Data-Driven Modeling Identifies TIRAP-Independent MyD88 Activation Complex and Myddosome Assembly Strategy in LPS/TLR4 Signaling
Abstract
1. Introduction
2. Results
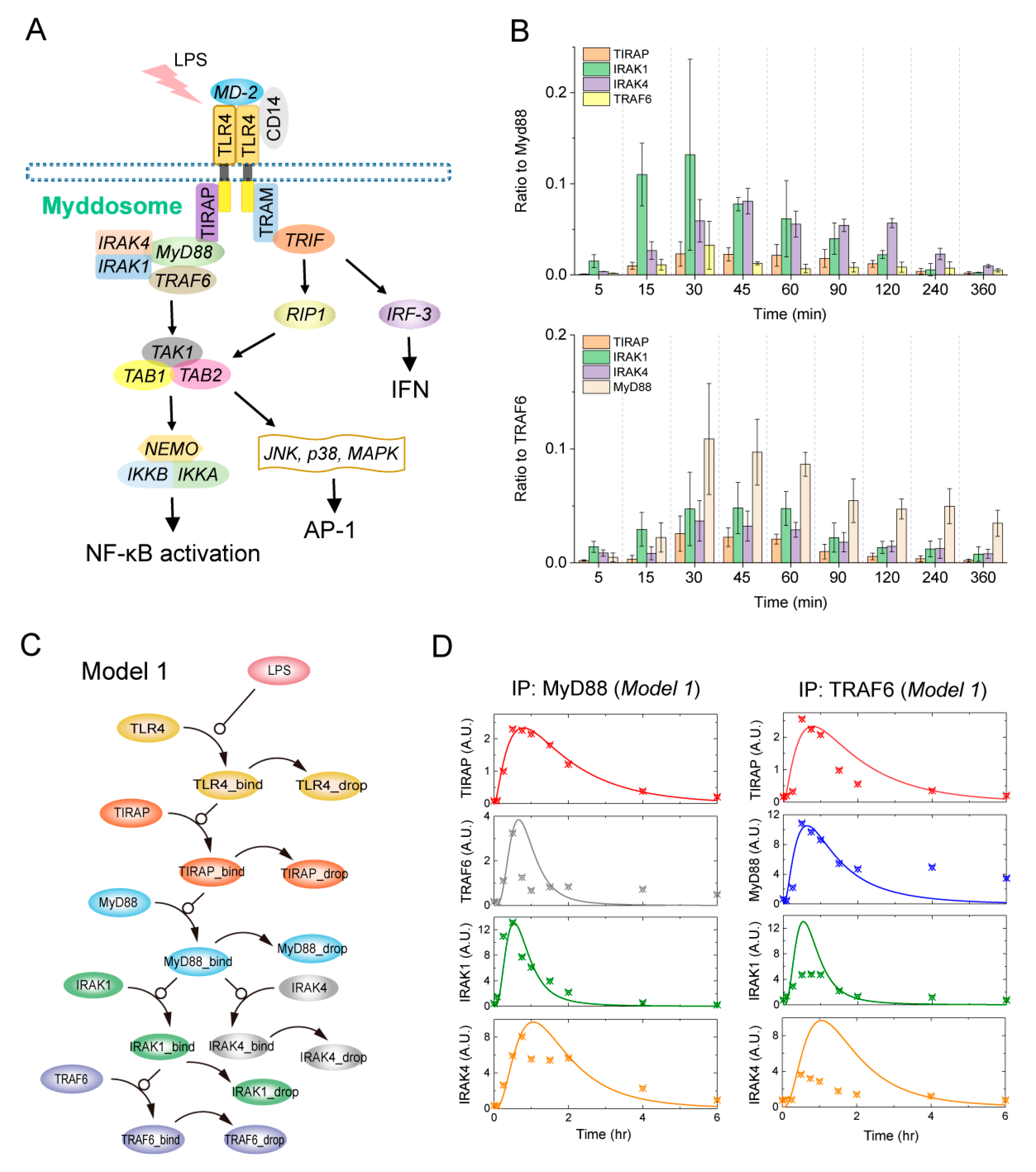
2.1. SWATH-MS Data-Based Modeling of LPS-Induced Myddosome Assembly
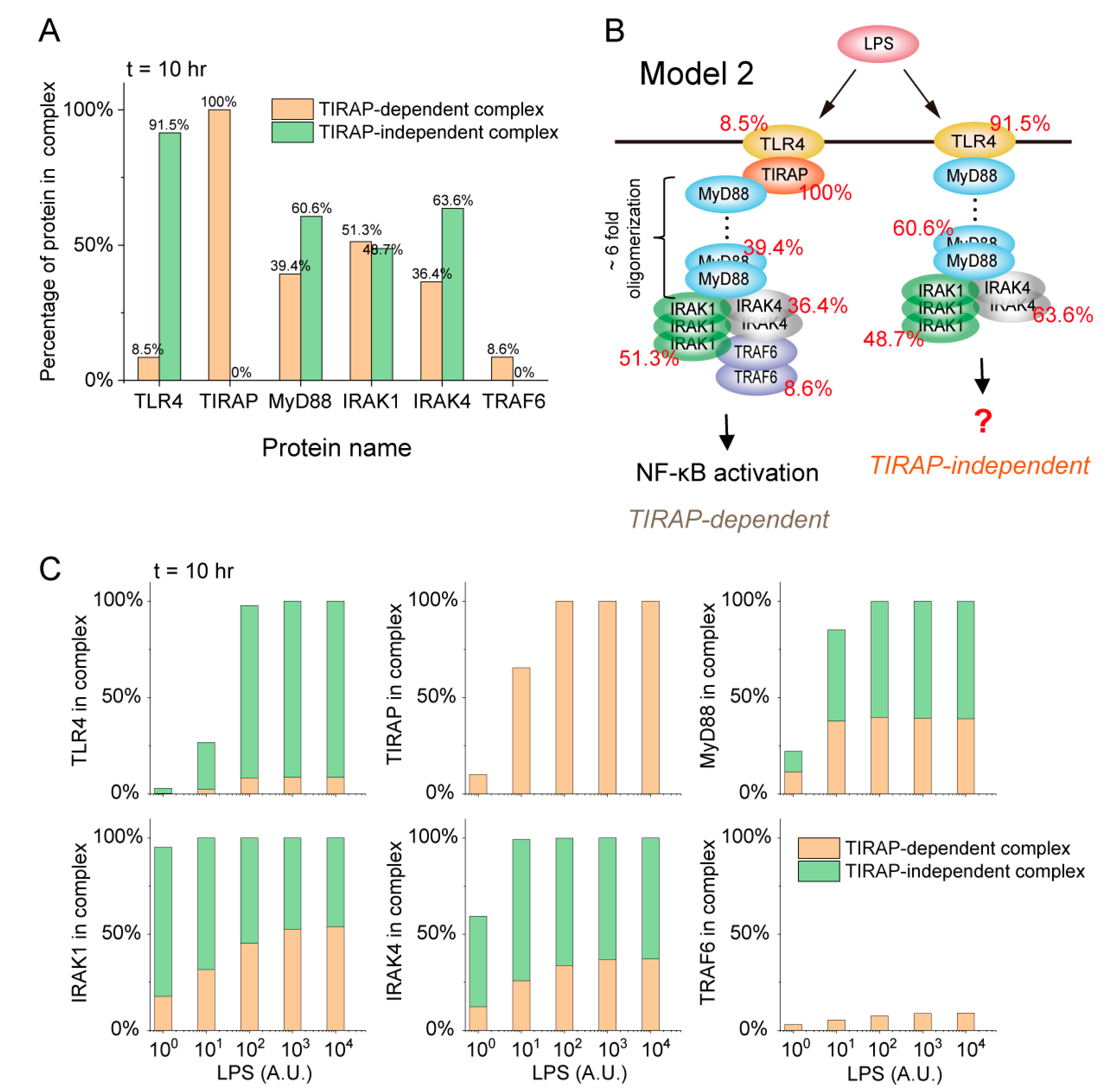
2.2. TIRAP-Independent MyD88 Activation Complex Formation upon LPS Stimulation
2.3. Distribution Strategy of Proteins in Complexes Determined by LPS Stimulation Strength
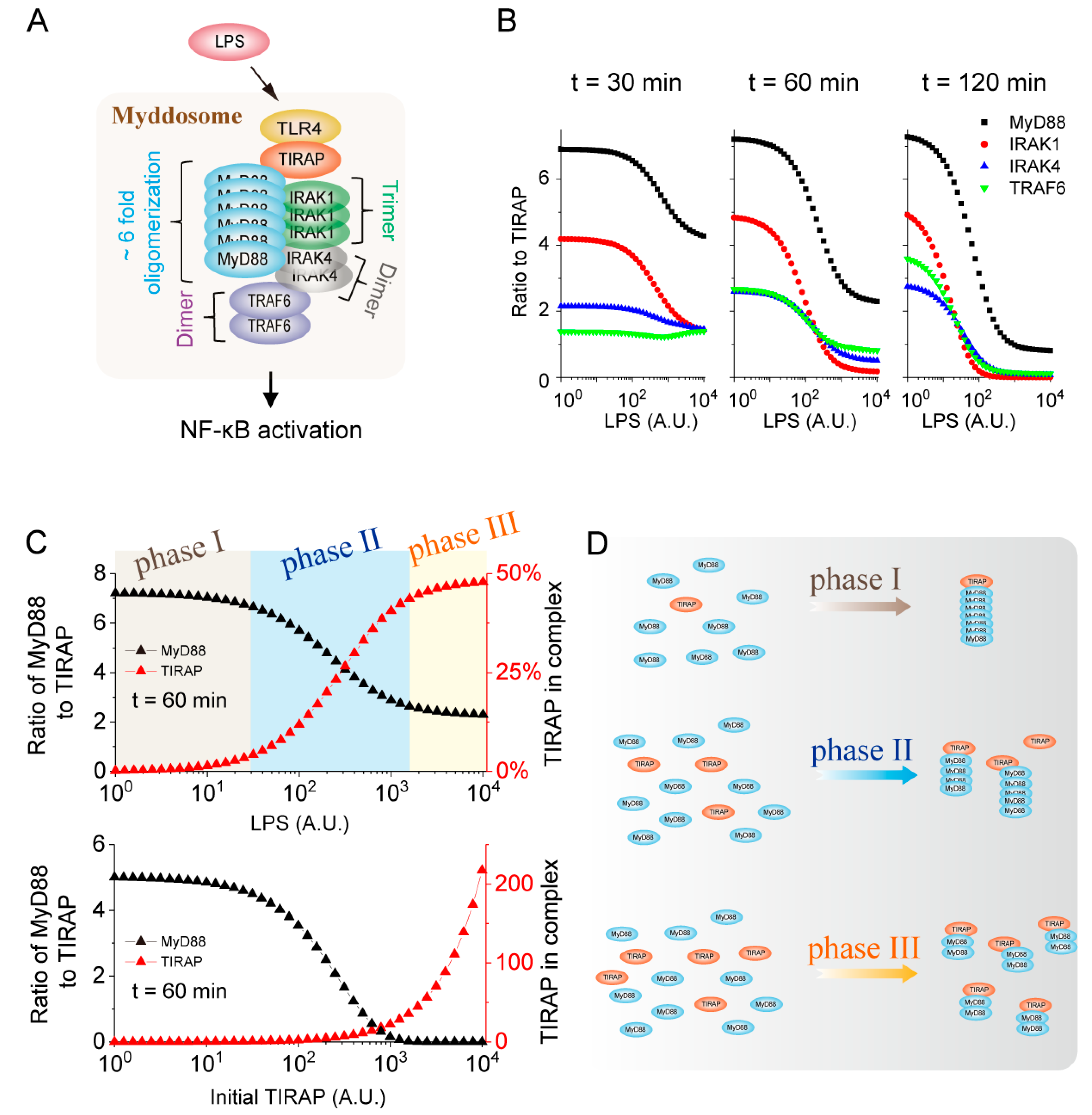
2.4. Higher-Order Assembly Strategy of MyD88 Determined by the TIRAP Level in the Myddosome
3. Discussion
4. Materials and Methods
4.1. Modeling Principles
4.2. Parameter Values Determination
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Beutler, B.; Rietschel, E.T. Innate immune sensing and its roots: The story of endotoxin. Nat. Rev. Immunol. 2003, 3, 169–176. [Google Scholar] [CrossRef]
- Iwasaki, A.; Medzhitov, R. Toll-like receptor control of the adaptive immune responses. Nat. Immunol. 2004, 5, 987–995. [Google Scholar] [CrossRef]
- Tan, Y.; Kagan, J.C. A cross-disciplinary perspective on the innate immune responses to bacterial lipopolysaccharide. Mol. Cell 2014, 54, 212–223. [Google Scholar] [CrossRef] [PubMed]
- Poltorak, A.; He, X.; Smirnova, I.; Liu, M.Y.; Van Huffel, C.; Du, X.; Birdwell, D.; Alejos, E.; Sliva, M.; Galanos, C.; et al. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: Mutations in Tlr4 gene. Science 1998, 282, 2085–2088. [Google Scholar] [CrossRef] [PubMed]
- Akira, S.; Takeda, K. Toll-like receptor signalling. Nat. Rev. Immunol. 2004, 4, 499–511. [Google Scholar] [CrossRef] [PubMed]
