Role of Bone Targeting Agents in the Prevention of Bone Metastases from Breast Cancer
Abstract
1. Introduction
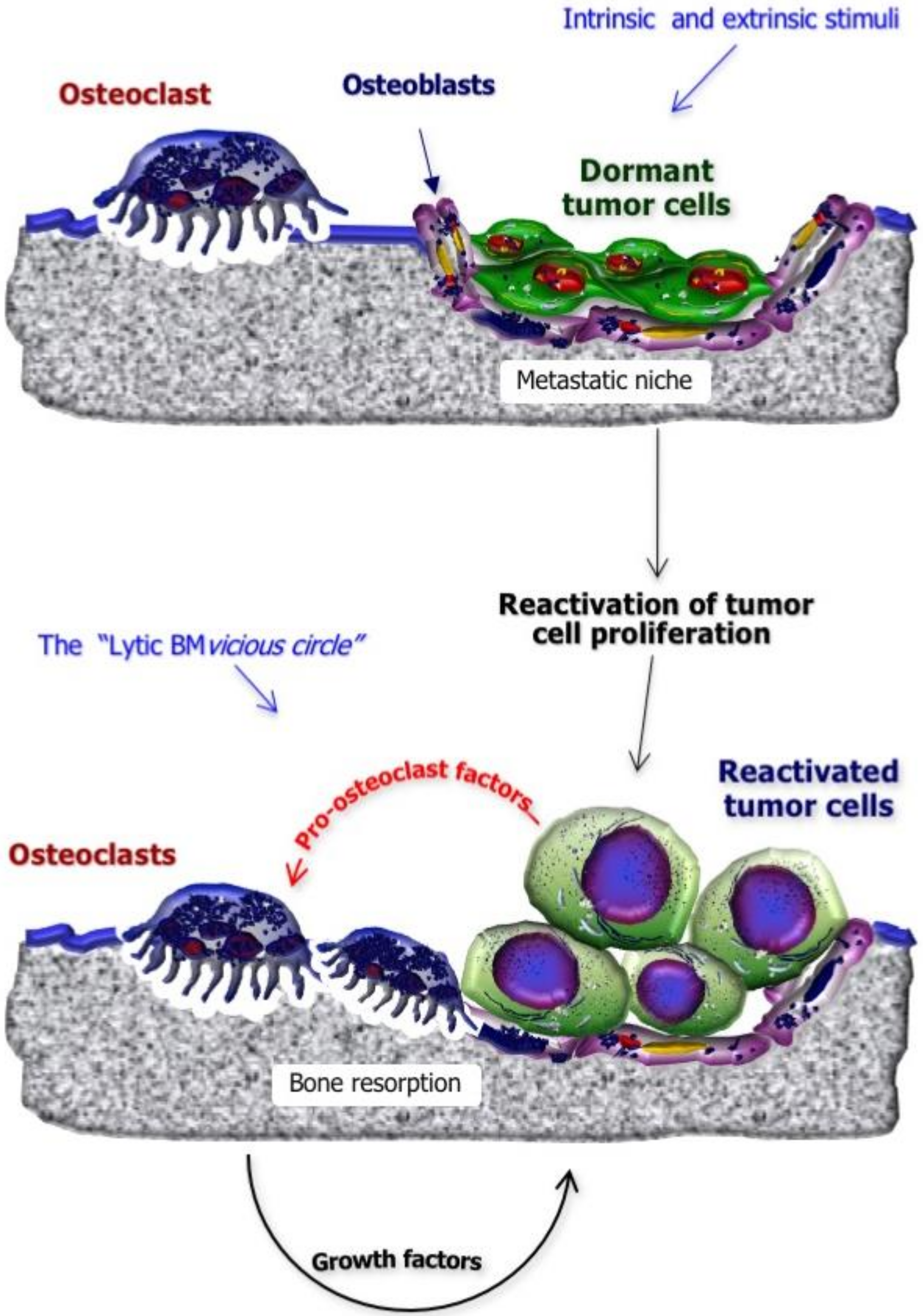
2. Mechanisms of BM Formation in BC
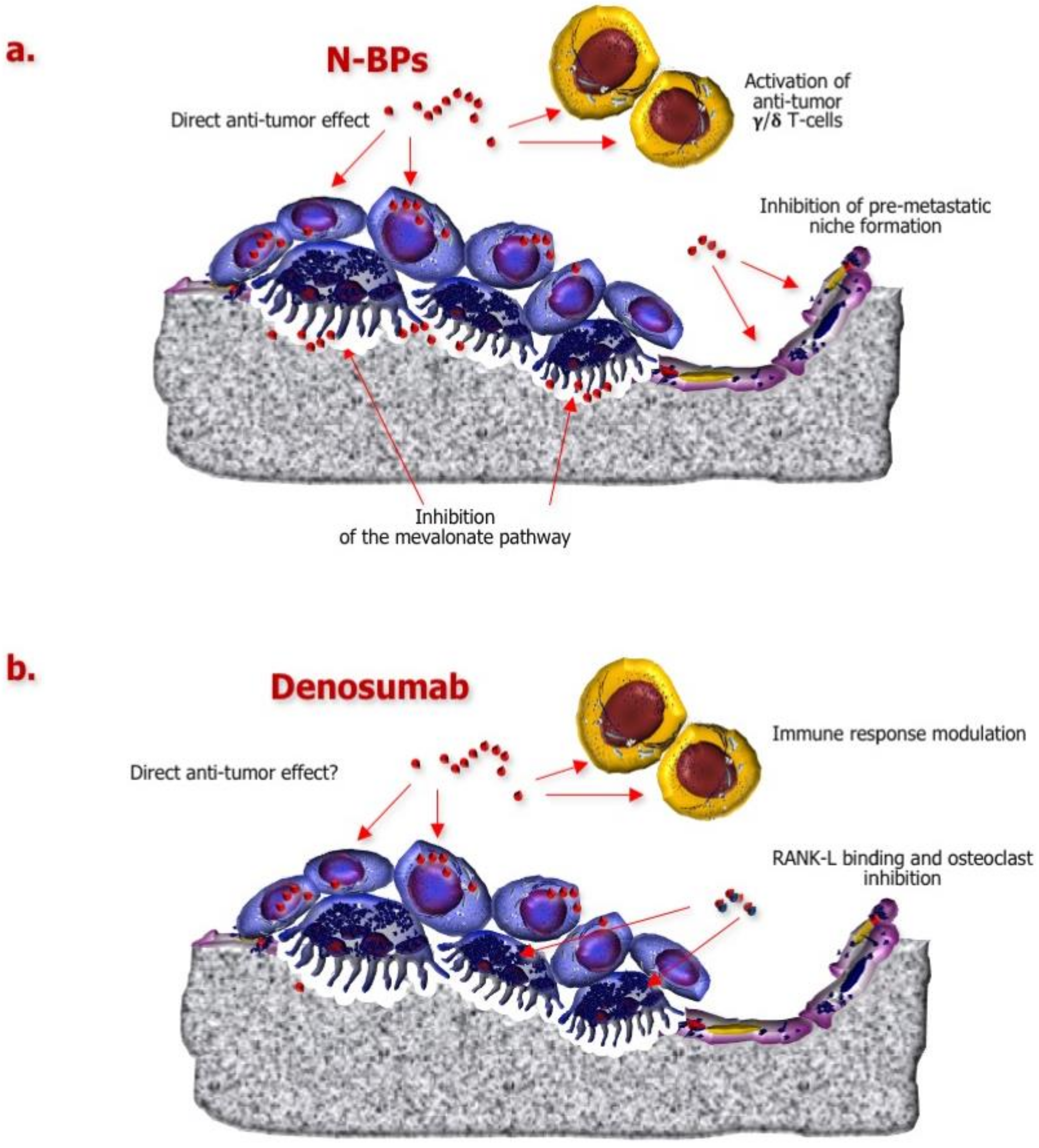
3. Role of Currently Approved BTAs in the Disruption of the “BM Cascade”
4. Impact of Adjuvant/Neoadjuvant BTAs on BC Relapse
4.1. Adjuvant BPs
4.2. Neoadjuvant BPs
4.3. Studies Focused on Denosumab
5. Seeking Prognostic and Predictive Biomarkers
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Ferlay, J.; Colombeta, M.; Soerjomataram, I.; Dyba, T.; Randi, G.; Bettio, M.; Gavin, A.; Visser, O.; Bray, F. Cancer incidence and mortality patterns in Europe: Estimates for 40 countries and 25 major cancers in 2018. Eur. J. Cancer 2018, 103, 356–387. [Google Scholar] [CrossRef] [PubMed]
- Carioli, G.; Malvezzi, M.; Rodriguez, T.; Bertuccio, P.; Negri, E.; La Vecchia, C. Trends and predictions to 2020 in breast cancer mortality in Europe. Breast 2017, 36, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Macedo, F.; Ladeira, K.; Pinho, F.; Saraiva, N.; Bonito, N.; Pinto, L.; Goncalves, F. Bone metastases: An overview. Oncol. Rev. 2017, 11, 321. [Google Scholar] [CrossRef]
- Kennecke, H.; Yerushalmi, R.; Woods, R.; Cheang, M.C.; Voduc, D.; Speers, C.H.; Nielsen, T.O.; Gelmon, K. Metastatic behavior of breast cancer subtypes. J. Clin. Oncol. 2010, 10, 3271–3277. [Google Scholar] [CrossRef]
- Coleman, R.E. Clinical features of metastatic bone disease and risk of skeletal morbidity. Clin. Cancer Res. 2006, 12 (Suppl. 20), 6243–6249. [Google Scholar] [CrossRef]
- D’Oronzo, S.; Coleman, R.; Brown, J.; Silvestris, F. Metastatic bone disease: Pathogenesis and therapeutic options. Up-date on bone metastasis management. J. Bone Oncol. 2018, 15. [Google Scholar] [CrossRef]
- Tanaka, R.; Yonemori, K.; Hirakawa, A.; Kinoshita, F.; Takahashi, N.; Hashimoto, J.; Kodaira, M.; Yamamoto, H.; Yunokawa, M.; Shimizu, C.; et al. Risk factors for developing skeletal-related events in breast cancer patients with bone metastases undergoing treatment with bone-modifying agents. Oncologist 2016, 21, 508–513. [Google Scholar] [CrossRef]
- Coleman, R. Clinical benefits of bone targeted agents in early breast cancer. Breast 2019, 48, S92–S96. [Google Scholar] [CrossRef]
- D’Oronzo, S.; Brown, J.; Coleman, R. The value of biomarkers in bone metastasis. Eur. J. Cancer Care 2017, 26, 6. [Google Scholar] [CrossRef]
- Kan, C.; Vargas, G.; Pape, F.L.; Clézardin, P. Cancer cell colonisation in the bone microenvironment. Int. J. Mol. Sci. 2016, 17, E1674. [Google Scholar] [CrossRef] [PubMed]
- Meng, F.; Wu, G. The rejuvenated scenario of epithelial–mesenchymal transition (EMT) and cancer metastasis. Cancer Metastasis Rev. 2012, 31, 455–467. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Cao, X. Characteristics and significance of the pre-metastatic niche. Cancer Cell 2016, 30, 668–681. [Google Scholar] [CrossRef] [PubMed]
- Puisieux, A.; Brabletz, T.; Caramel, J. Oncogenic roles of EMT-inducing transcription factors. Nat. Cell Biol. 2014, 16, 488–494. [Google Scholar] [CrossRef]
- Paget, S. The distribution of secondary growths in cancer of the breast. Lancet 1889, 571–573. [Google Scholar] [CrossRef]
