Comparing Molecular Mechanisms in Solar NH3 Production and Relations with CO2 Reduction
Abstract
1. Introduction
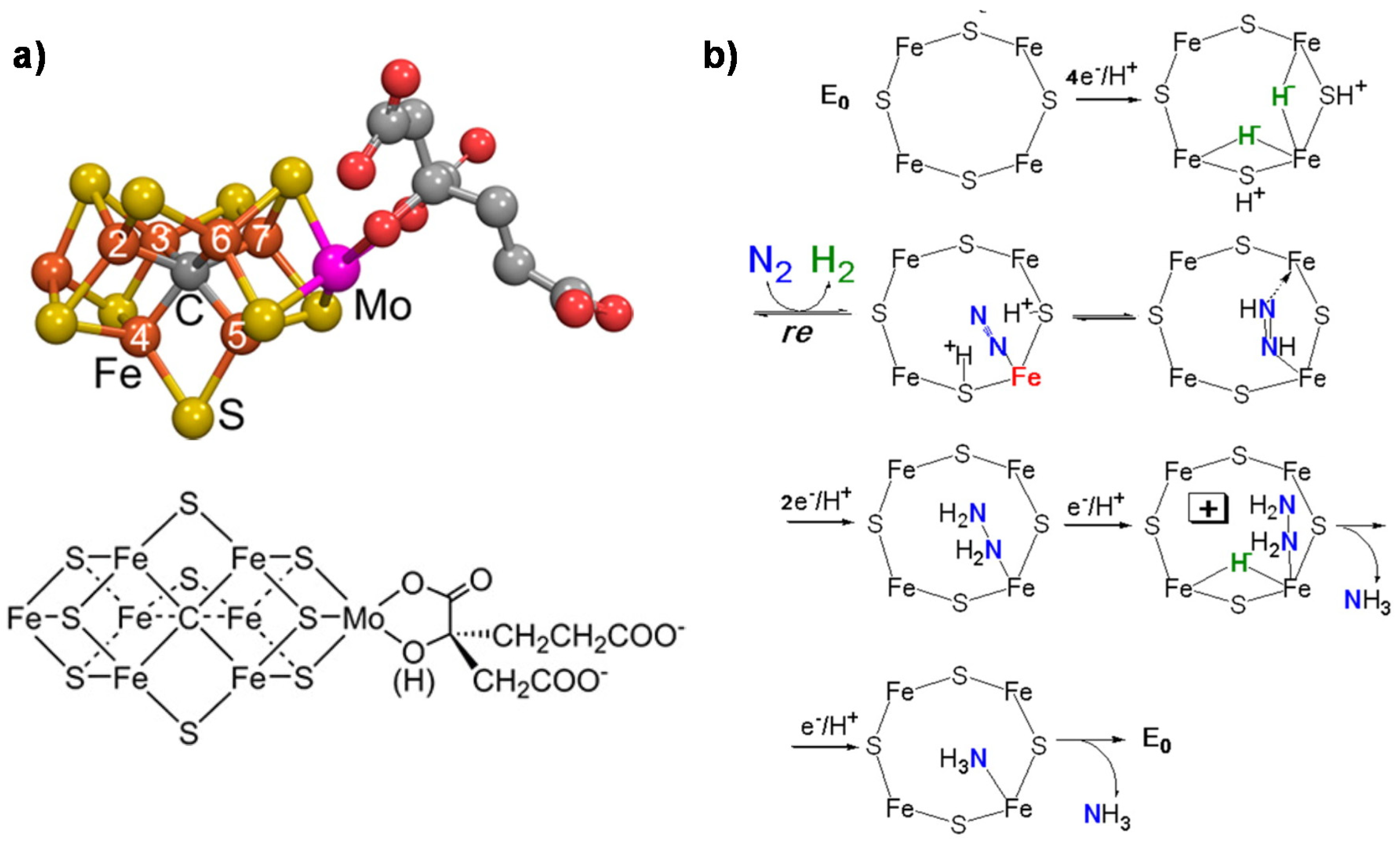
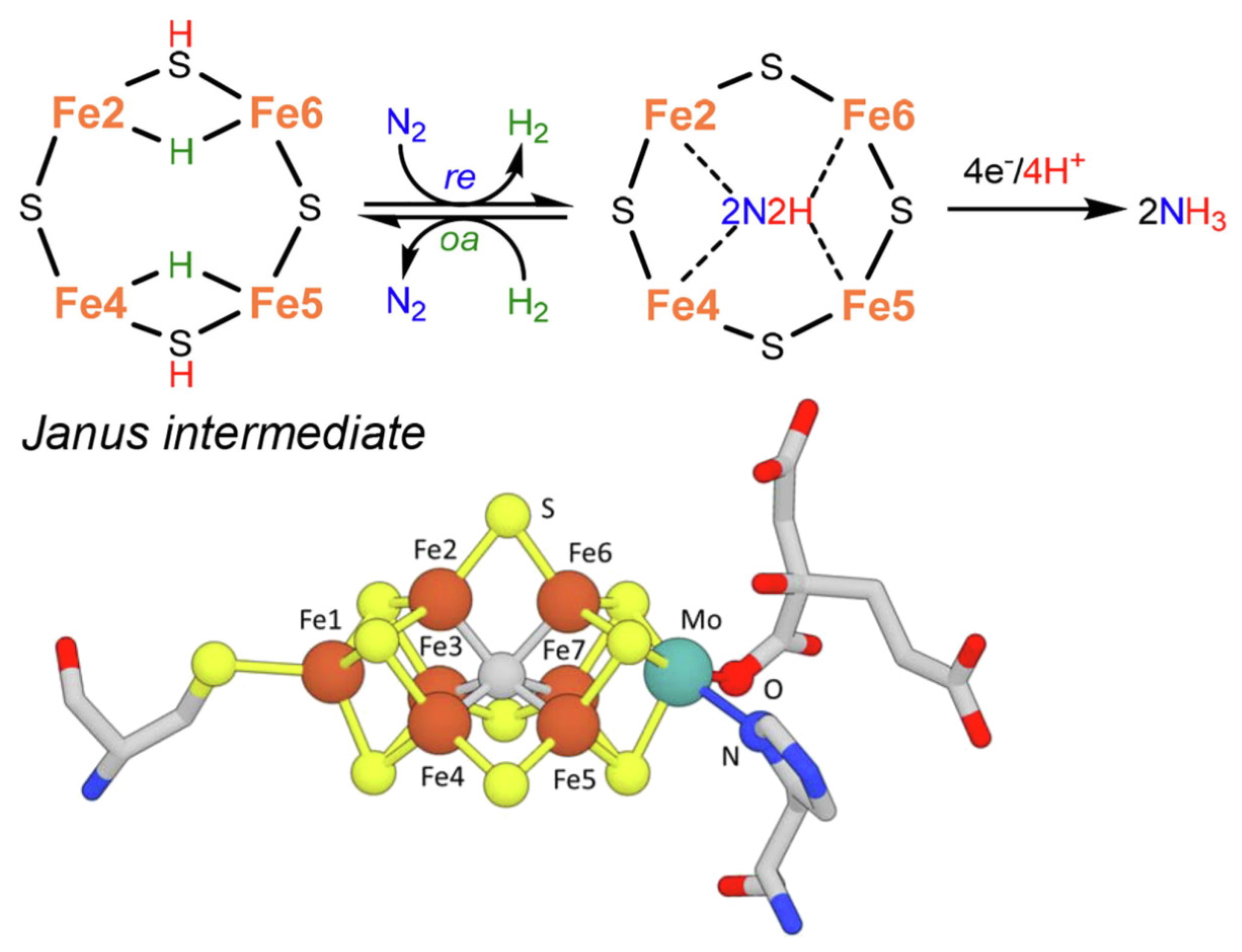
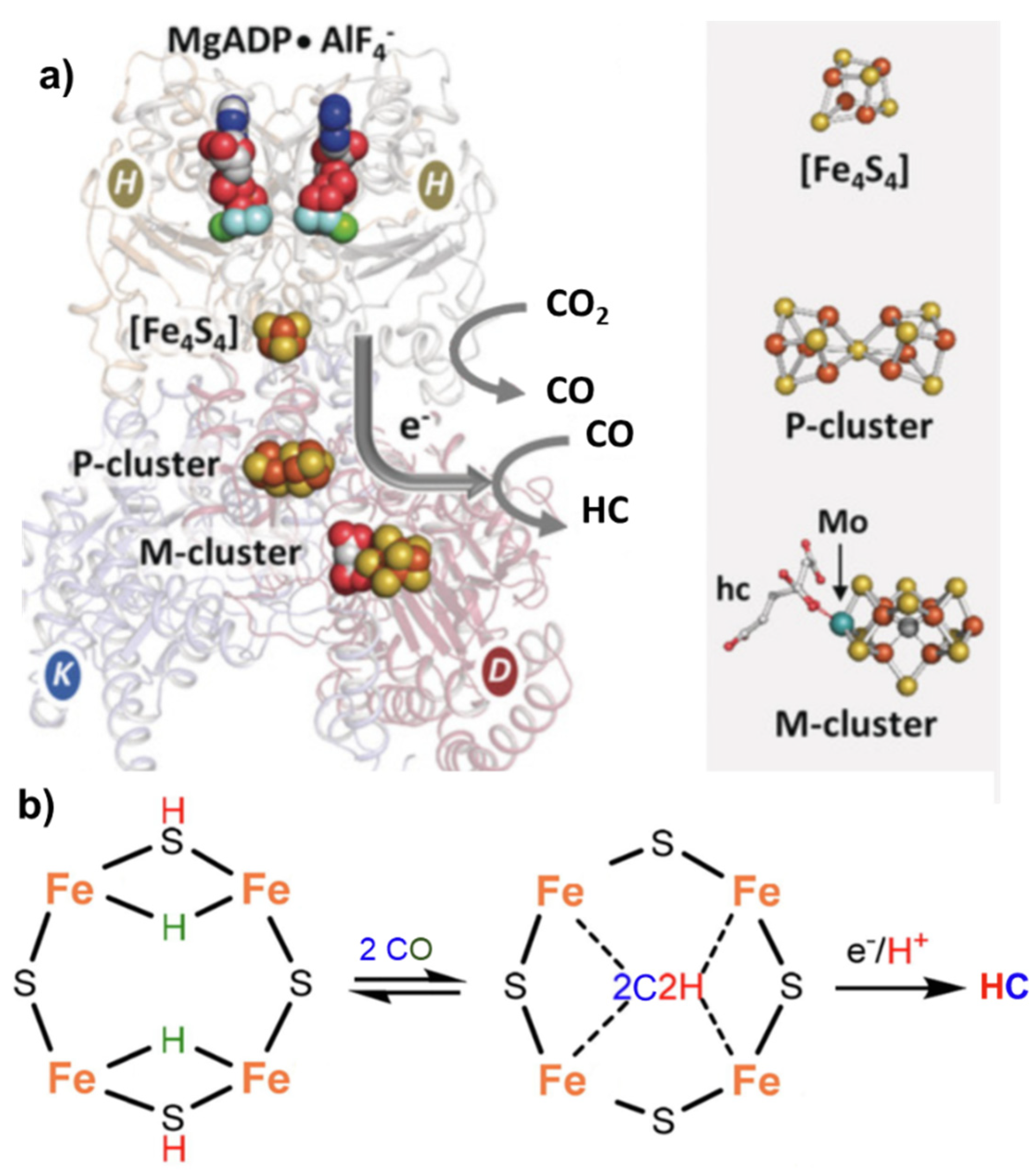
2. Mechanisms in Nitrogenase
- (i)
- A specific binding site for N2 able to first accept four electrons/protons to form two [Fe–H–Fe] bridging hydrides,
- (ii)
- coordination of N2 on two iron atoms with simultaneous reductive elimination of H2,
- (iii)
- multi-electron/proton transfer to a coordinated undissociated N2 molecule to form a N2H2 molecule stabilized by interaction with two iron atoms,
- (iv)
- further multi H+/e− transfer to form an end-on N2H4 coordinated molecule,
- (v)
- further steps of H+/e− transfer with stepwise release of ammonia.
3. Bioinspired Approaches
4. From Electrocatalysis to Biomimicking Mechanisms
5. Other Proposed Molecular Mechanisms of Reaction
- presence of C=O and O-C=O groups in graphene sheets [80];
- electron-deficient environment at the B-doping positions in boron-doped graphene [81];
- P atoms substituting C atoms in phosphorus-doped carbon nanotubes [82];
- pyridinic and pyrrolic N in N-doped porous carbons [83];
- defective nature of carbon-doped boron nitride nanosheets [84];
- sp2-hybridized B, due to substitution of an edge N atom in the cavity of C2N [85];
- double boron atom species in defect cavities of C2N [86];
- atomically dispersed Ni sites in a carbon matrix [87];
- heteronuclear dual-atom catalytic elements in FeMo/g-C3N4 [88];
- iron atoms in iron-phthalocyanine (FePc) grafted on oxygen-functionalized multiwalled carbon nanotubes (O-MWCNTs) [89];
- oxygen vacancies in NiCo2O4 on hollow N-carbon polyhedra [90];
- copper atoms on activated carbon functionalized with sulfonated groups [91].
6. Molecular Mechanisms in Plasma Catalysis
- -
- in vacuum plasma, the atomic N species (generated by N2 dissociation in the plasma) are adsorbed on the catalyst surface and react with atomic H from the gas phase to form NH(s) species and finally ammonia; the rate limiting step is the surface reaction;
- -
- in atmospheric pressure plasmas, the mechanism is still controversial. One possibility is the dissociative adsorption of excited N2 as the first reaction step but followed by surface dissociation and then surface hydrogenation by chemisorbed atomic hydrogen species. The other possibility is that atomic nitrogen directly generated in the plasma is chemisorbed and hydrogenated on the surface.