- Takeda, K.; Akira, S. TLR signaling pathways. Semin. Immunol. 2004, 16, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Horng, T.; Barton, G.M.; Flavell, R.A.; Medzhitov, R. The adaptor molecule TIRAP provides signalling specificity for Toll-like receptors. Nature 2002, 420, 329–333. [Google Scholar] [CrossRef]
- Yamamoto, M.; Sato, S.; Hemmi, H.; Sanjo, H.; Uematsu, S.; Kaisho, T.; Hoshino, K.; Takeuchi, O.; Kobayashi, M.; Fujita, T.; et al. Essential role for TIRAP in activation of the signalling cascade shared by TLR2 and TLR4. Nature 2002, 420, 324–329. [Google Scholar] [CrossRef]
- Nagpal, K.; Plantinga, T.S.; Wong, J.; Monks, B.G.; Gay, N.J.; Netea, M.G.; Fitzgerald, K.A.; Golenbock, D.T. A TIR domain variant of MyD88 adapter-like (Mal)/TIRAP results in loss of MyD88 binding and reduced TLR2/TLR4 signaling. J. Biol. Chem. 2009, 284, 25742–25748. [Google Scholar] [CrossRef]
- Lu, Y.C.; Yeh, W.C.; Ohashi, P.S. LPS/TLR4 signal transduction pathway. Cytokine 2008, 42, 145–151. [Google Scholar] [CrossRef]
- Gay, N.J.; Symmons, M.F.; Gangloff, M.; Bryant, C.E. Assembly and localization of Toll-like receptor signalling complexes. Nat. Rev. Immunol. 2014, 14, 546–558. [Google Scholar] [CrossRef] [PubMed]
- Ve, T.; Vajjhala, P.R.; Hedger, A.; Croll, T.; DiMaio, F.; Horsefield, S.; Yu, X.; Lavrencic, P.; Hassan, Z.; Morgan, G.P.; et al. Structural basis of TIR-domain-assembly formation in MAL-and MyD88-dependent TLR4 signaling. Nat. Struct. Mol. Biol. 2017, 24, 743–751. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.C.; Lo, Y.C.; Wu, H. Helical assembly in the MyD88-IRAK4-IRAK2 complex in TLR/IL-1R signalling. Nature 2010, 465, 885–890. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Yang, D.; Zhao, F.; Yang, Z.H.; Wang, D.; Qiao, M.; Fang, Y.; Li, W.; Wu, R.; He, P.; et al. Quantification of dynamic protein interactions and phosphorylation in LPS signaling pathway by SWATH-MS. Mol. Cell. Proteom. 2019, 18, 1054–1069. [Google Scholar] [CrossRef]
- Wu, H. Higher-order assemblies in a new paradigm of signal transduction. Cell 2013, 153, 287–292. [Google Scholar] [CrossRef]
- Wu, H.; Fuxreiter, M. The structure and dynamics of higher-order assemblies: Amyloids, signalosomes, and granules. Cell 2016, 165, 1055–1066. [Google Scholar] [CrossRef]
- Nakakuki, T.; Birtwistle, M.R.; Saeki, Y.; Yumoto, N.; Ide, K.; Nagashima, T.; Brusch, L.; Ogunnaike, B.A.; Okada-Hatakeyama, M.; Kholodenko, B.N. Ligand-specific c-Fos expression emerges from the spatiotemporal control of ErbB network dynamics. Cell 2010, 141, 884–896. [Google Scholar] [CrossRef]
- Shinohara, H.; Behar, M.; Inoue, K.; Hiroshima, M.; Yasuda, T.; Nagashima, T.; Kimura, S.; Sanjo, H.; Maeda, S.; Yumoto, N.; et al. Positive feedback within a kinase signaling complex functions as a switch mechanism for NF-κB activation. Science 2014, 344, 760–764. [Google Scholar] [CrossRef]
- Neumann, L.; Pforr, C.; Beaudouin, J.; Pappa, A.; Fricker, N.; Krammer, P.H.; Lavrik, I.N.; Eils, R. Dynamics within the CD95 death-inducing signaling complex decide life and death of cells. Mol. Syst. Biol. 2010, 6. [Google Scholar] [CrossRef]
