Sildenafil Reduces Expression and Release of IL-6 and IL-8 Induced by Reactive Oxygen Species in Systemic Sclerosis Fibroblasts
Abstract
1. Introduction
2. Results
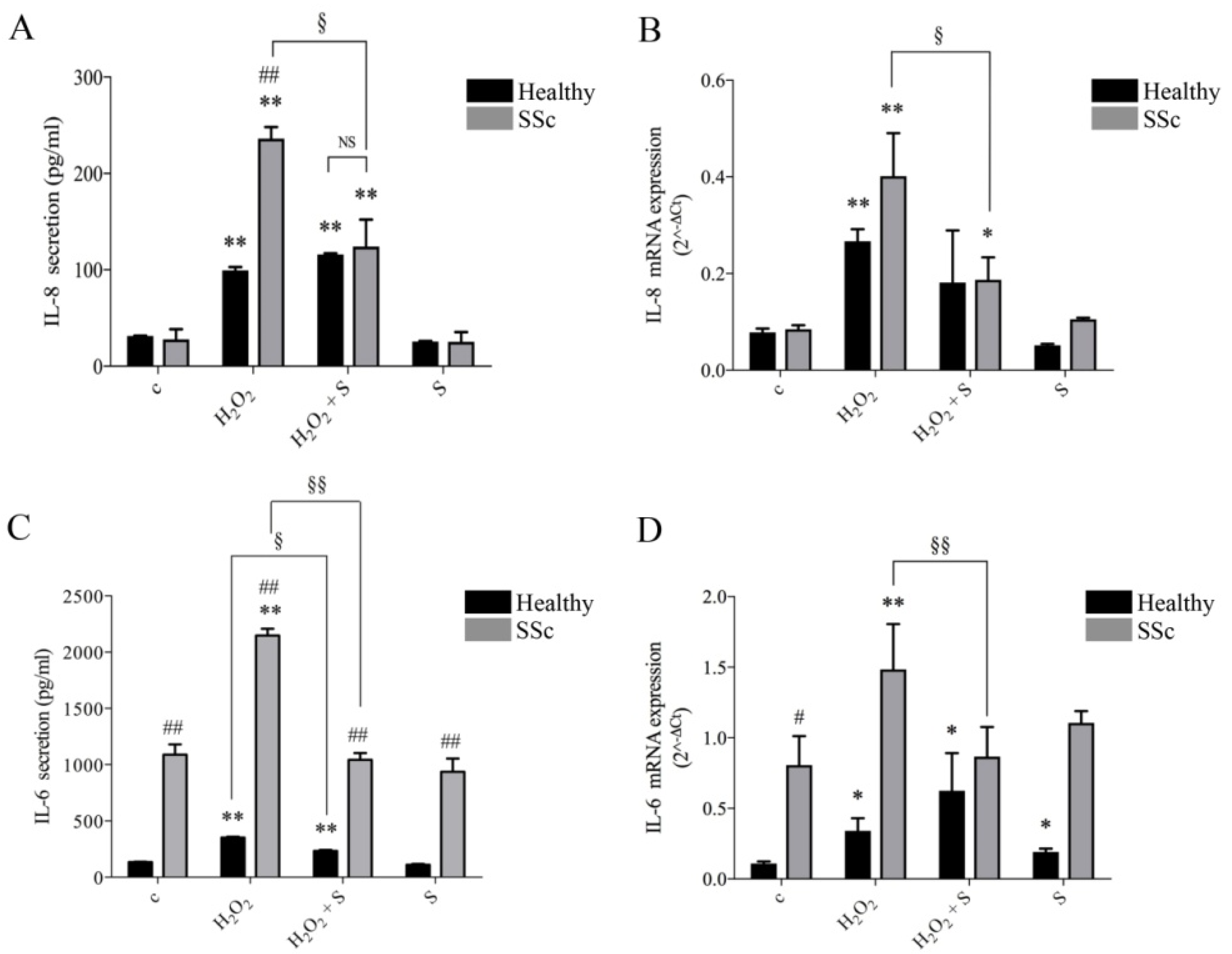
2.1. Sildenafil Inhibited Secretion and Gene Transcription of IL-6 and IL-8 Induced by Hydrogen Peroxide
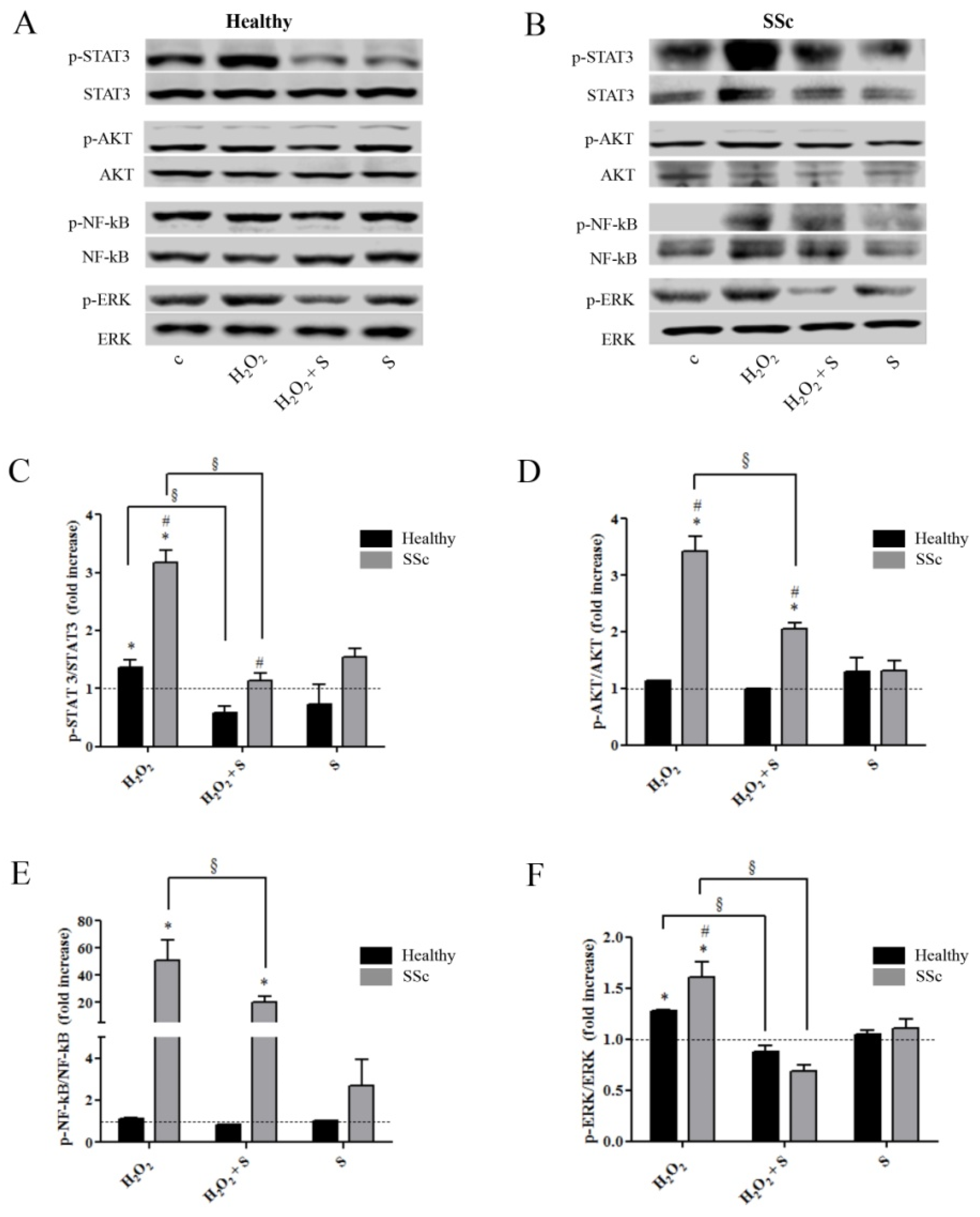
2.2. Sildenafil Suppressed the Activation of Intracellular Pathways Induced by the Pro-Oxidant Condition in Healthy and SSc Fibroblasts
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Cell Cultures and Treatments
4.3. Cytokine Secretion Assay
4.4. RNA Extraction, Reverse Transcription, and Real-Time Quantitative PCR
4.5. Protein Expression Analysis
4.6. Statistical Analysis
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| SSc | systemic sclerosis |
| RP | Raynaud’s phenomenon |
| PDE5i | phosphodiesterase type 5 inhibitor |
| PDEi | phosphodiesterase inhibitors |
| ROS | reactive oxygen species |
| IL | interleukin |
| ANA | Antinuclear Antibodies |
| Hfb | human dermal fibroblasts |
| H2O2 | Hydrogen peroxide |
References
- Crescioli, C.; Corinaldesi, C.; Riccieri, V.; Raparelli, V.; Vasile, M.; Del Galdo, F.; Valesini, G.; Lenzi, A.S.; Antinozzi, C. Association of circulating CXCL10 and CXCL11 with systemic sclerosis. Ann. Rheum. Dis. 2018, 77, 1845–1846. [Google Scholar] [CrossRef] [PubMed]
- DeForge, L.E.; Preston, A.M.; Takeuchi, E.; Kenney, J.; Boxer, L.A.; Remick, D.G. Regulation of interleukin 8 gene expression by oxidant stress. Int. J. Biol. Chem. 1993, 268, 25568–25576. [Google Scholar]
- Gabrielli, A.; Svegliati, S.; Moroncini, G.; Pomponio, G.; Santillo, M.; Avvedimento, E.V. Oxidative stress and the pathogenesis of scleroderma: The Murrell’s hypothesis revisited. Semin. Immunopathol. 2008, 30, 329–337. [Google Scholar] [CrossRef] [PubMed]