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ATP | Adenosine triphosphate |
| CNT | Carbon nanotube |
| CO2RR | CO2 reduction reaction |
| DFT | Density functional theory |
| GHG | Greenhouse gas |
| HB | Haber–Bosch |
| NHE | Normal hydrogen electrode |
| NTP | Non-thermal plasma |
| NRR | Nitrogen reduction reaction |
| PCET | Proton-coupled electron transfer |
| PEC | Photoelectrocatalytic |
| RE | Renewable energy |
| TON | Turnover number |
References
- Liu, A.; Yang, Y.; Ren, X.; Zhao, Q.; Gao, M.; Guan, W.; Meng, F.; Gao, L.; Yang, Q.; Liang, X.; et al. Current Progress of Electrocatalysts for Ammonia Synthesis Through Electrochemical Nitrogen Reduction Under Ambient Conditions. ChemSusChem 2020, 13, 3766–3788. [Google Scholar] [CrossRef]
- MacFarlane, D.R.; Cherepanov, P.V.; Choi, J.; Suryanto, B.H.R.; Hodgetts, R.Y.; Bakker, J.M.; Ferrero Vallana, F.M.; Simonov, A.N. A Roadmap to the Ammonia Economy. Joule 2020, 4, 1186–1205. [Google Scholar] [CrossRef]
- Qing, G.; Ghazfar, R.; Jackowski, S.T.; Habibzadeh, F.; Ashtiani, M.M.; Chen, C.-P.; Smith, M.R.; Hamann, T.W. Recent Advances and Challenges of Electrocatalytic N2 Reduction to Ammonia. Chem. Rev. 2020, 120, 5437–5516. [Google Scholar] [CrossRef] [PubMed]
- Hochman, G.; Goldman, A.S.; Felder, F.A.; Mayer, J.M.; Miller, A.J.M.; Holland, P.L.; Goldman, L.A.; Manocha, P.; Song, Z.; Aleti, S. Potential Economic Feasibility of Direct Electrochemical Nitrogen Reduction as a Route to Ammonia. ACS Sustainable Chem. Eng. 2020, 8, 8938–8948. [Google Scholar] [CrossRef]
- Lv, X.-W.; Weng, C.-C.; Yuan, Z.-Y. Ambient Ammonia Electrosynthesis: Current Status, Challenges, and Perspectives. ChemSusChem 2020, 13, 3061–3078. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Ithisuphalap, K.; Li, Y.; Mukherjee, S.; Lattimer, J.; Soloveichik, G.; Wu, G. Electrochemical ammonia synthesis through N2 and H2O under ambient conditions: Theory, practices, and challenges for catalysts and electrolytes. Nano Energy 2020, 69, 104469. [Google Scholar] [CrossRef]
- Smith, C.; Hill, A.K.; Torrente-Murciano, L. Current and future role of Haber–Bosch ammonia in a carbon-free energy landscape. Energy Env. Sci. 2020, 13, 331–344. [Google Scholar] [CrossRef]
- Global Alliance Powerfuels. Powerfuels: Missing Link to a Successful Energy Transition; Global Alliance Powerfuels: Berlin, Germany, June 2019. [Google Scholar]
- Li, M.; Huang, H.; Low, J.; Gao, C.; Long, R.; Xiong, Y. Recent Progress on Electrocatalyst and Photocatalyst Design for Nitrogen Reduction. Small Methods 2019, 3, 1800388. [Google Scholar] [CrossRef]
- Wang, Z.; Hu, X.; Liu, Z.; Zou, G.; Wang, G.; Zhang, K. Recent Developments in Polymeric Carbon Nitride-Derived Photocatalysts and Electrocatalysts for Nitrogen Fixation. ACS Catal. 2019, 9, 10260–10278. [Google Scholar] [CrossRef]
- Guo, C.; Ran, J.; Vasileff, A.; Qiao, S.-Z. Rational design of electrocatalysts and photo(electro)catalysts for nitrogen reduction to ammonia (NH3) under ambient conditions. Energy Env. Sci. 2018, 11, 45–56. [Google Scholar] [CrossRef]
- Hou, J.; Yang, M.; Zhang, J. Recent advances in catalysts, electrolytes and electrode engineering for the nitrogen reduction reaction under ambient condition. Nanoscale 2020, 12, 6900–6920. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Chen, S.; Li, Z.; Li, G.; Liu, X. Recent Advances in Electrochemical Synthesis of Ammonia through Nitrogen Reduction under Ambient Conditions. ChemElectroChem 2020, 7, 1067–1079. [Google Scholar] [CrossRef]
- Qin, Q.; Oschatz, M. Overcoming Chemical Inertness under Ambient Conditions: A Critical View on Recent Developments in Ammonia Synthesis via Electrochemical N2 Reduction by Asking Five Questions. ChemElectroChem 2020, 7, 878–889. [Google Scholar] [CrossRef]
- Hu, L.; Xing, Z.; Feng, X. Understanding the Electrocatalytic Interface for Ambient Ammonia Synthesis. ACS Energy Lett. 2020, 5, 430–436. [Google Scholar] [CrossRef]
- Zhu, X.; Mou, S.; Peng, Q.; Liu, Q.; Luo, Y.; Chen, G.; Gao, S.; Sun, X. Aqueous electrocatalytic N2 reduction for ambient NH3 synthesis: Recent advances in catalyst development and performance improvement. J. Mater. Chem A 2020, 8, 1545–1556. [Google Scholar] [CrossRef]
- Guo, W.; Zhang, K.; Liang, Z.; Zou, R.; Xu, Q. Electrochemical nitrogen fixation and utilization: Theories, advanced catalyst materials and system design. Chem. Soc. Rev. 2019, 48, 5658–5716. [Google Scholar] [CrossRef]