- Hughes, M.A.; Powley, I.R.; Jukes-Jones, R.; Horn, S.; Feoktistova, M.; Fairall, L.; Fairall, L.; Schwabe, W.R.; Leverkus, M.; Cain, K.; et al. Co-operative and hierarchical binding of c-FLIP and caspase-8: A unified model defines how c-FLIP isoforms differentially control cell fate. Mol. Cell 2016, 61, 834–849. [Google Scholar] [CrossRef]
- Ram, D.R.; Ilyukha, V.; Volkova, T.; Buzdin, A.; Tai, A.; Smirnova, I.; Poltorak, A. Balance between short and long isoforms of cFLIP regulates Fas-mediated apoptosis in vivo. Proc. Natl. Acad. Sci. USA 2016, 113, 1606–1611. [Google Scholar] [CrossRef] [PubMed]
- Schleich, K.; Warnken, U.; Fricker, N.; Öztürk, S.; Richter, P.; Kammerer, K.; Schnolzer, M.; Krammer, P.H.; Lavrik, I.N. Stoichiometry of the CD95 death-inducing signaling complex: Experimental and modeling evidence for a death effector domain chain model. Mol. Cell 2012, 47, 306–319. [Google Scholar] [CrossRef]
- Dickens, L.S.; Boyd, R.S.; Jukes-Jones, R.; Hughes, M.A.; Robinson, G.L.; Fairall, L.; Schwabe, W.R.; Cain, K.; MacFarlane, M. A death effector domain chain DISC model reveals a crucial role for caspase-8 chain assembly in mediating apoptotic cell death. Mol. Cell 2012, 47, 291–305. [Google Scholar] [CrossRef]
- Bashor, C.J.; Patel, N.; Choubey, S.; Beyzavi, A.; Kondev, J.; Collins, J.J.; Khalil, A.S. Complex signal processing in synthetic gene circuits using cooperative regulatory assemblies. Science 2019, 364, 593–597. [Google Scholar] [CrossRef]
- Cox, J.; Mann, M. Quantitative, high-resolution proteomics for data-driven systems biology. Annu. Rev. Biochem. 2011, 80, 273–299. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, L.A.; Bowie, A.G. The family of five: TIR-domain-containing adaptors in Toll-like receptor signalling. Nat. Rev. Immunol. 