P. Method for Separating Lignin from a Lignin Containing Liquid/Slurry. U.S. Patent 8,815,052, 26 August 2006. [Google Scholar]
- Miettinen, M. Continuous Method for the Precipitation of Lignin from Black Liquor. U.S. Patent 9,139,606, 22 September 2015. [Google Scholar]
- Wells, K.; Pors, D.; Foan, J.; Maki, K.; Kouisni, L.; Paleologou, M. CO2 Impacts of Commercial Scale Lignin Extraction at Hinton Pulp Using the LignoForce Process & Lignin Substitution into Petroleum-Based Products; PACWEST Conference: Newport Beach, CA, USA, June 2015; pp. 10–13. [Google Scholar]
- Lake, M.A.; Blackburn, J.C. Lignin Product and Process for Making Same. U.S. Patent 9,879,119, 30 January 2018. [Google Scholar]
- Kleinert, T.N. Organosolv Pulping and Recovery Process. U.S. Patent 3,585,104, 15 June 1971. [Google Scholar]
- Belgacem, M.N.; Blayo, A.; Gandini, A. Organosolv Lignin as a Filler in Inks, Varnishes and Paints. Ind. Crop. Prod. 2003, 18, 145–153. [Google Scholar] [CrossRef]
- Anttila, J.; Tanskanen, J.; Rousu, P.; Rousu, P.; Hytönen, K. Process for Preparing a Sugar Product. U.S. Patent Application No.12/741,693, 23 September 2010. [Google Scholar]
- Diebold, V.B.; Cowan, W.F.; Walsh, J.K. Solvent Pulping Process. U.S. Patent 4,100,016, 11 July 1978. [Google Scholar]
- Nimz, H.; Casten, R. Holzaufschluss Mit Essigsaure. German Patent DE 34.45, 4 December 1986. [Google Scholar]
- Nimz, H.; Schoene, M. Non-Waste Pulping and Bleaching with Acetic Acid. In Proceedings of the 7th International Symposium on Wood and Pulping Chemistry, Beijing, China, 25 May 1993; Volume 1, pp. 258–265. [Google Scholar]
- Baumeister, M.; Edel, E. Process for the Continuous Extraction of Vegetable-Fiber Material in Two Stages. U.S. Patent 4,496,426, 29 January 1985. [Google Scholar]
- Glasner, A.D.-I.; Bobik, M.D. Process for Recovery of Chemicals from the Pulping Liquor. E.U. Patent EP0538576B1, 4 December 1995. [Google Scholar]
- Delmas, M.; Mlayah, B.B. Process for Producing Bioethanol from Lignocellulosic Plant Raw Material. U.S. Patent 8,551,747, 8 October 2013. [Google Scholar]
- Mlayah, B.B.; Delmas, M.; Avignon, G. Installation for Implementing a Method for Producing Paper Pulp, Lignins and Sugars and Production Method Using Such an Installation. U.S. Patent 8,157,964, 17 April 2012. [Google Scholar]
- Mikkonen, H.; Peltonen, S.; Kallioinen, A.; Suurnäkki, A.; Kunnari, V.; Malm, T. Process for Defibering a Fibrous Raw-Material; World Intellectual Property Organization. International Patent WO2009066007, 28 May 2009. [Google Scholar]
- Rousu, P.; Rousu, P.; Rousu, E. Process for Producing Pulp with a Mixture of Formic Acid and Acetic Acid as Cooking Chemical. U.S. Patent 6,562,191, 13 May 2003. [Google Scholar]
- Rousu, P.; Rousu, P.; Rousu, E. Method of Producing Pulp Using Single-Stage Cooking with Formic Acid and Washing with Performic Acid. U.S. Patent 6,156,156, 5 December 2000. [Google Scholar]
- Seisto, A.; Poppius-Levlin, K. Milox Pulping of Agricultural Plants. In Proceedings of the 8th International Symposium on Wood and Pulping Chemistry, Helsinki, Finland, 6–9 June 1995. [Google Scholar]
- Berlin, A.; Balakshin, M.Y.; Ma, R.; Gutman, V.M.; Ortiz, D. Organosolv Process. U.S. Patent Application No. 13/584,697, 15 August 2013. [Google Scholar]
- Berlin, A.; Balakshin, M.Y.; Ma, R.; Gutman, V.M.; Ortiz, D. Organosolv Process World Intellectual Property Organization. International Patent WO2011097720A1, 18 August 2011. [Google Scholar]
- Luterbacher, J.S.; Shuai, L. Production of Monomers from Lignin during Depolymerisation of Lignocellulose-Containing Composition. U.S. Patent Application No. 16/093,065, 2 May 2019. [Google Scholar]
- Manesh, A.; Hemyeri, R.; Mohapatra, S.; Guenther, J.; Zoborowski, E.; Manesh, M.A. System and Method for Extraction of Chemicals from Lignocellulosic Materials. U.S. Patent 9,365,525, 14 June 2016. [Google Scholar]
- Manesh, A.; Guenther, J.H.; Zoborowski, E.G.; Braenner, W.; Manesh, M.A.; Hawk, L.J. Oxygen Assisted Organosolv Process, System and Method for Delignification of Lignocellulosic Materials and Lignin Recovery. U.S. Patent 9,382,283, 5 July 2016. [Google Scholar]
- Abacherli, A.; Doppenberg, F. Verfahren zur Aufbereitung von Aromatische Polymere Enthaltenden Alkalischen Lösungen. German Patent DE59807559, 20 March 1998. [Google Scholar]
- Hussin, M.H.; Aziz, A.A.; Iqbal, A.; Ibrahim, M.N.M.; Abd Latif, N.H. Development and Characterization Novel Bio-Adhesive for Wood Using Kenaf Core (Hibiscus Cannabinus) Lignin and Glyoxal. Int. J. Biol. Macromol. 2019, 122, 713–722. [Google Scholar] [CrossRef] [PubMed]
- Deandrea, M.; Mitchell, W.D.; Narendranath, N. Lignin Compositions and Methods for Use in Fermentation and Animal Feed. U.S. Patent Application No. 15/486,837, 19 October 2017. [Google Scholar]
- Chen, J.; Eraghi Kazzaz, A.; AlipoorMazandarani, N.; Hosseinpour Feizi, Z.; Fatehi, P. Production of Flocculants, Adsorbents, and Dispersants from Lignin. Molecules 2018, 23, 868. [Google Scholar] [CrossRef]
- Stigsson, L. Method for the Production of High Yield Chemical Pulp from Softwood. U.S. Patent Application No. 10/759,047, 21 July 2005. [Google Scholar]
- Abacherli, A.; Doppenberg, F. Method for Preparing Alkaline Solutions Containing Aromatic Polymers. Canadian Patent No. CA2283698A1, 1 December 1998. [Google Scholar]
- Chakar, F.S.; Ragauskas, A.J. Review of Current and Future Softwood Kraft Lignin Process Chemistry. Ind. Crop. Prod. 2004, 20, 131–141. [Google Scholar] [CrossRef]
- Temler, J.S. High-Yield Semi-Chemical Carbonate Pulping Process. U.S. Patent 4,229,251, 21 October 1980. [Google Scholar]
- Baklanova, O.; Plaksin, G.; Drozdov, V.; Duplyakin, V.; Chesnokov, N.; Kuznetsov, B. Preparation of Microporous Sorbents from Cedar Nutshells and Hydrolytic Lignin. Carbon 2003, 41, 1793–1800. [Google Scholar] [CrossRef]
- Eyal, A.; Vitner, A.; Mali, R. Method for Preparing a Hydrolyzate. U.S. Patent Application No. 13/577,215, 31 January 2013. [Google Scholar]
- Nguyen, Q.A.; Tucker, M.P. Dilute Acid/Metal Salt Hydrolysis of Lignocellulosics. U.S. Patent 6,423,145, 23 July 2002. [Google Scholar]
- Zhang, J.; Chen, G.; Yang, N.W.; Wang, Y.G. Preparation and Evaluation of Sodium Hydroxymethyl Lignosulfonate as Eco-Friendly Drilling Fluid Additive. Adv. Mater. Res. 2012, 415, 629–632. [Google Scholar] [CrossRef]
