The Histological, Effectoromic, and Transcriptomic Analyses of Solanum pinnatisectum Reveal an Upregulation of Multiple NBS-LRR Genes Suppressing Phytophthora infestans Infection
Abstract
1. Introduction
2. Results
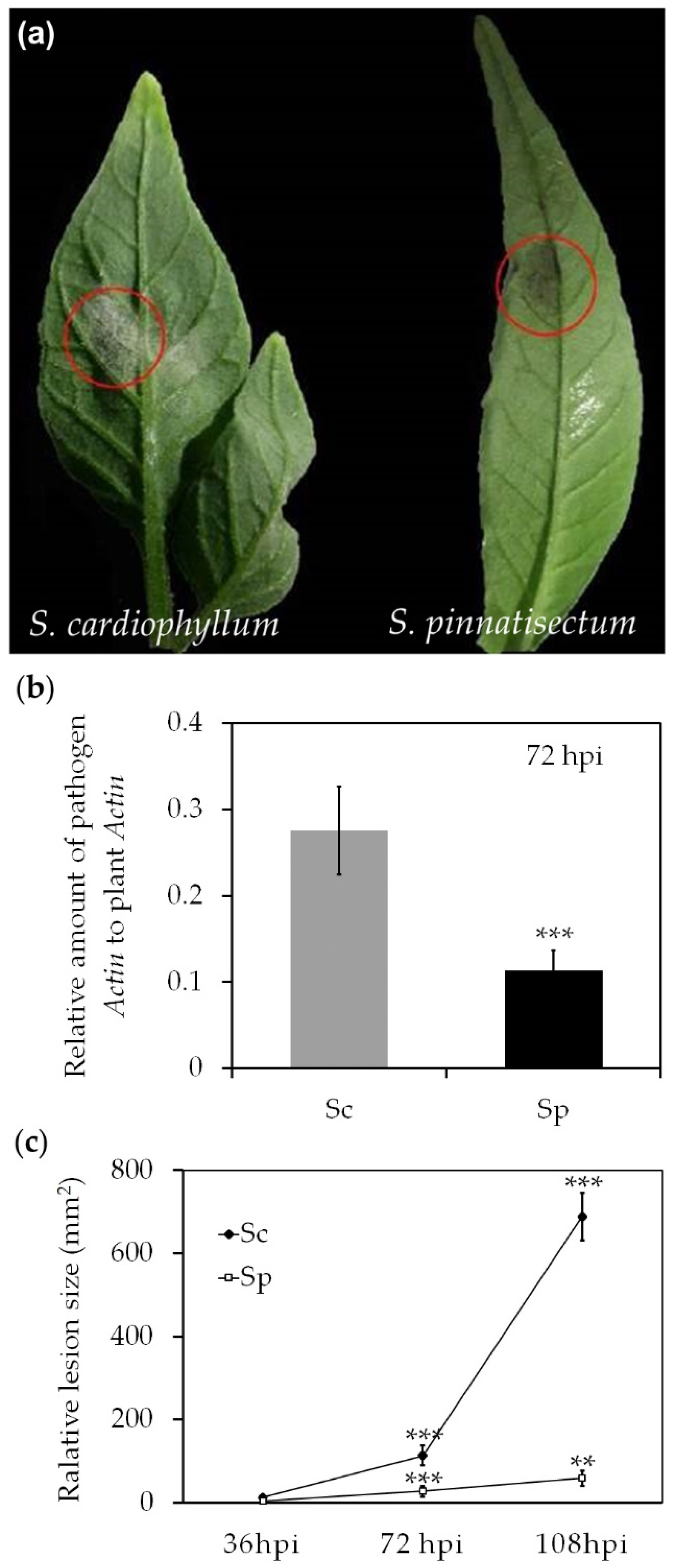
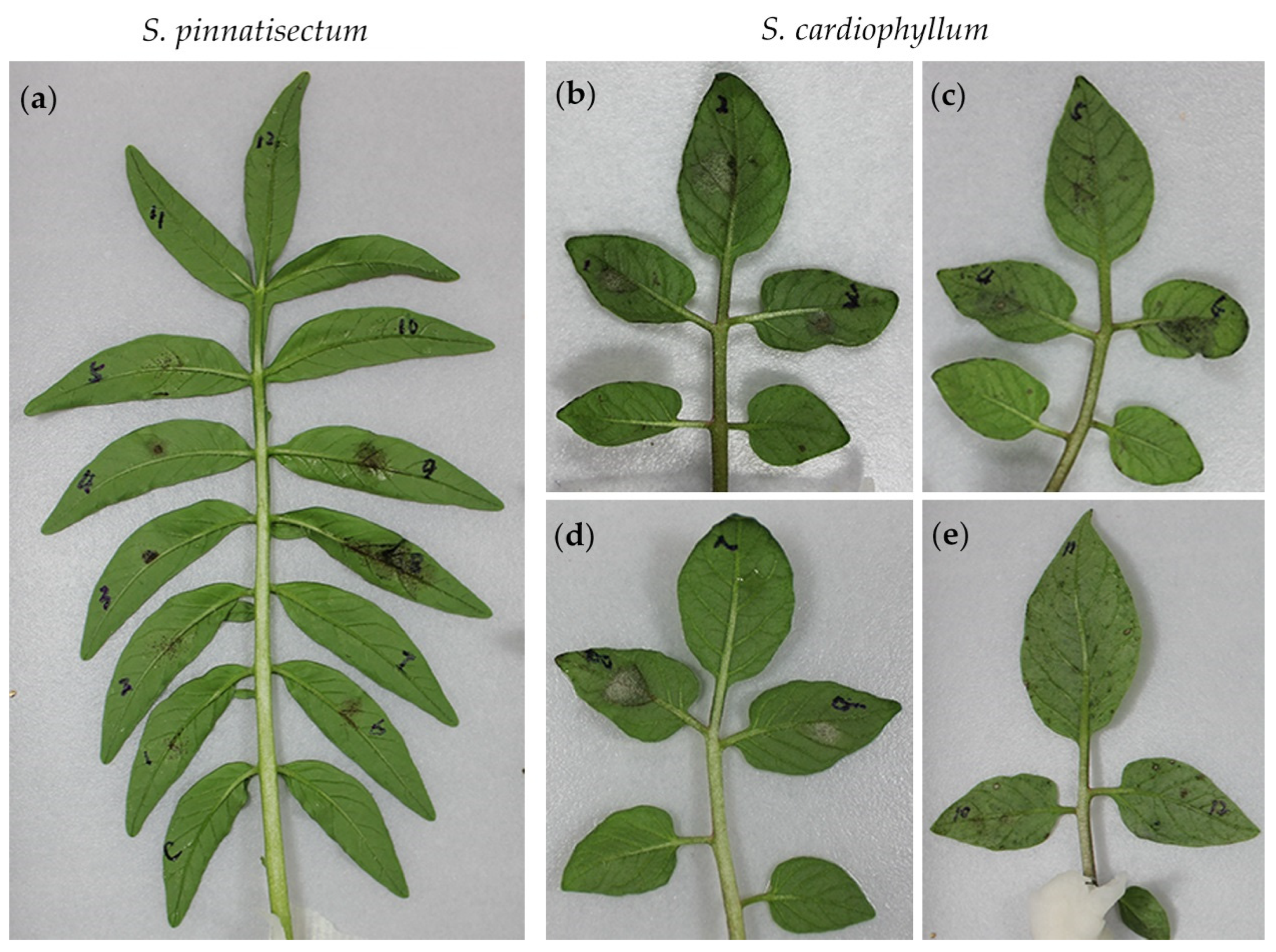
2.1. Infection with P. infestans on S. pinnatisectum is Evident
2.2. Histological Characteristics of S. pinnatisectum Infected by P. infestans
2.3. S. pinnatisectum Reveals Complicated Pathotypes Based on Multiple R Genes
2.4. RxLR Effector Screening Reveals Different R Protein Composition in S. pinnatisectum and S. cardiophyllum
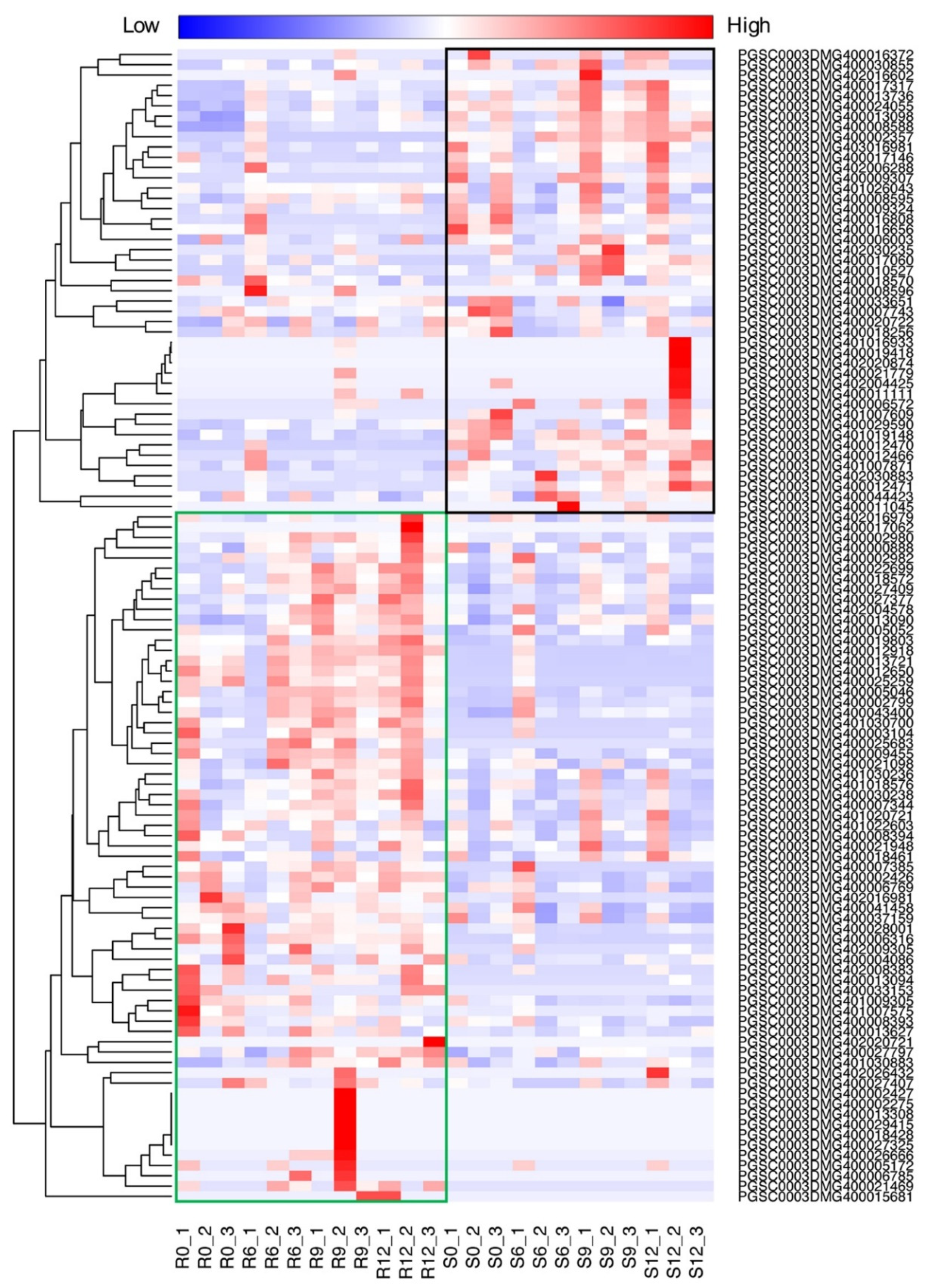
2.5. Transcriptome Dynamics of NLR Genes in Susceptible and Resistant Potatoes against P. infestans
3. Discussion
4. Materials and Methods
4.1. Plant and Pathogen Materials
4.2. Lesion Measurement and Biomass Analysis
4.3. Microscopic Characterization of S. pinnatisectum Infected by P. infestans
4.4. In planta Transient Expression of Known Avr Genes of P. infestans
4.5. RNA-seq and Profiling of the Expression of NLR Genes
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| Avr gene | Avirulence gene |
| EBN | Endosperm Balance Number |
| ETI | Effector Triggered Immunity |
| hpi | hours post inoculation |
| HR | Hypersensitive Response |
| NLR | NBS-LRR (nucleotide-binding site and leucine-rich repeat) |
| PRR | Pattern Recognition Receptor |
| PVX | Potato Virus X |
| QTL | Quantitative Trait Loci |
| RENseq | Resistance Gene Enrichment and Sequencing Method |
| R gene | Resistance gene |
| Rpi | Resistance (R) genes to P. infestans |
| RSA | Rye Sucrose Agar |
References
- Savary, S.; Willocquet, L.; Pethybridge, S.J.; Esker, P.; McRoberts, N.; Nelson, A. The global burden of pathogens and pests on major food crops. Nat. Ecol. Evol. 2019, 3, 430–439. [Google Scholar] [CrossRef] [PubMed]
- Fry, W. Phytophthora infestans: The plant (and R gene) destroyer. Mol. Plant. Pathol. 2008, 9, 385–402. [Google Scholar] [CrossRef] [PubMed]
