Insight into the bZIP Gene Family in Solanum tuberosum: Genome and Transcriptome Analysis to Understand the Roles of Gene Diversification in Spatiotemporal Gene Expression and Function
Abstract
1. Introduction
2. Results and Discussion
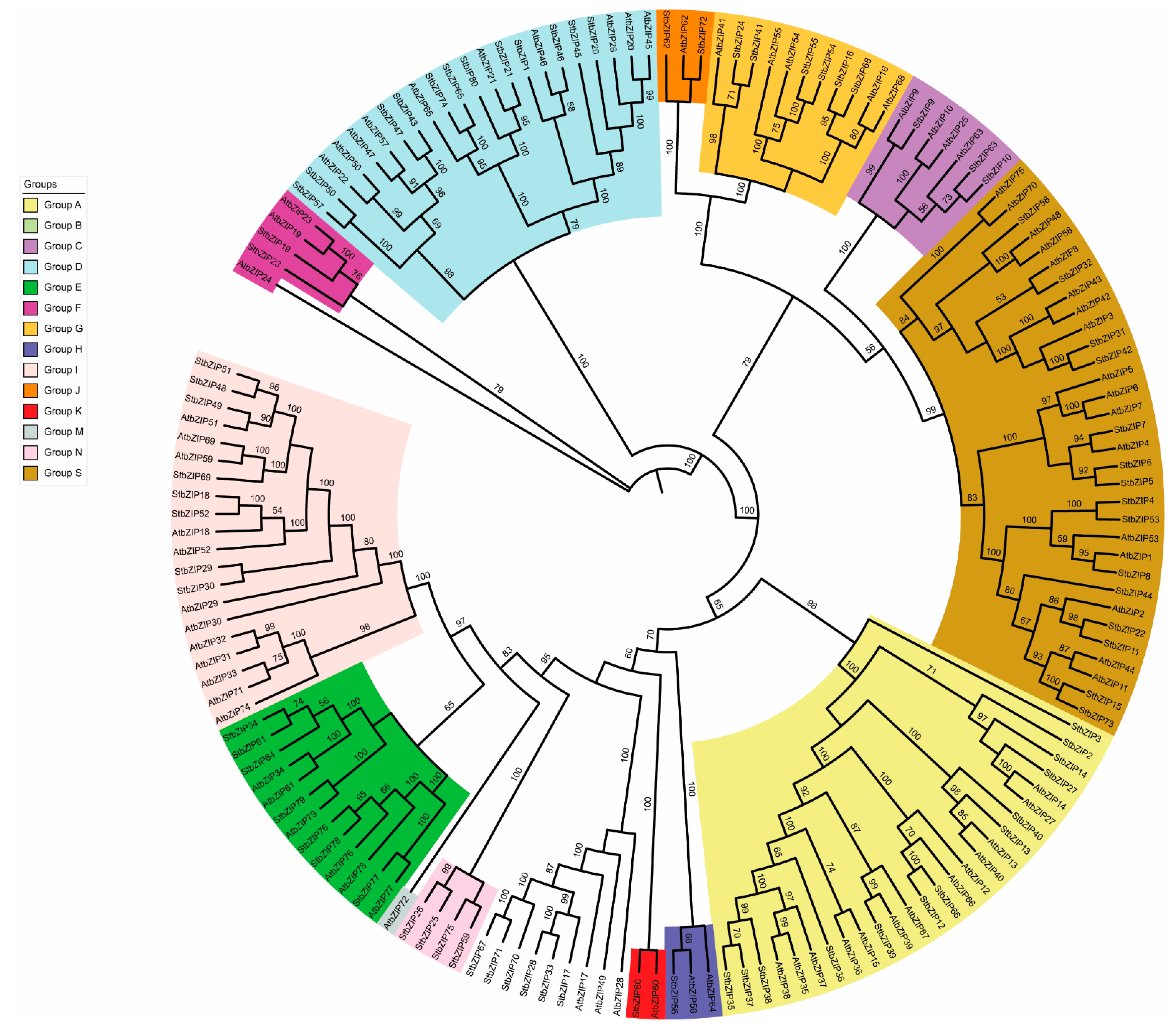
2.1. Identification and Phylogenetic Analysis of bZIP Family Members in S. tuberosum
2.2. Chromosomal Distribution of StbZIPs and Analysis of Gene Duplication Events
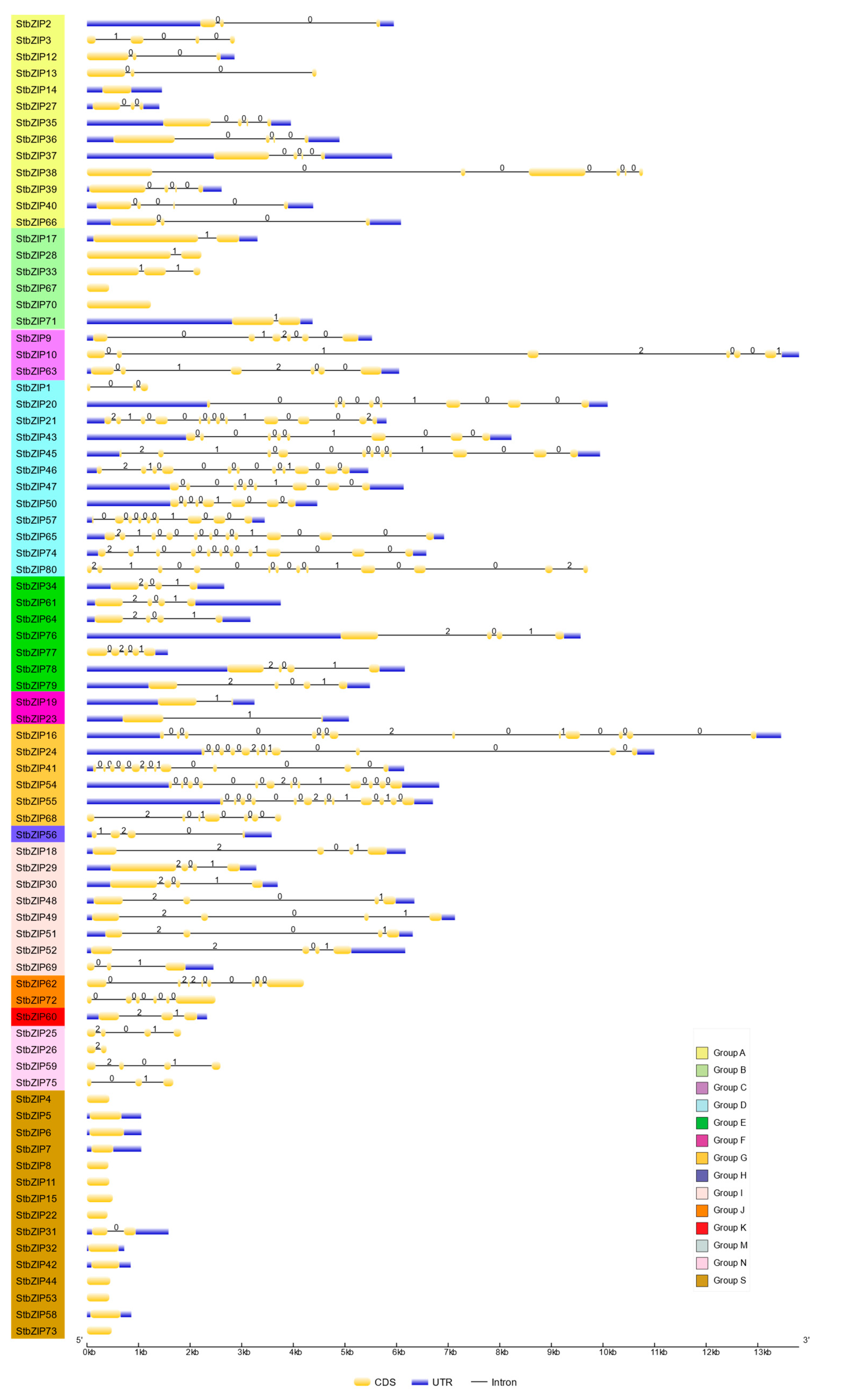
2.3. Gene Structure Analysis of StbZIP Genes
2.4. Analysis of the Amino Acid Composition Among Leucine-Rich Repeats belonging to S. tuberosum bZIP TFs
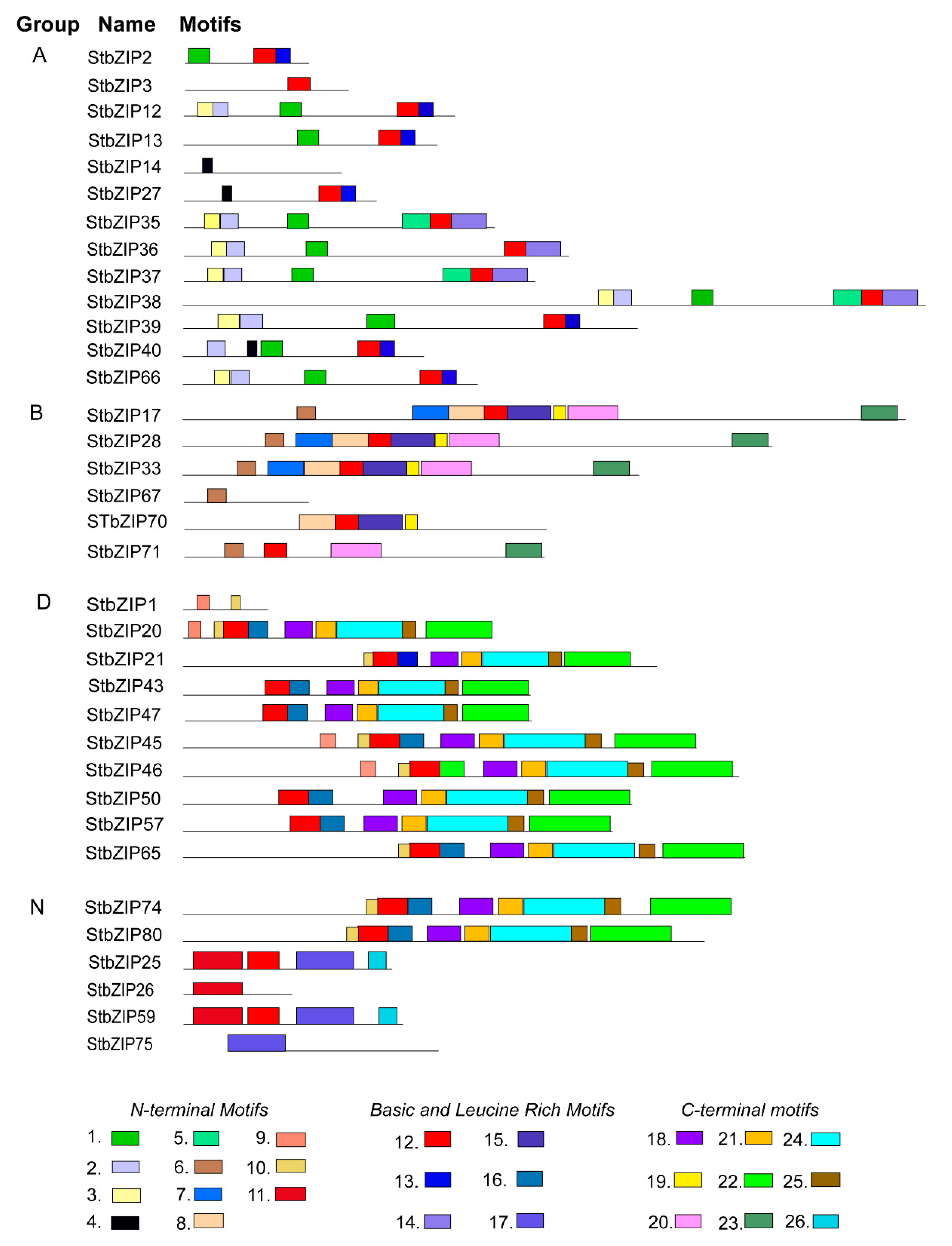
2.5. Analysis of Conserved Protein Motifs
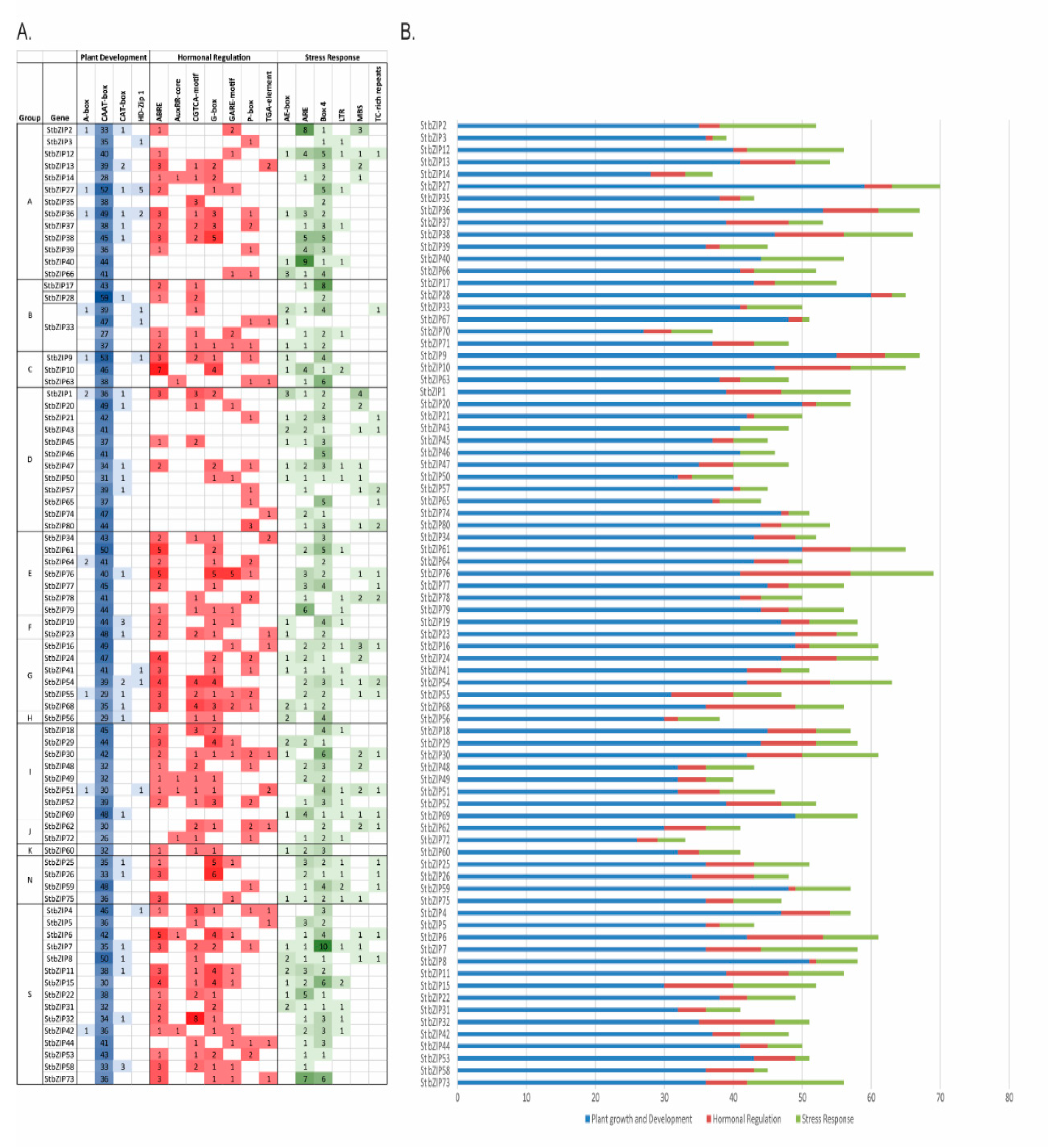
2.6. StbZIP Promoters Enriched with Developmental, Hormone-Response, and Stress-Related TF Binding Sites
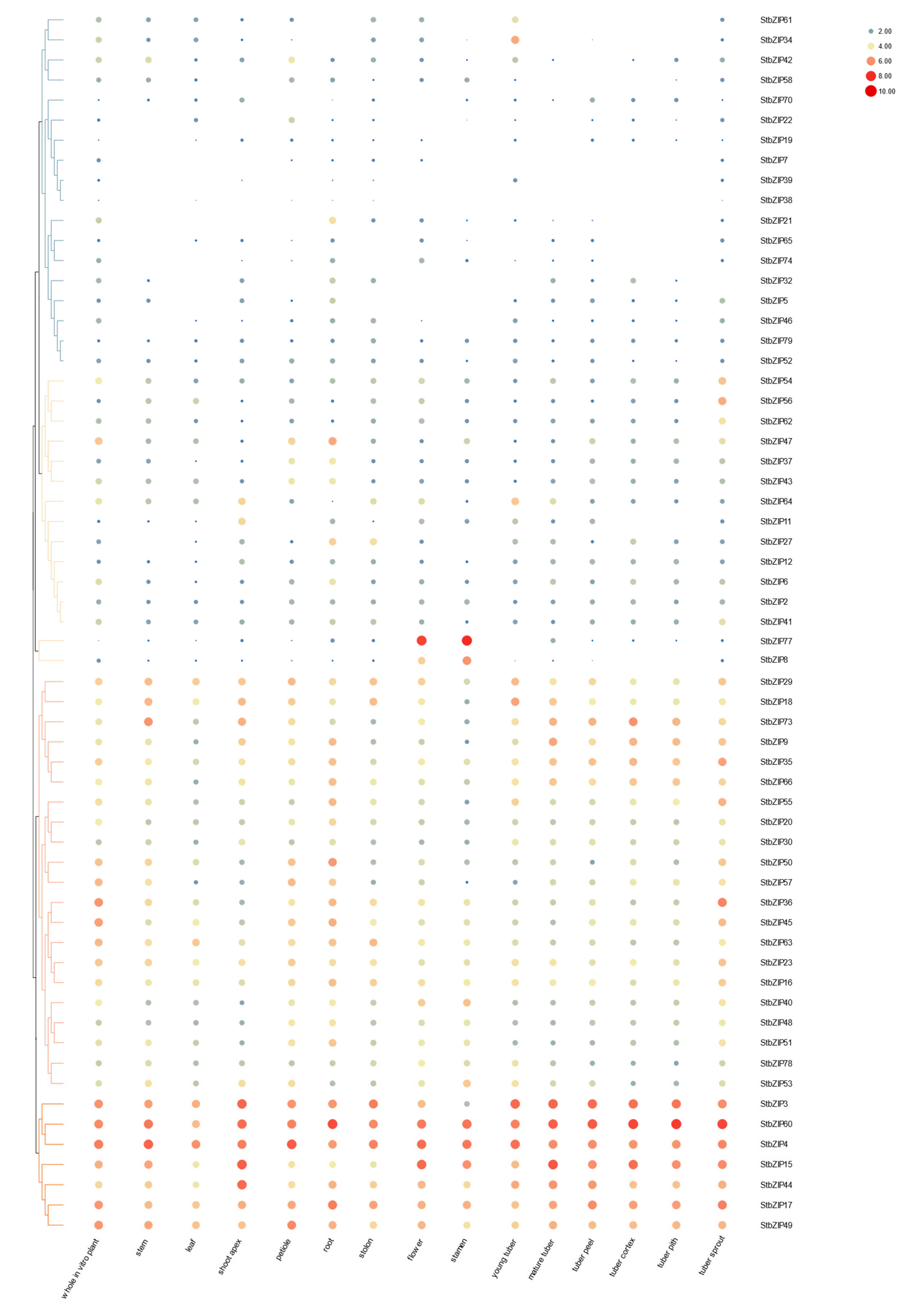
2.7. Differential Gene Expression in Plant Tissues
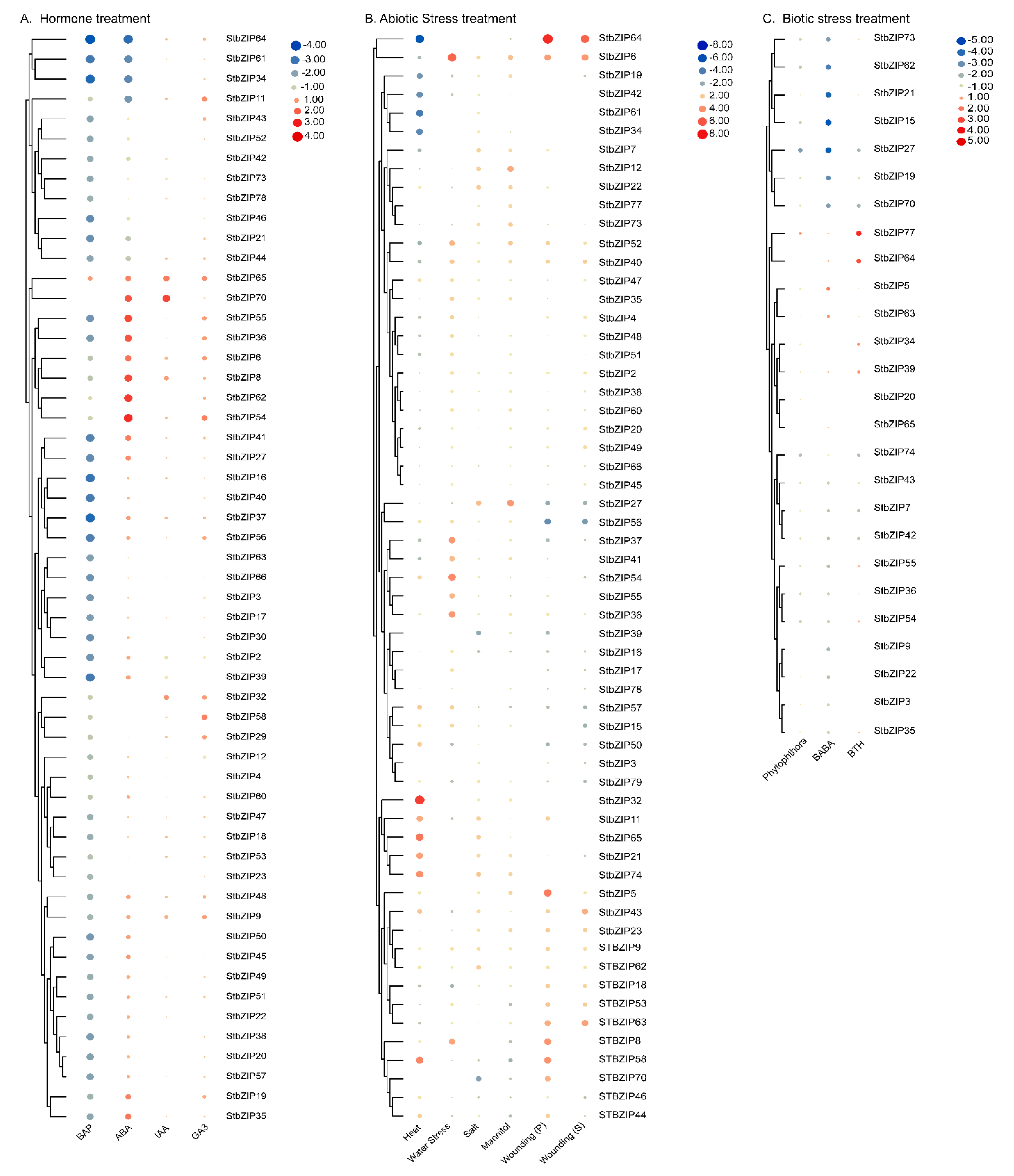
2.8. Expression of StbZIP Family Members Varies from Repression to Activation in Response to Five Hormones
2.9. Gene Expression Profiles Show Differential Responses to Various Abiotic and Biotic Stresses
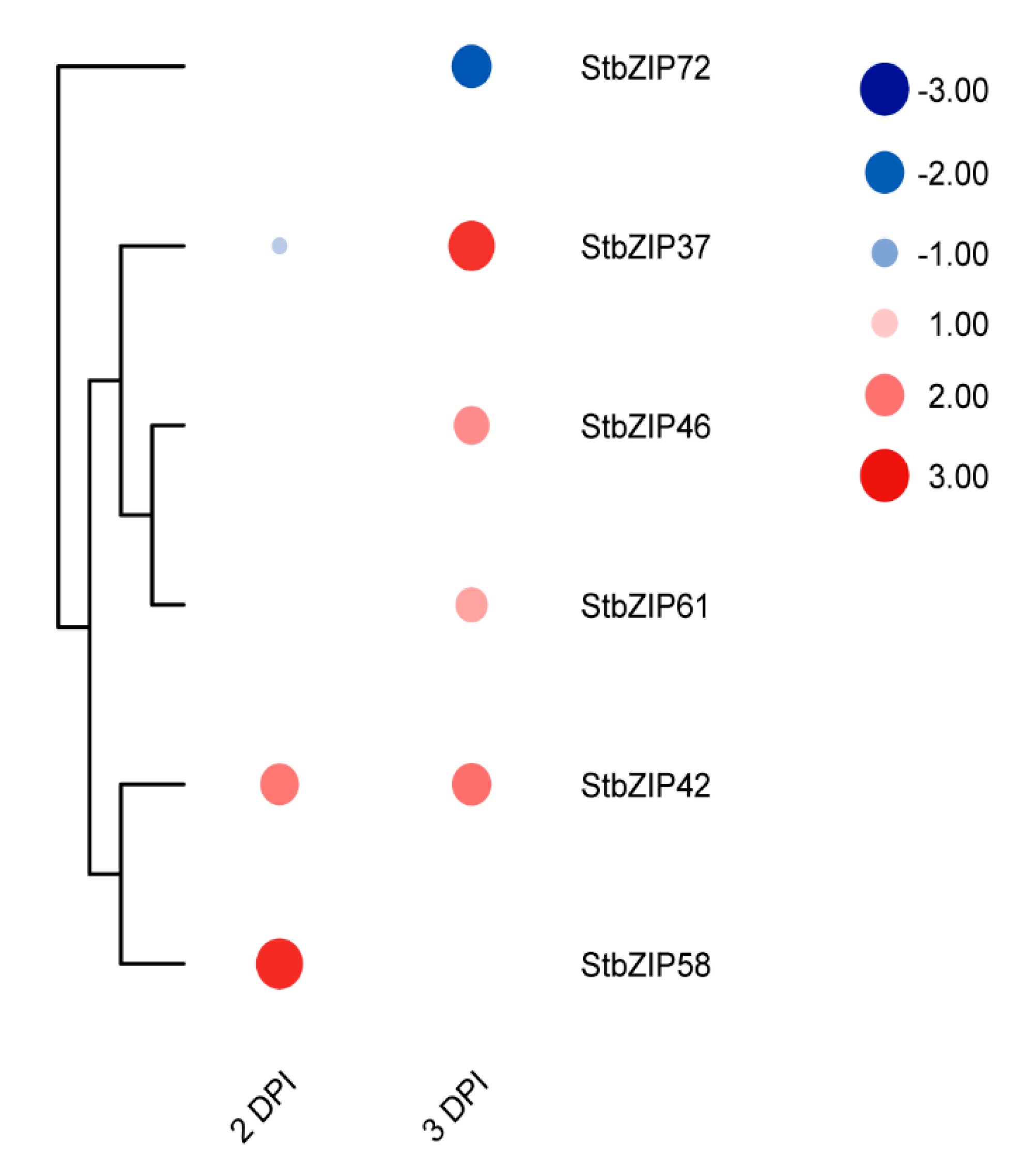
2.10. Gene Expression Profiles Show Differential Responses to Potato Virus X (PVX) Infection
3. Materials and Methods
3.1. Sequence Retrieval and Domain Characterization
3.2. Phylogeny, Chromosomal Locations, Gene Duplications, and Intron/Exon Gene Structure Analysis
3.3. Amino acid Sequence Alignments and Analysis of the Conserved basic and Leucine-rich Domain
3.4. Ab Initio Promoter Analysis
3.5. In Silico bZIP Gene Expression Analysis
3.6. PVX Inoculation of Potato Leaves for Transcriptomic Analysis
3.7. Transcriptomic Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rushton, P.J.; Bokowiec, M.T.; Han, S.; Zhang, H.; Brannock, J.F.; Chen, X.; Laudeman, T.W.; Timko, M.P. Tobacco Transcription Factors: Novel insights into transcriptional regulation in the Solanaceae. Plant Physiol. 2008, 147, 280–295. [Google Scholar] [CrossRef] [PubMed]
- Shiu, S.-H.; Shih, M.-C.; Li, W.-H. Transcription factor families have much higher expansion rates in plants than in animals. Plant Physiol. 2005, 139, 18–26. [Google Scholar] [CrossRef]
- Jindrich, K.; Degnan, B.M. The diversification of the basic leucine zipper family in eukaryotes correlates with the evolution of multicellularity. BMC Evol. Biol. 2016, 16, 28. [Google Scholar] [CrossRef]
- Dröge-Laser, W.; Snoek, B.L.; Snel, B.; Weiste, C. The Arabidopsis bZIP transcription factor family—An update. Curr. Opin. Plant Biol. 2018, 45, 36–49. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Xiong, L.; Gao, J.; Zhang, H.-Y. Molecular mechanisms of the protein-protein interaction–regulated binding specificity of basic-region leucine zipper transcription factors. J. Mol. Model. 2019, 25, 246. [Google Scholar] [CrossRef] [PubMed]
- Vinson, C.; Acharya, A.; Taparowsky, E.J. Deciphering B-ZIP transcription factor interactions in vitro and in vivo. Biochim. Biophys. Acta Gene Struct. Expr. 2006, 1759, 4–12. [Google Scholar] [CrossRef] [PubMed]
- Deppmann, C.D. Dimerization specificity of all 67 B-ZIP motifs in Arabidopsis thaliana: A comparison to Homo sapiens B-ZIP motifs. Nucleic Acids Res. 2004, 32, 3435–3445. [Google Scholar] [CrossRef]
- Jakoby, M.; Weisshaar, B.; Dröge-Laser, W.; Vicente-Carbajosa, J.; Tiedemann, J.; Kroj, T.; Parcy, F. bZIP transcription factors in Arabidopsis. Trends Plant Sci. 2002, 7, 106–111. [Google Scholar] [CrossRef]
- Foster, R.; Izawa, T.; Chua, N. Plant bZIP proteins gather at ACGT elements. FASEB J. 1994, 8, 192–200. [Google Scholar] [CrossRef]
- Corrêa, L.G.G.; Riaño-Pachón, D.M.; Schrago, C.G.; Vicentini dos Santos, R.; Mueller-Roeber, B.; Vincentz, M. The Role of bZIP Transcription factors in green plant evolution: Adaptive features emerging from four founder genes. PLoS ONE 2008, 3, e2944. [Google Scholar] [CrossRef]