- Wang, Q.; Lei, Y.; Wang, D.; Li, Y. Defect engineering in earth-abundant electrocatalysts for CO2 and N2 reduction. Energy Env. Sci. 2019, 12, 1730–1750. [Google Scholar] [CrossRef]
- Choi, J.; Suryanto Bryan, H.R.; Wang, D.; Du, H.-L.; Hodgetts, R.Y.; Ferrero Vallana, F.M.; MacFarlane, D.R.; Simonov, A.N.; Choi, J.; Du, H.-L.; et al. Identification and elimination of false positives in electrochemical nitrogen reduction studies. Nature Comm. 2020, 11, 5546. [Google Scholar] [CrossRef]
- Zheng, J.; Lyu, Y.; Wang, R.; Wang, S.; Zheng, J.; Lyu, Y.; Wang, R.; Wang, S.; Zheng, J.; Marco, R.D.; et al. Tuning the Electron Localization of Gold Enables the Control of Nitrogen-to-Ammonia Fixation. Angew. Chemie Int. Ed. 2019, 58, 18604–18609. [Google Scholar] [CrossRef]
- Bogaerts, A.; Tu, X.; Whitehead, J.C.; Centi, G.; Lefferts, L.; Guaitella, O.; Azzolina-Jury, F.; Kim, H.-H.; Murphy, A.B.; Schneider, W.F.; et al. The 2020 plasma catalysis roadmap. J. Phys. D: Appl. Phys. 2020, 53, 443001. [Google Scholar] [CrossRef]
- Neyts, E.C.; Ostrikov, K.; Sunkara, M.K.; Bogaerts, A. Plasma Catalysis: Synergistic Effects at the Nanoscale. Chem. Rev. 2015, 115, 13408–13446. [Google Scholar] [CrossRef] [PubMed]
- Bogaerts, A.; Centi, G. Plasma Technology for CO2 Conversion: A Personal Perspective on Prospects and Gaps. Frontiers in Energy Res. 2020, 8, 111. [Google Scholar] [CrossRef]
- Carreon, M.L. Plasma catalytic ammonia synthesis: State of the art and future directions. J. Physics D Appl. Phys. 2019, 52, 483001. [Google Scholar] [CrossRef]
- Rouwenhorst, K.H.R.; Kim, H.-H.; Lefferts, L. Vibrationally Excited Activation of N2 in Plasma-Enhanced Catalytic Ammonia Synthesis: A Kinetic Analysis. ACS Sustain. Chem. Eng. 2019, 7, 17515–17522. [Google Scholar] [CrossRef]
- Cherkasov, N.; Ibhadon, A.O.; Fitzpatrick, P. A review of the existing and alternative methods for greener nitrogen fixation. Chem. Eng. Proc. Process. Intensif. 2015, 90, 24–33. [Google Scholar] [CrossRef]
- Hoffman, B.M.; Lukoyanov, D.; Yang, Z.-Y.; Dean, D.R.; Seefeldt, L.C. Mechanism of Nitrogen Fixation by Nitrogenase: The Next Stage. Chem. Rev. 2014, 114, 4041–4062. [Google Scholar] [CrossRef]
- Hu, Y.; Ribbe, M.W. Nitrogenases—A Tale of Carbon Atom(s). Angew. Chemie Int. Ed. 2016, 55, 8216–8226. [Google Scholar] [CrossRef]
- Hu, Y.; Lee, C.C.; Ribbe, M.W. Extending the carbon chain: Hydrocarbon formation catalyzed by vanadium/molybdenum nitrogenases. Science 2011, 333, 753–755. [Google Scholar] [CrossRef]
- Hu, Y.; Lee, C.C.; Ribbe, M.W. Vanadium nitrogenase: A two-hit wonder? Dalton Trans. 2012, 41, 1118–1127. [Google Scholar] [CrossRef]
- Perathoner, S.; Centi, G. Catalysis for solar-driven chemistry: The role of electrocatalysis. Catal. Today 2019, 330, 157–170. [Google Scholar] [CrossRef]
- Perathoner, S.; Centi, G. Chemistry and energy beyond fossil fuels. A perspective view on the role of syngas from waste sources. Catal. Today 2020, 342, 4–12. [Google Scholar]
- Peng, H.; Tang, M.T.; Liu, X.; Lamoureux, S.P.; Bajdich, M.; Abild-Pedersen, F. The role of atomic carbon in directing electrochemical CO2 reduction to multicarbon products. Energy Environ. Sci. 2020. Accepted. [Google Scholar] [CrossRef]
- Todorova, T.K.; Schreiber, M.W.; Fontecave, M. Mechanistic Understanding of CO2 Reduction Reaction (CO2RR) Toward Multicarbon Products by Heterogeneous Copper-Based Catalysts. ACS Catal. 2020, 10, 1754–1768. [Google Scholar] [CrossRef]
- Lei, F.; Yingying, L.; Lei, F.; Chuan, X.; Haotian, W.; Chuan, X.; Fangqi, Y.; Jun, W.; Haotian, W. Strategies in catalysts and electrolyzer design for electrochemical CO2 reduction toward C2+ products. Sci. Adv. 2020, 6, eaay3111. [Google Scholar]
- Gao, D.; Sinev, I.; Scholten, F.; Aran-Ais, R.M.; Divins, N.J.; Timoshenko, J.; Roldan Cuenya, B.; Sinev, I.; Scholten, F.; Divins, N.J.; et al. Selective CO2 Electroreduction to Ethylene and Multicarbon Alcohols via Electrolyte-Driven Nanostructuring. Angew. Chemie Int. Ed. 2019, 58, 17047–17053. [Google Scholar] [CrossRef]
- Chang, X.; Malkani, A.; Yang, X.; Xu, B. Mechanistic Insights into Electroreductive C–C Coupling between CO and Acetaldehyde into Multicarbon Products. J. Am. Chem. Soc. 2020, 142, 2975–2983. [Google Scholar] [CrossRef]
- Birdja, Y.Y.; Pérez-Gallent, E.; Figueiredo, M.C.; Göttle, A.J.; Calle-Vallejo, F.; Koper, M.T.M. Advances and challenges in understanding the electrocatalytic conversion of carbon dioxide to fuels. Nat. Energy 2019, 4, 732–745. [Google Scholar] [CrossRef]