- Fry, W.E.; Birch, P.R.J.; Judelson, H.S.; Grunwald, N.J.; Danies, G.; Everts, K.L.; Gevens, A.J.; Gugino, B.K.; Johnson, D.A.; Johnson, S.B.; et al. Five reasons to consider Phytophthora infestans a reemerging pathogen. Phytopathology 2015, 105, 966–981. [Google Scholar] [CrossRef] [PubMed]
- Cooke, D.E.; Cano, L.M.; Raffaele, S.; Bain, R.A.; Cooke, L.R.; Etherington, G.J.; Deahl, K.L.; Farrer, R.A.; Gilroy, E.M.; Goss, E.M.; et al. Genome analyses of an aggressive and invasive lineage of the Irish potato famine pathogen. PLoS Pathog. 2012, 8, e1002940. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Yin, J.L.; Sun, J.P.; Ma, H.M.; Ma, Y.F.; Quan, J.L.; Shan, W.X. Population structure of the late blight pathogen Phytophthora infestans in a potato germplasm nursery in two consecutive years. Phytopathology 2015, 105, 771–777. [Google Scholar] [CrossRef]
- Nowicki, M.; Foolad, M.R.; Nowakowska, M.; Kozik, E.U. Potato and tomato late blight caused by Phytophthora infestans: An overview of pathology and resistance breeding. Plant. Dis. 2012, 96, 4–17. [Google Scholar] [CrossRef]
- Du, J.; Vleeshouwers, V.G.A.A. New strategies towards durable late blight resistance in potato. In The Potato Genome; Kumar Chakrabarti, S., Xie, C., Kumar Tiwari, J., Eds.; Springer: Cham, Switzerland, 2017; pp. 161–169. [Google Scholar]
- Vleeshouwers, V.G.A.A.; Finkers, R.; Budding, D.; Visser, M.; Jacobs, M.M.J.; van Berloo, R.; Pel, M.; Champouret, N.; Bakker, E.; Krenek, P.; et al. SolRgene: An online database to explore disease resistance genes in tuber-bearing Solanum species. BMC Plant. Biol. 2011, 11, 116. [Google Scholar] [CrossRef]
- Rodewald, J.; Trognitz, B. Solanum resistance genes against Phytophthora infestans and their corresponding avirulence genes. Mol. Plant. Pathol. 2013, 14, 740–757. [Google Scholar] [CrossRef]
- Marla, S.S. Structural analysis of resistance (R) genes in potato (Solanum species) genome. In The Potato Genome; Kumar Chakrabarti, S., Xie, C., Kumar Tiwari, J., Eds.; Springer: Cham, Switzerland, 2017; pp. 269–281. [Google Scholar]