- Ha, H.K.; Lee, W.; Park, H.J.; Lee, S.D.; Lee, J.Z.; Chung, M.K. Clinical significance of CXCL16/CXCR6 expression in patients with prostate cancer. Mol. Med. Rep. 2011, 4, 419–424. [Google Scholar] [PubMed]
- Lee, J.H.; Kim, H.N.; Kim, K.O.; Jin, W.J.; Lee, S.; Kim, H.H.; Ha, H.; Lee, Z.H. CXCL10 promotes osteolytic bone metastasis by enhancing cancer outgrowth and osteoclastogenesis. Cancer Res. 2012, 72, 3175–3186. [Google Scholar] [CrossRef] [PubMed]
- Cives, M.; Quaresmini, D.; Rizzo, F.M.; Felici, C.; D’Oronzo, S.; Simone, V.; Silvestris, F. Osteotropism of neuroendocrine tumors: Role of the CXCL12/CXCR4 pathway in promoting EMT in vitro. Oncotarget 2017, 8, 22534–22549. [Google Scholar] [CrossRef] [PubMed]
- Saidak, Z.; Boudot, C.; Abdoune, R.; Petit, L.; Brazier, M.; Mentaverri, R.; Kamel, S. Extracellular calcium promotes the migration of breast cancer cells through the activation of the calcium sensing receptor. Exp. Cell Res. 2009, 315, 2072–2080. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Clézardin, P.; Kamel, S.; Brazier, M.; Mentaverri, R. The CaSR in pathogenesis of breast cancer: A new target for early stage bone metastases. Front. Oncol. 2020, 10, 69. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.H.; Nakashima, T.; Sanchez, O.H.; Kozieradzki, I.; Komarova, S.V.; Sarosi, I.; Morony, S.; Rubin, E.; Sarao, R.; Hojilla, C.V.; et al. Regulation of cancer cell migration and bone metastasis by RANKL. Nature 2006, 440, 692–696. [Google Scholar] [CrossRef] [PubMed]
- Croucher, P.I.; McDonald, M.M.; Martin, T.J. Bone metastasis: The importance of the neighbourhood. Nat. Rev. Cancer 2016, 16, 373–386. [Google Scholar] [CrossRef] [PubMed]
- Sosa, M.S.; Bragado, P.; Aguirre-Ghiso, J.A. Mechanisms of disseminated cancer cell dormancy: An awakening field. Nat. Rev. Cancer 2014, 14, 611–622. [Google Scholar] [CrossRef] [PubMed]
- Shiozawa, Y.; Pedersen, E.A.; Patel, L.R.; Ziegler, A.M.; Havens, A.M.; Jung, Y.; Wang, J.; Zalucha, S.; Loberg, R.D.; Pienta, K.J.; et al. GAS6/AXL axis regulates prostate cancer invasion, proliferation, and survival in the bone marrow niche. Neoplasia 2010, 12, 116–127. [Google Scholar] [CrossRef]
- Kobayashi, A.; Okuda, H.; Xing, F.; Pandey, P.R.; Watabe, M.; Hirota, S.; Pai, S.K.; Liu, W.; Fukuda, K.; Chambers, C.; et al. Bone morphogenetic protein 7 in dormancy and metastasis of prostate cancer stem-like cells in bone. J. Exp. Med. 2011, 208, 2641–2655. [Google Scholar] [CrossRef]
- Salvador, F.; Llorente, A.; Gomis, R.R. From latency to overt bone metastasis in breast cancer: Potential for treatment and prevention. J. Pathol. 2019, 249, 6–18. [Google Scholar] [CrossRef]
- Wang, S.; Li, G.X.; Tan, C.C.; He, R.; Kang, L.J.; Lu, J.T.; Li, X.Q.; Wang, Q.S.; Liu, P.F.; Zhai, Q.L.; et al. FOXF2 reprograms breast cancer cells into bone metastasis seeds. Nat. Commun. 2019, 10, 2707. [Google Scholar] [CrossRef]
- Roodman, G.D. Mechanisms of bone metastasis. Discov. Med. 2004, 4, 144–148. [Google Scholar] [CrossRef]
- Guise, T.A.; Kozlow, W.M.; Heras-Herzig, A.; Padalecki, S.S.; Yin, J.J.; Chirgwin, J.M. Molecular Mechanisms of Breast Cancer Metastases to Bone. Clin. Breast Cancer 2005, 5, S46–S53. [Google Scholar] [CrossRef]