- Corey, A.; Wamsley, K.; Winowiski, T.; Moritz, J. Effects of Calcium Lignosulfonate, Mixer-Added Fat, and Feed Form on Feed Manufacture and Broiler Performance. J. Appl. Poult. Res. 2014, 23, 418–428. [Google Scholar] [CrossRef]
- Hemmilä, V.; Adamopoulos, S.; Hosseinpourpia, R.; Ahmed, S.A. Ammonium Lignosulfonate Adhesives for Particleboards with PMDI and Furfuryl Alcohol as Crosslinkers. Polymers 2019, 11, 1633. [Google Scholar] [CrossRef]
- Joensson, B.; Grundberg, H.; Gustafsson, A. Lignosulfonate of a Certain Quality and Method of Preparation of Lignosulfonate of a Certain Quality. U.S. Patent 9,447,131, 20 September 2016. [Google Scholar]
- Sjoede, A.; Froelander, A.; Lersch, M.; Roedsrud, G. Lignocellulosic Biomass Conversion. U.S. Patent 10,648,008, 12 May 2020. [Google Scholar]
- Reknes, K. Agglomerated Particulate Lignosulfonate. U.S. Patent 8,277,557, 2 February 2012. [Google Scholar]
- Reknes, K. Agglomerated Particulate Lignosulfonate. U.S. Patent Application 14/575,760, 4 June 2015. [Google Scholar]
- Lanthier, S.; Tassin, P.; Mahieu, E. Process for the Treatment of a Sulfonated Lignin-Based Liquor Containing Sulfite and Ammonium Ions with Formaldehyde. German Patent Number DE10107122A1, 26 September 2002. [Google Scholar]
- Lanthier, S.; Tassin, P.; Mahieu, E. Processing of Sulfonated Lignin-Based Liquor Containing Sulfite and Ammonium Ions, Obtained in Paper Production, for Re-Use in Building Industry Comprises Optional Replacement of Some Ammonium Ions and Treatment with Formaldehyde. French Patent Number FR2805263A1, 24 August 2001. [Google Scholar]
- Harada, H.; Hirota, M.; Nishijima, E.; Yashiro, J.; Yatsushiro, X.; Mahirota, H.; Nishijima, E. System for producing bioethanol using lignocellulose as raw material. Japanese Patent JP2009213389A, 24 September 2009. [Google Scholar]
- Ligninsulphonates|Burgo Group. Available online: https://www.burgo.com/en/group/figures/ls (accessed on 10 August 2020).
- Argyropoulos, D. Use of Lignocellulosics Solvated in Ionic Liquids for Production of Biofuels. U.S. Patent 8,182,557, 22 May 2012. [Google Scholar]
- Holbrey, J.; Swatloski, R.; Chen, J.; Daly, D.; Rogers, R. Polymer Dissolution and Blend Formation in Ionic Liquids. U.S. Patent 7,888,412, 15 February 2011. [Google Scholar]
- Fearon, O.; Kuitunen, S.; Vuorinen, T. Reaction Kinetics of Strong Nucleophiles with a Dimeric Non-Phenolic Lignin Model Compound with α-Carbonyl Functionality (Adleron) in Aqueous Alkali Solution. Holzforschung 2016, 70, 811–818. [Google Scholar] [CrossRef]
- Gierer, J. Chemical Aspects of Kraft Pulping. Wood Sci. Technol. 1980, 14, 241–266. [Google Scholar] [CrossRef]
- Sixta, H. Pulp Properties and Applications. Handb. Pulp 2006, 1009–1067. [Google Scholar]
- Demuner, I.F.; Colodette, J.L.; Demuner, A.J.; Jardim, C.M. Biorefinery Review: Wide-Reaching Products through Kraft Lignin. BioResources 2019, 14, 7543–7581. [Google Scholar]
- Yoon, S.-H.; Van Heiningen, A. Kraft Pulping and Papermaking Properties of Hot-Water Pre-Extracted Loblolly Pine in an Integrated Forest Products Biorefinery. Tappi J. 2008, 7, 22–27. [Google Scholar]
- Ragnar, M.; Lindgren, C.T.; Nilvebrant, N.-O. PKa-Values of Guaiacyl and Syringyl Phenols Related to Lignin. J. Wood Chem. Technol. 2000, 20, 277–305. [Google Scholar] [CrossRef]
- Sundin, J. Precipitation of Kraft Lignin under Alkaline Conditions. Doctoral Thesis, Royal Institute of Technology, Stockholm, Sweden, 1 December 2000. [Google Scholar]
- Zhu, W.; Theliander, H. Precipitation of Lignin from Softwood Black Liquor: An Investigation of the Equilibrium and Molecular Properties of Lignin. Bioresources 2015, 10, 1696–1714. [Google Scholar] [CrossRef]
- Jansen, R.; LAWSON, J.A.; Lapidot, N. Methods for Separating and Refining Lignin from Black Liquor and Compositions Thereof. U.S. Patent 10,767,308, 8 September 2020. [Google Scholar]
- Evstigneev, E. Factors Affecting Lignin Solubility. Russ. J. Appl. Chem. 2011, 84, 1040–1045. [Google Scholar] [CrossRef]
- Ohman, F.; Theliander, H.; Tomani, P.; Axegard, P. Method for Lignin Separation from Black Liquor. U.S. Patent 9,777,033, 3 October 2017. [Google Scholar]
- Kouisni, L.; Paleologou, M. Method for Separating Lignin from Black Liquor. U.S. Patent 8,771,464, 8 July 2014. [Google Scholar]
- Kouisni, L.; Gagné, A.; Maki, K.; Holt-Hindle, P.; Paleologou, M. LignoForce System for the Recovery of Lignin from Black Liquor: Feedstock Options, Odor Profile, and Product Characterization. ACS Sustain. Chem. Eng. 2016, 4, 5152–5159. [Google Scholar] [CrossRef]
- Lake, M.A.; Blackburn, J.C. SLRP-an Innovative Lignin-Recovery Technology. Cellul Chem. Technol. 2014, 48, 799–804. [Google Scholar]
- Lake, M.A.; Blackburn, J.C. Process for Recovering Lignin. U.S. Patent 9,260,464, 16 February 2016. [Google Scholar]
- Borand, M.N.; Karaosmanoğlu, F. Effects of Organosolv Pretreatment Conditions for Lignocellulosic Biomass in Biorefinery Applications: A Review. J. Renew. Sustain. Energy 2018, 10, 033104. [Google Scholar] [CrossRef]
- Schulze, P.; Seidel-Morgenstern, A.; Lorenz, H.; Leschinsky, M.; Unkelbach, G. Advanced Process for Precipitation of Lignin from Ethanol Organosolv Spent Liquors. Bioresour. Technol. 2016, 199, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Pandey, M.P.; Kim, C.S. Lignin Depolymerization and Conversion: A Review of Thermochemical Methods. Chem. Eng. Technol. 2011, 34, 29–41. [Google Scholar] [CrossRef]
- Pye, E.K.; Lora, J.H. The AlcellTM Process: A Proven Alternative to Kraft Pulping. Tappi J. 1991, 74, 113–118. [Google Scholar]
- Sarkanen, K.V. Chemistry of Solvent Pulping. Tappi J. 1990, 73, 215–219. [Google Scholar]
- Brosse, N.; Dufour, A.; Meng, X.; Sun, Q.; Ragauskas, A. Miscanthus: A Fast-Growing Crop for Biofuels and Chemicals Production. BiofuelsBioprod. Bioref. 2012, 6, 580–598. [Google Scholar] [CrossRef]
- Patt, R.; Kordsachia, O. Herstellung von Zellstoffen Unter Verwendung von Alkalischen Sulfitlösungen Mit Zusatz von Anthrachinon Und Methanol. Das Pap. (Darmstadt) 1986, 40, V1–V8. [Google Scholar]
- Kordsachia, O.; Patt, R.; Wandinger, B. ASAM Pulping and Chlorine Free Bleaching of Eucalyptus; Forest Institute (INFOR): Santiago, Chile, 1993. [Google Scholar]
- Kordsachia, O.; Wandinger, B.; Patt, R. Some Investigations on ASAM Pulping and Chlorine Free Bleaching of Eucalyptus from Spain. Holz Als Roh-Und Werkst. 1992, 50, 85–91. [Google Scholar] [CrossRef]
- Technologies & Solutions. Chempolis. Available online: https://chempolis.com/technologies-solutions/ (accessed on 8 March 2020).