- Zhu, S.X.; Li, Y.; Vossen, J.H.; Visser, R.G.F.; Jacobsen, E. Functional stacking of three resistance genes against Phytophthora infestans in potato. Transgenic Res. 2012, 21, 89–99. [Google Scholar] [CrossRef]
- Vleeshouwers, V.G.; Oliver, R.P. Effectors as tools in disease resistance breeding against biotrophic, hemibiotrophic, and necrotrophic plant pathogens. Mol. Plant. Microbe Interact. 2014, 27, 196–206. [Google Scholar] [CrossRef]
- Oh, S.K.; Young, C.; Lee, M.; Oliva, R.; Bozkurt, T.O.; Cano, L.M.; Win, J.; Bos, J.I.B.; Liu, H.Y.; van Damme, M.; et al. In planta expression screens of Phytophthora infestans RXLR effectors reveal diverse phenotypes, including activation of the Solanum bulbocastanum disease resistance protein Rpi-blb2. Plant Cell 2009, 21, 2928–2947. [Google Scholar] [CrossRef] [PubMed]
- Vleeshouwers, V.G.A.A.; Rietman, H.; Krenek, P.; Champouret, N.; Young, C.; Oh, S.K.; Wang, M.Q.; Bouwmeester, K.; Vosman, B.; Visser, R.G.F.; et al. Effector genomics accelerates discovery and functional profiling of potato disease resistance and Phytophthora infestans avirulence genes. PLoS ONE 2008, 3, e2875. [Google Scholar] [CrossRef] [PubMed]
- Rietman, H.; Bijsterbosch, G.; Cano, L.M.; Lee, H.R.; Vossen, J.H.; Jacobsen, E.; Visser, R.G.F.; Kamoun, S.; Vleeshouwers, V.G.A.A. Qualitative and quantitative late blight resistance in the potato cultivar Sarpo Mira is determined by the perception of five distinct RXLR effectors. Mol. Plant. Microbe 2012, 25, 910–919. [Google Scholar] [CrossRef] [PubMed]
- Lokossou, A.A.; Rietman, H.; Wang, M.Q.; Krenek, P.; van der Schoot, H.; Henken, B.; Hoekstra, R.; Vleeshouwers, V.G.A.A.; van der Vossen, E.A.G.; Visser, R.G.F.; et al. Diversity, distribution, and evolution of Solanum bulbocastanum late blight resistance genes. Mol. Plant. Microbe 2010, 23, 1206–1216. [Google Scholar] [CrossRef]