- Rosen, L.S.; Gordon, D.; Tchekmedyian, N.; Yanagihara, R.; Hirsh, V.; Krzakowski, M.; Pawlicki, M.; De Souza, P.; Zheng, M.; Urbanowitz, G.; et al. Long term efficacy and safety of zoledronic acid in the treatment of skeletal metastases in patients with non small cell lung carcinoma and other solid tumors: A randomized, phase III, double blind, placebo-controlled trial. Cancer 2004, 100, 2613–2621. [Google Scholar] [CrossRef]
- Rosen, L.S.; Gordon, D.; Kaminski, M.; Howell, A.; Belch, A.; Mackey, J.; Apffelstaedt, J.; Hussein, M.A.; Coleman, R.E.; Reitsma, D.J.; et al. Long-term efficacy and safety of zoledronic acid compared with pamidronate disodium in treatment of skeletal complications in patients with advanced multiple myeloma or breast cancer: A randomized, double-blind, multicenter, comparative trial. Cancer 2003, 98, 1735–1744. [Google Scholar] [CrossRef] [PubMed]
- Stopeck, A.T.; Lipton, A.; Body, J.J.; Steger, G.G.; Tonkin, K.; de Boer, R.H.; Lichinitser, M.; Fujiwara, Y.; Yardley, D.A.; Viniegra, M.; et al. Denosumab compared with zoledronic acid for the treatment of bone metastases in patients with advanced breast cancer: A randomized, double-blind study. J. Clin. Oncol. 2010, 28, 5132–5139. [Google Scholar] [CrossRef] [PubMed]
- Stopeck, A.T.; Fizazi, K.; Body, J.J.; Brown, J.E.; Carducci, M.; Diel, I.; Fujiwara, Y.; Martín, M.; Paterson, A.; Tonkin, K.; et al. Safety of long-term denosumab therapy: Results from the open label extension phase of two phase 3 studies in patients with metastatic breast and prostate cancer. Support. Care Cancer 2016, 24, 447–455. [Google Scholar] [CrossRef]
- Handforth, C.; D’Oronzo, S.; Coleman, R.; Brown, J. Cancer Treatment and Bone Health. Calcif. Tissue Int. 2018, 102, 251–264. [Google Scholar] [CrossRef] [PubMed]
- D’Oronzo, S.; Stucci, S.; Tucci, M.; Silvestris, F. Cancer treatment-induced bone loss (CTIBL): Pathogenesis and clinical implications. Cancer Treat. Rev. 2015, 41, 798–808. [Google Scholar] [CrossRef] [PubMed]
- Perez, E.A.; Josse, R.G.; Pritchard, K.I.; Ingle, J.N.; Martino, S.; Findlay, B.P.; Shenkier, T.N.; Tozer, R.G.; Palmer, M.J.; Shepherd, L.E.; et al. Effect of letrozole versus placebo on bone mineral density in women with primary breast cancer completing 5 or more years of adjuvant tamoxifen: A companion study to NCI CCTG MA.17. J.Clin.Oncol. 2006, 24, 3629–3635. [Google Scholar] [CrossRef]
- Roelofs, A.J.; Thompson, K.; Ebetino, H.; Rogers, M.J.; Coxon, F.P. Bisphosphonates: Molecular mechanisms of action and effects on bone cells, monocytes and macrophages. Curr. Pharm. Des. 2010, 16, 2950–2960. [Google Scholar] [CrossRef]
- Sousa, S.; Clézardin, P. Bone-targeted therapies in cancer-induced bone disease. Calcif. Tissue Int. 2018, 102, 227–250. [Google Scholar] [CrossRef]
- Cabillic, F.; Toutirais, O.; Lavoue, V.; de La Pintière, C.T.; Daniel, P.; Rioux-Leclerc, N.; Turlin, B.; Mönkkönen, H.; Mönkkönen, J.; Boudjema, K.; et al. Aminobisphosphonate-pretreated dendritic cells trigger successful Vc9Vd2T cell amplification for immunotherapy in advanced cancer patients. Cancer Immunol. Immunother. 2010, 59, 1611–1619. [Google Scholar] [CrossRef]
- Kalyan, S.; Chandrasekaran, V.; Quabius, E.S.; Lindhorst, T.K.; Kabelitz, D. Neutrophil uptake of nitrogen-bisphosphonates leads to the suppression of human peripheral blood cd T cells. Cell Mol. Life Sci. 2014, 71, 2335–2346. [Google Scholar] [CrossRef]