- Sridach, W. The Environmentally Benign Pulping Process of Non-Wood Fibers. Suranaree J. Sci. Technol. 2010, 17, 105–123. [Google Scholar]
- Leponiemi, A. Non-Wood Pulping Possibilities-a Challenge for the Chemical Pulping Industry. Appita Technol. Innov. Manuf. Environ. 2008, 61, 234–243. [Google Scholar]
- Zhao, X.; Dai, L.; Liu, D. Characterization and Comparison of Acetosolv and Milox Lignin Isolated from Crofton Weed Stem. J. Appl. Polym. Sci. 2009, 114, 1295–1302. [Google Scholar] [CrossRef]
- Ligero, P.; Vega, A.; Villaverde, J. Delignification of Miscanthus× Giganteus by the Milox Process. Bioresour. Technol. 2010, 101, 3188–3193. [Google Scholar] [CrossRef] [PubMed]
- Delmas, G.; Benjelloun-Mlayah, B.; Bigot, Y.L.; Delmas, M. Functionality of Wheat Straw Lignin Extracted in Organic Acid Media. J. Appl. Polym. Sci. 2011, 121, 491–501. [Google Scholar] [CrossRef]
- Snelders, J.; Dornez, E.; Benjelloun-Mlayah, B.; Huijgen, W.J.J.; de Wild, P.J.; Gosselink, R.J.A.; Gerritsma, J.; Courtin, C.M. Biorefining of Wheat Straw Using an Acetic and Formic Acid Based Organosolv Fractionation Process. Bioresour. Technol. 2014, 156, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Kangas, H.; Hakala, T.; Tamminen, T.; Määttänen, M.; Rovio, S.; Liitiä, T.; Poppius-Levlin, K. Optimisation of Acetic Acid Lignofibre Organosolv Process. Bioresources 2015, 10, 2699–2718. [Google Scholar] [CrossRef]
- Kangas, H.; Liitiä, T.; Rovio, S.; Ohra-Aho, T.; Heikkinen, H.; Tamminen, T.; Poppius-Levlin, K. Characterization of Dissolved Lignins from Acetic Acid Lignofibre (LGF) Organosolv Pulping and Discussion of Its Delignification Mechanisms. Holzforschung 2015, 69, 247–256. [Google Scholar] [CrossRef]
- Pan, X.; Arato, C.; Gilkes, N.; Gregg, D.; Mabee, W.; Pye, K.; Xiao, Z.; Zhang, X.; Saddler, J. Biorefining of Softwoods Using Ethanol Organosolv Pulping: Preliminary Evaluation of Process Streams for Manufacture of Fuel-Grade Ethanol and Co-Products. Biotechnol. Bioeng. 2005, 90, 473–481. [Google Scholar] [CrossRef]
- Arato, C.; Pye, E.K.; Gjennestad, G. The Lignol Approach to Biorefining of Woody Biomass to Produce Ethanol and Chemicals. Appl. Biochem. Biotechnol. 2005, 123, 871–882. [Google Scholar] [CrossRef]
- Mupondwa, E.; Li, X.; Tabil, L.; Sokhansanj, S.; Adapa, P. Status of Canada’s Lignocellulosic Ethanol: Part I: Pretreatment Technologies. Renew. Sustain. Energy Rev. 2017, 72, 178–190. [Google Scholar] [CrossRef]
- Shuai, L.; Amiri, M.T.; Questell-Santiago, Y.M.; Héroguel, F.; Li, Y.; Kim, H.; Meilan, R.; Chapple, C.; Ralph, J.; Luterbacher, J.S. Formaldehyde Stabilization Facilitates Lignin Monomer Production during Biomass Depolymerization. Science 2016, 354, 329–333. [Google Scholar] [CrossRef]
- Aro, T.; Fatehi, P. Production and Application of Lignosulfonates and Sulfonated Lignin. ChemSusChem 2017, 10, 1861–1877. [Google Scholar] [CrossRef]
- FAN, J.; ZHAN, H. Optimization of Synthesis of Spherical Lignosulphonate Resin and Its Structure Characterization* *Supported by the Ph.D. Programs Foundation of Ministry of Education of China (20020561001). Chin. J. Chem. Eng. 2008, 16, 407–410. [Google Scholar] [CrossRef]
- Calvo-Flores, F.G.; Dobado, J.A.; Isac-García, J.; Martín-Martínez, F.J. Lignin and Lignans as Renewable Raw Materials: Chemistry, Technology and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2015; ISBN 1-118-68351-X. [Google Scholar]
- Doherty, W.O.; Mousavioun, P.; Fellows, C.M. Value-Adding to Cellulosic Ethanol: Lignin Polymers. Ind. Crop. Prod. 2011, 33, 259–276. [Google Scholar] [CrossRef]
- Matsushita, Y. Conversion of Technical Lignins to Functional Materials with Retained Polymeric Properties. J. Wood Sci. 2015, 61, 230–250. [Google Scholar] [CrossRef]
- Areskogh, D.; Li, J.; Gellerstedt, G.; Henriksson, G. Investigation of the Molecular Weight Increase of Commercial Lignosulfonates by Laccase Catalysis. Biomacromolecules 2010, 11, 904–910. [Google Scholar] [CrossRef] [PubMed]
- Howard, G.C. Process of Utilizing Waste Sulphite Liquor. U.S. Patent 1,551,882, 1 September 1925. [Google Scholar]
- Howard, G.C. Utilization of Sulfite, Liquor. Ind. Eng. Chem. 1934, 26, 614–617. [Google Scholar] [CrossRef]
- Sandborn, L.T.; Richter, S.J.; Clemens, H.G. Process of Making Vanillin. U.S. Patent 2,057,117, 13 October 1936. [Google Scholar]
- Li, T.; Takkellapati, S. The Current and Emerging Sources of Technical Lignins and Their Applications. BiofuelsBioprod. Biorefining 2018, 12, 756–787. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, A.; Sánchez, R.; Requejo, A.; Ferrer, A. Feasibility of Rice Straw as a Raw Material for the Production of Soda Cellulose Pulp. J. Clean. Prod. 2010, 18, 1084–1091. [Google Scholar] [CrossRef]
- Heitner, C.; Dimmel, D.; Schmidt, J. Lignin and Lignans: Advances in Chemistry; CRC Press: Boca Raton, FL, USA, 2016; ISBN 1-4200-1580-X. [Google Scholar]