- Jupe, F.; Pritchard, L.; Etherington, G.J.; Mackenzie, K.; Cock, P.J.; Wright, F.; Sharma, S.K.; Bolser, D.; Bryan, G.J.; Jones, J.D.; et al. Identification and localisation of the NB-LRR gene family within the potato genome. BMC Genom. 2012, 13, 75. [Google Scholar] [CrossRef]
- Kuhl, J.C.; Hanneman, R.E.; Havey, M.J. Characterization and mapping of Rpi1, a late-blight resistance locus from diploid (1EBN) Mexican Solanum pinnatisectum. Mol. Genet. Genom. 2001, 265, 977–985. [Google Scholar] [CrossRef]
- Chen, Q.; Kawchuk, L.M.; Lynch, D.R.; Goettel, M.S.; Fujimoto, D.K. Identification of late blight, Colorado potato beetle, and blackleg resistance in three Mexican and two south American wild 2x (1EBN) Solanum species. Am. J. Potato Res. 2003, 80, 9–19. [Google Scholar] [CrossRef]
- Yang, L.; Wang, D.D.; Xu, Y.; Zhao, H.; Wang, L.; Cao, X.L.; Chen, Y.; Chen, Q. A new resistance gene against potato late blight originating from Solanum pinnatisectum located on potato chromosome 7. Front. Plant. Sci. 2017, 8, 1729. [Google Scholar] [CrossRef]
- Park, T.H.; Vleeshouwers, V.G.A.A.; Jacobsen, E.; van der Vossen, E.; Visser, R.G.F. Molecular breeding for resistance to Phytophthora infestans (Mont.) de Bary in potato (Solanum tuberosum L.): A perspective of cisgenesis. Plant Breed 2009, 128, 109–117. [Google Scholar] [CrossRef]
- Ghislain, M.; Trognitz, B.; Herrera, M.D.; Solis, J.; Casallo, G.; Vasquez, C.; Hurtado, O.; Castillo, R.; Portal, L.; Orrillo, M. Genetic loci associated with field resistance to late blight in offspring of Solanum phureja and S. tuberosum grown under short-day conditions. Appl. Genet. 2001, 103, 433–442. [Google Scholar] [CrossRef]
- Lokossou, A.A.; Park, T.H.; van Arkel, G.; Arens, M.; Ruyter-Spira, C.; Morales, J.; Whisson, S.C.; Birch, P.R.J.; Visser, R.G.F.; Jacobsen, E.; et al. Exploiting knowledge of R/Avr genes to rapidly clone a new LZ-NBS-LRR family of late blight resistance genes from potato linkage group IV. Mol. Plant. Microbe 2009, 22, 630–641. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.L. Research on Late Blight Resistance and Amino Acid Transporter Genes in Potato. Master’s Thesis, Northwest A&F University, Yangling, China, June 2017. [Google Scholar]