- Panchy, N.; Lehti-Shiu, M.; Shiu, S.-H. Evolution of gene duplication in plants. Plant Physiol. 2016, 171, 2294–2316. [Google Scholar] [CrossRef] [PubMed]
- Qiao, X.; Li, Q.; Yin, H.; Qi, K.; Li, L.; Wang, R.; Zhang, S.; Paterson, A.H. Gene duplication and evolution in recurring polyploidization–diploidization cycles in plants. Genome Biol. 2019, 20, 38. [Google Scholar] [CrossRef] [PubMed]
- Nijhawan, A.; Jain, M.; Tyagi, A.K.; Khurana, J.P. Genomic survey and gene expression analysis of the basic leucine zipper transcription factor family in rice. Plant Physiol. 2008, 146, 333–350. [Google Scholar] [CrossRef] [PubMed]
- Wei, K.; Chen, J.; Wang, Y.; Chen, Y.; Chen, S.; Lin, Y.; Pan, S.; Zhong, X.; Xie, D. Genome-wide analysis of bZIP-encoding genes in maize. DNA Res. 2012, 19, 463–476. [Google Scholar] [CrossRef] [PubMed]
- Pourabed, E.; Ghane Golmohamadi, F.; Soleymani Monfared, P.; Razavi, S.M.; Shobbar, Z.-S. Basic leucine zipper family in barley: Genome-wide characterization of members and expression analysis. Mol. Biotechnol. 2015, 57, 12–26. [Google Scholar] [CrossRef] [PubMed]
- Ji, Q.; Zhang, L.; Wang, Y.; Wang, J. Genome-wide analysis of basic leucine zipper transcription factor families in Arabidopsis thaliana, Oryza sativa and Populus trichocarpa. J. Shanghai Univ. 2009, 13, 174–182. [Google Scholar] [CrossRef]
- Zhou, Y.; Xu, D.; Jia, L.; Huang, X.; Ma, G.; Wang, S.; Zhu, M.; Zhang, A.; Guan, M.; Lu, K.; et al. Genome-wide identification and structural analysis of bZIP transcription factor genes in Brassica napus. Genes 2017, 8, 288. [Google Scholar] [CrossRef]
- Wang, J.; Zhou, J.; Zhang, B.; Vanitha, J.; Ramachandran, S.; Jiang, S.-Y. Genome-wide expansion and expression divergence of the basic leucine zipper transcription factors in higher plants with an emphasis on Sorghum f. J. Integr. Plant Biol. 2011, 53, 212–231. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, Y.; Shi, H.; Guo, M.; Chai, M.; He, Q.; Yan, M.; Cao, D.; Zhao, L.; Cai, H.; et al. Evolutionary and expression analyses of soybean basic Leucine zipper transcription factor family. BMC Genom. 2018, 19, 159. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Pan, S.; Cheng, S.; Zhang, B.; Mu, D.; Ni, P.; Zhang, G.; Yang, S.; Li, R.; Wang, J.; et al. Genome sequence and analysis of the tuber crop potato. Nature 2011, 475, 189–195. [Google Scholar] [CrossRef]
- Pham, G.M.; Hamilton, J.P.; Wood, J.C.; Burke, J.T.; Zhao, H.; Vaillancourt, B.; Ou, S.; Jiang, J.; Robin Buell, C. Construction of a chromosome-scale long-read reference genome assembly for potato. Gigascience 2020, 9, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Zhou, T.; Lian, M.; Liu, T.; Hou, J.; Ijaz, R.; Song, B. Genome-wide identification and characterization of the AREB/ABF/ABI5 subfamily members from Solanum tuberosum. Int. J. Mol. Sci. 2019, 20, 311. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, T.; Fujita, Y.; Maruyama, K.; Mogami, J.; Todaka, D.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Four Arabidopsis AREB/ABF transcription factors function predominantly in gene expression downstream of SnRK2 kinases in abscisic acid signalling in response to osmotic stress. Plant Cell Environ. 2015, 38, 35–49. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, A.; Roychoudhury, A. Abscisic-acid-dependent basic leucine zipper (bZIP) transcription factors in plant abiotic stress. Protoplasma 2017, 254, 3–16. [Google Scholar] [CrossRef]
- Srivastava, R.; Li, Z.; Russo, G.; Tang, J.; Bi, R.; Muppirala, U.; Chudalayandi, S.; Severin, A.; He, M.; Vaitkevicius, S.I.; et al. Response to persistent er stress in plants: A multiphasic process that transitions cells from prosurvival activities to cell death. Plant Cell 2018, 30, 1220–1242. [Google Scholar] [CrossRef]
- Afrin, T.; Diwan, D.; Sahawneh, K.; Pajerowska-Mukhtar, K. Multilevel regulation of endoplasmic reticulum stress responses in plants: Where old roads and new paths meet. J. Exp. Bot. 2020, 71, 1659–1667. [Google Scholar] [CrossRef]