- Junankar, S.; Shay, G.; Jurczyluk, J.; Ali, N.; Down, J.; Pocock, N.; Parker, A.; Nguyen, A.; Sun, S.; Kashemirov, B.; et al. Real-time intravital imaging establishes tumor-associated macrophages as the extraskeletal target of bisphosphonate action in cancer. Cancer Discov. 2015, 5, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Wood, J.; Bonjean, K.; Ruetz, S.; Bellahcène, A.; Devy, L.; Foidart, J.M.; Castronovo, V.; Green, J.R. Novel antiangiogenic effects of the bisphosphonate compound zoledronic acid. J. Pharm. Exp. 2002, 302, 1055–1061. [Google Scholar] [CrossRef] [PubMed]
- Santini, D.; Vincenzi, B.; Dicuonzo, G.; Avvisati, G.; Massacesi, C.; Battistoni, F.; Gavasci, M.; Rocci, L.; Tirindelli, M.C.; Altomare, V.; et al. Zoledronic acid induces significant and long-lasting modifications of circulating angiogenic factors in cancer patients. Clin. Cancer Res. 2003, 9, 2893–2897. [Google Scholar] [PubMed]
- Kim, B.S.; Yang, S.S.; Kim, C.S.; Lee, J. Zoledronate suppresses VEGF-induced capillary tube formation and inhibits expression of interferon-induced transmembrane protein-1 in human umbilical vein endothelial cells. Int. J. Mol. Med. 2018, 41, 2879–2884. [Google Scholar] [CrossRef] [PubMed]
- Senaratne, S.G.; Pirianov, G.; Mansi, J.L.; Arnett, T.R.; Colston, K.W. Bisphosphonates induce apoptosis in human breast cancer cell lines. Br. J. Cancer 2000, 82, 1459–1468. [Google Scholar] [CrossRef] [PubMed]
- Boissier, S.; Ferreras, M.; Peyruchaud, O.; Magnetto, S.; Ebetino, F.H.; Colombel, M.; Delmas, P.; Delaissé, J.M.; Clézardin, P. Bisphosphonates inhibit breast and prostate carcinoma cell invasion, an early event in the formation of bone metastases. Cancer Res. 2000, 60, 2949–2954. [Google Scholar]
- Dedes, P.; Gialeli, C.; Tsonis, A.; Kanakis, I.; Theocharis, A.D.; Kletsas, D.; Tzanakakis, G.N.; Karamanos, N.K. Expression of matrix macromolecules and functional properties of breast cancer cells are modulated by the bisphosphonate zoledronic acid. Biochim. Biophys. Acta 2012, 1820, 1926–1939. [Google Scholar] [CrossRef]
- Clezardin, P. Mechanisms of action of bisphosphonates in oncology: A scientific concept evolving from antiresorptive to anticancer activities. Bone Key Rep. 2013, 2, 267. [Google Scholar] [CrossRef]
- Naoe, M.; Ogawa, Y.; Takeshita, K.; Morita, J.; Shichijo, T.; Fuji, K.; Fukagai, T.; Iwamoto, S.; Terao, S. Zoledronate stimulates gamma delta T cells in prostate cancer patients. Oncol. Res. 2010, 18, 493–501. [Google Scholar] [CrossRef]
- Ferrari-Lacraz, S.; Ferrari, S. Do RANKL inhibitors (denosumab) affect inflammation and immunity? Osteoporos. Int. 2011, 22, 435–446. [Google Scholar] [CrossRef]
- Cheng, M.L.; Fong, L. Effects of RANKL-targeted therapy in immunity and cancer. Front. Oncol. 2014, 3, 329. [Google Scholar] [CrossRef] [PubMed]
- Hofbauer, L.C.; Rachner, T.D.; Hamann, C. From bone to breast and back-the bone cytokine RANKL and breast cancer. Breast Cancer Res. 2011, 13, 107. [Google Scholar] [CrossRef] [PubMed]
- Sisay, M.; Mengistu, G.; Edessa, D. The RANK/RANKL/OPG system in tumorigenesis and metastasis of cancer stem cell: Potential targets for anticancer therapy. Onco Targets Therapy 2017, 10, 3801–3810. [Google Scholar] [CrossRef] [PubMed]