- Centi, G.; Perathoner, S. Nanocarbon for Energy Material Applications: N2 Reduction Reaction. Small 2020. submitted. [Google Scholar]
- Khare, E.; Yadav, A. The Role of Microbial Enzyme Systems in Plant Growth Promotion. Clim. Chang. Envir. Sustain. 2017, 5, 122–145. [Google Scholar] [CrossRef]
- Burgess, B.K.; Lowe, D.J. Mechanism of Molybdenum Nitrogenase. Chem. Rev. 1996, 96, 2983–3011. [Google Scholar] [CrossRef]
- Burgess, B.K. The iron-molybdenum cofactor of nitrogenase. Chem. Rev. 1990, 90, 1377–1406. [Google Scholar] [CrossRef]
- Kim, J.; Rees, D.C. Structural models for the metal centers in the nitrogenase molybdenum-iron protein. Science 1992, 257, 1677–1682. [Google Scholar] [CrossRef] [PubMed]
- Spatzal, T.; Aksoyoglu, M.; Zhang, L.; Andrade, S.L.A.; Schleicher, E.; Weber, S.; Rees, D.C.; Einsle, O. Evidence for Interstitial Carbon in Nitrogenase FeMo Cofactor. Science 2011, 334, 940. [Google Scholar] [CrossRef]
- Khadka, N.; Milton, R.D.; Shaw, S.; Lukoyanov, D.; Dean, D.R.; Minteer, S.D.; Raugei, S.; Hoffman, B.M.; Seefeldt, L.C. Mechanism of Nitrogenase H2 Formation by Metal-Hydride Protonation Probed by Mediated Electrocatalysis and H/D Isotope Effects. JACS 2017, 139, 13518–13524. [Google Scholar] [CrossRef] [PubMed]
- Lukoyanov, D.; Yang, Z.-Y.; Khadka, N.; Dean, D.R.; Seefeldt, L.C.; Hoffman, B.M. Identification of a Key Catalytic Intermediate Demonstrates That Nitrogenase Is Activated by the Reversible Exchange of N2 for H2. JACS 2015, 137, 3610–3615. [Google Scholar] [CrossRef]
- Garden, A.L.; Skúlason, E. The Mechanism of Industrial Ammonia Synthesis Revisited: Calculations of the Role of the Associative Mechanism. J. Phys. Chem. C 2015, 119, 26554–26559. [Google Scholar] [CrossRef]
- Fuller, J.; Fortunelli, A.; Goddard III, W.A.; An, Q. Reaction mechanism and kinetics for ammonia synthesis on the Fe(211) reconstructed surface. Phys. Chem. Chem. Phys. 2019, 21, 11444–11454. [Google Scholar] [CrossRef]
- Schlögl, R. Catalytic synthesis of ammonia-a “never-ending story”? Angew. Chem. Int. Ed. 2003, 42, 2004–2008. [Google Scholar] [CrossRef]
- Schütze, J.; Mahdi, W.; Herzog, B.; Schlögl, R. On the structure of the activated iron catalyst for ammonia synthesis. Topics Catal. 1994, 1, 195–214. [Google Scholar] [CrossRef]
- Bazhenova, T.; Shilov, A. Nitrogen fixation in solution. Coord. Chem. Rev. 1995, 144, 69–145. [Google Scholar] [CrossRef]
- Ghosh, A.C.; Duboc, C.; Gennari, M. Synergy between metals for small molecule activation: Enzymes and bio-inspired complexes. Coord. Chem. Rev. 2021, 428, 213606. [Google Scholar] [CrossRef]
- Rebelein, J.G.; Hu, Y.; Ribbe, M.W. Differential Reduction of CO2 by Molybdenum and Vanadium Nitrogenases. Angew. Chem. Int. Ed. 2014, 53, 11543–11546. [Google Scholar] [CrossRef] [PubMed]
- Seefeldt, L.C.; Yang, Z.-Y.; Lukoyanov, D.A.; Harris, D.F.; Dean, D.R.; Raugei, S.; Hoffman, B.M. Reduction of Substrates by Nitrogenases. Chem. Rev. 2020, 120, 5082–5106. [Google Scholar] [CrossRef] [PubMed]
- Rettberg, L.A.; Stiebritz, M.T.; Kang, W.; Lee, C.C.; Ribbe, M.W.; Hu, Y. Structural and Mechanistic Insights into CO2 Activation by Nitrogenase Iron Protein. Chem. A Europ. J. 2019, 25, 13078–13082. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-I.; Sorlie, M.; Christiansen, J.; Yang, T.-C.; Shao, J.; Dean, D.R.; Hales, B.J.; Hoffman, B.M. Electron Inventory, Kinetic Assignment (En), Structure, and Bonding of Nitrogenase Turnover Intermediates with C2H2 and CO. JACS 2005, 127, 15880–15890. [Google Scholar] [CrossRef]
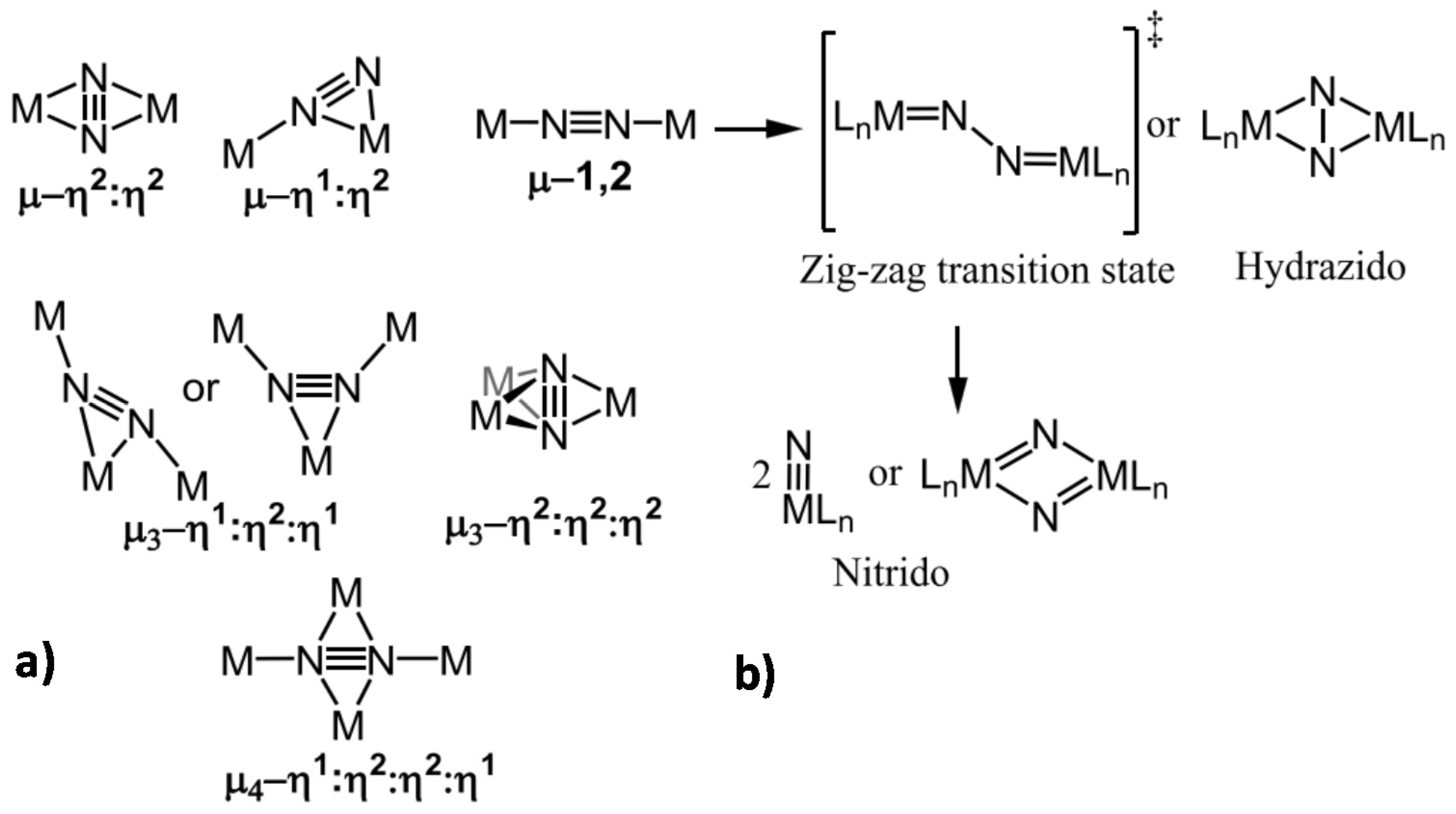
- Jia, H.-P.; Quadrelli, E.A. Mechanistic aspects of dinitrogen cleavage and hydrogenation to produce ammonia in catalysis and organometallic chemistry: Relevance of metal hydride bonds and dihydrogen. Chem. Soc. Rev. 2014, 43, 547–564. [Google Scholar] [CrossRef]
- Lukoyanov, D.; Khadka, N.; Yang, Z.-Y.; Dean, D.R.; Seefeldt, L.C.; Hoffman, B.M. Reductive Elimination of H2 Activates Nitrogenase to Reduce the N≡N Triple Bond: Characterization of the E4(4H) Janus Intermediate in Wild-Type Enzyme. JACS 2016, 138, 10674–10683. [Google Scholar] [CrossRef]
- Seefeldt, L.C.; Hoffman, B.M.; Dean, D.R. Mechanism of Mo-dependent nitrogenase. Annu. Rev. Biochem. 2009, 78, 701–722. [Google Scholar] [CrossRef]
- Bjornsson, R.; Lima, F.A.; Spatzal, T.; Weyhermueller, T.; Glatzel, P.; Bill, E.; Einsle, O.; Neese, F.; DeBeer, S. Identification of a spin-coupled Mo(iii) in the nitrogenase iron–molybdenum cofactor. Chem. Sci. 2014, 5, 3096–3103. [Google Scholar] [CrossRef]