- Gayral, M.; Arias Gaguancela, O.; Vasquez, E.; Herath, V.; Flores, F.J.; Dickman, M.B.; Verchot, J. Multiple ER-to-nucleus stress signaling pathways are activated during Plantago asiatica mosaic virus and Turnip mosaic virus infection in Arabidopsis thaliana. Plant J. 2020, 103, 1233–1245. [Google Scholar] [CrossRef]
- Tajima, H.; Iwata, Y.; Iwano, M.; Takayama, S.; Koizumi, N. Identification of an Arabidopsis transmembrane bZIP transcription factor involved in the endoplasmic reticulum stress response. Biochem. Biophys. Res. Commun. 2008, 374, 242–247. [Google Scholar] [CrossRef]
- Liu, J.X.; Howell, S.H. bZIP28 and NF-Y transcription factors are activated by ER stress and assemble into a transcriptional complex to regulate stress response genes in Arabidopsis. Plant Cell 2010, 22, 782–796. [Google Scholar] [CrossRef]
- Williams, B.; Kabbage, M.; Britt, R.; Dickman, M.B. AtBAG7, an Arabidopsis Bcl-2-associated athanogene, resides in the endoplasmic reticulum and is involved in the unfolded protein response. Proc. Natl. Acad. Sci. USA 2010, 107, 6088–6093. [Google Scholar] [CrossRef]
- Srivastava, R.; Deng, Y.; Shah, S.; Rao, A.G.; Howell, S.H. Binding protein is a master regulator of the endoplasmic reticulum stress sensor/transducer bZIP28 in Arabidopsis. Plant Cell 2013, 25, 1416–1429. [Google Scholar] [CrossRef]
- Alonso, R.; Oñate-Sánehez, L.; Weltmeier, F.; Ehlert, A.; Diaz, I.; Dietrich, K.; Vicente-Carbajosa, J.; Dröge-Laser, W. A Pivotal Role of the basic leucine zipper transcription factor bZIP53 in the regulation of Arabidopsis seed maturation gene expression based on heterodimerization and protein complex formation. Plant Cell 2009, 21, 1747–1761. [Google Scholar] [CrossRef] [PubMed]
- Ehlert, A.; Weltmeier, F.; Wang, X.; Mayer, C.S.; Smeekens, S.; Vicente-Carbajosa, J.; Dröge-Laser, W. Two-hybrid protein-protein interaction analysis in Arabidopsis protoplasts: Establishment of a heterodimerization map of group C and group S bZIP transcription factors. Plant J. 2006, 46, 890–900. [Google Scholar] [CrossRef] [PubMed]
- Weltmeier, F.; Rahmani, F.; Ehlert, A.; Dietrich, K.; Schütze, K.; Wang, X.; Chaban, C.; Hanson, J.; Teige, M.; Harter, K.; et al. Expression patterns within the Arabidopsis C/S1 bZIP transcription factor network: Availability of heterodimerization partners controls gene expression during stress response and development. Plant Mol. Biol. 2009, 69, 107–119. [Google Scholar] [CrossRef]
- Peviani, A.; Lastdrager, J.; Hanson, J.; Snel, B. The phylogeny of C/S1 bZIP transcription factors reveals a shared algal ancestry and the pre-angiosperm translational regulation of S1 transcripts. Sci. Rep. 2016, 6, 30444. [Google Scholar] [CrossRef]
- Gatz, C. From pioneers to team players: TGA transcription factors provide a molecular link between different stress pathways. Mol. Plant-Microbe Interact. 2013, 26, 151–159. [Google Scholar] [CrossRef]
- Zhou, X.T.; Jia, L.J.; Wang, H.Y.; Zhao, P.; Wang, W.Y.; Liu, N.; Song, S.W.; Wu, Y.; Su, L.; Zhang, J.; et al. The potato transcription factor StbZIP61 regulates dynamic biosynthesis of salicylic acid in defense against Phytophthora infestans infection. Plant J. 2018, 95, 1055–1068. [Google Scholar] [CrossRef]
- Llorca, C.M.; Berendzen, K.W.; Malik, W.A.; Mahn, S.; Piepho, H.P.; Zentgraf, U. The elucidation of the interactome of 16 arabidopsis bZIP factors reveals three independent functional networks. PLoS ONE 2015, 10, e0139884. [Google Scholar] [CrossRef] [PubMed]
- Long, M.; Rosenberg, C.; Gilbert, W. Intron phase correlations and the evolution of the intron/exon structure of genes. Proc. Natl. Acad. Sci. USA 1995, 92, 12495–12499. [Google Scholar] [CrossRef] [PubMed]
- Dröge-Laser, W.; Weiste, C. The C/S1 bZIP Network: A Regulatory Hub Orchestrating Plant Energy Homeostasis. Trends Plant Sci. 2018, 23, 422–433. [Google Scholar] [CrossRef] [PubMed]