2007, 7, 353–364. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Taylor, B.; Ourthiague, D.R.; Hoffmann, A. Distinct single-cell signaling characteristics are conferred by the MyD88 and TRIF pathways during TLR4 activation. Sci. Signal. 2015, 8, ra69. [Google Scholar] [CrossRef] [PubMed]
- Horng, T.; Barton, G.M.; Medzhitov, R. TIRAP: An adapter molecule in the Toll signaling pathway. Nat. Immunol. 2001, 2, 835–841. [Google Scholar] [CrossRef]
- Fitzgerald, K.A.; Palsson-McDermott, E.M.; Bowie, A.G.; Jefferies, C.A.; Mansell, A.S.; Brady, G.; Brint, E.; Dunne, A.; Gray, P.; Harte, M.T.; et al. Mal (MyD88-adapter-like) is required for Toll-like receptor-4 signal transduction. Nature 2001, 413, 78–83. [Google Scholar] [CrossRef]
- Cole, L.E.; Laird, M.H.; Seekatz, A.; Santiago, A.; Jiang, Z.; Barry, E.; Shirey, K.A.; Fitzgerald, K.A.; Vogel, S.N. Phagosomal retention of Francisella tularensis results in TIRAP/Mal-independent TLR2 signaling. J. Leukoc. Biol. 2010, 87, 275–281. [Google Scholar] [CrossRef][Green Version]
- Kuwabara, T.; Imajoh-Ohmi, S. LPS-induced apoptosis is dependent upon mitochondrial dysfunction. Apoptosis 2004, 9, 467–474. [Google Scholar] [CrossRef]
- Wu, X.N.; Yang, Z.H.; Wang, X.K.; Zhang, Y.; Wan, H.; Song, Y.; Chen, X.; Shao, J.; Han, J. Distinct roles of RIP1–RIP3 hetero-and RIP3–RIP3 homo-interaction in mediating necroptosis. Cell Death Differ. 2014, 21, 1709–1720. [Google Scholar] [CrossRef]
- Marzahn, M.R.; Marada, S.; Lee, J.; Nourse, A.; Kenrick, S.; Zhao, H.; Ben-Nissan, G.; Kolaitis, R.; Peters, J.L.; Pounds, S.; et al. Higher-order oligomerization promotes localization of SPOP to liquid nuclear speckles. EMBO J. 2016, 35, 1254–1275. [Google Scholar] [CrossRef]