- Nagelski, A.L.; Fataftah, M.S.; Bollmeyer, M.M.; McWilliams, S.F.; MacMillan, S.N.; Mercado, B.Q.; Lancaster, K.M.; Holland, P.L. The influences of carbon donor ligands on biomimetic multi-iron complexes for N2 reduction. Chem. Sci. 2020, 11, 12710–12720. [Google Scholar] [CrossRef]
- Chalkley, M.J.; Drover, M.W.; Peters, J.C. Catalytic N2-to-NH3 (or -N2H4) Conversion by Well-Defined Molecular Coordination Complexes. Chem. Rev. 2020, 120, 5582–5636. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.; Buratto, W.R.; Torres, J.F.; Murray, L.J. Activation of Dinitrogen by Polynuclear Metal Complexes. Chem. Rev. 2020, 120, 5517–5581. [Google Scholar] [CrossRef]
- Tanifuji, K.; Ohki, Y. Metal-Sulfur Compounds in N2 Reduction and Nitrogenase-Related Chemistry. Chem. Rev. 2020, 120, 5194–5251. [Google Scholar] [CrossRef]
- Yandulov, D.V.; Schrock, R.R. Catalytic reduction of dinitrogen to ammonia at a single molybdenum center. Science 2003, 301, 76–78. [Google Scholar] [CrossRef]
- Anderson, J.S.; Moret, M.E.; Peters, J.C. Conversion of Fe–NH2 to Fe–N2 with release of NH3. JACS 2013, 135, 534–537. [Google Scholar] [CrossRef]
- Centi, G.; Perathoner, S.; Winè, G.; Gangeri, M. Electrocatalytic conversion of CO2 to long carbon-chain hydrocarbons. Green Chem. 2007, 9, 671–678. [Google Scholar] [CrossRef]
- Gangeri, M.; Perathoner, S.; Caudo, S.; Centi, G.; Amadou, J.; Begin, D.; Pham-Huu, C.; Ledoux, M.J.; Tessonnier, J.-P.; Su, D.S.; et al. Fe and Pt carbon nanotubes for the electrocatalytic conversion of carbon dioxide to oxygenates. Catal. Today 2009, 143, 57–63. [Google Scholar] [CrossRef]
- Huang, Y.; Handoko, A.D.; Hirunsit, P.; Yeo, B.S. Electrochemical Reduction of CO2 Using Copper Single-Crystal Surfaces: Effects of CO* Coverage on the Selective Formation of Ethylene. ACS Catal. 2017, 7, 1749–1756. [Google Scholar] [CrossRef]
- Schouten, K.J.P.; Qin, Z.; Gallent, E.P.; Koper, M.T.M. Two Pathways for the Formation of Ethylene in CO Reduction on Single-Crystal Copper Electrodes. JACS 2012, 134, 9864–9867. [Google Scholar] [CrossRef]
- Kortlever, R.; Shen, J.; Schouten, K.J.P.; Calle-Vallejo, F.; Koper, M.T.M. Catalysts and Reaction Pathways for the Electrochemical Reduction of Carbon Dioxide. J. Phys. Chem. Lett. 2015, 6, 4073–4082. [Google Scholar] [CrossRef]
- Montoya, J.H.; Shi, C.; Chan, K.; Nørskov, J.K. Theoretical Insights into a CO Dimerization Mechanism in CO2 Electroreduction. J. Phys. Chem. Lett. 2015, 6, 2032–2037. [Google Scholar] [CrossRef] [PubMed]
- Goodpaster, J.D.; Bell, A.T.; Head-Gordon, M. Identification of Possible Pathways for C–C Bond Formation during Electrochemical Reduction of CO2: New Theoretical Insights from an Improved Electrochemical Model. J. Phys. Chem. Lett. 2016, 7, 1471–1477. [Google Scholar] [CrossRef] [PubMed]
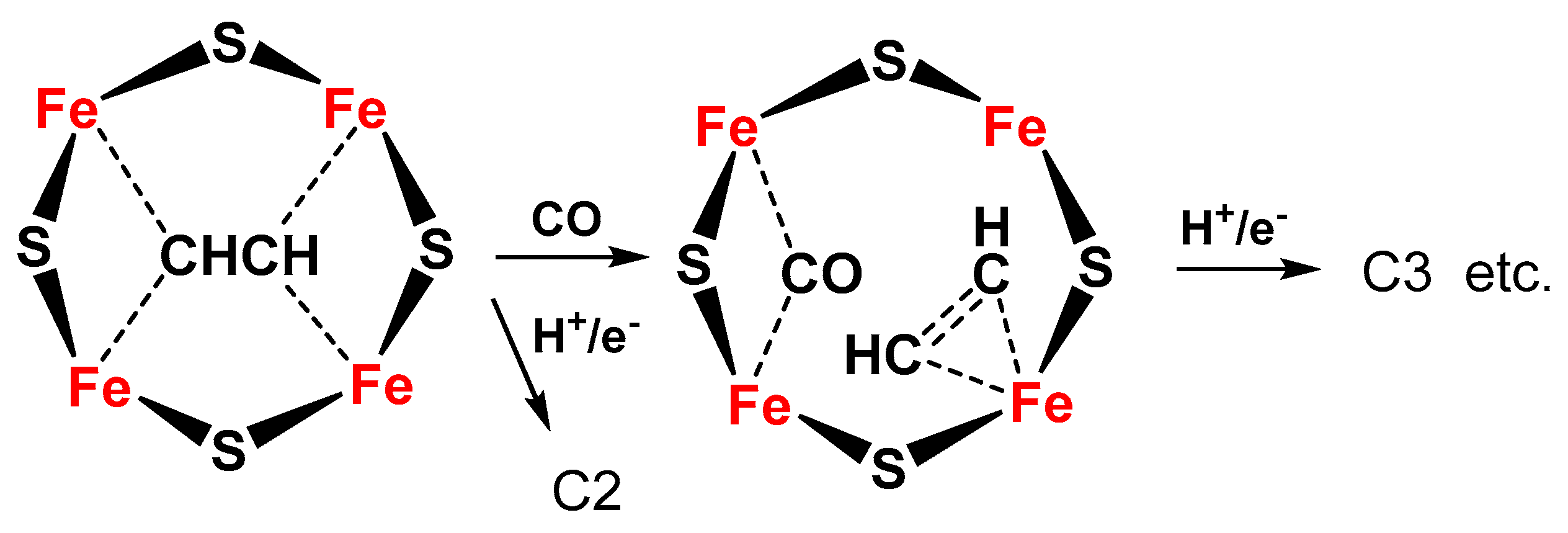
- Chan, Y.-T.; Huang, I.-S.; Tsai, M.-K. Enhancing C–C bond formation by surface strain: A computational investigation for C2 and C3 intermediate formation on strained Cu surfaces. Phys. Chem. Chem. Phys. 2019, 21, 22704–22710. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Perathoner, S.; Ampelli, C.; Mebrahtu, C.; Su, D.S.; Centi, G. Electrocatalytic Synthesis of Ammonia at Room Temperature and Atmospheric Pressure from Water and Nitrogen on a Carbon-Nanotube-Based Electrocatalyst. Angew. Chem. Int. Ed. 2017, 56, 2699–2703. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Perathoner, S.; Ampelli, C.; Mebrahtu, C.; Su, D.S.; Centi, G. Room-Temperature Electrocatalytic Synthesis of NH3 from H2O and N2 in a Gas–Liquid–Solid Three-Phase Reactor. ACS Sustain. Chem. Eng. 2017, 5, 7393–7400. [Google Scholar] [CrossRef]
- Chen, S.; Perathoner, S.; Ampelli, C.; Wei, H.; Abate, S.; Zhang, B.; Centi, G. Direct Synthesis of Ammonia from N2 and H2O on Different Iron Species Supported on Carbon Nanotubes using a Gas-Phase Electrocatalytic Flow Reactor. ChemElectroChem 2020, 7, 3028–3037. [Google Scholar] [CrossRef]
- Genovese, C.; Schuster, M.E.; Gibson, E.K.; Gianolio, D.; Posligua, V.; Grau-Crespo, R.; Cibin, G.; Wells, P.P.; Garai, D.; Solokha, V.; et al. Operando spectroscopy study of the carbon dioxide electro-reduction by iron species on nitrogen-doped carbon. Nat. Comm. 2018, 9, 935. [Google Scholar] [CrossRef]
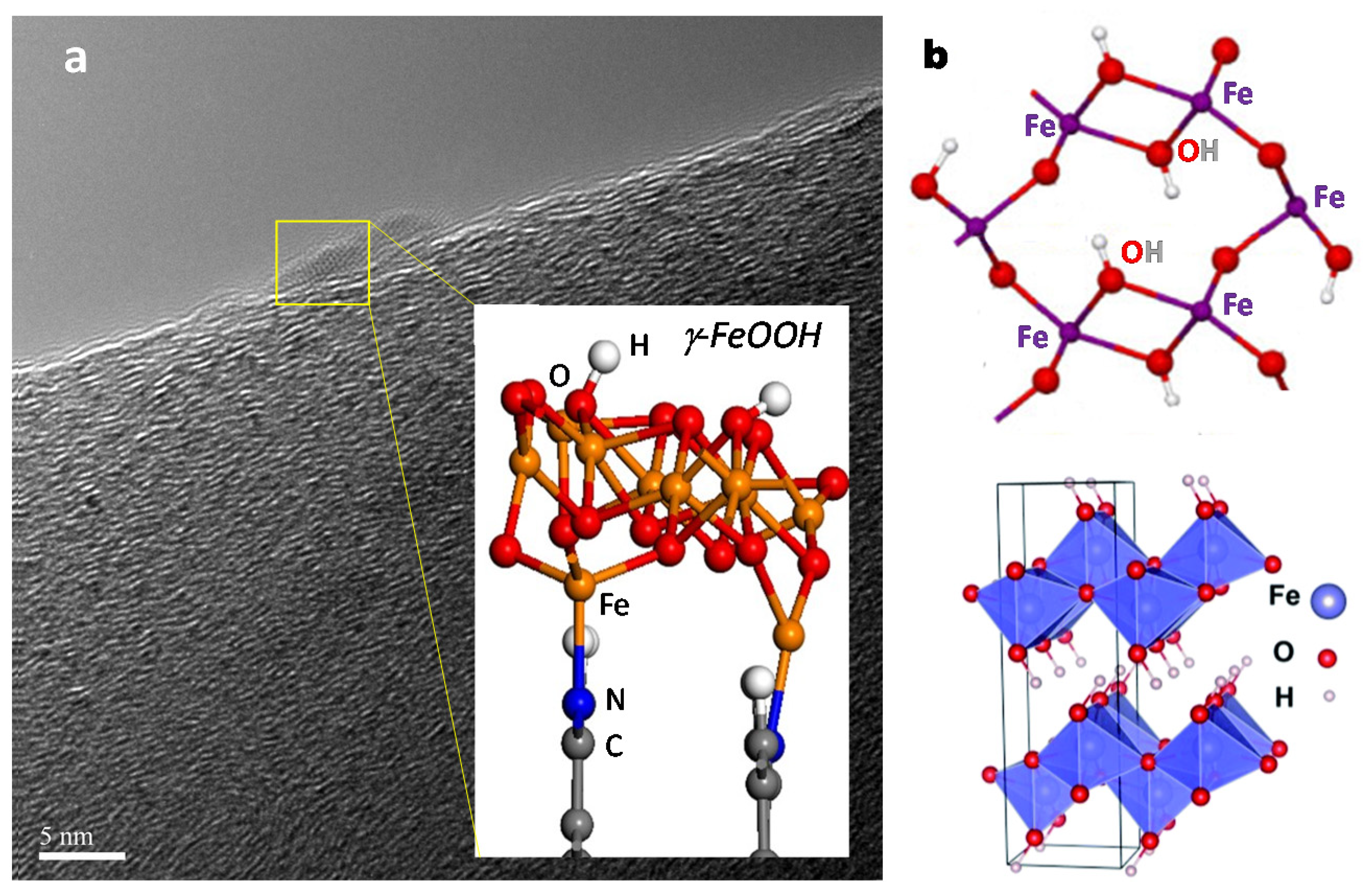