- Newman, J.R.S.; Keating, A.E. Comprehensive identification of human bZIP interactions with coiled-coil arrays. Science 2003, 300, 2097–2101. [Google Scholar] [CrossRef] [PubMed]
- Yun, K.Y.; Park, M.R.; Mohanty, B.; Herath, V.; Xu, F.; Mauleon, R.; Wijaya, E.; Bajic, V.B.; Bruskiewich, R.; de los Reyes, B.G. Transcriptional regulatory network triggered by oxidative signals configures the early response mechanisms of japonica rice to chilling stress. BMC Plant Biol. 2010, 10, 16. [Google Scholar] [CrossRef]
- Ye, C.; Dickman, M.B.; Whitham, S.A.; Payton, M.; Verchot, J. The unfolded protein response is triggered by a plant viral movement protein. Plant Physiol. 2011, 156, 741–755. [Google Scholar] [CrossRef] [PubMed]
- Noshi, M.; Mori, D.; Tanabe, N.; Maruta, T.; Shigeoka, S. Arabidopsis clade IV TGA transcription factors, TGA10 and TGA9, are involved in ROS-mediated responses to bacterial PAMP flg22. Plant Sci. 2016, 252, 12–21. [Google Scholar] [CrossRef]
- Murmu, J.; Bush, M.J.; de Long, C.; Li, S.; Xu, M.; Khan, M.; Malcolmson, C.; Fobert, P.R.; Zachgo, S.; Hepworth, S.R. Arabidopsis basic leucine-zipper transcription factors TGA9 and TGA10 interact with floral glutaredoxins ROXY1 and ROXY2 and are redundantly required for anther development. Plant Physiol. 2010, 154, 1492–1504. [Google Scholar] [CrossRef] [PubMed]
- Goyer, A.; Hamlin, L.; Crosslin, J.M.; Buchanan, A.; Chang, J.H. RNA-Seq analysis of resistant and susceptible potato varieties during the early stages of potato virus Y infection. BMC Genom. 2015, 16, 472. [Google Scholar] [CrossRef] [PubMed]
- Gaguancela, O.A.; Źuñiga, L.P.; Arias, A.V.; Halterman, D.; Flores, F.J.; Johansen, I.E.; Wang, A.; Yamaji, Y.; Verchot, J. The IRE1/bZIP60 pathway and bax inhibitor 1 suppress systemic accumulation of potyviruses and potexviruses in Arabidopsis and Nicotiana benthamiana plants. Mol. Plant-Microbe Interact. 2016, 29, 750–766. [Google Scholar] [CrossRef]
- Rolly, N.K.; Imran, Q.M.; Shahid, M.; Imran, M.; Khan, M.; Lee, S.U.; Hussain, A.; Lee, I.J.; Yun, B.W. Drought-induced AtbZIP62 transcription factor regulates drought stress response in Arabidopsis. Plant Physiol. Biochem. 2020, 156, 384–395. [Google Scholar] [CrossRef]
- Finkelstein, R.; Gampala, S.S.L.; Lynch, T.J.; Thomas, T.L.; Rock, C.D. Redundant and distinct functions of the ABA response loci ABA-insensitive(ABI)5 and ABRE-binding factor (ABF)3. Plant Mol. Biol. 2005, 59, 253–267. [Google Scholar] [CrossRef]
- Abdeen, A.; Schnell, J.; Miki, B. Transcriptome analysis reveals absence of unintended effects in drought-tolerant transgenic plants overexpressing the transcription factor ABF3. BMC Genom. 2010, 11, 69. [Google Scholar] [CrossRef]
- Howe, K.L.; Contreras-Moreira, B.; De Silva, N.; Maslen, G.; Akanni, W.; Allen, J.; Alvarez-Jarreta, J.; Barba, M.; Bolser, D.M.; Cambell, L.; et al. Ensembl Genomes 2020-enabling non-vertebrate genomic research. Nucleic Acids Res. 2020, 48, D689–D695. [Google Scholar] [CrossRef] [PubMed]
- Kersey, P.J.; Allen, J.E.; Allot, A.; Barba, M.; Boddu, S.; Bolt, B.J.; Carvalho-Silva, D.; Christensen, M.; Davis, P.; Grabmueller, C.; et al. Ensembl Genomes 2018: An integrated omics infrastructure for non-vertebrate species. Nucleic Acids Res. 2018, 46, D802–D808. [Google Scholar] [CrossRef] [PubMed]
- Zerbino, D.R.; Achuthan, P.; Akanni, W.; Amode, M.R.; Barrell, D.; Bhai, J.; Billis, K.; Cummins, C.; Gall, A.; Girón, C.G.; et al. Ensembl 2018. Nucleic Acids Res. 2018, 46, D754–D761. [Google Scholar] [CrossRef] [PubMed]
- El-Gebali, S.; Mistry, J.; Bateman, A.; Eddy, S.R.; Luciani, A.; Potter, S.C.; Qureshi, M.; Richardson, L.J.; Salazar, G.A.; Smart, A.; et al. The Pfam protein families database in 2019. Nucleic Acids Res. 2019, 47, D427–D432. [Google Scholar] [CrossRef]
- Sigrist, C.J.A.; De Castro, E.; Cerutti, L.; Cuche, B.A.; Hulo, N.; Bridge, A.; Bougueleret, L.; Xenarios, I. New and continuing developments at PROSITE. Nucleic Acids Res. 2013, 41, D344–D347. [Google Scholar] [CrossRef]