- Pu, Y.; Hu, F.; Huang, F.; Ragauskas, A.J. Lignin Structural Alterations in Thermochemical Pretreatments with Limited Delignification. Bioenergy Res. 2015, 8, 992–1003. [Google Scholar] [CrossRef]
- Sturgeon, M.R.; Kim, S.; Lawrence, K.; Paton, R.S.; Chmely, S.C.; Nimlos, M.; Foust, T.D.; Beckham, G.T. A Mechanistic Investigation of Acid-Catalyzed Cleavage of Aryl-Ether Linkages: Implications for Lignin Depolymerization in Acidic Environments. ACS Sustain. Chem. Eng. 2014, 2, 472–485. [Google Scholar] [CrossRef]
- Van den Bosch, S.; Koelewijn, S.-F.; Renders, T.; Van den Bossche, G.; Vangeel, T.; Schutyser, W.; Sels, B.F. Catalytic Strategies Towards Lignin-Derived Chemicals. Top Curr Chem (Z) 2018, 376, 36. [Google Scholar] [CrossRef]
- Constant, S.; Wienk, J.H.L.; Frissen, E.A.; de Peinder, P.; Boelens, R.; van Es, D.S.; Grisel, H.R.J.; Weckhuysen, M.B.; Huijgen, J.W.J.; Gosselink, A.R.J.; et al. New Insights into the Structure and Composition of Technical Lignins: A Comparative Characterisation Study. Green Chem. 2016, 18, 2651–2665. [Google Scholar] [CrossRef]
- Mishra, P.K.; Wimmer, R. Aerosol Assisted Self-Assembly as a Route to Synthesize Solid and Hollow Spherical Lignin Colloids and Its Utilization in Layer by Layer Deposition. Ultrason Sonochem 2017, 35, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Feng, X.; Yang, D.; Yi, C.; Qiu, X. Pi-Pi stacking of the aromatic groups in lignosulfonates. Bioresources 2012, 7, 1145–1156. [Google Scholar] [CrossRef]
- Yang, M.; Zhao, W.; Singh, S.; Simmons, B.; Cheng, G. On the Solution Structure of Kraft Lignin in Ethylene Glycol and Its Implication for Nanoparticle Preparation. Nanoscale Adv. 2018, 1, 299–304. [Google Scholar] [CrossRef]
- Iravani, S.; Varma, R.S. Greener Synthesis of Lignin Nanoparticles and Their Applications. Green Chemis. 2020, 22, 612–636. [Google Scholar] [CrossRef]
- Wang, B.; Sun, D.; Wang, H.-M.; Yuan, T.-Q.; Sun, R.-C. Green and Facile Preparation of Regular Lignin Nanoparticles with High Yield and Their Natural Broad-Spectrum Sunscreens. ACS Sustain. Chem. Eng. 2019, 7, 2658–2666. [Google Scholar] [CrossRef]
- Yin, H.; Liu, L.; Wang, X.; Wang, T.; Zhou, Y.; Liu, B.; Shan, Y.; Wang, L.; Lü, X. A Novel Flocculant Prepared by Lignin Nanoparticles-Gelatin Complex from Switchgrass for the Capture of Staphylococcus Aureus and Escherichia Coli. Colloids Surf. A Physicochem. Eng. Asp. 2018, 545, 51–59. [Google Scholar] [CrossRef]
- Azimvand, J.; Didehban, K.; Mirshokraie, S. Safranin-O Removal from Aqueous Solutions Using Lignin Nanoparticle-g-Polyacrylic Acid Adsorbent: Synthesis, Properties, and Application. Adsorpt. Sci. Technol. 2018, 36, 1422–1440. [Google Scholar] [CrossRef]
- Dai, L.; Liu, R.; Hu, L.-Q.; Zou, Z.-F.; Si, C.-L. Lignin Nanoparticle as a Novel Green Carrier for the Efficient Delivery of Resveratrol. ACS Sustain. Chem. Eng. 2017, 5, 8241–8249. [Google Scholar] [CrossRef]
- Li, Y.; Qiu, X.; Qian, Y.; Xiong, W.; Yang, D. PH-Responsive Lignin-Based Complex Micelles: Preparation, Characterization and Application in Oral Drug Delivery. Chem. Eng. J. 2017, 327, 1176–1183. [Google Scholar] [CrossRef]
- Sipponen, M.H.; Smyth, M.; Leskinen, T.; Johansson, L.-S.; Österberg, M. All-Lignin Approach to Prepare Cationic Colloidal Lignin Particles: Stabilization of Durable Pickering Emulsions. Green Chem. 2017, 19, 5831–5840. [Google Scholar] [CrossRef]
- Sipponen, M.H.; Farooq, M.; Koivisto, J.; Pellis, A.; Seitsonen, J.; Österberg, M. Spatially Confined Lignin Nanospheres for Biocatalytic Ester Synthesis in Aqueous Media. Nat. Commun. 2018, 9, 2300. [Google Scholar] [CrossRef] [PubMed]
- Mattinen, M.-L.; Valle-Delgado, J.J.; Leskinen, T.; Anttila, T.; Riviere, G.; Sipponen, M.; Paananen, A.; Lintinen, K.; Kostiainen, M.; Österberg, M. Enzymatically and Chemically Oxidized Lignin Nanoparticles for Biomaterial Applications. Enzym. Microb. Technol. 2018, 111, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Mattinen, M.-L.; Riviere, G.; Henn, A.; Nugroho, R.W.N.; Leskinen, T.; Nivala, O.; Valle-Delgado, J.J.; Kostiainen, M.A.; Österberg, M. Colloidal Lignin Particles as Adhesives for Soft Materials. Nanomaterials 2018, 8, 1001. [Google Scholar] [CrossRef]
- Gonzalez, G.; Nelly, M.; Levi, M.; Turri, S.; Griffini, G. Lignin Nanoparticles by Ultrasonication and Their Incorporation in Waterborne Polymer Nanocomposites. J. Appl. Polym. Sci. 2017, 134, 45318. [Google Scholar] [CrossRef]
- Figueiredo, P.; Lintinen, K.; Kiriazis, A.; Hynninen, V.; Liu, Z.; Bauleth-Ramos, T.; Rahikkala, A.; Correia, A.; Kohout, T.; Sarmento, B. In Vitro Evaluation of Biodegradable Lignin-Based Nanoparticles for Drug Delivery and Enhanced Antiproliferation Effect in Cancer Cells. Biomaterials 2017, 121, 97–108. [Google Scholar] [CrossRef]
