Feasibility of Imaging EpCAM Expression in Ovarian Cancer Using Radiolabeled DARPin Ec1
Abstract
1. Introduction
2. Results
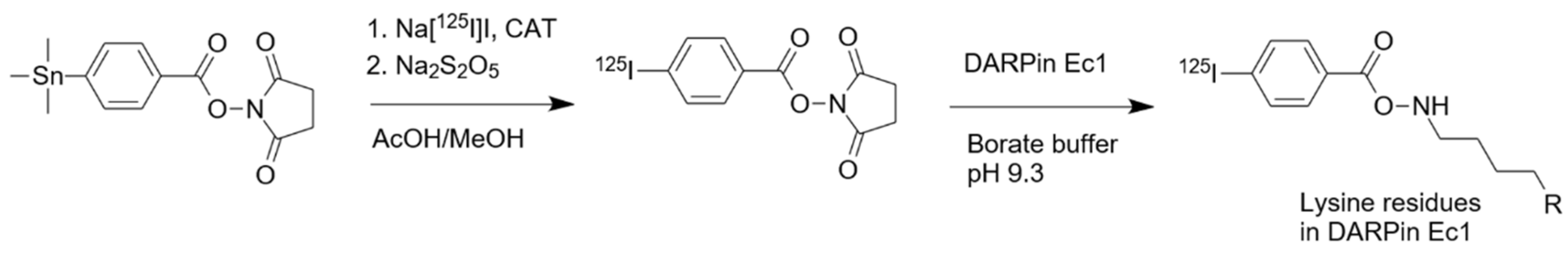
2.1. Radiolabeling and Stability
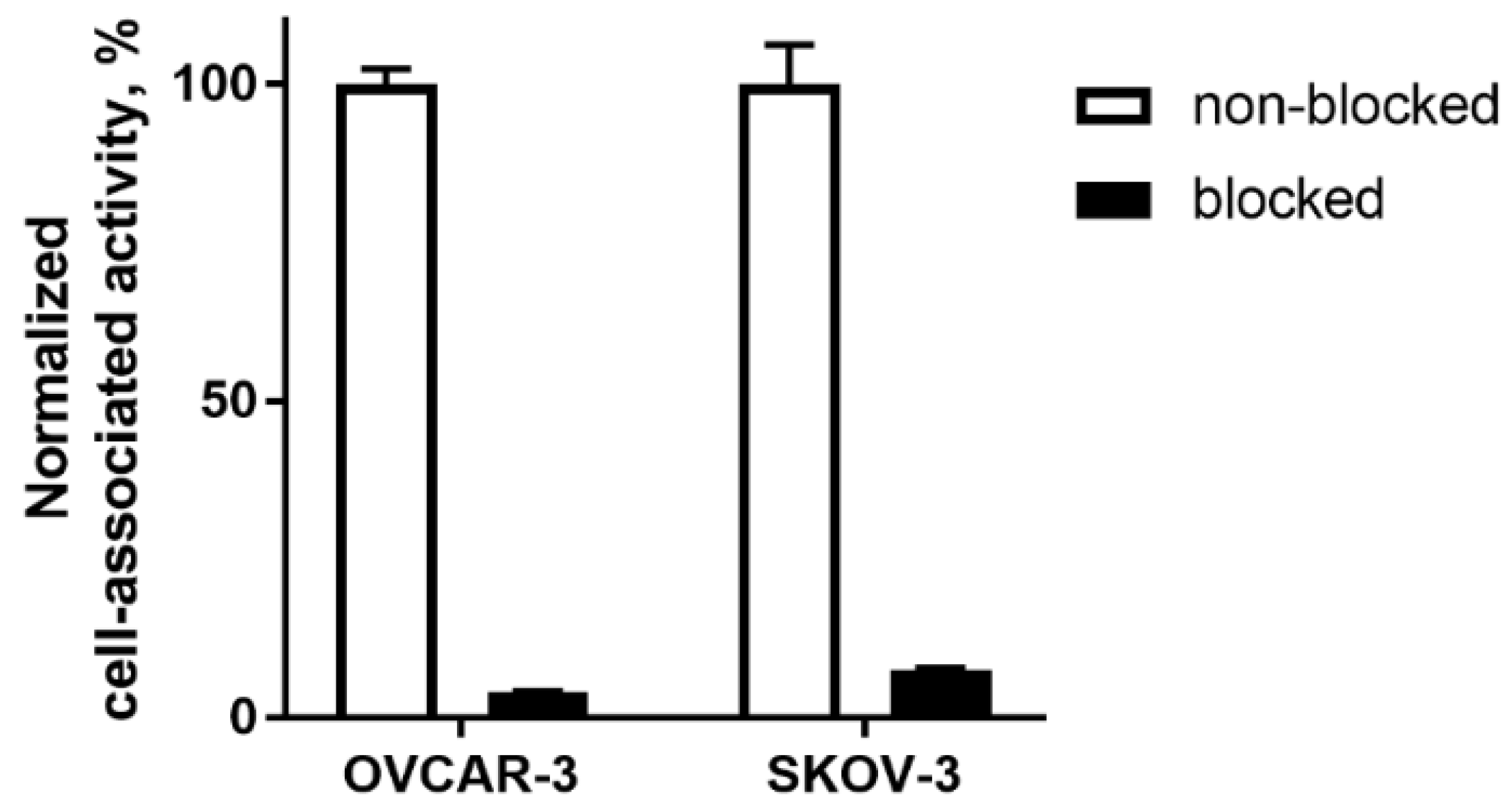
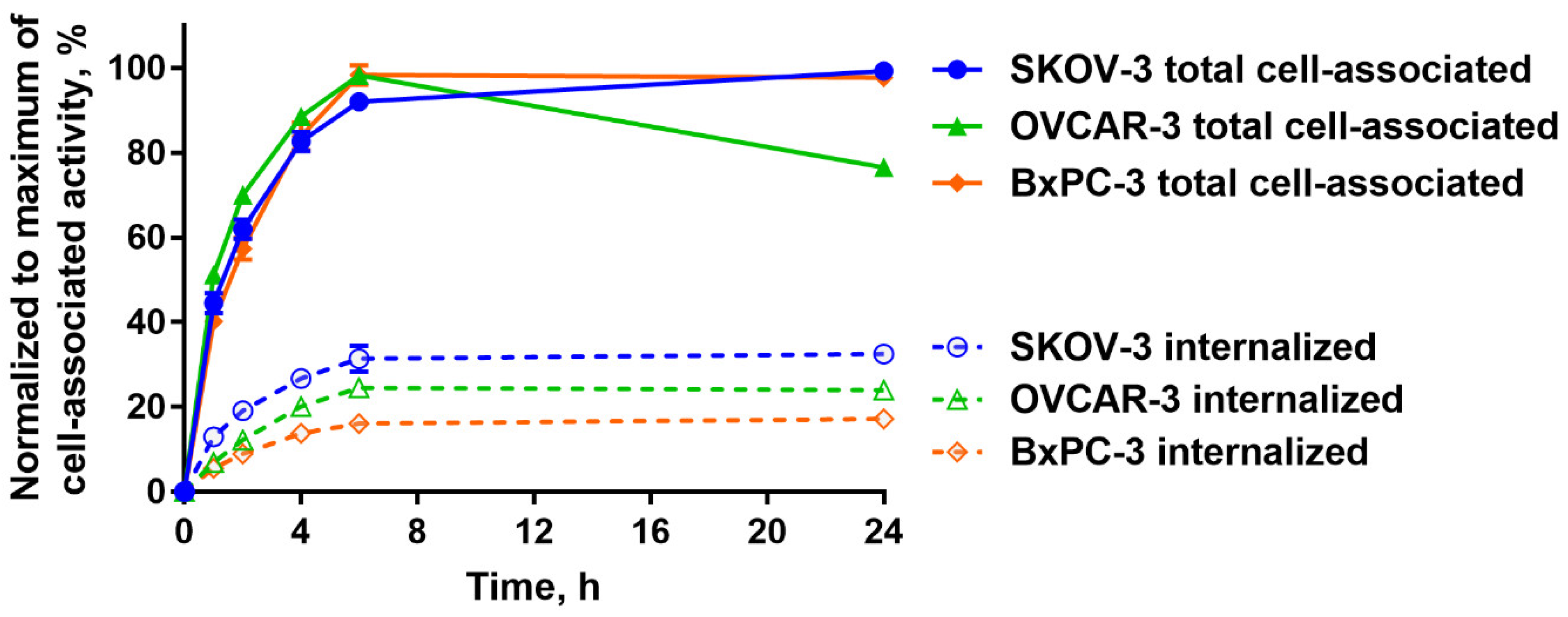
2.2. In Vitro Studies
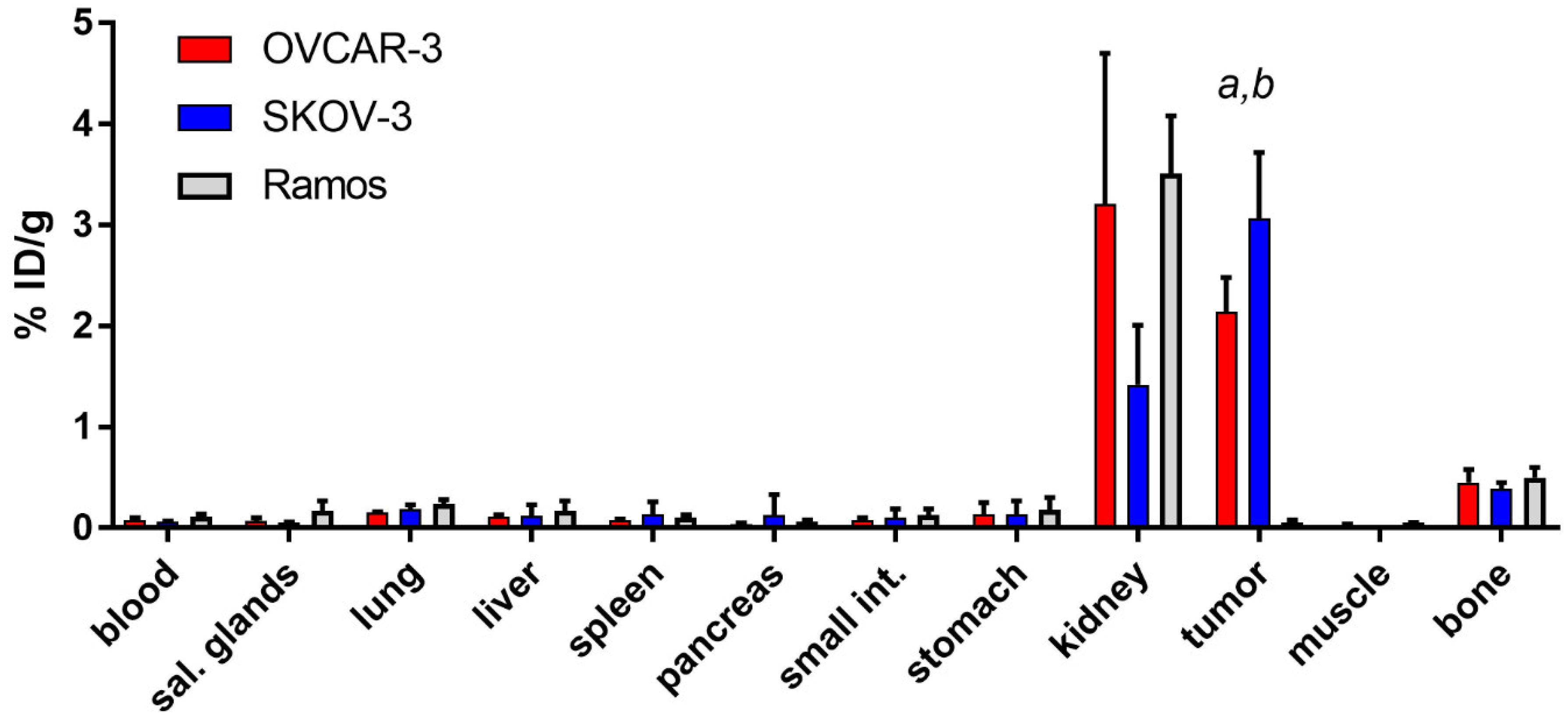
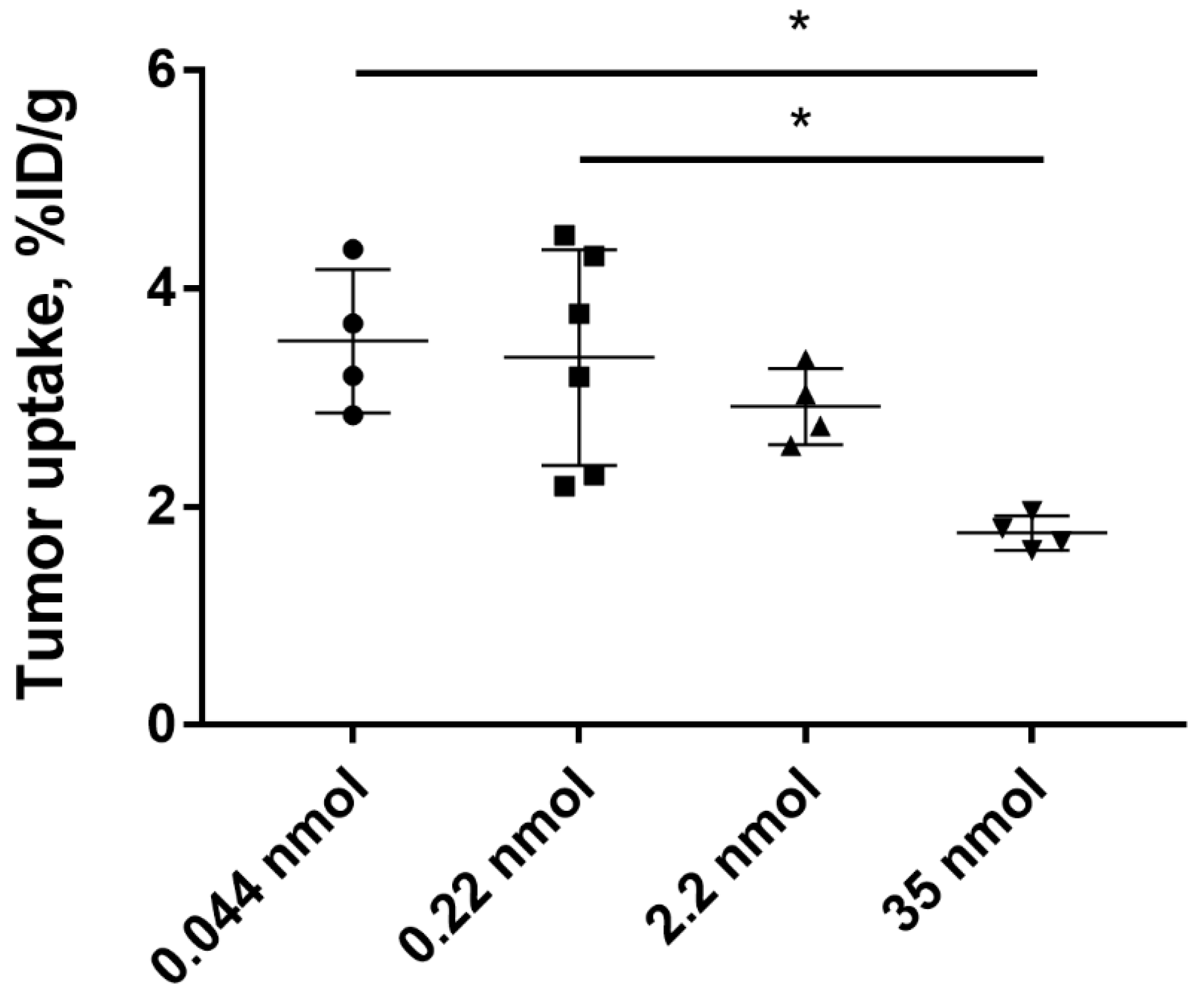
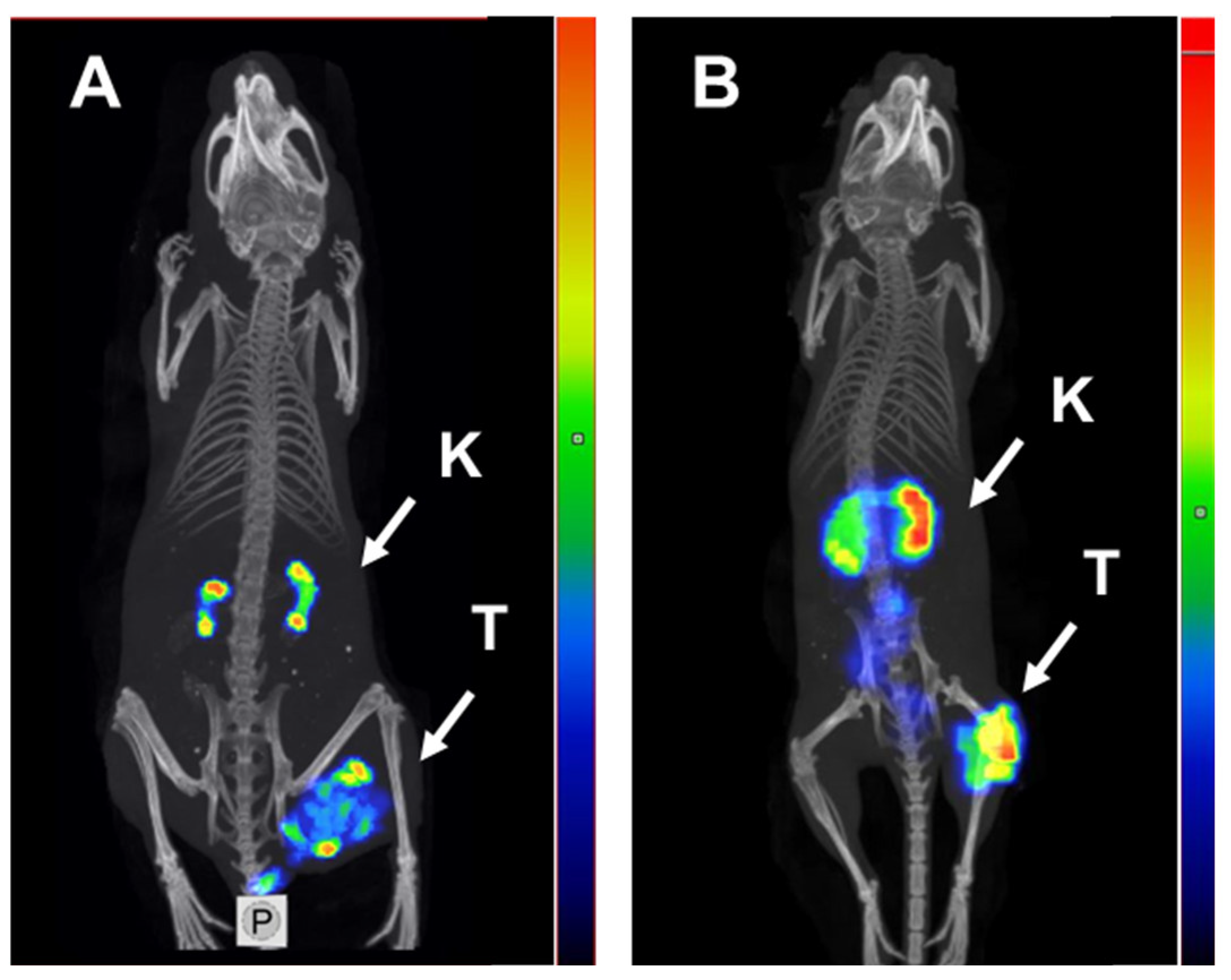
2.3. In Vivo Studies
3. Discussion
4. Materials and Methods
4.1. General Materials and Instruments
4.2. Protein Production and Radiolabeling
4.3. Binding Specificity and Cellular Processing Assays
4.4. Affinity Measurements Using LigandTracer
4.5. Animal Studies