- Mitchell, A.L.; Attwood, T.K.; Babbitt, P.C.; Blum, M.; Bork, P.; Bridge, A.; Brown, S.D.; Chang, H.Y.; El-Gebali, S.; Fraser, M.I.; et al. InterPro in 2019: Improving coverage, classification and access to protein sequence annotations. Nucleic Acids Res. 2019, 47, D351–D360. [Google Scholar] [CrossRef]
- Letunic, I.; Bork, P. 20 years of the SMART protein domain annotation resource. Nucleic Acids Res. 2018, 46, D493–D496. [Google Scholar] [CrossRef]
- Trifinopoulos, J.; Nguyen, L.T.; von Haeseler, A.; Minh, B.Q. W-IQ-TREE: A fast online phylogenetic tool for maximum likelihood analysis. Nucleic Acids Res. 2016, 44, W232–W235. [Google Scholar] [CrossRef]
- Nguyen, L.T.; Schmidt, H.A.; Von Haeseler, A.; Minh, B.Q. IQ-TREE: A fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol. Biol. Evol. 2015, 32, 268–274. [Google Scholar] [CrossRef]
- Minh, B.Q.; Nguyen, M.A.T.; Von Haeseler, A. Ultrafast approximation for phylogenetic bootstrap. Mol. Biol. Evol. 2013, 30, 1188–1195. [Google Scholar] [CrossRef]
- Hoang, D.T.; Chernomor, O.; Von Haeseler, A.; Minh, B.Q.; Vinh, L.S. UFBoot2: Improving the ultrafast bootstrap approximation. Mol. Biol. Evol. 2018, 35, 518–522. [Google Scholar] [CrossRef] [PubMed]
- Letunic, I.; Bork, P. Interactive Tree of Life (iTOL) v4: Recent updates and new developments. Nucleic Acids Res. 2019, 47, W256–W259. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools: An integrative toolkit developed for interactive analyses of big biological data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Tang, H.; Debarry, J.D.; Tan, X.; Li, J.; Wang, X.; Lee, T.H.; Jin, H.; Marler, B.; Guo, H.; et al. MCScanX: A toolkit for detection and evolutionary analysis of gene synteny and collinearity. Nucleic Acids Res. 2012, 40, e49. [Google Scholar] [CrossRef]
- Hu, B.; Jin, J.; Guo, A.Y.; Zhang, H.; Luo, J.; Gao, G. GSDS 2.0: An upgraded gene feature visualization server. Bioinformatics 2015, 31, 1296–1297. [Google Scholar] [CrossRef]
- Omasits, U.; Ahrens, C.H.; Müller, S.; Wollscheid, B. Protter: Interactive protein feature visualization and integration with experimental proteomic data. Bioinformatics 2014, 30, 884–886. [Google Scholar] [CrossRef]
- Tsirigos, K.D.; Peters, C.; Shu, N.; Käll, L.; Elofsson, A. The TOPCONS web server for consensus prediction of membrane protein topology and signal peptides. Nucleic Acids Res. 2015, 43, W401–W407. [Google Scholar] [CrossRef]
- Kumar, M.; Thakur, V.; Raghava, G.P.S. COPid: Composition based protein identification. In Silico Biol. 2008, 8, 121–128. [Google Scholar]
- Kozlowski, L.P. IPC - Isoelectric Point Calculator. Biol. Direct 2016, 11, 55. [Google Scholar] [CrossRef]
- Bailey, T.L.; Boden, M.; Buske, F.A.; Frith, M.; Grant, C.E.; Clementi, L.; Ren, J.; Li, W.W.; Noble, W.S. MEME Suite: Tools for motif discovery and searching. Nucleic Acids Res. 2009, 37, W202–W208. [Google Scholar] [CrossRef]
- Lescot, M.; Déhais, P.; Thijs, G.; Marchal, K.; Moreau, Y.; Van De Peer, Y.; Rouzé, P.; Rombauts, S. PlantCARE, a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucleic Acids Res. 2002, 30, 325–327. [Google Scholar] [CrossRef] [PubMed]
- Papatheodorou, I.; Fonseca, N.A.; Keays, M.; Tang, Y.A.; Barrera, E.; Bazant, W.; Burke, M.; Füllgrabe, A.; Fuentes, A.M.-P.; George, N.; et al. Expression Atlas: Gene and protein expression across multiple studies and organisms. Nucleic Acids Res. 2018, 46, D246–D251. [Google Scholar] [CrossRef] [PubMed]
- Massa, A.N.; Childs, K.L.; Lin, H.; Bryan, G.J.; Giuliano, G.; Buell, C.R. The transcriptome of the reference potato genome solanum tuberosum group Phureja clone DM1-3 516R44. PLoS ONE 2011, 6, e26801. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Tu, Z.J.; Millett, B.P.; Bradeen, J.M. Insights into organ-specific pathogen defense responses in plants: RNA-seq analysis of potato tuber-Phytophthora infestans interactions. BMC Genom. 2013, 14, 340. [Google Scholar] [CrossRef]