| Number | Reactions | ki | Names | Initial Values (A.U.) |
|---|---|---|---|---|
| 1 | LPS + TLR4 → LPS_TLR4 | 1.28×10−6 s−1 | LPS | 500 |
| 2 | LPS_TLR4 → LPS_bind + TLR4_bind | 1.0 s−1 | TLR4 | 20 |
| 3 | TLR4_bind + TIRAP → TLR4_bind_TIRAP | 9.3×10−6 s−1 | TIRAP | 100 |
| 4 | TLR4_bind_TIRAP → TLR4_bind + TIRAP_bind | 1.0 s−1 | MyD88 | 1000 |
| 5 | TIRAP_bind + MyD88 → TIRAP_bind_MyD88 | 5.6×10−5 s−1 | IRAK1 | 100 |
| 6 | TIRAP_bind_MyD88 → TIRAP_bind + MyD88_bind | 1.0 s−1 | IRAK4 | 100 |
| 7 | MyD88_bind + IRAK1 → MyD88_bind_IRAK1 | 4.75×10−5 s−1 | TRAF6 | 100 |
| 8 | MyD88_bind_IRAK1 → MyD88_bind + IRAK1_bind | 1.0 s−1 | ||
| 9 | MyD88_bind + IRAK4 → MyD88_bind_IRAK4 | 7.98×10−6 s−1 | ||
| 10 | MyD88_bind_IRAK4 → MyD88_bind + IRAK4_bind | 1.0 s−1 | ||
| 11 | IRAK1_bind + TRAF6 → IRAK1_bind_TRAF6 | 7.0×10−6 s−1 | ||
| 12 | IRAK1_bind_TRAF6 → IRAK1_bind + TRAF6_bind | 1.0 s−1 | ||
| 13 | TIRAP_bind → TIRAP_drop | 5.0×10−3 s−1 | ||
| 14 | MyD88_bind → MyD88_drop | 1.0×10−2 s−1 | ||
| 15 | IRAK1_bind → IRAK1_drop | 2.2×10−3 s−1 | ||
| 16 | IRAK4_bind → IRAK4_drop | 5.62×10−4 s−1 | ||
| 17 | TRAF6_bind → TRAF6_drop | 1.96×10−3 s−1 |
| Number | Reactions | ki | Names | Initial Values (A.U.) |
|---|---|---|---|---|
| 1 | LPS + TLR4 → LPS_TLR4 | 1.28×10−6 s−1 | LPS | 500 |
| 2 | LPS_TLR4 → LPS_bind + TLR4_bind | 1.0 s−1 | TLR4 | 20 |
| 3 | TLR4_bind + TIRAP → TLR4_bind_TIRAP | 9.3×10−6 s−1 | TIRAP | 100 |
| 4 | TLR4_bind_TIRAP → TLR4_bind + TIRAP_bind | 1.0 s−1 | MyD88 | 1000 |
| 5 | TIRAP_bind + MyD88 → TIRAP_bind_MyD88 | 6.0×10−5 s−1 | IRAK1 | 100 |