- Yin, J.L.; Gu, B.; Huang, G.Y.; Tian, Y.; Quan, J.L.; Lindqvist-Kreuze, H.; Shan, W.X. Conserved RXLR effector genes of Phytophthora infestans expressed at the early stage of potato infection are suppressive to host defense. Front. Plant Sci. 2017, 8, 2155. [Google Scholar] [CrossRef] [PubMed]
- Caplan, J.; Dinesh-Kumar, S.P. Recognition and signal transduction associated with R gene-mediated resistance. In Natural Resistance Mechanisms of Plants to Viruses; Loebenstein, G., Carr, J.P., Eds.; Springer: Dordrecht, Switzerland, 2006; pp. 73–98. [Google Scholar] [CrossRef]
- Han, Q.M.; Thieme, R.; Gao, X.N.; Kang, Z.S.; Huang, L.L. Investigation of host responses of different potato genotypes at tissue, cellular and subcellular levels after infection with Phytophthora infestans. Am. J. Potato Res. 2013, 90, 524–532. [Google Scholar] [CrossRef]
- Lenman, M.; Ali, A.; Muhlenbock, P.; Carlson-Nilsson, U.; Liljeroth, E.; Champouret, N.; Vleeshouwers, V.G.; Andreasson, E. Effector-driven marker development and cloning of resistance genes against Phytophthora infestans in potato breeding clone SW93-1015. Appl. Genet. 2016, 129, 105–115. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Liu, Z.; Halterman, D.A. Molecular determinants of resistance activation and suppression by Phytophthora infestans effector IPI-O. PLoS Pathog. 2012, 8, e1002595. [Google Scholar] [CrossRef]
- Dussault-Benoit, C.; Arsenault-Labrecque, G.; Sonah, H.; Belzile, F.; Belanger, R.R. Discriminant haplotypes of avirulence genes of Phytophthora sojae lead to a molecular assay to predict phenotypes. Mol Plant. Pathol. 2020, 21, 318–329. [Google Scholar] [CrossRef]
- Gao, C.; Xu, H.; Huang, J.; Sun, B.; Zhang, F.; Savage, Z.; Duggan, C.; Yan, T.; Wu, C.H.; Wang, Y.; et al. Pathogen manipulation of chloroplast function triggers a light-dependent immune recognition. Proc. Natl. Acad. Sci. USA 2020, 117, 9613–9620. [Google Scholar] [CrossRef]