- Sprenger, H.; Kurowsky, C.; Horn, R.; Erban, A.; Seddig, S.; Rudack, K.; Fischer, A.; Walther, D.; Zuther, E.; Köhl, K.; et al. The drought response of potato reference cultivars with contrasting tolerance. Plant Cell Environ. 2016, 39, 2370–2389. [Google Scholar] [CrossRef]
- Verchot-Lubicz, J.; Ye, C.M.; Bamunusinghe, D. Molecular biology of potexviruses: Recent advances. J. Gen. Virol. 2007, 88, 1643–1655. [Google Scholar] [CrossRef]
- Dijkstra, J.; de Jager, C.P. Practical Plant Virology; Springer: Berlin/Heidelberg, Germany, 1998; ISBN 978-3-642-48981-5. [Google Scholar]
- Chen, Y.; Chen, Y.; Shi, C.; Huang, Z.; Zhang, Y.; Li, S.; Li, Y.; Ye, J.; Yu, C.; Li, Z.; et al. SOAPnuke: A MapReduce acceleration-supported software for integrated quality control and preprocessing of high-throughput sequencing data. Gigascience 2018, 7. [Google Scholar] [CrossRef]
- Li, H.; Handsaker, B.; Wysoker, A.; Fennell, T.; Ruan, J.; Homer, N.; Marth, G.; Abecasis, G.; Durbin, R. The Sequence Alignment/Map format and SAMtools. Bioinformatics 2009, 25, 2078–2079. [Google Scholar] [CrossRef]
- Pertea, M.; Pertea, G.M.; Antonescu, C.M.; Chang, T.-C.; Mendell, J.T.; Salzberg, S.L. StringTie enables improved reconstruction of a transcriptome from RNA-seq reads. Nat. Biotechnol. 2015, 33, 290–295. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Massa, A.N.; Manrique-Carpintero, N.C.; Coombs, J.; Haynes, K.G.; Bethke, P.C.; Brandt, T.L.; Gupta, S.K.; Yencho, G.C.; Novy, R.G.; Douches, D.S. Linkage analysis and QTL mapping in a tetraploid russet mapping population of potato. BMC Genet. 2018, 19, 87. [Google Scholar] [CrossRef] [PubMed]
- Pagny, G.; Paulstephenraj, P.S.; Poque, S.; Sicard, O.; Cosson, P.; Eyquard, J.P.; Caballero, M.; Chague, A.; Gourdon, G.; Negrel, L.; et al. Family-based linkage and association mapping reveals novel genes affecting Plum pox virus infection in Arabidopsis thaliana. New Phytol. 2012, 196, 873–886. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Leach, L.; Yang, J.; Zhang, F.; Tao, Q.; Dang, Z.; Chen, Y.; Luo, Z. A tetrasomic inheritance model and likelihood-based method for mapping quantitative trait loci in autotetraploid species. New Phytol. 2020, nph.16413. [Google Scholar] [CrossRef] [PubMed]
- Plich, J.; Zimnoch-Guzowska, E.; Tatarowska, B.; Śliwka, J. Quantitative trait loci analysis of potato tuber greening. Mol. Biol. Rep. 2020, 47, 1713–1722. [Google Scholar] [CrossRef]
- Li, L.; Zhang, Q.; Huang, D. A review of imaging techniques for plant phenotyping. Sensors 2014, 14, 20078–20111. [Google Scholar] [CrossRef]


| Group | Gene Name | Locus ID | Synonym | GenBank | Group | Gene Name | Locus ID | Synonym | GenBank |
|---|---|---|---|---|---|---|---|---|---|
| A | AtbZIP12 | AT2G41070 | DPBF4 | AF334209 | G | AtbZIP16 | AT2G35530 | NM179917 | |
| AtbZIP13 | AT5G44080 | AtbZIP41 | AT4G36730 | GBF1 | NM179179.3 | ||||
| AtbZIP14 | AT4G35900 | FD | NM119756.5 | AtbZIP54 | AT4G01120 | GBF2 | NM116342.3 | ||
| ABA Responsive | AtbZIP15 | AT5G42910 | MBD2 | NM123656.2 | AtbZIP55 | AT2G46270 | GBF3 | NM001337182.1 | |
| Flowering time | AtbZIP27 | AT2G17770 | FD paralog | NM127331.3 | AtbZIP68 | AT1G32150 | NM102948.4 | ||
| Seed germination | AtbZIP35 | AT1G49720 | ABF1 | NM001198254.2 | StbZIP16 | Soltu.DM.08G003670 | |||
| Abiotic Stress | AtbZIP36 | AT1G45249 | ABF2, ATAREB1 | NM001333256.1 | StbZIP24 | Soltu.DM.02G006750 | |||
| AtbZIP37 | AT4G34000 | ABF3 | NM001342246.1 | StbZIP41 | Soltu.DM.02G025470 | ||||
| AtbZIP38 | AT3G19290 | ABF4, AREB2 | NM001203005.2 | StbZIP54 | Soltu.DM.05G019830 | ||||
| AtbZIP39 | AT2G36270 | ABI5 | NM129185.4 | StbZIP55 | Soltu.DM.01G034570 | ||||
| AtbZIP40 | AT1G03970 | GBF4 | NM100278.3 | StbZIP68 | Soltu.DM.08G021830 | ||||
| AtbZIP66 | AT3G56850 | AREB3 | NM115544.3 | H | AtbZIP56 | AT5G11260 | HY5 | NM001343175.1 | |
| AtbZIP67 | AT3G44460 | DPBF2 | NM114314.5 | Anthocyanin Accum. | AtbZIP64 | AT3G17609 | HYH | NM001084700.2 | |
