Removing Critical Gaps in Chemical Test Methods by Developing New Assays for the Identification of Thyroid Hormone System-Disrupting Chemicals—The ATHENA Project
Abstract
1. Introduction
2. Results
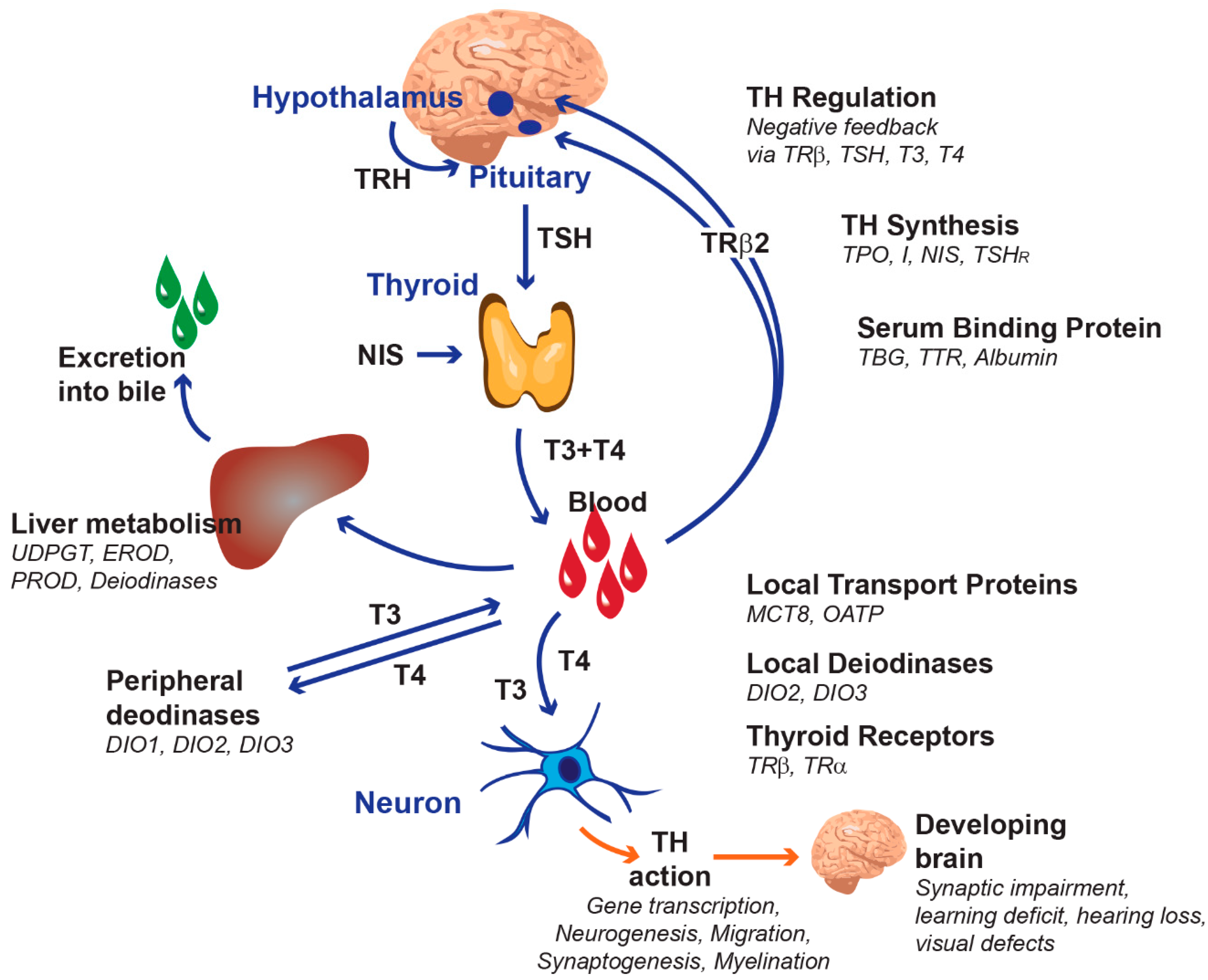
2.1. Terminology, Concept and Rationale
- The development of quantitative structure–activity relationships (QSARs) for molecular initiating events leading to diminished TH action, to allow for the improved screening of candidate TH system disruptors;
- The scaling up of in vitro methods for the inhibition of deiodinases, dehalogenases and cell membrane TH transporters to a high throughput format;
- The development of new assays to capture the disruption of the delivery of THs across physiological barriers (cell membrane transporters in the blood–brain barrier, the blood–cerebrospinal fluid barrier, and the placenta);
- The development of new assays based on human pluripotent stem cell-derived brain cells and brain organoids to complement and, to a certain extent, replace current animal models;