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ADAPT BSA CT | Albumin-binding domain derived affinity protein Bovine serum albumin Computed tomography |
| DARPin | Designed ankyrin repeat protein |
| EpCAM | Epithelial cell adhesion molecule |
| HER2 | Human epidermal growth factor receptor 2 |
| iTLC PBS PET PIB | Instant thin layer chromatography Phosphate buffered saline Positron emission tomography Para-iodobenzoate |
| RGB SPECT | Red, green and blue color scale Single photon emission computed tomography |
References
- Narod, S. Can advanced-stage ovarian cancer be cured? Nat. Rev. Clin. Oncol. 2016, 13, 255–261. [Google Scholar] [CrossRef]
- Went, P.T.; Lugli, A.; Meier, S.; Bundi, M.; Mirlacher, M.; Sauter, G.; Dirnhofer, S. Frequent EpCam protein expression in human carcinomas. Hum. Pathol. 2004, 35, 122–128. [Google Scholar] [CrossRef]
- Spizzo, G.; Fong, D.; Wurm, M.; Ensinger, C.; Obrist, P.; Hofer, C.; Mazzoleni, G.; Gastl, G.; Went, P. EpCAM expression in primary tumour tissues and metastases: An immunohistochemical analysis. J. Clin. Pathol. 2011, 64, 415–420. [Google Scholar] [CrossRef] [PubMed]
- Spizzo, G.; Went, P.; Dirnhofer, S.; Obrist, P.; Moch, H.; Baeuerle, P.A.; Mueller-Holzner, E.; Marth, C.; Gastl, G.; Zeimet, A.G. Overexpression of epithelial cell adhesion molecule (Ep-CAM) is an independent prognostic marker for reduced survival of patients with epithelial ovarian cancer. Gynecol. Oncol. 2006, 103, 483–488. [Google Scholar] [CrossRef] [PubMed]