- Nolan, E.; Vaillant, F.; Branstetter, D.; Pal, B.; Giner, G.; Whitehead, L.; Lok, S.W.; Mann, G.B.; Kathleen Cuningham Foundation Consortium for Research into Familial Breast Cancer (kConFab); Rohrbach, K.; et al. RANK ligand as a potential target for breast cancer prevention in BRCA1-mutation carriers. Nat. Med. 2016, 22, 933–942. [Google Scholar] [CrossRef]
- Vetter, M.; Landin, J.; Szczerba, B.M.; Castro-Giner, F.; Gkountela, S.; Donato, C.; Krol, I.; Scherrer, R.; Balmelli, C.; Malinovska, A.; et al. Denosumab treatment is associated with the absence of circulating tumor cells in patients with breast cancer. Breast Cancer Res. 2018, 20, 141. [Google Scholar] [CrossRef]
- Strobl, S.; Wimmer, K.; Exner, R.; Devyatko, Y.; Bolliger, M.; Fitzal, F.; Gnant, M. Adjuvant bisphosphonate therapy in postmenopausal breast cancer. Curr. Treat. Options Oncol. 2018, 19, 18. [Google Scholar] [CrossRef]
- Diel, I.J.; Solomayer, E.F.; Costa, S.D.; Gollan, C.; Goerner, R.; Wallwiener, D.; Kaufmann, M.; Bastert, G. Reduction in new metastases in breast cancer with adjuvant clodronate treatment. New Engl. J. Med. 1998, 339, 357–363. [Google Scholar] [CrossRef]
- Diel, I.J.; Jaschke, A.; Solomayer, E.F.; Gollan, C.; Bastert, G.; Sohn, C.; Schuetz, F. Adjuvant oral clodronate improves the overall survival of primary breast cancer patients with micrometastases to the bone marrow: A long-term follow-up. Ann. Oncol. 2008, 19, 2007–2011. [Google Scholar] [CrossRef]
- Powles, T.; Paterson, A.; McCloskey, E.; Schein, P.; Scheffler, B.; Alwynne, T.; Ashley, S.; Smith, I.; Ottestad, L.; Kanis, J. Reduction in bone relapse and improved survival with oral clodronate for adjuvant treatment of operable breastcancer. Breast Cancer Res. 2006, 8, R13. [Google Scholar] [CrossRef] [PubMed]
- Paterson, A.H.; Anderson, S.J.; Lembersky, B.C.; Paterson, A.H.; Anderson, S.J.; Lembersky, B.C.; Fehrenbacher, L.; Falkson, C.I.; King, K.M.; Weir, L.M.; et al. Oral clodronate for adjuvant treatment of operable breast cancer (National Surgical Adjuvant Breast and Bowel Project protocol B-34): A multicentre, placebo-controlled, randomised trial. Lancet Oncol. 2012, 13, 734–742. [Google Scholar] [CrossRef]
- Von Minckwitz, G.; Möbus, V.; Schneeweiss, A. German Adjuvant Intergroup Node-Positive Study: A phase III trial to compare oral ibandronate versus observation in patients with high-risk early breast cancer. J. Clin. Oncol. 2013, 31, 3531–3539. [Google Scholar] [CrossRef]
- Gnant, M.; Mlineritsch, B.; Stoeger, H.; Luschin-Ebengreuth, G.; Knauer, M.; Moik, M.; Jakesz, R.; Seifert, M.; Taucher, S.; Bjelic-Radisic, V.; et al. Zoledronic acid combined with adjuvant endocrine therapy of tamoxifen versus anastrozole plus ovarian function suppression in premenopausal early breast cancer: Final analys is of the Austrian Breast and Colorectal Cancer Study Group Trial 12. Ann. Oncol. 2015, 26, 313–320. [Google Scholar] [CrossRef]
- Coleman, R.; Cameron, D.; Dodwell, D.; Bell, R.; Wilson, C.; Rathbone, E.; Keane, M.; Gil, M.; Burkinshaw, R.; Grieve, R.; et al. Adjuvant zoledronic acid in patients with early breast cancer: Final efficacy analysis of the AZURE (BIG01/04) randomised open-label phase 3 trial. Lancet Oncol. 2014, 15, 997–1006. [Google Scholar] [CrossRef]