| StbZIP2 | Soltu.DM.01G005870 | Light Responsive, | StbZIP56 | Soltu.DM.08G011730 | |||||
| StbZIP3 | Soltu.DM.01G006940 | I | AtbZIP18 | AT2G40620 | NM129624.5 | ||||
| StbZIP12 | Soltu.DM.10G030340 | ABL2 | AtbZIP29 | AT4G38900 | NM001036733.4 | ||||
| StbZIP13 | Soltu.DM.04G027170 | AtbZIP30 | AT2G21230 | NM001335735.1 | |||||
| StbZIP14 | Soltu.DM.02G023460 | AtbZIP31 | AT2G13150 | NM126912.1 | |||||
| StbZIP27 | Soltu.DM.02G005680 | AtbZIP32 | AT2G12940 | UNE4 | NM126904.6 | ||||
| StbZIP35 | Soltu.DM.11G016910 | AREB4 | AtbZIP33 | AT2G12900 | NM126901.1 | ||||
| StbZIP36 | Soltu.DM.04G033590 | AREB2 | AtbZIP51 | AT1G43700 | NM103495.4 | ||||
| StbZIP37 | Soltu.DM.10G015000 | AREB3 | AtbZIP52 | AT1G06850 | NM001331671.1 | ||||
| StbZIP38 | Soltu.DM.01G047570 | AREB1 | AtbZIP59 | AT2G31370 | NM001336329.1 | ||||
| StbZIP39 | Soltu.DM.09G003620 | ABI5 | AtbZIP69 | AT1G06070 | NM100488.4 | ||||
| StbZIP40 | Soltu.DM.01G043800 | AtbZIP71 | AT2G24340 | NM127996.1 | |||||
| StbZIP66 | Soltu.DM.10G025990 | ABL1 | AtbZIP74 | AT2G21235 | NM179679.2 | ||||
| B | AtbZIP17 | AT2G40950 | NM129659.3 | StbZIP18 | Soltu.DM.06G011870 | ||||
| ER Stress | AtbZIP28 | AT3G10800 | NM111917.5 | StbZIP29 | Soltu.DM.01G050330 | ||||
| Responsive | AtbZIP49 | AT3G56660 | NM115525.2 | StbZIP30 | Soltu.DM.04G036300 | ||||
| StbZIP17 | Soltu.DM.10G029910 | StbZIP48 | Soltu.DM.04G026570 | ||||||
| StbZIP28 | Soltu.DM.04G021020 | StbZIP49 | Soltu.DM.06G017610 | ||||||
| StbZIP33 | Soltu.DM.04G020920 | StbZIP51 | Soltu.DM.04G026750 | ||||||
| StbZIP67 | Soltu.DM.08G019490 | StbZIP52 | Soltu.DM.06G011430 | ||||||
| StbZIP70 | Soltu.DM.08G019530 | StbZIP69 | Soltu.DM.06G014230 | ||||||
| StbZIP71 | Soltu.DM.08G019590 | J | AtbZIP62 | AT1G19490 | NM101806.3 | ||||
| C | AtbZIP9 | AT5G24800 | NM122389.4 | StbZIP62 | Soltu.DM.10G024960 | ||||
| C/S1 Regulatory Network, Energy Starvation, Seed Development | AtbZIP10 | AT4G02640 | NM001340389.1 | StbZIP72 | Soltu.DM.09G003280 | ||||
| AtbZIP25 | AT3G54620 | NM115319.4 | K | AtbZIP60 | AT1G42990 | NM103458.3 | |||
| AtbZIP63 | AT5G28770 | NM122760.4 | ER stress Responsive | StbZIP60 | Soltu.DM.04G038150 | ||||
| StbZIP9 | Soltu.DM.08G002700 | M | AtbZIP72 | AT5G07160 | NM120798.2 | ||||
| StbZIP10 | Soltu.DM.08G008380 | N | StbZIP25 | Soltu.DM.03G005150 | |||||
| StbZIP63 | Soltu.DM.01G036510 | StbZIP26 | Soltu.DM.03G005200 | ||||||
| D | AtbZIP20 | AT5G06950 | AHBP-1B; TGA2 | NM001036768.2 | StbZIP59 | Soltu.DM.06G032460 | |||
| SA Responsive | AtbZIP21 | AT1G08320 | TGA9 | NM001331769.1 | StbZIP75 | Soltu.DM.11G017940 | |||
| or | AtbZIP22 | AT1G22070 | TGA3 | NM102057.4 | S | AtbZIP1 | AT5G49450 | NM124322.3 | |
| Early Flowering | AtbZIP26 | AT5G06960 | OBF5; TGA5 | NM203016.2 | Includes the C/S1 Regulatory Network | AtbZIP2 | AT2G18160 | NM127373.2 | |
| AtbZIP45 | AT3G12250 | TGA6 | NM202564.2 | AtbZIP3 | AT5G15830 | NM121588.3 | |||
| AtbZIP46 | AT1G68640 | PAN | NM105536 | AtbZIP4 | AT1G59530 | NM104646 | |||
| AtbZIP47 | AT5G65210 | TGA1 | NM125919.3 | AtbZIP5 | AT3G49760 | NM114836.3 | |||
| AtbZIP50 | AT1G77920 | TGA7 | NM106441.4 | AtbZIP6 | AT2G22850 | NM127850.3 | |||
| AtbZIP57 | AT5G10030 | TGA4 | NM001343084.1 | AtbZIP7 | AT4G37730 | NM119935.3 | |||
| AtbZIP65 | AT5G06839 | TGA10 | NM001203315.2 | AtbZIP8 | AT1G68880 | NM105562.3 | |||
| StbZIP1 | Soltu.DM.01G005540 | AtbZIP11 | AT4G34590 | NM119625.3 | |||||
| StbZIP20 | Soltu.DM.11G019010 | AtbZIP42 | AT3G30530 | NM113954.2 | |||||
| StbZIP21 | Soltu.DM.06G029750 | AtbZIP43 | AT5G38800 | NM123241.3 | |||||
| StbZIP43 | Soltu.DM.04G022450 | AtbZIP44 | AT1G75390 | NM001084357.2 | |||||
| StbZIP45 | Soltu.DM.10G026630 | AtbZIP48 | AT2G04038 | NM126441.1 | |||||
| StbZIP46 | Soltu.DM.05G002740 | AtbZIP53 | AT3G62420 | NM116107.2 | |||||
| StbZIP47 | Soltu.DM.04G007700 | AtbZIP58 | AT1G13600 | NM101230.4 | |||||
| StbZIP50 | Soltu.DM.12G007270 | AtbZIP70 | AT5G60830 | NM125476.1 | |||||
| StbZIP57 | Soltu.DM.04G028540 | AtbZIP75 | AT5G08141 | NM125476.1 | |||||