- Chen, S.; Perathoner, S.; Ampelli, C.; Wei, H.; Abate, S.; Zhang, B.; Centi, G. Enhanced performance in the direct electrocatalytic synthesis of ammonia from N2 and H2O by an in-situ electrochemical activation of CNT-supported iron oxide nanoparticles. J. Energy Chem. 2020, 49, 22–32. [Google Scholar] [CrossRef]
- Wang, T.; Xia, L.; Yang, J.-J.; Wang, H.; Fang, W.-H.; Chen, H.; Tang, D.; Asiri, A.M.; Luo, Y.; Cui, G.; et al. Electrocatalytic N2-to-NH3 conversion using oxygen-doped graphene: Experimental and theoretical studies. Chem. Commun. 2019, 55, 7502–7505. [Google Scholar] [CrossRef]
- Yu, X.; Han, P.; Wei, Z.; Huang, L.; Gu, Z.; Peng, S.; Ma, J.; Zheng, G. Boron-Doped Graphene for Electrocatalytic N2 Reduction. Joule 2018, 2, 1610–1622. [Google Scholar] [CrossRef]
- Yuan, L.-P.; Wu, Z.-Y.; Jiang, W.-J.; Tang, T.; Niu, S.; Hu, J.-S. Phosphorus-doping activates carbon nanotubes for efficient electroreduction of nitrogen to ammonia. Nano Res. 2020, 13, 1376–1382. [Google Scholar] [CrossRef]
- Liu, Y.; Su, Y.; Quan, X.; Fan, X.; Chen, S.; Yu, H.; Zhao, H.; Zhang, Y.; Zhao, J. Facile Ammonia Synthesis from Electrocatalytic N2 Reduction under Ambient Conditions on N-Doped Porous Carbon. ACS Catal. 2018, 8, 1186–1191. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, M.; Wang, H.; Cang, D.; Ji, X.; Liu, B.; Yang, W.; Li, D.; Liu, J. Defective Carbon-Doped Boron Nitride Nanosheets for Highly Efficient Electrocatalytic Conversion of N2 to NH3. ACS Sustain. Chem. Eng. 2020, 8, 5278–5286. [Google Scholar] [CrossRef]
- Yin, H.; Gan, L.-Y.; Wang, P. The identification of optimal active boron sites for N2 reduction. J. Mater. Chem. A 2020, 8, 3910–3917. [Google Scholar] [CrossRef]
- Cao, Y.; Deng, S.; Fang, Q.; Sun, X.; Zhao, C.X.; Zheng, J.; Gao, Y.; Zhuo, H.; Li, Y.; Yao, Z. Single and double boron atoms doped nanoporous C2 N-h2D electrocatalysts for highly efficient N2 reduction reaction: A density functional theory study. Nanotechn. 2019, 30, 335403. [Google Scholar] [CrossRef]
- Mukherjee, S.; Yang, X.; Shan, W.; Samarakoon, W.; Karakalos, S.; Cullen, D.A.; More, K.; Wang, M.; Feng, Z.; Wang, G.; et al. Atomically Dispersed Single Ni Site Catalysts for Nitrogen Reduction toward Electrochemical Ammonia Synthesis Using N2 and H2O. Small Methods 2020, 4, 1900821. [Google Scholar] [CrossRef]
- Wang, S.; Shi, L.; Bai, X.; Li, Q.; Ling, C.; Wang, J. Highly Efficient Photo-/Electrocatalytic Reduction of Nitrogen into Ammonia by Dual-Metal Sites. ACS Cent. Sci. 2020, 6, 1762–1771. [Google Scholar] [CrossRef]
- Xu, F.; Zhang, L.; Ding, X.; Cong, M.; Jin, Y.; Chen, L.; Gao, Y. Selective electroreduction of dinitrogen to ammonia on a molecular iron phthalocyanine/O-MWCNT catalyst under ambient conditions. Chem. Commun. 2019, 55, 14111–14114. [Google Scholar] [CrossRef]
- Lai, F.; Feng, J.; Ye, X.; Zong, W.; He, G.; Yang, C.; Wang, W.; Miao, Y.-E.; Pan, B.; Yan, W.; et al. Oxygen vacancy engineering in spinel-structured nanosheet wrapped hollow polyhedra for electrochemical nitrogen fixation under ambient conditions. J. Mater. Chem. A 2020, 8, 1652–1659. [Google Scholar] [CrossRef]
- Zhang, S.; Li, W.; Liu, Y.; Wang, J.; Wang, G.; Zhang, Y.; Han, M.; Zhang, H. A sulfonate group functionalized active carbon-based Cu catalyst for electrochemical ammonia synthesis under ambient conditions. Inorg. Chem. Front. 2019, 6, 2832–2836. [Google Scholar] [CrossRef]
- Eilert, A.; Roberts, F.S.; Friebel, D.; Nilsson, A. Formation of Copper Catalysts for CO2 Reduction with High Ethylene/Methane Product Ratio Investigated with In Situ X-ray Absorption Spectroscopy. J. Phys. Chem. Lett. 2016, 7, 1466–1470. [Google Scholar] [CrossRef] [PubMed]
- Munir, S.; Varzeghani, A.R.; Kaya, S. Electrocatalytic reduction of CO2 to produce higher alcohols. Sustain. Energy Fuels 2018, 2, 2532–2541. [Google Scholar] [CrossRef]
- Li, Y.; Kim, D.; Louisia, S.; Xie, C.; Kong, Q.; Yu, S.; Lin, T.; Aloni, S.; Fakra, S.C.; Yang, P. Electrochemically scrambled nanocrystals are catalytically active for CO2-to-multicarbons. Proc. Natl. Acad. Sci. USA 2020, 117, 9194–9201. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, T.-T.; Pang, Y.; Liang, Z.-Q.; Wang, Z.; Li, Y.; Tan, C.-S.; Li, J.; Dinh, C.T.; De Luna, P.; Hsieh, P.-L.; et al. Copper nanocavities confine intermediates for efficient electrosynthesis of C3 alcohol fuels from carbon monoxide. Nat. Catal. 2018, 1, 946–951. [Google Scholar] [CrossRef]