- Figueiredo, P.; Ferro, C.; Kemell, M.; Liu, Z.; Kiriazis, A.; Lintinen, K.; Florindo, H.F.; Yli-Kauhaluoma, J.; Hirvonen, J.; Kostiainen, M.A. Functionalization of Carboxylated Lignin Nanoparticles for Targeted and PH-Responsive Delivery of Anticancer Drugs. Nanomedicine 2017, 12, 2581–2596. [Google Scholar] [CrossRef]
- Lievonen, M.; Valle-Delgado, J.J.; Mattinen, M.-L.; Hult, E.-L.; Lintinen, K.; Kostiainen, M.A.; Paananen, A.; Szilvay, G.R.; Setälä, H.; Österberg, M. A Simple Process for Lignin Nanoparticle Preparation. Green Chem. 2016, 18, 1416–1422. [Google Scholar] [CrossRef]
- Silmore, K.S.; Gupta, C.; Washburn, N.R. Tunable Pickering Emulsions with Polymer-Grafted Lignin Nanoparticles (PGLNs). J. Colloid Interface Sci. 2016, 466, 91–100. [Google Scholar] [CrossRef]
- Liu, Z.-H.; Hao, N.; Shinde, S.; Pu, Y.; Kang, X.; Ragauskas, J.A.; Yuan, S.J. Defining Lignin Nanoparticle Properties through Tailored Lignin Reactivity by Sequential Organosolv Fragmentation Approach (SOFA). Green Chem. 2019, 21, 245–260. [Google Scholar] [CrossRef]
- Tian, D.; Hu, J.; Bao, J.; Chandra, R.P.; Saddler, J.N.; Lu, C. Lignin Valorization: Lignin Nanoparticles as High-Value Bio-Additive for Multifunctional Nanocomposites. Biotechnol. Biofuels 2017, 10, 192. [Google Scholar] [CrossRef] [PubMed]
- Tian, D.; Hu, J.; Chandra, R.P.; Saddler, J.N.; Lu, C. Valorizing Recalcitrant Cellulolytic Enzyme Lignin via Lignin Nanoparticles Fabrication in an Integrated Biorefinery. ACS Sustain. Chem. Eng. 2017, 5, 2702–2710. [Google Scholar] [CrossRef]
- Gutiérrez-Hernández, J.M.; Escalante, A.; Murillo-Vázquez, R.N.; Delgado, E.; González, F.J.; Toríz, G. Use of Agave Tequilana-Lignin and Zinc Oxide Nanoparticles for Skin Photoprotection. J. Photochem. Photobiol. B Biol. 2016, 163, 156–161. [Google Scholar] [CrossRef] [PubMed]
- Zikeli, F.; Vinciguerra, V.; D’Annibale, A.; Capitani, D.; Romagnoli, M.; Scarascia Mugnozza, G. Preparation of Lignin Nanoparticles from Wood Waste for Wood Surface Treatment. Nanomaterials 2019, 9, 281. [Google Scholar] [CrossRef]
- Gong, W.; Ran, Z.; Ye, F.; Zhao, G. Lignin from Bamboo Shoot Shells as an Activator and Novel Immobilizing Support for α-Amylase. Food Chem. 2017, 228, 455–462. [Google Scholar] [CrossRef]
- Xiong, F.; Han, Y.; Wang, S.; Li, G.; Qin, T.; Chen, Y.; Chu, F. Preparation and Formation Mechanism of Size-Controlled Lignin Nanospheres by Self-Assembly. Ind. Crop. Prod. 2017, 100, 146–152. [Google Scholar] [CrossRef]
- Xing, Q.; Buono, P.; Ruch, D.; Dubois, P.; Wu, L.; Wang, W.-J. Biodegradable UV-Blocking Films through Core–Shell Lignin–Melanin Nanoparticles in Poly(Butylene Adipate-Co-Terephthalate). ACS Sustain. Chem. Eng. 2019, 7, 4147–4157. [Google Scholar] [CrossRef]
- Xiao, D.; Ding, W.; Zhang, J.; Ge, Y.; Wu, Z.; Li, Z. Fabrication of a Versatile Lignin-Based Nano-Trap for Heavy Metal Ion Capture and Bacterial Inhibition. Chem. Eng. J. 2019, 358, 310–320. [Google Scholar] [CrossRef]
- Yang, W.; Fortunati, E.; Bertoglio, F.; Owczarek, J.S.; Bruni, G.; Kozanecki, M.; Kenny, J.M.; Torre, L.; Visai, L.; Puglia, D. Polyvinyl Alcohol/Chitosan Hydrogels with Enhanced Antioxidant and Antibacterial Properties Induced by Lignin Nanoparticles. Carbohydr. Polym. 2018, 181, 275–284. [Google Scholar] [CrossRef]
- Yang, W.; Rallini, M.; Wang, D.-Y.; Gao, D.; Dominici, F.; Torre, L.; Kenny, J.M.; Puglia, D. Role of Lignin Nanoparticles in UV Resistance, Thermal and Mechanical Performance of PMMA Nanocomposites Prepared by a Combined Free-Radical Graft Polymerization/Masterbatch Procedure. Compos. Part A Appl. Sci. Manuf. 2018, 107, 61–69. [Google Scholar] [CrossRef]
- Juikar, S.J.; Vigneshwaran, N. Extraction of Nanolignin from Coconut Fibers by Controlled Microbial Hydrolysis. Ind. Crop. Prod. 2017, 109, 420–425. [Google Scholar] [CrossRef]
- Wurm, F.; Landfester, K.; Yiam-Sawas, D.; Thines, E.; Fischer, J. Lignin Biomaterial as Agricultural Drug Carrier. U.S. Patent Application No. 16/075,503, 7 February 2019. [Google Scholar]
- Falsini, S.; Clemente, I.; Papini, A.; Tani, C.; Schiff, S.; Salvatici, M.C.; Petruccelli, R.; Benelli, C.; Giordano, C.; Gonnelli, C. When Sustainable Nanochemistry Meets Agriculture: Lignin Nanocapsules for Bioactive Compound Delivery to Plantlets. ACS Sustain. Chem. Eng. 2019, 7, 19935–19942. [Google Scholar] [CrossRef]
- Datta, R.; Kelkar, A.; Baraniya, D.; Molaei, A.; Moulick, A.; Meena, R.S.; Formanek, P. Enzymatic Degradation of Lignin in Soil: A Review. Sustainability 2017, 9, 1163. [Google Scholar] [CrossRef]
- Pang, Y.; Wang, S.; Qiu, X.; Luo, Y.; Lou, H.; Huang, J. Preparation of Lignin/SDS Composite Nanoparticles and Its Application in Pickering Emulsion Template Based Microencapsulation. J. Agric. Food Chem. 2017, 65, 11011–11019. [Google Scholar] [CrossRef] [PubMed]
- Borregaard. Available online: https://www.lignotech.com (accessed on 18 December 2020).