| 6 | TIRAP_bind_MyD88 → TIRAP_bind + MyD88_bind | 1.0 s−1 | IRAK4 | 100 |
| 7 | MyD88_bind + IRAK1 → MyD88_bind_IRAK1 | 2.92×10−5 s−1 | TRAF6 | 100 |
| 8 | MyD88_bind_IRAK1 → MyD88_bind + IRAK1_bind | 1.0 s−1 | ||
| 9 | MyD88_bind + IRAK4 → MyD88_bind_IRAK4 | 1.2×10−5 s−1 | ||
| 10 | MyD88_bind_IRAK4 → MyD88_bind + IRAK4_bind | 1.0 s−1 | ||
| 11 | IRAK1_bind + TRAF6 → IRAK1_bind_TRAF6 | 7.0×10−6 s−1 | ||
| 12 | IRAK1_bind_TRAF6 → IRAK1_bind + TRAF6_bind | 1.0 s−1 | ||
| 13 | TIRAP_bind → TIRAP_drop | 5.0×10−3 s−1 | ||
| 14 | MyD88_bind → MyD88_drop | 8.0×10−3 s−1 | ||
| 15 | IRAK1_bind → IRAK1_drop | 4.0×10−3 s−1 | ||
| 16 | IRAK4_bind → IRAK4_drop | 3.0×10−3 s−1 | ||
| 17 | TRAF6_bind → TRAF6_drop | 8.0×10−4 s−1 | ||
| 18 | MyD88_drop + TRAF6_drop → MyD88_drop_TRAF6_drop | 1.0×10−7 s−1 | ||
| 19 | MyD88_drop_TRAF6_drop → MyD88_drop + TRAF6_BIND | 1.0 s−1 | ||
| 20 | TRAF6_BIND + MyD88_drop → TRAF6_BIND_ MyD88_drop | 1.07×10−5 s−1 | ||
| 21 | TRAF6_BIND_ MyD88_drop → TRAF6_BIND + MyD88_BIND | 1.0 s−1 | ||
| 22 | TRAF6_BIND + IRAK1_drop → TRAF6_BIND_IRAK1_drop | 6.0×10−5 s−1 | ||
| 23 | TRAF6_BIND_IRAK1_drop → TRAF6_BIND + IRAK1_BIND | 1.0 s−1 | ||
| 24 | TRAF6_BIND + IRAK4_drop → TRAF6_BIND_IRAK4_drop | 2.92×10−5 s−1 | ||
| 25 | TRAF6_BIND_IRAK4_drop → TRAF6_BIND + IRAK4_BIND | 1.0 s−1 | ||
| 26 | TRAF6_BIND → TRAF6_DROP | 4.0×10−4 s−1 | ||
| 27 | MyD88_BIND → MyD88_DROP | 5.0×10−4 s−1 | ||
| 28 | IRAK1_BIND → IRAK1_DROP | 1.5×10−3 s−1 | ||
| 29 | IRAK4_BIND → IRAK4_DROP | 1.0×10−3 s−1 | ||
| 30 | TLR4_bind + MyD88 → TLR4_bind_MyD88 | 1.0×10−5 s−1 | ||
| 31 | TLR4_bind_MyD88 → TLR4_bind + MyD88_binda | 1.0 s−1 | ||