- Zhang, B.-B.; Wang, Y.-H.; Xu, S.-M.; Chen, K.; Yang, Y.-G.; Kong, Q.-H. Tuning nanocavities of Au@Cu2O yolk–shell nanoparticles for highly selective electroreduction of CO2 to ethanol at low potential. RSC Adv. 2020, 10, 19192–19198. [Google Scholar] [CrossRef]
- Sen, S.; Liu, D.; Palmore, G.T.R. Electrochemical Reduction of CO2 at Copper Nanofoams. ACS Catal. 2014, 4, 3091–3095. [Google Scholar] [CrossRef]
- Lum, Y.; Yue, B.; Lobaccaro, P.; Bell, A.T.; Ager, J.W. Optimizing C–C Coupling on Oxide-Derived Copper Catalysts for Electrochemical CO2 Reduction. J. Phys. Chem. C 2017, 121, 14191–14203. [Google Scholar] [CrossRef]
- Genovese, C.; Ampelli, C.; Perathoner, S.; Centi, G. Mechanism of C–C bond formation in the electrocatalytic reduction of CO2 to acetic acid. A challenging reaction to use renewable energy with chemistry. Green Chem. 2017, 19, 2406–2415. [Google Scholar] [CrossRef]
- Marepally, B.C.; Ampelli, C.; Genovese, C.; Tavella, F.; Quadrelli, E.A.; Perathoner, S.; Centi, G. Electrocatalytic reduction of CO2 over dendritic-type Cu- and Fe-based electrodes prepared by electrodeposition. J. CO2 Utiliz. 2020, 35, 194–204. [Google Scholar] [CrossRef]
- Han, H.; Noh, Y.; Kim, Y.; Park, S.; Yoon, W.; Jang, D.; Choi, S.M.; Kim, W.B. Selective electrochemical CO2 conversion to multicarbon alcohols on highly efficient N-doped porous carbon-supported Cu catalysts. Green Chem. 2020, 22, 71–84. [Google Scholar] [CrossRef]
- Eren, B.; Zherebetskyy, D.; Patera, L.L.; Wu, C.H.; Bluhm, H.; Africh, C.; Wang, L.W.; Somorjai, G.A.; Salmeron, M. Activation of Cu(111) surface by decomposition into nanoclusters driven by CO adsorption. Science 2016, 351, 475–478. [Google Scholar] [CrossRef] [PubMed]
- Kuhl, K.P.; Cave, E.R.; Abram, D.N.; Jaramillo, T.F. New insights into the electrochemical reduction of carbon dioxide on metallic copper surfaces. Energy Envir. Sci. 2012, 5, 7050–7059. [Google Scholar] [CrossRef]
- Genovese, C.; Ampelli, C.; Perathoner, S.; Centi, G. Electrocatalytic conversion of CO2 on carbon nanotube-based electrodes for producing solar fuels. J. Catal. 2013, 308, 237–249. [Google Scholar] [CrossRef]
- Ampelli, C.; Centi, G.; Passalacqua, R.; Perathoner, S. Synthesis of solar fuels by a novel photoelectrocatalytic approach. Energy Envir. Sci. 2010, 3, 292–301. [Google Scholar] [CrossRef]
- Ampelli, C.; Genovese, C.; Marepally, B.C.; Papanikolaou, G.; Perathoner, S.; Centi, G. Electrocatalytic conversion of CO2 to produce solar fuels in electrolyte or electrolyte-less configurations of PEC cells. Faraday Discuss. 2015, 183, 125–145. [Google Scholar] [CrossRef]
- Marepally, B.C.; Ampelli, C.; Genovese, C.; Saboo, T.; Perathoner, S.; Wisser, F.M.; Veyre, L.; Canivet, J.; Quadrelli, E.A.; Centi, G. Enhanced formation of >C1 Products in Electroreduction of CO2 by Adding a CO2 Adsorption Component to a Gas-Diffusion Layer-Type Catalytic Electrode. ChemSusChem 2017, 10, 4442–4446. [Google Scholar] [CrossRef]
- Shi, R.; Zhang, X.; Waterhouse, G.I.N.; Zhao, Y.; Zhang, T. The Journey toward Low Temperature, Low Pressure Catalytic Nitrogen Fixation. Adv. Energy Mater. 2020, 10, 2000659. [Google Scholar] [CrossRef]
- Hollevoet, L.; Jardali, F.; Gorbanev, Y.; Creel, J.; Bogaerts, A.; Martens, J.A. Towards Green Ammonia Synthesis through Plasma-Driven Nitrogen Oxidation and Catalytic Reduction. Angew. Chem. Int. Ed. 2020, 59, 23825–23829. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mallamace, D.; Papanikolaou, G.; Perathoner, S.; Centi, G.; Lanzafame, P. Comparing Molecular Mechanisms in Solar NH3 Production and Relations with CO2 Reduction. Int. J. Mol. Sci. 2021, 22, 139. https://doi.org/10.3390/ijms22010139
Mallamace D, Papanikolaou G, Perathoner S, Centi G, Lanzafame P. Comparing Molecular Mechanisms in Solar NH3 Production and Relations with CO2 Reduction. International Journal of Molecular Sciences. 2021; 22(1):139. https://doi.org/10.3390/ijms22010139
Chicago/Turabian StyleMallamace, Domenico, Georgia Papanikolaou, Siglinda Perathoner, Gabriele Centi, and Paola Lanzafame. 2021. "Comparing Molecular Mechanisms in Solar NH3 Production and Relations with CO2 Reduction" International Journal of Molecular Sciences 22, no. 1: 139. https://doi.org/10.3390/ijms22010139
APA StyleMallamace, D., Papanikolaou, G., Perathoner, S., Centi, G., & Lanzafame, P. (2021). Comparing Molecular Mechanisms in Solar NH3 Production and Relations with CO2 Reduction. International Journal of Molecular Sciences, 22(1), 139. https://doi.org/10.3390/ijms22010139