- Zhao, H.; Shao, G.D.; Gao, W.X.; Gu, B. Double-barreled particle bombardment mediated transient gene expression in Arabidopsis. Chin. Bull. Bot. 2020, 55. Available online: http://www.chinbullbotany.com/CN/10.11983/CBB19169 (accessed on 28 November 2019). [CrossRef]
- Saunders, D.G.O.; Breen, S.; Win, J.; Schornack, S.; Hein, I.; Bozkurt, T.O.; Champouret, N.; Vleeshouwers, V.G.A.A.; Birch, P.R.J.; Gilroy, E.M.; et al. Host protein BSL1 associates with Phytophthora infestans RXLR effector AVR2 and the Solanum demissum immune receptor R2 to mediate disease resistance. Plant. Cell 2012, 24, 3420–3434. [Google Scholar] [CrossRef]
- Orbegozo, J.; Roman, M.L.; Rivera, C.; Gamboa, S.; Tovar, J.C.; Forbes, G.A.; Lindqvist-Kreuze, H.; Kreuze, J.F.; Ghislain, M. Rpi-blb2 gene from Solanum bulbocastanum confers extreme resistance to late blight disease in potato. Plant. Cell Tiss. Org. 2016, 125, 269–281. [Google Scholar] [CrossRef]
- Jo, K.R.; Kim, C.J.; Kim, S.J.; Kim, T.Y.; Bergervoet, M.; Jongsma, M.A.; Visser, R.G.; Jacobsen, E.; Vossen, J.H. Development of late blight resistant potatoes by cisgene stacking. BMC Biotechnol. 2014, 14, 50. [Google Scholar] [CrossRef] [PubMed]
- Vleeshouwers, V.G.A.A.; Raffaele, S.; Vossen, J.H.; Champouret, N.; Oliva, R.; Segretin, M.E.; Rietman, H.; Cano, L.M.; Lokossou, A.; Kessel, G.; et al. Understanding and exploiting late blight resistance in the age of effectors. Annu. Rev. Phytopathol. 2011, 49, 507–531. [Google Scholar] [CrossRef] [PubMed]
- Andolfo, G.; Jupe, F.; Witek, K.; Etherington, G.J.; Ercolano, M.R.; Jones, J.D.G. Defining the full tomato NB-LRR resistance gene repertoire using genomic and cDNA RenSeq. BMC Plant. Biol. 2014, 14, 120. [Google Scholar] [CrossRef] [PubMed]
- Witek, K.; Jupe, F.; Witek, A.I.; Baker, D.; Clark, M.D.; Jones, J.D.G. Accelerated cloning of a potato late blight-resistance gene using RenSeq and SMRT sequencing. Nat. Biotechnol. 2016, 34, 656–660. [Google Scholar] [CrossRef]