- Tenhaeff, W.E.; Rios, O.; More, K.; McGuire, M.A. Highly Robust Lithium Ion Battery Anodes from Lignin: An Abundant, Renewable, and Low-Cost Material. Adv. Funct. Mater. 2014, 24, 86–94. [Google Scholar] [CrossRef]
- Qin, Y.; Yang, D.; Qiu, X. Hydroxypropyl Sulfonated Lignin as Dye Dispersant: Effect of Average Molecular Weight. ACS Sustain. Chem. Eng. 2015, 3, 3239–3244. [Google Scholar] [CrossRef]
- Snowdon, M.R.; Mohanty, A.K.; Misra, M. A Study of Carbonized Lignin as an Alternative to Carbon Black. ACS Sustain. Chem. Eng. 2014, 2, 1257–1263. [Google Scholar] [CrossRef]
- Cerrutti, B.; De Souza, C.; Castellan, A.; Ruggiero, R.; Frollini, E. Carboxymethyl Lignin as Stabilizing Agent in Aqueous Ceramic Suspensions. Ind. Crop. Prod. 2012, 36, 108–115. [Google Scholar] [CrossRef]
- Greil, P. Biomorphous Ceramics from Lignocellulosics. J. Eur. Ceram. Soc. 2001, 21, 105–118. [Google Scholar] [CrossRef]
- Kalliola, A.; Vehmas, T.; Liitiä, T.; Tamminen, T. Alkali-O2 Oxidized Lignin–A Bio-Based Concrete Plasticizer. Ind. Crop. Prod. 2015, 74, 150–157. [Google Scholar] [CrossRef]
- Kamoun, A.; Jelidi, A.; Chaabouni, M. Evaluation of the Performance of Sulfonated Esparto Grass Lignin as a Plasticizer–Water Reducer for Cement. Cem. Concr. Res. 2003, 33, 995–1003. [Google Scholar] [CrossRef]
- Zhang, T.; Cai, G.; Liu, S. Application of Lignin-Based by-Product Stabilized Silty Soil in Highway Subgrade: A Field Investigation. J. Clean. Prod. 2017, 142, 4243–4257. [Google Scholar] [CrossRef]
- Grossman, A.; Vermerris, W. Lignin-Based Polymers and Nanomaterials. Curr. Opin. Biotechnol. 2019, 56, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Chang, X.; Sun, J.; Xu, Z.; Zhang, F.; Wang, J.; Lv, K.; Dai, Z. A Novel Nano-Lignin-Based Amphoteric Copolymer as Fluid-Loss Reducer in Water-Based Drilling Fluids. Colloids Surf. A Physicochem. Eng. Asp. 2019, 583, 123979. [Google Scholar] [CrossRef]
- Pishnamazi, M.; Casilagan, S.; Clancy, C.; Shirazian, S.; Iqbal, J.; Egan, D.; Edlin, C.; Croker, D.M.; Walker, G.M.; Collins, M.N. Microcrystalline Cellulose, Lactose and Lignin Blends: Process Mapping of Dry Granulation via Roll Compaction. Powder Technol. 2019, 341, 38–50. [Google Scholar] [CrossRef]
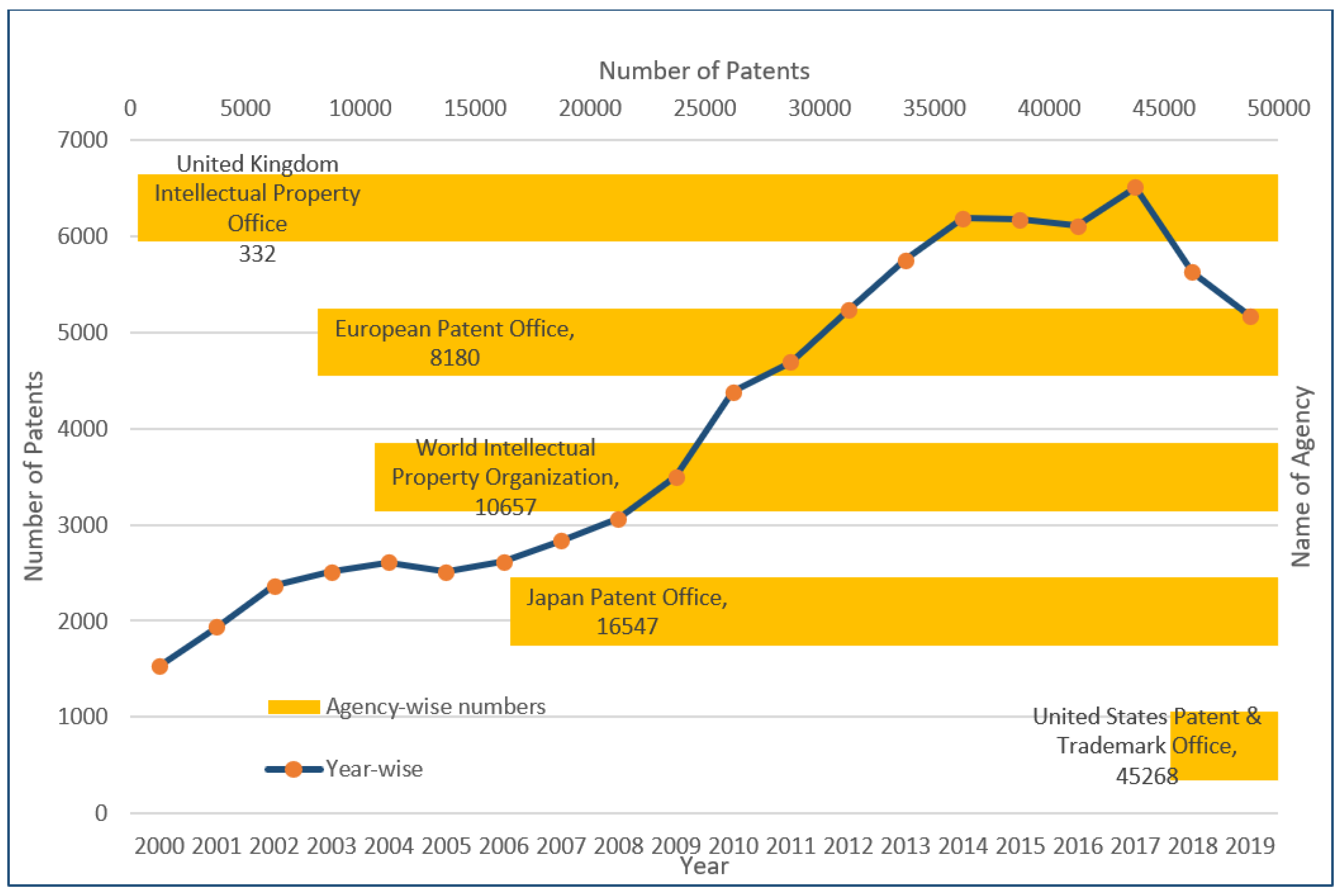
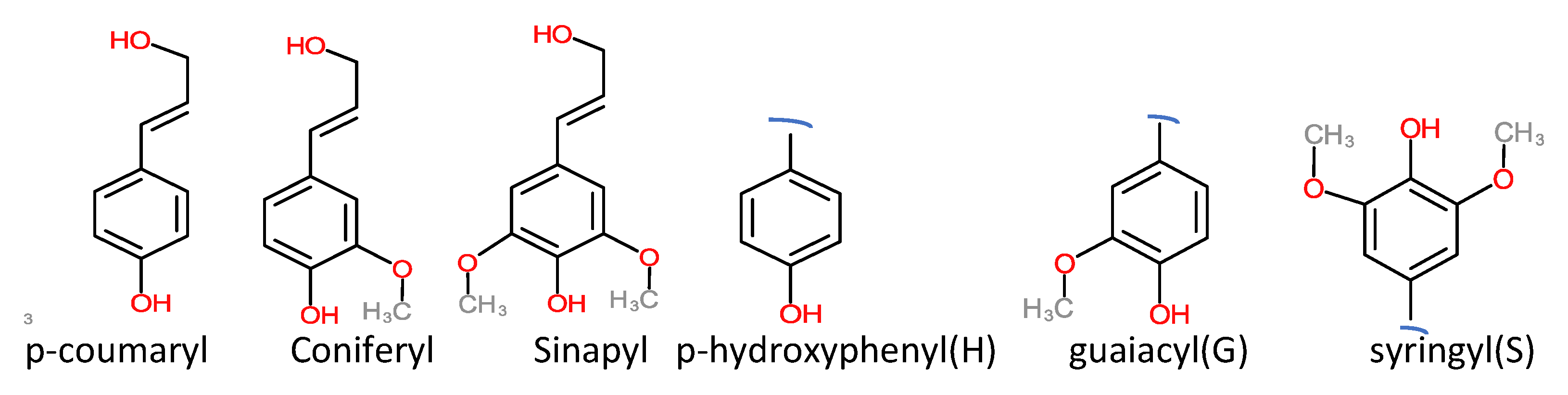
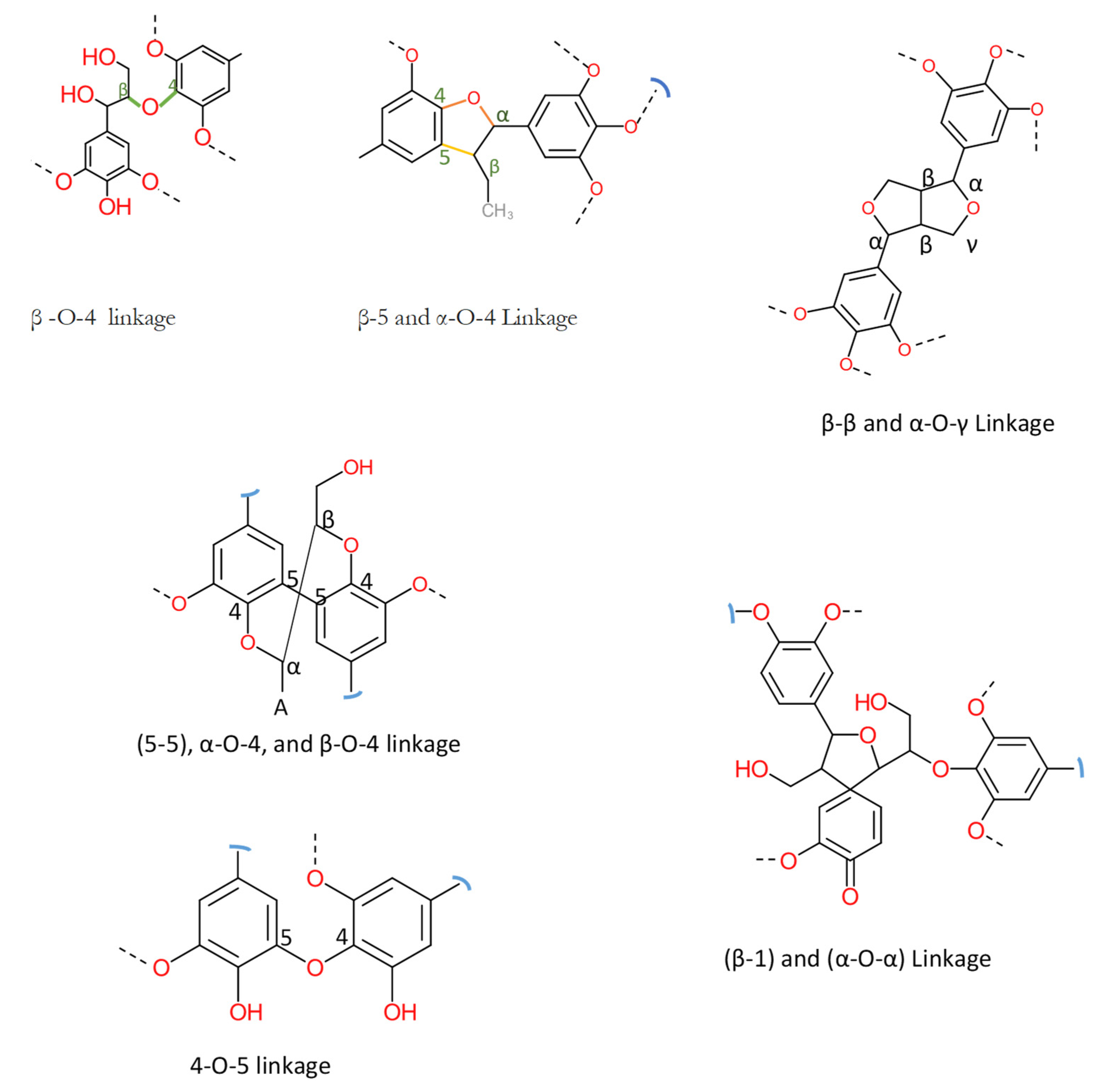
| Bonding | Softwood | Hardwood | Grassess |
|---|---|---|---|
| β-O-4 linkage | 45–60 | 60–62 | 74–84 |
| β-5 and α-O-4 Linkage | 9–12 | 3–11 | 5–11 |
| β-β and α-O-γ Linkage | 2–6 | 3–12 | 1–7 |
| (5-5), α-O-4, and β-O-5 linkage | 5–7 | <1 | -- |
| β-1) and (α-O-α) Linkage | 2 | 2 | -- |
| 4-O-5 linkage (E) | 1–9 | 1–7 | -- |
| Technical Lignin | Remarks | Remarks and Applications |
|---|---|---|
| Kraft | Discovered by Carl F, Dahl in 1879 [26], sulphur content- 1–3% | Fertilizers and pesticides [27,28], Carbon fibers [29], Blend with thermoplastics [30], Resins [31], Ion-exchange resins [32], Activated carbons [33], Preparation of low molecular weight compounds [34] |
| Indulin | Based on acid precipitation. Marketed since the 1950s by Ingenivity, Virginia, USA. Classical technical Kraft-lignin in the market [35]. | |
| Lignoboost | Developed by Inventia and Chalmers Technical University in 2002 [36], Nordic Paper Backhammer (8000 tons per annum) in 2015 [37] | |
| Bio-choice lignin | In 2013, Lignoboost by Domtar Plymouth Mill, North Carolina, USA, Marketed as Bio-choice lignin. | |
| Lignoforce | Developed by FPinnovations group and NORAM, Hindon pulp mill Alberta, Canada (30 tons per day) [38]. | |