- Bellone, S.; Siegel, E.R.; Cocco, E.; Cargnelutti, M.; Silasi, D.A.; Azodi, M.; Schwartz, P.E.; Rutherford, T.J.; Pecorelli, S.; Santin, A.D. Overexpression of epithelial cell adhesion molecule in primary, metastatic, and recurrent/chemotherapy-resistant epithelial ovarian cancer: Implications for epithelial cell adhesion molecule-specific immunotherapy. Int. J. Gynecol. Cancer 2009, 19, 860–866. [Google Scholar] [CrossRef] [PubMed]
- ClinicalTrials.gov. Study in EpCAM Positive Patients with Symptomatic Malignant Ascites Using Removab Versus an Untreated Control Group; Identifier NCT00836654; National Library of Medicine: Bethesda, MD, USA, 2005. Available online: https://clinicaltrials.gov/ct2/show/NCT00836654 (accessed on 17 April 2020).
- ClinicalTrials.gov. Identifier NCT00635596, Phase I Study of MT110 in Lung Cancer (Adenocarcinoma and Small Cell), Gastric Cancer or Adenocarcinoma of the Gastro-Esophageal Junction, Colorectal Cancer, Breast Cancer, Hormone-Refractory Prostate Cancer, and Ovarian Cancer (MT110-101). Available online: https://clinicaltrials.gov/ct2/show/NCT00635596 (accessed on 17 April 2020).
- ClinicalTrials.gov. Study of the Safety of VB6-845 in Patients with Advanced Solid Tumours of Epithelial Origin; Identifier NCT00481936; National Library of Medicine: Bethesda, MD, USA, 2005. Available online: https://clinicaltrials.gov/ct2/show/NCT00481936 (accessed on 17 April 2020).