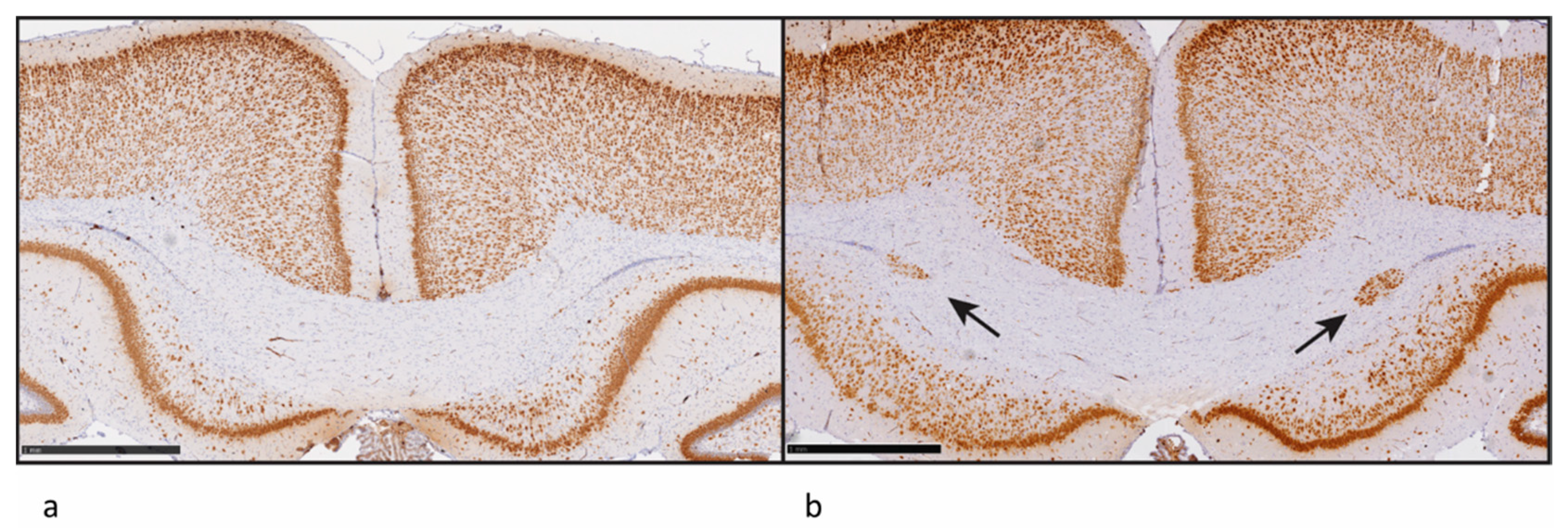
- The identification of endpoints reflective of brain morphology and markers of brain development for possible inclusion in existing Organisation for Economic Co-operation and Development (OECD) test guidelines;
- The integration of new and existing test methods into a coherent testing strategy;
- The validation of the human relevance of new test methods.
2.2. Regulatory and Societal Needs for New Test Methods for Thyroid Hormone System Disruption
2.3. Overall Approach and Methodology
- Domain 1: Mobilising basic research for the development of novel assays for TH system disruption;
- Domain 2: The development of test methods towards assay pre-validation and ring-testing;
- Domain 3: Testing strategies and testing in a multi-causal context;
- Domain 4: Enhanced international collaboration and development of international strategies for TH system disruptor identification and regulation.