- Gabrielli, A.; Svegliati, S.; Moroncini, G.; Amico, D. New insights into the role of oxidative stress in scleroderma fibrosis. Open Rheumatol. J. 2012, 6, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Abdulle, A.E.; Diercks, G.F.; Feelisch, M.; Mulder, D.J.; Goor, H.V. The Role of Oxidative Stress in the Development of Systemic Sclerosis Related Vasculopathy. Front. Physiol. 2018, 9, 1177. [Google Scholar] [CrossRef] [PubMed]
- Muangchan, C.; Pope, J.E. Interleukin 6 in systemic sclerosis and potential implications for targeted therapy. J. Rheumatol. 2012, 39, 1120–1124. [Google Scholar] [CrossRef]
- Crestani, B.; Seta, N.; Palazzo, E.; Rolland, C.; Venembre, P.; Dehoux, M.; Boutten, A.; Soler, P.; Dombret, M.-C.; Kahn, M.-F. Interleukin-8 and Neutrophils in Systemic Sclerosis with Lung Involvement. Am. J. Respir. Crit. Care Med. 1994, 150, 1363–1367. [Google Scholar] [CrossRef]
- Hoffmann-Vold, A.M.; Maher, T.M.; Philpot, E.E.; Ashrafzadeh, A.; Barake, R.; Barsotti, S.; Bruni, C.; Carducci, P.; Carreira, P.E.; Castellv, I. The identification and management of interstitial lung disease in systemic sclerosis: Evidence-based European consensus statements. Lancet Rheumatol. 2020, 2, 71–83. [Google Scholar] [CrossRef]
- Sierra-Sepúlveda, A.; Esquinca-González, A.; Benavides-Suárez, S.A.; Sordo-Lima, D.E.; Caballero-Islas, A.E.; Cabral-Castañeda, A.R.; Rodríguez-Reyna, T.S. Systemic Sclerosis Pathogenesis and Emerging Therapies, beyond the Fibroblast. BioMed Res. Int. 2019, 2019, 4569826. [Google Scholar] [CrossRef]
- Kitaba, S.; Murota, H.; Terao, M.; Azukizawa, H.; Terabe, F.; Shima, Y.; Fujimoto, M.; Tanaka, T.; Naka, T.; Kishimoto, T. Blockade of interleukin-6 receptor alleviates disease in mouse model of scleroderma. Am. J. Pathol. 2012, 180, 165–176. [Google Scholar] [CrossRef]
- Shima, Y.; Kuwahara, Y.; Murota, H.; Kitaba, S.; Kawai, M.; Hirano, T.; Arimitsu, J.; Narazaki, M.; Hagihara, K.; Ogata, A. The skin of patients with systemic sclerosis softened during the treatment with anti-IL-6 receptor antibody tocilizumab. Rheumatology 2010, 49, 2408–2412. [Google Scholar] [CrossRef] [PubMed]
- Matsumori, A.; Ono, K.; Sato, Y.; Shioi, T.; Nose, Y.; Sasayama, S. Differential modulation of cytokine production by drugs: Implications for therapy in heart failure. J. Mol. Cell Cardiol. 1996, 28, 2491–2499. [Google Scholar] [CrossRef] [PubMed]