- Burges, A.; Wimberger, P.; Kümper, C.; Gorbounova, V.; Sommer, H.; Schmalfeldt, B.; Pfisterer, J.; Lichinitser, M.; Makhson, A.; Moiseyenko, V.; et al. Effective relief of malignant ascites in patients with advanced ovarian cancer by a trifunctional anti-EpCAM x anti-CD3 antibody: A phase I/II study. Clin. Cancer Res. 2007, 13, 3899–3905. [Google Scholar] [CrossRef]
- Frøysnes, I.S.; Andersson, Y.; Larsen, S.G.; Davidson, B.; Øien, J.T.; Julsrud, L.; Fodstad, Ø.; Dueland, S.; Flatmark, K. ImmunoPeCa trial: Long-term outcome following intraperitoneal MOC31PE immunotoxin treatment in colorectal peritoneal metastasis. Eur. J. Surg. Oncol. 2019. [Google Scholar] [CrossRef]
- Andersson, Y.; Inderberg, E.M.; Kvalheim, G.; Herud, T.M.; Engebraaten, O.; Flatmark, K.; Dueland, S.; Fodstad, Ø. Immune stimulatory effect of anti-EpCAM immunotoxin—Improved overall survival of metastatic colorectal cancer patients. Acta Oncol. 2020, 59, 404–409. [Google Scholar] [CrossRef]
- Andersson, Y.; Haavardtun, S.I.; Davidson, B.; Dørum, A.; Fleten, K.G.; Fodstad, Ø.; Flatmark, K. MOC31PE immunotoxin—Targeting peritoneal metastasis from epithelial ovarian cancer. Oncotarget 2017, 8, 61800–61809. [Google Scholar] [CrossRef]
- Schmidt, M.; Scheulen, M.E.; Dittrich, C.; Obrist, P.; Marschner, N.; Dirix, L.; Schmidt, M.; Rüttinger, D.; Schuler, M.; Reinhardt, C.; et al. An open-label, randomized phase II study of adecatumumab, a fully human anti-EpCAM antibody, as monotherapy in patients with metastatic breast cancer. Ann. Oncol. 2010, 21, 275–282. [Google Scholar] [CrossRef]
- Kosterink, J.G.; de Jonge, M.W.; Smit, E.F.; Piers, D.A.; Kengen, R.A.; Postmus, P.E.; Shochat, D.; Groen, H.J.; The, H.T.; de Leij, L. Pharmacokinetics and scintigraphy of indium-111-DTPA-MOC-31 in small-cell lung carcinoma. J. Nucl. Med. 1995, 36, 2356–2362. [Google Scholar] [PubMed]
- De Bree, R.; Roos, J.C.; Quak, J.J.; Den Hollander, W.; Snow, G.B.; Van Dongen, G.A. Clinical screening of monoclonal antibodies 323/A3, cSF-25 and K928 for suitability of targetting tumours in the upper aerodigestive and respiratory tract. Nucl. Med. Commun. 1994, 15, 613–627. [Google Scholar] [CrossRef] [PubMed]
- Breitz, H.B.; Tyler, A.; Bjorn, M.J.; Lesley, T.; Weiden, P.L. Clinical experience with Tc-99m nofetumomab merpentan (Verluma) radioimmunoscintigraphy. Clin. Nucl. Med. 1997, 22, 615–620. [Google Scholar] [CrossRef] [PubMed]
- Krasniqi, A.; D’Huyvetter, M.; Devoogdt, N.; Frejd, F.Y.; Sörensen, J.; Orlova, A.; Keyaerts, M.; Tolmachev, V. Same-Day Imaging Using Small Proteins: Clinical Experience and Translational Prospects in Oncology. J. Nucl. Med. 2018, 59, 885–891. [Google Scholar] [CrossRef]
- Sörensen, J.; Sandberg, D.; Sandström, M.; Wennborg, A.; Feldwisch, J.; Tolmachev, V.; Åström, G.; Lubberink, M.; Garske-Román, U.; Carlsson, J.; et al. First-in-human molecular imaging of HER2 expression in breast cancer metastases using the 111In-ABY-025 affibody molecule. J. Nucl. Med. 2014, 55, 730–735. [Google Scholar] [CrossRef]
- Sörensen, J.; Velikyan, I.; Sandberg, D.; Wennborg, A.; Feldwisch, J.; Tolmachev, V.; Orlova, A.; Sandström, M.; Lubberink, M.; Olofsson, H.; et al. Measuring HER2-Receptor Expression In Metastatic Breast Cancer Using [68Ga]ABY-025 Affibody PET/CT. Theranostics 2016, 6, 262–271. [Google Scholar] [CrossRef]
- Plückthun, A. Designed ankyrin repeat proteins (DARPins): Binding proteins for research, diagnostics, and therapy. Annu. Rev. Pharmacol. Toxicol. 2015, 55, 489–511. [Google Scholar] [CrossRef]