- Anderson, R.G.; McDowell, J.M. A PCR assay for the quantification of growth of the oomycete pathogen Hyaloperonospora arabidopsidis in Arabidopsis thaliana. Mol. Plant Pathol. 2015, 16, 893–898. [Google Scholar] [CrossRef]
- Wang, Y.; Meng, Y.L.; Zhang, M.; Tong, X.M.; Wang, Q.H.; Sun, Y.Y.; Quan, J.L.; Govers, F.; Shan, W.X. Infection of Arabidopsis thaliana by Phytophthora parasitica and identification of variation in host specificity. Mol. Plant. Pathol. 2011, 12, 187–201. [Google Scholar] [CrossRef]
- Kim, D.; Landmead, B.; Salzberg, S.L. HISAT: A fast spliced aligner with low memory requirements. Nat. Methods 2015, 12, 357–360. [Google Scholar] [CrossRef]
- Liao, Y.; Smyth, G.K.; Shi, W. featureCounts: An efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 2014, 30, 923–930. [Google Scholar] [CrossRef]
- Xu, X.; Pan, S.K.; Cheng, S.F.; Zhang, B.; Mu, D.S.; Ni, P.X.; Zhang, G.Y.; Yang, S.; Li, R.Q.; Wang, J.; et al. Genome sequence and analysis of the tuber crop potato. Nature 2011, 475, 189–194. [Google Scholar] [CrossRef]
- Mistry, J.; Finn, R.D.; Eddy, S.R.; Bateman, A.; Punta, M. Challenges in homology search: HMMER3 and convergent evolution of coiled-coil regions. Nucleic Acids Res. 2013, 41. [Google Scholar] [CrossRef]
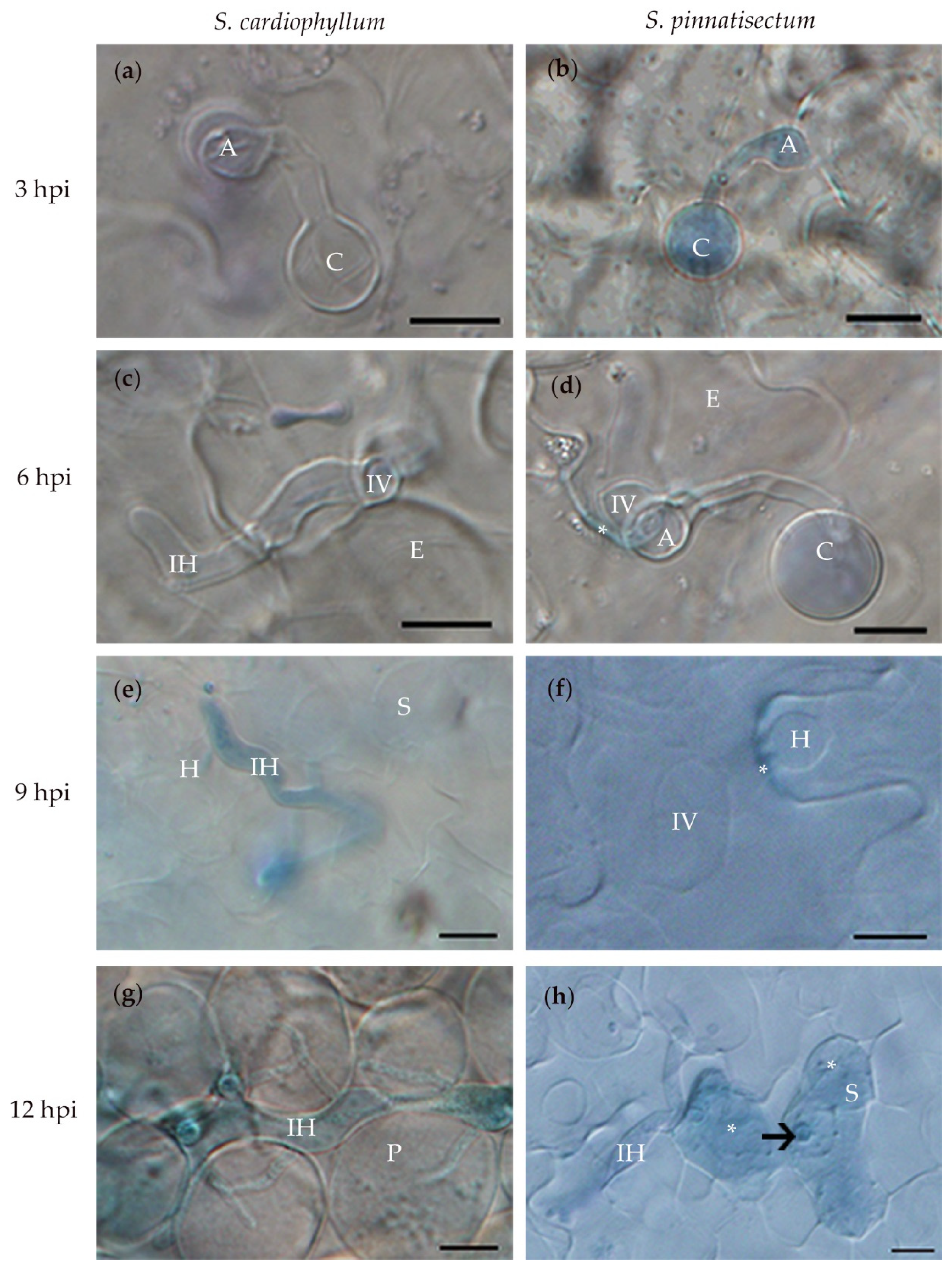

| Time/hpi* | Solanum spp. | Cysts (C) | Hyphae Infected (HI) | ||||
|---|---|---|---|---|---|---|---|
| GC/C | A/C | HI/C | HE/HI | HS/HI | HP/HI | ||
| 3 | S. c | 159/177 | 159/177 | 33/177 | 33/33 | 0/33 | 0/33 |
| S. p | 77/94 | 61/94 | 5/94 | 5/5 | 0/5 | 0/5 | |
| 6 | S. c | 162/162 | 155/162 | 134/162 | 109/134 | 25/134 | 0/134 |
| S. p | 147/148 | 138/148 | 113/148 | 112/113 | 1/113 | 0/113 | |
| 9 | S. c | 272/272 | 272/272 | 272/272 | 162/272 | 110/272 | 0/272 |
| S. p | 119/119 | 119/119 | 116/119 | 109/116 | 7/116 | 0/116 | |
| 12 | S. c | 145/145 | 145/145 | 145/145 | 25/145 | 84/145 | 36/145 |
| S. p | 84/84 | 84/84 | 83/84 | 77/83 | 5/83 | 1/83 | |