| 32 | MyD88_binda + IRAK1 → MyD88_binda_IRAK1 | 1.8×10−6 s−1 | ||
| 33 | MyD88_binda_IRAK1 → MyD88_binda + IRAK1_binda | 1.0 s−1 | ||
| 34 | MyD88_binda + IRAK4 → MyD88_binda_IRAK4 | 5.0×10−7 s−1 | ||
| 35 | MyD88_binda_IRAK4 → MyD88_binda + IRAK4_binda | 1.0 s−1 | ||
| 36 | IRAK1_binda → IRAK1_dropa | 1.4×10−3 s−1 | ||
| 37 | IRAK4_binda → IRAK4_dropa | 1.2×10−3 s−1 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Zhong, C.-Q.; Yin, Z.; Qi, H.; Xu, F.; He, Q.; Shuai, J. Data-Driven Modeling Identifies TIRAP-Independent MyD88 Activation Complex and Myddosome Assembly Strategy in LPS/TLR4 Signaling. Int. J. Mol. Sci. 2020, 21, 3061. https://doi.org/10.3390/ijms21093061
Li X, Zhong C-Q, Yin Z, Qi H, Xu F, He Q, Shuai J. Data-Driven Modeling Identifies TIRAP-Independent MyD88 Activation Complex and Myddosome Assembly Strategy in LPS/TLR4 Signaling. International Journal of Molecular Sciences. 2020; 21(9):3061. https://doi.org/10.3390/ijms21093061
Chicago/Turabian StyleLi, Xiang, Chuan-Qi Zhong, Zhiyong Yin, Hong Qi, Fei Xu, Qingzu He, and Jianwei Shuai. 2020. "Data-Driven Modeling Identifies TIRAP-Independent MyD88 Activation Complex and Myddosome Assembly Strategy in LPS/TLR4 Signaling" International Journal of Molecular Sciences 21, no. 9: 3061. https://doi.org/10.3390/ijms21093061
APA StyleLi, X., Zhong, C.-Q., Yin, Z., Qi, H., Xu, F., He, Q., & Shuai, J. (2020). Data-Driven Modeling Identifies TIRAP-Independent MyD88 Activation Complex and Myddosome Assembly Strategy in LPS/TLR4 Signaling. International Journal of Molecular Sciences, 21(9), 3061. https://doi.org/10.3390/ijms21093061