- Shilova, O.N.; Deyev, S.M. DARPins: Promising Scaffolds for Theranostics. Acta Naturae 2019, 11, 42–53. [Google Scholar] [CrossRef]
- Goldstein, R.; Sosabowski, J.; Livanos, M.; Leyton, J.; Vigor, K.; Bhavsar, G.; Nagy-Davidescu, G.; Rashid, M.; Miranda, E.; Yeung, J.; et al. Development of the Designed Ankyrin Repeat Protein (DARPin) G3 for HER2 Molecular Imaging. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 288–301. [Google Scholar] [CrossRef]
- Vorobyeva, A.; Bragina, O.; Altai, M.; Mitran, B.; Orlova, A.; Shulga, A.; Proshkina, G.; Chernov, V.; Tolmachev, V.; Deyev, S. Comparative Evaluation of Radioiodine and Technetium-Labeled DARPin 9_29 for Radionuclide Molecular Imaging of HER2 Expression in Malignant Tumors. Contrast Media Mol. Imaging 2018, 2018, 6930425. [Google Scholar] [CrossRef]
- Deyev, S.; Vorobyeva, A.; Schulga, A.; Proshkina, G.; Güler, R.; Löfblom, J.; Mitran, B.; Garousi, J.; Altai, M.; Buijs, J.; et al. Comparative Evaluation of Two DARPin Variants: Effect of Affinity, Size, and Label on Tumor Targeting Properties. Mol. Pharm. 2019, 3, 995–1008. [Google Scholar] [CrossRef] [PubMed]
- Vorobyeva, A.; Schulga, A.; Rinne, S.S.; Günther, T.; Orlova, A.; Deyev, S.; Tolmachev, V. Indirect radioiodination of DARPin G3 using N-succinimidyl-para-iodobenzoate improves the contrast of HER2 molecular imaging. Int. J. Mol. Sci. 2019, 20, 3047. [Google Scholar] [CrossRef] [PubMed]
- Stefan, N.; Martin-Killias, P.; Wyss-Stoeckle, S.; Honegger, A.; Zangemeister-Wittke, U.; Plückthun, A. DARPins recognizing the tumor-associated antigen EpCAM selected by phage and ribosome display and engineered for multivalency. J. Mol. Biol. 2011, 413, 826–843. [Google Scholar] [CrossRef] [PubMed]
- Martin-Killias, P.; Stefan, N.; Rothschild, S.; Plückthun, A.; Zangemeister-Wittke, U. A novel fusion toxin derived from an EpCAM-specific designed ankyrin repeat protein has potent antitumor activity. Clin. Cancer Res. 2011, 17, 100–110. [Google Scholar] [CrossRef]
- Simon, M.; Stefan, N.; Borsig, L.; Plückthun, A.; Zangemeister-Wittke, U. Increasing the antitumor effect of an EpCAM-targeting fusion toxin by facile click PEGylation. Mol. Cancer Ther. 2014, 13, 375–385. [Google Scholar] [CrossRef]
- Deyev, S.M.; Vorobyeva, A.; Schulga, A.; Abouzayed, A.; Günther, T.; Garousi, J.; Konovalova, E.; Ding, H.; Gräslund, T.; Orlova, A.; et al. Effect of a radiolabel biochemical nature on tumor-targeting properties of EpCAM-binding engineered scaffold protein DARPin Ec1. Int. J. Biol. Macromol. 2020, 145, 216–225. [Google Scholar] [CrossRef]
- Tolmachev, V.; Orlova, A.; Lundqvist, H. Approaches to improve cellular retention of radiohalogen labels delivered by internalising tumour-targeting proteins and peptides. Curr. Med. Chem. 2003, 10, 2447–2460. [Google Scholar] [CrossRef]
- Tolmachev, V.; Orlova, A. Affibody Molecules as Targeting Vectors for PET Imaging. Cancers 2020, 12, 651. [Google Scholar] [CrossRef]
- Orlova, A.; Magnusson, M.; Eriksson, T.L.; Nilsson, M.; Larsson, B.; Höidén-Guthenberg, I.; Widström, C.; Carlsson, J.; Tolmachev, V.; Ståhl, S.; et al. Tumor imaging using a picomolar affinity HER2 binding affibody molecule. Cancer Res. 2006, 66, 4339–4348. [Google Scholar] [CrossRef]
- Andersson, K.G.; Oroujeni, M.; Garousi, J.; Mitran, B.; Ståhl, S.; Orlova, A.; Löfblom, J.; Tolmachev, V. Feasibility of imaging of epidermal growth factor receptor expression with ZEGFR:2377 affibody molecule labeled with 99mTc using a peptide-based cysteine-containing chelator. Int. J. Oncol. 2016, 49, 2285–2293. [Google Scholar] [CrossRef]