| StbZIP65 | Soltu.DM.10G029320 | StbZIP4 | Soltu.DM.01G024850 | ||||||
| StbZIP74 | Soltu.DM.10G027000 | StbZIP5 | Soltu.DM.03G005290 | ||||||
| StbZIP80 | Soltu.DM.11G021800 | StbZIP6 | Soltu.DM.02G027390 | ||||||
| E | AtbZIP34 | AT2G42380 | NM001336969.1 | StbZIP7 | Soltu.DM.04G032270 | ||||
| AtbZIP61 | AT3G58120 | NM115674.4 | StbZIP8 | Soltu.DM.01G040220 | |||||
| AtbZIP76 | AT1G58110 | NM001036128.2 | StbZIP11 | Soltu.DM.02G024680 | |||||
| AtbZIP77 | AT1G35490 | NM001333158.1 | StbZIP15 | Soltu.DM.01G049720 | |||||
| AtbZIP78 | AT4G06598 | NM001340558.1 | StbZIP22 | Soltu.DM.02G006290 | |||||
| AtbZIP79 | AT5G04840 | NM120566.6 | StbZIP31 | Soltu.DM.03G006600 | |||||
| StbZIP34 | Soltu.DM.12G028390 | StbZIP32 | Soltu.DM.04G000200 | ||||||
| StbZIP61 | Soltu.DM.07G020410 | XP_006348282 | StbZIP42 | Soltu.DM.02G030370 | |||||
| StbZIP64 | Soltu.DM.07G023890 | StbZIP44 | Soltu.DM.04G035840 | ||||||
| StbZIP76 | Soltu.DM.10G011180 | StbZIP53 | Soltu.DM.06G000140 | ||||||
| StbZIP77 | Soltu.DM.11G012470 | StbZIP58 | Soltu.DM.05G001710 | ||||||
| StbZIP78 | Soltu.DM.01G042790 | StbZIP73 | Soltu.DM.10G016240 | ||||||
| StbZIP79 | Soltu.DM.11G007430 | ||||||||
| F | AtbZIP19 | AT4G35040 | NM001342322.1 | ||||||
| AtbZIP23 | AT2G16770 | NM001335489.1 | |||||||
| AtbZIP24 | AT3G51960 | NM001339543.1 | |||||||
| StbZIP19 | Soltu.DM.10G017560 | ||||||||
| StbZIP23 | Soltu.DM.01G051130 | ||||||||
| Functional Groups, Gene ID, Locus ID, Synonyms and NCBI accessions are provided for all genes used in this study. The color coding for the functional groups correspond to the colors in the legends in Figure 1. | |||||||||
| Duplicated Gene Pair | Ka | Ks | Ka/Ks |
|---|---|---|---|
| StbZIP55/StbZIP54 | 0.18 | 0.63 | 0.29 |
| StbZIP38/StbZIP37 | 0.19 | 0.67 | 0.29 |
| StbZIP38/StbZIP36 | 0.39 | 2.86 | 0.14 |
| StbZIP29/StbZIP30 | 0.36 | 1.88 | 0.19 |
| StbZIP23/StbZIP19 | 0.17 | 0.67 | 0.26 |
| StbZIP27/StbZIP14 | 0.4 | 1.33 | 0.30 |
| StbZIP14/StbZIP37 | 0.69 | 1.43 | 0.48 |
| StbZIP11/StbZIP44 | 0.26 | 1.17 | 0.23 |
| StbZIP6/StbZIP7 | 0.44 | 1.39 | 0.32 |
| StbZIP6/StbZIP5 | 0.26 | 0.87 | 0.30 |
| StbZIP5/StbZIP6 | 0.26 | 0.87 | 0.30 |
| StbZIP7/StbZIP6 | 0.44 | 1.39 | 0.32 |
| StbZIP36/StbZIP38 | 0.39 | 2.86 | 0.14 |
| StbZIP44/StbZIP11 | 0.26 | 1.17 | 0.23 |
| StbZIP30/StbZIP29 | 0.36 | 1.88 | 0.19 |
| StbZIP54/StbZIP55 | 0.18 | 0.63 | 0.29 |
| StbZIP61/StbZIP34 | 0.25 | 1.1 | 0.23 |
| StbZIP37/StbZIP38 | 0.19 | 0.67 | 0.29 |
| StbZIP19/StbZIP23 | 0.17 | 0.67 | 0.26 |
| StbZIP66/StbZIP12 | 0.15 | 0.57 | 0.26 |
| StbZIP12/StbZIP66 | 0.15 | 0.57 | 0.26 |
| StbZIP34/StbZIP61 | 0.25 | 1.1 | 0.23 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Herath, V.; Verchot, J. Insight into the bZIP Gene Family in Solanum tuberosum: Genome and Transcriptome Analysis to Understand the Roles of Gene Diversification in Spatiotemporal Gene Expression and Function. Int. J. Mol. Sci. 2021, 22, 253. https://doi.org/10.3390/ijms22010253
Herath V, Verchot J. Insight into the bZIP Gene Family in Solanum tuberosum: Genome and Transcriptome Analysis to Understand the Roles of Gene Diversification in Spatiotemporal Gene Expression and Function. International Journal of Molecular Sciences. 2021; 22(1):253. https://doi.org/10.3390/ijms22010253
Chicago/Turabian StyleHerath, Venura, and Jeanmarie Verchot. 2021. "Insight into the bZIP Gene Family in Solanum tuberosum: Genome and Transcriptome Analysis to Understand the Roles of Gene Diversification in Spatiotemporal Gene Expression and Function" International Journal of Molecular Sciences 22, no. 1: 253. https://doi.org/10.3390/ijms22010253
APA StyleHerath, V., & Verchot, J. (2021). Insight into the bZIP Gene Family in Solanum tuberosum: Genome and Transcriptome Analysis to Understand the Roles of Gene Diversification in Spatiotemporal Gene Expression and Function. International Journal of Molecular Sciences, 22(1), 253. https://doi.org/10.3390/ijms22010253