- Giannattasio, S.; Corinaldesi, C.; Colletti, M.; Di Luigi, L.; Antinozzi, C.; Filardi, T.; Scolletta, S.; Basili, S.; Lenzi, A.; Morano, S. The phosphodiesterase 5 inhibitor S decreases the proinflammatory chemokine IL-8 in diabetic cardiomyopathy: In vivo and in vitro evidence. J. Endocrinol. Investig. 2019, 42, 715–725. [Google Scholar] [CrossRef] [PubMed]
- Kadono, T.; Kikuchi, K.; Ihn, H.; Takehara, K.; Tamaki, K. Increased production of interleukin 6 and interleukin 8 in scleroderma fibroblasts. J. Rheumatol. 1998, 25, 296–301. [Google Scholar] [CrossRef]
- Phatak, S.; Ajmani, S.; Agarwal, V.; Misra, D.P. Phosphodiesterase-5 inhibitors: Raynaud’s and beyond. Indian J. Rheumatol. 2017, 12, 227–231. [Google Scholar]
- Jeon, Y.H.; Heo, Y.S.; Kim, C.M.; Hyun, Y.L.; Lee, T.G.; Ro, S.; Cho, J.M. Phosphodiesterase: Overview of protein structures, potential therapeutic applications and recent progress in drug development. Cell Mol. Life Sci. 2005, 62, 1198–1220. [Google Scholar] [CrossRef]
- Higuchi, T.; Kawaguchi, Y.; Takagi, K.; Tochimoto, A.; Ota, Y.; Katsumata, Y.; Ichida, H.; Hanaoka, M.; Kawasumi, H.; Tochihara, M. Sildenafil attenuates the fibrotic phenotype of skin fibroblasts in patients with systemic sclerosis. Clin. Immunol. 2015, 161, 333–338. [Google Scholar] [CrossRef]
- Bickel, M. The Role of interleukin-8 in Inflammation and Mechanisms of Regulation. J. Periodontol. 1993, 64, 456–460. [Google Scholar]
- Wang, L.; Tang, C.; Cao, H.; Li, K.; Pang, X.; Zhong, L.; Dang, W.; Tang, H.; Huang, Y.; Wei, L. Activation of IL-8 via PI3K/Akt-dependent pathway is involved in leptin-mediated epithelial-mesenchymal transition in human breast cancer cells. Cancer Biol. Ther. 2015, 16, 1220–1230. [Google Scholar] [CrossRef]
- Skaug, B.; Khanna, D.; Swindell, W.R.; Hinchcliff, M.E.; Frech, T.M.; Steen, V.D.; Hant, F.N.; Gordon, J.K.; Shah, A.A.; Zhu, L. Global skin gene expression analysis of early diffuse cutaneous systemic sclerosis shows a prominent innate and adaptive inflammatory profile. Ann. Rheum. Dis. 2020, 79, 379–386. [Google Scholar] [CrossRef]