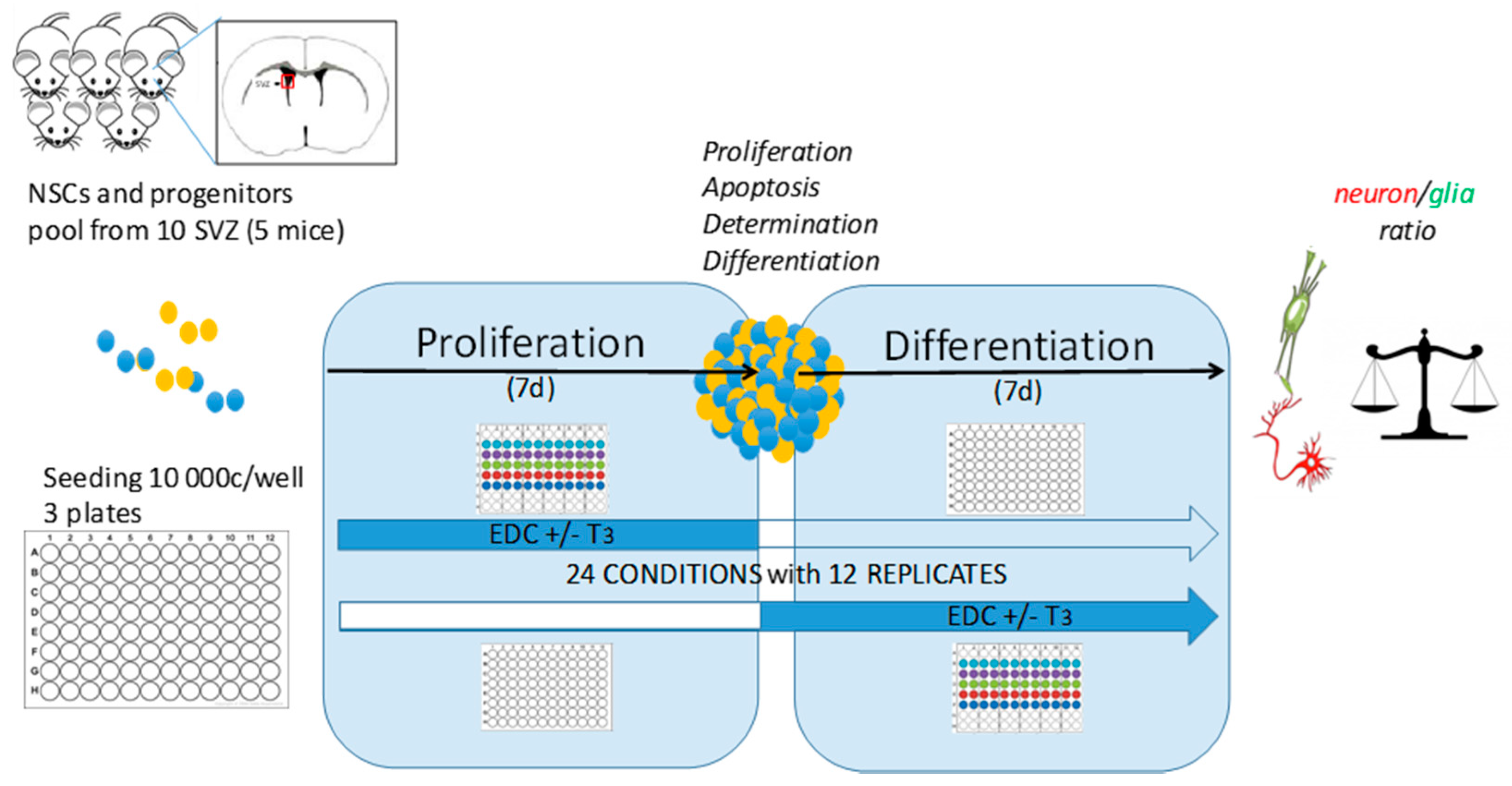
2.3.1. Domain 1: Mobilising Basic Research for the Development of Novel TH System Disruption Assays
2.3.2. Domain 2: Development of Test Methods towards Assay Pre-Validation and Ring-Testing
2.3.3. Domain 3: Thyroid Hormone System Disruptor Testing in a Multi-Factorial Context and the Development of Comprehensive Testing Strategies
2.3.4. Enhanced International Collaboration, Development of International Strategies for TH System Disruptor Identification and Regulation
3. Discussion
- Show adverse effects in an intact organism or its progeny, which is a change in the morphology, physiology, growth, development, reproduction or life span of an organism, system or (sub)population that results in an impairment of functional capacity, an impairment of the capacity to compensate for additional stress or an increase in susceptibility to other influences;
- Have an endocrine mode of action (MOA), i.e., alter the function(s) of the endocrine system;
- Exhibit adverse effects because of an endocrine MOA.
- Substances inducing histopathological changes in the thyroid (focal hyperplasia and/or neoplasia), with or without changes in the circulating levels of TH, are regarded as posing a hazard for human TH insufficiency in adults, as well as the pre- and post-natal neurological development of offspring;
- Substances that alter circulating levels of T3 and/or T4 without histopathological findings are also seen as presenting a potential concern for neurodevelopment;
- In the absence of substance-specific data which provide proof of the contrary, humans and rodents are considered equally sensitive to TH system disruption (including cases where liver enzyme induction is responsible for increased TH clearance).