- Coleman, R.E.; Collinson, M.; Gregory, W.; Marshall, H.; Bell, R.; Dodwell, D.; Keane, M.; Gil, M.; Barrett-Lee, P.; Ritchie, D.; et al. Benefits and risks of adjuvant treatment with zoledronic acid in stage II/III breast cancer. 10 years follow-up of the AZURE randomized clinical trial (BIG01/04). J. Bone Oncol. 2018, 13, 123–135. [Google Scholar] [CrossRef]
- Pan, K.; Bosserman, L.D.; Chlebowski, R.T. Ovarian suppression in adjuvant endocrine therapy for premenopausal breast cancer. J. Clin. Oncol. 2019, 37, 858–862. [Google Scholar] [CrossRef]
- Nakamura, T.; Imai, Y.; Matsumoto, T.; Sato, S.; Takeuchi, K.; Igarashi, K.; Harada, Y.; Azuma, Y.; Krust, A.; Yamamoto, Y.; et al. Estrogen prevents bone loss via estrogen receptor alpha and induction of Fas ligand in osteoclasts. Cell 2007, 130, 811–823. [Google Scholar] [CrossRef]
- Perrone, F.; DeLaurentiis, M.; DePlacido, S.; Orditura, M.; Cinieri, S.; Riccardi, F.; Ribecco, A.S.; Putzu, C.; Del Mastro, L.; Rossi, E.; et al. Adjuvant zoledronic acid and letrozole plus ovarian function suppression in premenopausal breast cancer: HOBOE phase 3 randomised trial. Eur. J. Cancer 2019, 118, 178–186. [Google Scholar] [CrossRef]
- Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). Adjuvant bisphosphonate treatment in early breast cancer: Meta-analyses of individual patient data from randomised trials. Lancet 2015, 386, 1353–1361. [Google Scholar] [CrossRef]
- Dhesy-Thind, S.; Fletcher, G.G.; Blanchette, P.S.; Clemons, M.J.; Dillmon, M.S.; Frank, E.S.; Gandhi, S.; Gupta, R.; Mates, M.; Moy, B.; et al. Use of adjuvant bisphosphonates and other bone-modifying agents in breast cancer: A cancer care Ontario and American society of clinical oncology clinical practice guideline. J. Clin. Oncol. 2017, 35, 2062–2081. [Google Scholar] [CrossRef]
- Hadji, P.; Coleman, R.E.; Wilson, C.; Powles, T.J.; Clézardin, P.; Aapro, M.; Costa, L.; Body, J.J.; Markopoulos, C.; Santini, D.; et al. Adjuvant bisphosphonates in early breast cancer: Consensus guidance for clinical practice from a European Panel. Ann. Oncol. 2016, 27, 379–390. [Google Scholar] [CrossRef]
- Aft, R.; Naughton, M.; Trinkaus, K.; Watson, M.; Ylagan, L.; Chavez-MacGregor, M.; Zhai, J.; Kuo, S.; Shannon, W.; Diemer, K.; et al. Effect of zoledronic acid on disseminated tumour cells in women with locally advanced breast cancer: An open label, randomised, phase 2 trial. Lancet Oncol. 2010, 11, 421–428. [Google Scholar] [CrossRef]
- Aft, R.; Naughton, M.; Trinkaus, K.; Weilbaecher, K. Effect of (Neo)adjuvant zoledronic acid on disease-free and overall survival in clinical stage II/III breastcancer. Br. J. Cancer 2012, 107, 7–11. [Google Scholar] [CrossRef]
- Coleman, R.E.; Winter, M.C.; Cameron, D.; Bell, R.; Dodwell, D.; Keane, M.M.; Gil, M.; Ritchie, D.; Passos-Coelho, J.L.; Wheatley, D.; et al. The effects of adding zoledronic acid to neoadjuvant chemotherapy on tumour response: Exploratory evidence for direct anti-tumour activity in breast cancer. Br. J. Cancer 2010, 102, 1099–1105. [Google Scholar] [CrossRef]
- Horiguchi, J.; Hasegawa, Y.; Miura, D.; Miura, D.; Ishikawa, T.; Hayashi, M.; Takao, S.; Kim, S.J.; Yamagami, K.; Miyashita, M.; et al. A randomized controlled trial comparing zoledronic acid plus chemotherapy with chemotherapy alone as a neoadjuvant treatment in patients with HER2-negative primary breast cancer. J. Clin. Oncol. 2013, 31, 1029. [Google Scholar] [CrossRef]