- Garret, S.M.; Frost, D.B.; Feghali-Bostwick, C. The mighty fibroblast and its utility in scleroderma research. J. Scleroderma Related Disord. 2017, 2, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Pilz, R.B.; Casteel, D.E. Regulation of gene expression by cyclic GMP. Circ. Res. 2003, 93, 1034–1046. [Google Scholar] [CrossRef] [PubMed]
- Galdo, F.D.; Sotgia, F.; de Almeida, C.J.; Jasmin, J.F.; Musick, M.; Lisanti, M.P.; Jiménez, S.A. Decreased expression of caveolin 1 in patients with systemic sclerosis: Crucial role in the pathogenesis of tissue fibrosis. Arthritis Rheum. 2008, 58, 2854–2865. [Google Scholar] [CrossRef] [PubMed]
- Marampon, F.; Antinozzi, C.; Corinaldesi, C.; Vannelli, G.B.; Sarchielli, E.; Migliaccio, S.; Di Luigi, L.; Lenzi, A.; Crescioli, C. The phosphodiesterase 5 inhibitor tadalafil regulates lipidic homeostasis in human skeletal muscle cell metabolism. Endocrine 2018, 59, 602–661. [Google Scholar] [CrossRef] [PubMed]
- Fittipaldi, S.; Mercatelli, N.; Dimauro, I.; Jackson, M.J.; Paronetto, M.P.; Caporossi, D. Alpha B-crystallin induction in skeletal muscle cells under redox imbalance is mediated by a JNK-dependent regulatory mechanism. Free Radic. Biol. Med. 2015, 86, 331–342. [Google Scholar] [CrossRef]
- Testa, E.; Nardozi, D.; Antinozzi, C.; Faieta, M.; Di Cecca, S.; Caggiano, C.; Fukuda, T.; Bonanno, E.; Zhenkun, L.; Maldonado, A.; et al. H2AFX and MDC1 promote maintenance of genomic integrity in male germ cells. J. Cell Sci. 2018, 20, 1–16. [Google Scholar] [CrossRef]
- May, L.T.; Sehgal, P.B. Phosphorylation of interleukin-6 at serine54: An early event in the secretory pathway in human fibroblasts. Biochem. Biophys. Res. Commun. 1992, 15, 524–530. [Google Scholar] [CrossRef]
- Dimauro, I.; Scalabrin, M.; Fantini, C.; Grazioli, E.; Valls, M.R.B.; Mercatelli, N.; Parisi, A.; Sabatini, S.; Di Luigi, L.; Caporossi, D. Resistance training and redox homeostasis: Correlation with age-associated genomic changes. Redox Biol. 2016, 10, 34–44. [Google Scholar] [CrossRef]