| SLRP | Sequential liquid-lignin recovery and purification [39]. | |
| Organosolv | Discovered in 1968 by Kleinert [40], sulphur-free. | High number of reactive sites (supports further modification), low mol weight (not material of choice for binder and adhesives) and high purity. Additive to inks, coatings and paints [41] |
| Formico process | Formic acid and/or acetic acid based method [42]. | |
| Alcell process | Alcohol based pulping and recovery (APR) process before 1987, later named as alcell process [43]. | |
| Acetosolv and Acetocell process | Acetic acid based pulping without (Acetocell) or with catalyst (Acetosolv) process [44]. | |
| Formacell process | Based on Acetosolv (mixture of formic and acetic acid) [45]. | |
| Organocell process | Sodium hydroxide, methanol and catalytic amount of anthraquinone as cooking medium [46]. | |
| ASAM process | Alkaline Sulfite Anthraquinone and Methanol based cooking medium [47]. | |
| CIMV process | Mixture of acetic acid, formic acid and water as cooking medium [48,49], trademark-Biolignin. | |
| Lignofibre process | Organic solvent (Ethanol or acetic acid) with phosphinic acid [50]. | |
| Milox Process | Peroxyformic and peroxyacetic acid based process [51,52,53]. | |
| Lignol technology | Derived from Alcell process, Ethanol-based process [54,55]. | |
| Bloom Process | Formaldehyde-based protection chemistry [56]. | |
| AST process | Acid, lignin dissolving solvent, water with or without oxidant [57,58] | |
| Soda lignin | Sulphur-free, [59]. | Reduced toxicity and increased biocompatibility Phenolic resins [60], animal feed [61], dispersants [62] |
| NovaFiber Process | Soda-AQ precooking followed by carbonate buffered oxygen delignification [63]. | |
| Protobind products | Aq. NaOH based method, mainly non-wood/grass based, Tradename for the family of lignin products by Greenvalue [64]. | |
| Northway lignin chemicals | aq. sodium carbonate treatment of woody biomass under pressure [65,66]. | |
| Acid Hydrolytic Lignin | Developed as pretreatment method, sulphur may be present or absent. | Good sorption properties, used as sorbants [67] |
| Bergius-Rheinau Process | Concentrated hydrochloric acid based method, used by HCl cleantech (later Virdia Inc and now Stora Enso) [68]. | |
| DAWN technology by Avantium | Bergius-Rheinau Process based method developed by Avantium [69]. | |
| Lignosulfonate | Sulphite process, sulphur- 3.5–8.0%. | Unique colloidal properties due to variety (hydroxyl, carboxylic and sulphur containing) of functional groups. Binder and drilling agent [70], animal feed [71], glue and particles boards [72] |
| Domsjo Lignin. Now Aditya Birla group | Sodium lignosulfonate [73]. | |
| Borregaard Lignotech | Calcium lignosulfonate [74,75,76]. | |
| La Rochette venizel, now Saica | Ammonium lignosulfonate [77,78]. | |
| Nippon paper chemical | San XTM, VanillexTM, and PearllexTM (Ca, Na, Mg salts) [79]. | |
| Cartiere Burgo | Lignin solubilized as calcium salt of sulphonic acid from Norway spruce, commercial names-Bretax and Sartex [80]. | |
| TEMBEC, now Rayonier Advanced Materials | Ammonium and sodium lignosulfonates, commercially available as ARBO- range of products. | |
| Others | Green method | |
| Ionic liquids, Molten salt hydrates, | Several patents exist [81,82], Industrially produced material absent |
| Technical Lignin Type | Kraft (Indulin) | Soda (P1000) | Organosolv (Alcell) | Organosolv (Wheat Straw) | Organosolv (Poplar) | Organosolv (Spruce) |