- Garousi, J.; Lindbo, S.; Borin, J.; von Witting, E.; Vorobyeva, A.; Oroujeni, M.; Mitran, B.; Orlova, A.; Buijs, J.; Tolmachev, V.; et al. Comparative evaluation of dimeric and monomeric forms of ADAPT scaffold protein for targeting of HER2-expressing tumours. Eur. J. Pharm. Biopharm. 2019, 134, 37–48. [Google Scholar] [CrossRef]
- Altai, M.; Leitao, C.D.; Rinne, S.S.; Vorobyeva, A.; Atterby, C.; Ståhl, S.; Tolmachev, V.; Löfblom, J.; Orlova, A. Influence of Molecular Design on the Targeting Properties of ABD-Fused Mono- and Bi-Valent Anti-HER3 Affibody Therapeutic Constructs. Cells 2018, 7, 164. [Google Scholar] [CrossRef] [PubMed]
- Rinne, S.S.; Xu, T.; Dahlsson Leitao, C.; Ståhl, S.; Löfblom, J.; Orlova, A.; Tolmachev, V.; Vorobyeva, A. Influence of Residualizing Properties of the Radiolabel on Radionuclide Molecular Imaging of HER3 Using Affibody Molecules. Int. J. Mol. Sci. 2020, 21, 1312. [Google Scholar] [CrossRef] [PubMed]
- Altai, M.; Liu, H.; Ding, H.; Mitran, B.; Edqvist, P.H.; Tolmachev, V.; Orlova, A.; Gräslund, T. Affibody-derived drug conjugates: Potent cytotoxic molecules for treatment of HER2 over-expressing tumors. J. Control. Release 2018, 288, 84–95. [Google Scholar] [CrossRef] [PubMed]
- Wållberg, H.; Orlova, A. Slow Internalization of Anti-HER2 Synthetic Affibody Monomer 111In-DOTA-ZHER2:342-pep2: Implications for Development of Labeled Tracers. Cancer Biother. Radiopharm. 2008, 23, 435–442. [Google Scholar] [CrossRef]
- Tolmachev, V.; Orlova, A.; Andersson, K. Methods for Radiolabelling of Monoclonal Antibodies. Methods Mol. Biol. 2014, 1060, 309–330. [Google Scholar] [CrossRef]

| Cell Line | KD(pM) |
|---|---|
| OVCAR-3 | 35 ± 1 |
| SKOV-3 | 80 ± 10 |
| Tissue/Organ | OVCAR-3 | SKOV-3 |
|---|---|---|
| Blood | 30 ± 11 a | 48 ± 12 |
| Salivary glands | 36 ± 19 a | 59 ± 8 |
| Lung | 15 ± 4 | 16 ± 2 |
| Liver | 20 ± 4 a | 45 ± 10 |
| Spleen | 28 ± 6 | 40 ± 9 |
| Pancreas | 57 ± 17 a | 102 ± 17 |
| Small intestine | 30 ± 8 | 55 ± 16 |
| Stomach | 20 ± 10 a | 42 ± 7 |
| Kidney | 0.8 ± 0.4 a | 2.4 ± 0.8 |
| Muscle | 71 ± 25 | 97 ± 28 |
| Bone | 5 ± 2 | 8 ± 2 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vorobyeva, A.; Konovalova, E.; Xu, T.; Schulga, A.; Altai, M.; Garousi, J.; Rinne, S.S.; Orlova, A.; Tolmachev, V.; Deyev, S. Feasibility of Imaging EpCAM Expression in Ovarian Cancer Using Radiolabeled DARPin Ec1. Int. J. Mol. Sci. 2020, 21, 3310. https://doi.org/10.3390/ijms21093310
Vorobyeva A, Konovalova E, Xu T, Schulga A, Altai M, Garousi J, Rinne SS, Orlova A, Tolmachev V, Deyev S. Feasibility of Imaging EpCAM Expression in Ovarian Cancer Using Radiolabeled DARPin Ec1. International Journal of Molecular Sciences. 2020; 21(9):3310. https://doi.org/10.3390/ijms21093310
Chicago/Turabian StyleVorobyeva, Anzhelika, Elena Konovalova, Tianqi Xu, Alexey Schulga, Mohamed Altai, Javad Garousi, Sara S. Rinne, Anna Orlova, Vladimir Tolmachev, and Sergey Deyev. 2020. "Feasibility of Imaging EpCAM Expression in Ovarian Cancer Using Radiolabeled DARPin Ec1" International Journal of Molecular Sciences 21, no. 9: 3310. https://doi.org/10.3390/ijms21093310
APA StyleVorobyeva, A., Konovalova, E., Xu, T., Schulga, A., Altai, M., Garousi, J., Rinne, S. S., Orlova, A., Tolmachev, V., & Deyev, S. (2020). Feasibility of Imaging EpCAM Expression in Ovarian Cancer Using Radiolabeled DARPin Ec1. International Journal of Molecular Sciences, 21(9), 3310. https://doi.org/10.3390/ijms21093310