- Charehbili, A.; van de Ven, S.; Smit, V.T.; Meershoek-Klein Kranenbarg, E.; Hamdy, N.A.; Putter, H.; Heijns, J.B.; van Warmerdam, L.J.; Kessels, L.; Dercksen, M.; et al. Addition of zoledronic acid to neoadjuvant chemotherapy does not enhance tumor response in patients with HER2 negative stage II/III breast cancer: The NEOZOTAC trial (BOOG2010-01). Ann. Oncol. 2014, 25, 998–1004. [Google Scholar] [CrossRef]
- Kroep, J.R.; Charehbili, A.; Coleman, R.E.; Aft, R.L.; Hasegawa, Y.; Winter, M.C.; Weilbaecher, K.; Akazawa, K.; Hinsley, S.; Putter, H.; et al. Effects of neoadjuvant chemotherapy with or without zoledronic acid on pathological response: A meta-analysis of randomized trials. Eur. J. Cancer 2016, 54, 57–63. [Google Scholar] [CrossRef]
- Lelièvre, L.; Clézardin, P.; Magaud, L.; Roche, L.; Tubiana-Mathieu, N.; Tigaud, J.D.; Topart, D.; Raban, N.; Mouret-Reynier, M.A.; Mathevet, P. Comparative Study of Neoadjuvant Chemotherapy with and Without Zometa for Management of Locally Advanced Breast Cancer with Serum VEGF as Primary Endpoint: The NEOZOL Study. Clin. Breast Cancer 2018, 18, e1311–e1321. [Google Scholar] [CrossRef]
- De Groot, S.; Pijl, H.; Charehbili, A.; van de Ven, S.; Smit, V.T.H.B.M.; Meershoek-Klein Kranenbarg, E.; Heijns, J.B.; van Warmerdam, L.J.C.; Kessels, L.W.; Dercksen, M.W.; et al. Addition of zoledronic acid to neoadjuvant chemotherapy is not beneficial in patients with HER2-negative stage II/III breast cancer: 5-year survival analysis of the NEOZOTAC trial (BOOG2010-01). Breast Cancer Res. 2019, 21, 97. [Google Scholar]
- Stopeck, A.; Martin, M.; Ritchie, D.; Body, J.J.; Paterson, A.; Viniegra, M.; Jassem, J.; Takano, T.; Van Poznak, C.; Bourgeois, H.; et al. Effect of Denosumab Versus Zoledronic Acid Treatment in Patients with Breast Cancer and Bone Metastases: Results from the Extended Blinded Treatment Phase. CancerRes. 2010, 70 (Suppl. 24), 6–14. [Google Scholar]
- Gnant, M.; Pfeiler, G.; Steger, G.G.; Egle, D.; Greil, R.; Fitzal, F.; Wette, V.; Balic, M.; Haslbauer, F.; Melbinger-Zeinitzer, E.; et al. Adjuvant denosumab in postmenopausal patients with hormone receptor-positive breast cancer (ABCSG-18): Disease-free survival results from a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2019, 20, 339–351. [Google Scholar] [CrossRef]
- Coleman, R.; Finkelstein, D.M.; Barrios, C.; Martin, M.; Iwata, H.; Hegg, R.; Glaspy, J.; Periañez, A.M.; Tonkin, K.; Deleu, I.; et al. Adjuvant denosumab in early breast cancer (D-CARE): An international, multicentre, randomised, controlled, phase 3 trial. Lancet Oncol. 2020, 21, 60–72. [Google Scholar] [CrossRef]
- D’Oronzo, S.; Brown, J.; Coleman, R. The role of biomarkers in the management of bone-homing malignancies. J. Bone Oncol. 2017, 9, 1–9. [Google Scholar] [CrossRef]
- Santoro, N. The menopausal transition. Am.J.Med. 2005, 118, 8S–13S. [Google Scholar] [CrossRef]
- Pavlovic, M.; Arnal-Estapé, A.; Rojo, F.; Bellmunt, A.; Tarragona, M.; Guiu, M.; Planet, E.; Garcia-Albéniz, X.; Morales, M.; Urosevic, J.; et al. Enhanced MAF oncogene expression and breast cancer bone metastasis. J. Natl. Cancer Inst. 2015, 107, djv256. [Google Scholar] [CrossRef]