|---|---|---|---|---|---|---|
| Chemical composition: weight percent per unit dry weight | ||||||
| Arabinan | 0.1 | 0.2 | <0.1 | 0.1 | <0.1 | <0.1 |
| Xylan | 0.6 | 1.5 | 0.1 | 0.2 | 0.2 | 0.2 |
| Galactan | 0.6 | 0.2 | <0.1 | <0.1 | <0.1 | <0.1 |
| Glucan | 0.1 | 0.5 | 0.1 | 0.2 | 0.1 | 0.3 |
| Mannan | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 | 0.6 |
| Sum | 1.4 | 2.4 | 0.2 | 0.5 | 0.3 | 1.1 |
| Ash | 2.6 | 2.5 | <0.1 | <0.1 | <0.1 | <0.1 |
| Sulphur | 1.7 | 1.1 | 0.0 | 0.1 | 0.0 | 0.0 |
| AIL | 90.3 | 85.1 | 94.3 | 94.1 | 94.3 | 95.5 |
| ASL | 1.9 | 5.4 | 1.9 | 0.9 | 1.6 | 1.8 |
| Hydroxyl group content: | ||||||
| Aliphatic-OH | 1.79 | 1.26 | 1.04 | 1.27 | 0.80 | 1.43 |
| 5-OH | 1.31 | 1.73 | 1.68 | 1.24 | 1.89 | 1.21 |
| G-OH | 1.30 | 0.73 | 0.58 | 0.92 | 0.58 | 1.44 |
| p-hp-OH | 0.16 | 0.40 | 0.11 | 0.38 | 0.18 | 0.08 |
| Total Ar-OH | 2.77 | 2.86 | 3.30 | 2.54 | 2.59 | 2.73 |
| Molecular weight: | ||||||
| Mw (g mol−1) | 4290 | 3270 | 2580 | 1960 | 2180 | 2030 |
| MN (g mol−1) | 530 | 620 | 600 | 450 | 570 | 420 |
| PD | 8.1 | 5.2 | 4.3 | 4.4 | 3.8 | 4.9 |
| Technical Lignin | Reported by | Application |
|---|---|---|
| Alkali Lignin | Wang et al. 2019 [140] | Cosmetics |
| Yin et al. 2018 [141] | Wastewater treatment | |
| Azimwand et al. 2018 [142] | Wastewater treatment | |
| Dai et al. 2017 [143] | Biomedicine | |
| Li et al. 2017 [144] | Biomedicine | |
| Siddiqui et al.2017 & 2020 [9,10] | Biomedicine | |
| Mishra and Wimmer, 2017 [136] | Coatings | |
| Kraft Lignin | Sipponen et al. 2017 [145] | Emulsion stabilization |
| Sipponen et al. 2018 [146] | Biocatalyst | |
| Mattinen et al. 2018 [147] | Biomedicine | |
| Mattinen et al. 2018 [148] | Biomedicine | |
| Gonzalez et al. 2017 [149] | Wastewater treatment | |
| Figueiredo et al. 2017a,b [150,151] | Biomedicine | |
| Lievonen et al. 2016 [152] | Novel Method | |
| Silmore et al. 2016 [153] | Dispersants | |
| Organosolv | Liu. et al. 2019 [154] | Biorefinery |
| Tian et al. 2017 [155] | Nanocomposites | |
| Tian et al. 2017 [156] | Nanocomposites | |
| Gutiérrez-Hernández et al. 2016 [157] | Cosmetics | |
| Hydrolytic lignin | Zikeli et al. 2019 [158] (Acid) | Paint and coating |
| Gong et al. 2017 [159] (Acid) | Enzyme immobilization | |
| Yu et al. 2018 [160] (Enzymatic) | Activated carbon | |
| Soda lignin | Xing at al. 2019 [161] | Packaging, Agriculture |
| Xiao et al. 2019 [162] | Wastewater treatment | |
| Chen et al. 2018 | Biomedicine | |
| Yang et al. 2018 [163] | Biomedicine | |
| Yang et al. 2018 [164] | Coatings | |
| Juikar and Vigneshwaran, 2017 [165] | Biomedicine | |
| Gutiérrez-Hernández et al. 2016 [157] | Cosmetics |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ekielski, A.; Mishra, P.K. Lignin for Bioeconomy: The Present and Future Role of Technical Lignin. Int. J. Mol. Sci. 2021, 22, 63. https://doi.org/10.3390/ijms22010063
Ekielski A, Mishra PK. Lignin for Bioeconomy: The Present and Future Role of Technical Lignin. International Journal of Molecular Sciences. 2021; 22(1):63. https://doi.org/10.3390/ijms22010063
Chicago/Turabian StyleEkielski, Adam, and Pawan Kumar Mishra. 2021. "Lignin for Bioeconomy: The Present and Future Role of Technical Lignin" International Journal of Molecular Sciences 22, no. 1: 63. https://doi.org/10.3390/ijms22010063
APA StyleEkielski, A., & Mishra, P. K. (2021). Lignin for Bioeconomy: The Present and Future Role of Technical Lignin. International Journal of Molecular Sciences, 22(1), 63. https://doi.org/10.3390/ijms22010063