- Illert, A.L.; Kawaguchi, H.; Antinozzi, C.; Bassermann, F.; Quintanilla-Martinez, L.; von Klitzing, C.; Hiwatari, M.; Peschel, C.; de Rooij, D.G.; Morris, S.W. Targeted inactivation of nuclear interaction partner of ALK disrupts meiotic prophase. Development 2012, 139, 2523–2534. [Google Scholar] [CrossRef]
- Ceci, R.; Duranti, G.; Rossi, A.; Savini, I.; Sabatini, S. Skeletal muscle differentiation: Role of dehydroepiandrosterone sulfate. Horm. Metab. Res. 2011, 43, 702–707. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Luigi, L.; Sgrò, P.; Duranti, G.; Sabatini, S.; Caporossi, D.; Del Galdo, F.; Dimauro, I.; Antinozzi, C. Sildenafil Reduces Expression and Release of IL-6 and IL-8 Induced by Reactive Oxygen Species in Systemic Sclerosis Fibroblasts. Int. J. Mol. Sci. 2020, 21, 3161. https://doi.org/10.3390/ijms21093161
Di Luigi L, Sgrò P, Duranti G, Sabatini S, Caporossi D, Del Galdo F, Dimauro I, Antinozzi C. Sildenafil Reduces Expression and Release of IL-6 and IL-8 Induced by Reactive Oxygen Species in Systemic Sclerosis Fibroblasts. International Journal of Molecular Sciences. 2020; 21(9):3161. https://doi.org/10.3390/ijms21093161
Chicago/Turabian StyleDi Luigi, Luigi, Paolo Sgrò, Guglielmo Duranti, Stefania Sabatini, Daniela Caporossi, Francesco Del Galdo, Ivan Dimauro, and Cristina Antinozzi. 2020. "Sildenafil Reduces Expression and Release of IL-6 and IL-8 Induced by Reactive Oxygen Species in Systemic Sclerosis Fibroblasts" International Journal of Molecular Sciences 21, no. 9: 3161. https://doi.org/10.3390/ijms21093161
APA StyleDi Luigi, L., Sgrò, P., Duranti, G., Sabatini, S., Caporossi, D., Del Galdo, F., Dimauro, I., & Antinozzi, C. (2020). Sildenafil Reduces Expression and Release of IL-6 and IL-8 Induced by Reactive Oxygen Species in Systemic Sclerosis Fibroblasts. International Journal of Molecular Sciences, 21(9), 3161. https://doi.org/10.3390/ijms21093161