- Coleman, R.; Hall, A.; Albanell, J.; Hanby, A.; Bell, R.; Cameron, D.; Dodwell, D.; Marshall, H.; Jean-Mairet, J.; Tercero, J.C.; et al. Effect of MAF amplification on treatment outcomes with adjuvant zoledronic acid in early breast cancer: A secondary analysis of the international, open-label, randomised, controlled, phase 3 AZURE (BIG01/04) trial. Lancet Oncol. 2017, 18, 1543–1552. [Google Scholar] [CrossRef]
- Westbrook, J.A.; Cairns, D.A.; Peng, J.; Speirs, V.; Hanby, A.M.; Holen, I.; Wood, S.L.; Ottewell, P.D.; Marshall, H.; Banks, R.E.; et al. CAPG and GIPC1: Breast Cancer Biomarkers for Bone Metastasis Development and Treatment. J. Natl. Cancer Inst. 2016, 108, djv360. [Google Scholar] [CrossRef]
- Westbrook, J.A.; Wood, S.L.; Cairns, D.A.; Mc Mahon, K.; Gahlaut, R.; Thygesen, H.; Shires, M.; Roberts, S.; Marshall, H.; Oliva, M.R.; et al. Identification and validation of DOCK4 as a potential biomarker for risk of bone metastasis development in patients with early breast cancer. J. Pathol. 2019, 247, 381–391. [Google Scholar] [CrossRef]
- Brown, J.; Rathbone, E.; Hinsley, S.; Gregory, W.; Gossiel, F.; Marshall, H.; Burkinshaw, R.; Shulver, H.; Thandar, H.; Bertelli, G.; et al. Associations Between Serum Bone Biomarkers in Early Breast Cancer and Development of Bone Metastasis: Results from the AZURE (BIG01/04) Trial. J. Natl. Cancer Inst. 2018, 110, djx280. [Google Scholar] [CrossRef]
- Handforth, C.; D’Oronzo, S.; Brown, J. Medical prevention and treatment of bone metastases. In Primer on the Metabolic Bone Diseases and Disorders of Mineral Metabolism, 9th ed.; Bilezikian, J.P., Bouillon, R., Eds.; John Wiley and Sons: New Jersey, NJ, USA, 2018; Chapter 105; pp. 799–808. [Google Scholar]
- Swami, S.; Johnson, J.; Bettinson, L.A.; Kimura, T.; Zhu, H.; Albertelli, M.A.; Johnson, R.W.; Wu, J.Y. Prevention of breast cancer skeletal metastases with parathyroid hormone. JCI Insight 2017, 2, e90874. [Google Scholar] [CrossRef]
- Vahle, J.L.; Long, G.G.; Sandusky, G.; Westmore, M.; Ma, Y.L.; Sato, M. Bone neoplasms in F344 rats given teriparatide [rhPTH(1–34)] are dependent on duration of treatment and dose. Toxicol. Pathol. 2004, 32, 426–438. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
D’Oronzo, S.; Silvestris, E.; Paradiso, A.; Cives, M.; Tucci, M. Role of Bone Targeting Agents in the Prevention of Bone Metastases from Breast Cancer. Int. J. Mol. Sci. 2020, 21, 3022. https://doi.org/10.3390/ijms21083022
D’Oronzo S, Silvestris E, Paradiso A, Cives M, Tucci M. Role of Bone Targeting Agents in the Prevention of Bone Metastases from Breast Cancer. International Journal of Molecular Sciences. 2020; 21(8):3022. https://doi.org/10.3390/ijms21083022
Chicago/Turabian StyleD’Oronzo, Stella, Erica Silvestris, Angelo Paradiso, Mauro Cives, and Marco Tucci. 2020. "Role of Bone Targeting Agents in the Prevention of Bone Metastases from Breast Cancer" International Journal of Molecular Sciences 21, no. 8: 3022. https://doi.org/10.3390/ijms21083022
APA StyleD’Oronzo, S., Silvestris, E., Paradiso, A., Cives, M., & Tucci, M. (2020). Role of Bone Targeting Agents in the Prevention of Bone Metastases from Breast Cancer. International Journal of Molecular Sciences, 21(8), 3022. https://doi.org/10.3390/ijms21083022