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AOP | Adverse Outcome Pathway |
| DIO | Deiodinase |
| ECHA | European Chemicals Agency |
| ECVAM | European Centre for the Validation of Alternative Methods |
| EFSA | European Food Safety Authority |
| EOGRTS | Extended One-Generation Reproductive Toxicity Study |
| HTS | High Throughput Screening |
| EROD | 7-Ethoxyresorufin O-dealkylase |
| IYD | Iodotyrosine dehalogenase 1 |
| MCT8 | Monocarboxylate transporter 8 |
| MOA | Mode of Action |
| NIS | Sodium iodide symporter |
| OECD | Organisation for Economic Cooperation and Development |
| PROD | Pentoxyresorufin O-dealkylase |
| QSAR | Quantitative Structure–Activity Relationship |
| T3 | Triiodothyronine |
| T4 | Thyroxine |
| TBG | Thyroxin-Binding Globulin |
| TG | Test Guideline |
| TR | Thyroid Receptor |
| TRH | Thyrotropin-Releasing Hormone |
| TSH | Thyroid Stimulating Hormone |
| TSHR | Thyroid Stimulating Hormone Receptor |
| TTR | Transthyretin |
| UDPGT | Uridine 5′diphospho-glucuronosyltransferase |
References
- Kortenkamp, A.; Martin, O.; Baynes, A.; Silva, S.; Axelstad, M.; Hass, U. Supporting the Organization of a Workshop on Thyroid Disruption—Final Report. Framework Contract ENV.A.3/FRA/2014/0029 on Implementation of the Community Strategy on Endocrine Disrupters. 2017. Available online: https://op.europa.eu/lv/publication-detail/-/publication/472d2c88-a8b1-11e7-837e-01aa75ed71a1 (accessed on 24 March 2020).
- Gilbert, M.; Rovet, J.; Chen, Z.; Koibuchi, N. Developmental thyroid hormone disruption: Prevalence, environmental contaminants and neurodevelopmental consequences. Neurotox 2012, 33, 842–852. [Google Scholar] [CrossRef] [PubMed]
- Korevaar, T.I.M.; Muetzel, R.; Medici, M.; Chaker, L.; Jaddoe, V.W.V.; DeRijke, Y.B.; Steegers, E.A.P.; Visser, T.J.; Tiemeier, H.; Peeters, R.P. Association of maternal thyroid function during early pregnancy with offspring IQ and brain morphology in childhood: A population-based prospective cohort study. Lancet Diabetes Endocrinol. 2016, 4, 35–43. [Google Scholar] [CrossRef]
- ATHENA—Assays for the Identification of Thyroid Hormone Axis-Disrupting Chemicals: Elaborating Novel Assessment Strategies. Available online: https://athenaedctestmethods.net/ (accessed on 24 March 2020).
- Murk, A.J.; Rijntjes, E.; Blaauboer, B.J.; Clewell, R.; Crofton, K.M.; Dingemans, M.M.L.; Furlow, J.D.; Kavlock, R.; Köhrle, J.; Opitz, R.; et al. Mechanism-based testing strategy using in vitro approaches for identification of thyroid hormone disrupting chemicals. Toxicol. In Vitro 2013, 27, 1320–1346. [Google Scholar] [CrossRef] [PubMed]
- Derakhshan, A.; Shu, H.; Peeters, R.P.; Kortenkamp, A.; Lindh, C.; Demeinex, B.; Bornehag, C.; Korevaar, T. Association of urinary bisphenols and triclosans with thyroid function during early pregnancy. Environ. Int. 2019, 133, 105123. [Google Scholar] [CrossRef] [PubMed]
- Wilpert, N.M.; Krueger, M.; Opitz, R.; Sebinger, D.; Paisdzior, S.; Mages, B.; Schulz, A.; Spranger, J.; Wirth, E.K.; Stachelscheid, H.; et al. Spatiotemporal Changes of Cerebral Monocarboxylate Transporter 8 Expression. Thyroid 2020. [Google Scholar] [CrossRef] [PubMed]
- Renko, K.; Hoefig, C.S.; Dupuy, C.; Harder, L.; Schwiebert, C.; Kohrle, J.; Schomburg, L. A non-radioactive DEHAL assay for testing substrates, inhibitors, and monitoring endogenous activity. Endocrinology 2016, 157, 4516–4525. [Google Scholar] [CrossRef] [PubMed]
- Jayarama-Naidu, R.; Johannes, J.; Meyer, F.; Wirth, E.K.; Schomburg, L.; Kohrle, J.; Renko, K. A non-radioactive uptake assay for rapid analysis of thyroid hormone transporter function. Endocrinology 2015, 156, 2739–2745. [Google Scholar] [CrossRef] [PubMed]
- Hamers, T.; Kortenkamp, A.; Scholze, M.; Molenaar, D.; Cenijn, P.; Weiss, J.M. Transthyretin-binding activity of complex mixtures representing the composition of thyroid-hormone disrupting contaminants in house dust and human serum. Environ. Health Perspect. 2020, 128, 017015. [Google Scholar] [CrossRef] [PubMed]
- Goodman, J.H.; Gilbert, M.E. Modest thyroid hormone insufficiency during development induces a cellular malformation in the corpus callosum: A model for cortical dysplasia. Endocrinology 2007, 148, 2593–2597. [Google Scholar] [CrossRef] [PubMed]
- OECD, Validation of the Xenopus Eleutheroembryonic Thyroid Assay (XETA) for the Detection of Thyroid Active Substances. Draft Report. 2018. Available online: http://www.oecd.org/chemicalsafety/testing/draft-validation-report-xeta.pdf (accessed on 25 March 2020).