| Isolate | Avirulence on R1-R11 Differential Host | Virulence Phenotype | |
|---|---|---|---|
| S. pinnatisectum | S. cardiophyllum | ||
| 1 | 1 | + | +++ |
| 2 | 2, 5, 9 | + | +++ |
| 3 | 1, 2, 4, 5, 7, 9, 10, 11 | +/- | ++ |
| 4 | 5 | +/- | +++ |
| 5 | 1, 2, 5, 6, 7, 9, 10, 11 | +/- | + |
| 6 | 1, 2, 5, 6, 7, 8, 9, 10, 11 | + | +++ |
| 7 | 7 | - | - |
| 8 | 1, 5, 8, 9, 10, 11 | +++ | +++ |
| 9 | 2, 5, 8, 9 | ++ | +++ |
| 10 | 9 | +/- | ++ |
| 11 | 1, 7, 9, 11 | - | ++ |
| 12 | 0 | +/- | ++ |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gu, B.; Cao, X.; Zhou, X.; Chen, Z.; Wang, Q.; Liu, W.; Chen, Q.; Zhao, H. The Histological, Effectoromic, and Transcriptomic Analyses of Solanum pinnatisectum Reveal an Upregulation of Multiple NBS-LRR Genes Suppressing Phytophthora infestans Infection. Int. J. Mol. Sci. 2020, 21, 3211. https://doi.org/10.3390/ijms21093211
Gu B, Cao X, Zhou X, Chen Z, Wang Q, Liu W, Chen Q, Zhao H. The Histological, Effectoromic, and Transcriptomic Analyses of Solanum pinnatisectum Reveal an Upregulation of Multiple NBS-LRR Genes Suppressing Phytophthora infestans Infection. International Journal of Molecular Sciences. 2020; 21(9):3211. https://doi.org/10.3390/ijms21093211
Chicago/Turabian StyleGu, Biao, Xiaoli Cao, Xiaoli Zhou, Zhaodan Chen, Qinhu Wang, Wei Liu, Qin Chen, and Hua Zhao. 2020. "The Histological, Effectoromic, and Transcriptomic Analyses of Solanum pinnatisectum Reveal an Upregulation of Multiple NBS-LRR Genes Suppressing Phytophthora infestans Infection" International Journal of Molecular Sciences 21, no. 9: 3211. https://doi.org/10.3390/ijms21093211
APA StyleGu, B., Cao, X., Zhou, X., Chen, Z., Wang, Q., Liu, W., Chen, Q., & Zhao, H. (2020). The Histological, Effectoromic, and Transcriptomic Analyses of Solanum pinnatisectum Reveal an Upregulation of Multiple NBS-LRR Genes Suppressing Phytophthora infestans Infection. International Journal of Molecular Sciences, 21(9), 3211. https://doi.org/10.3390/ijms21093211