- OECD Guidelines for the Testing of Chemicals. Test No. 248: Xenopus Eleutheroembryonic Thyroid Assay (XETA), ISBN 9789264916784. 2019. Available online: https://doi.org/10.1787/a13f80ee-en (accessed on 25 March 2020).
- Kortenkamp, A.; Martin, O.; Christiansen, S.; Hass, U. Mapping Commonalities and Differences in Approaches for Testing and Assessment of Endocrine Disruptors within the EU and among Relevant International Trading Partners—Final Report; Framework Contract ENV.A.3/FRA/2014/0029 on Implementation of the Community Strategy on Endocrine Disrupters; European Commission: Brussels, Belgium, 2017; Available online: https://bura.brunel.ac.uk/bitstream/2438/15517/1/FullText.pdf (accessed on 25 March 2020).
- ECHA. EFSA Guidance for the identification of endocrine disruptors in the context of Regulations (EU) No 528/2012 and (EC) No 1107/2009. EFSA J. 2018. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kortenkamp, A.; Axelstad, M.; Baig, A.H.; Bergman, Å.; Bornehag, C.-G.; Cenijn, P.; Christiansen, S.; Demeneix, B.; Derakhshan, A.; Fini, J.-B.; et al. Removing Critical Gaps in Chemical Test Methods by Developing New Assays for the Identification of Thyroid Hormone System-Disrupting Chemicals—The ATHENA Project. Int. J. Mol. Sci. 2020, 21, 3123. https://doi.org/10.3390/ijms21093123
Kortenkamp A, Axelstad M, Baig AH, Bergman Å, Bornehag C-G, Cenijn P, Christiansen S, Demeneix B, Derakhshan A, Fini J-B, et al. Removing Critical Gaps in Chemical Test Methods by Developing New Assays for the Identification of Thyroid Hormone System-Disrupting Chemicals—The ATHENA Project. International Journal of Molecular Sciences. 2020; 21(9):3123. https://doi.org/10.3390/ijms21093123
Chicago/Turabian StyleKortenkamp, Andreas, Marta Axelstad, Asma H. Baig, Åke Bergman, Carl-Gustaf Bornehag, Peter Cenijn, Sofie Christiansen, Barbara Demeneix, Arash Derakhshan, Jean-Baptiste Fini, and et al. 2020. "Removing Critical Gaps in Chemical Test Methods by Developing New Assays for the Identification of Thyroid Hormone System-Disrupting Chemicals—The ATHENA Project" International Journal of Molecular Sciences 21, no. 9: 3123. https://doi.org/10.3390/ijms21093123
APA StyleKortenkamp, A., Axelstad, M., Baig, A. H., Bergman, Å., Bornehag, C.-G., Cenijn, P., Christiansen, S., Demeneix, B., Derakhshan, A., Fini, J.-B., Frädrich, C., Hamers, T., Hellwig, L., Köhrle, J., Korevaar, T. I. M., Lindberg, J., Martin, O., Meima, M. E., Mergenthaler, P., ... Zoeller, R. T. (2020). Removing Critical Gaps in Chemical Test Methods by Developing New Assays for the Identification of Thyroid Hormone System-Disrupting Chemicals—The ATHENA Project. International Journal of Molecular Sciences, 21(9), 3123. https://doi.org/10.3390/ijms21093123




