Interleukin-17 Reduces βENaC via MAPK Signaling in Vascular Smooth Muscle Cells
Abstract
1. Introduction
2. Methods
2.1. Cell Culture
2.2. Cell Treatment
2.3. Protein Isolation
2.4. Western Blot on A10 Protein Isolates
2.5. Live/Dead Viability/Cytotoxicity Assay
2.6. Statistical Analysis.
3. Results
3.1. IL-17 Reduces βENaC Expression in a Concentration-Dependent Fashion in Cultured VSMCs
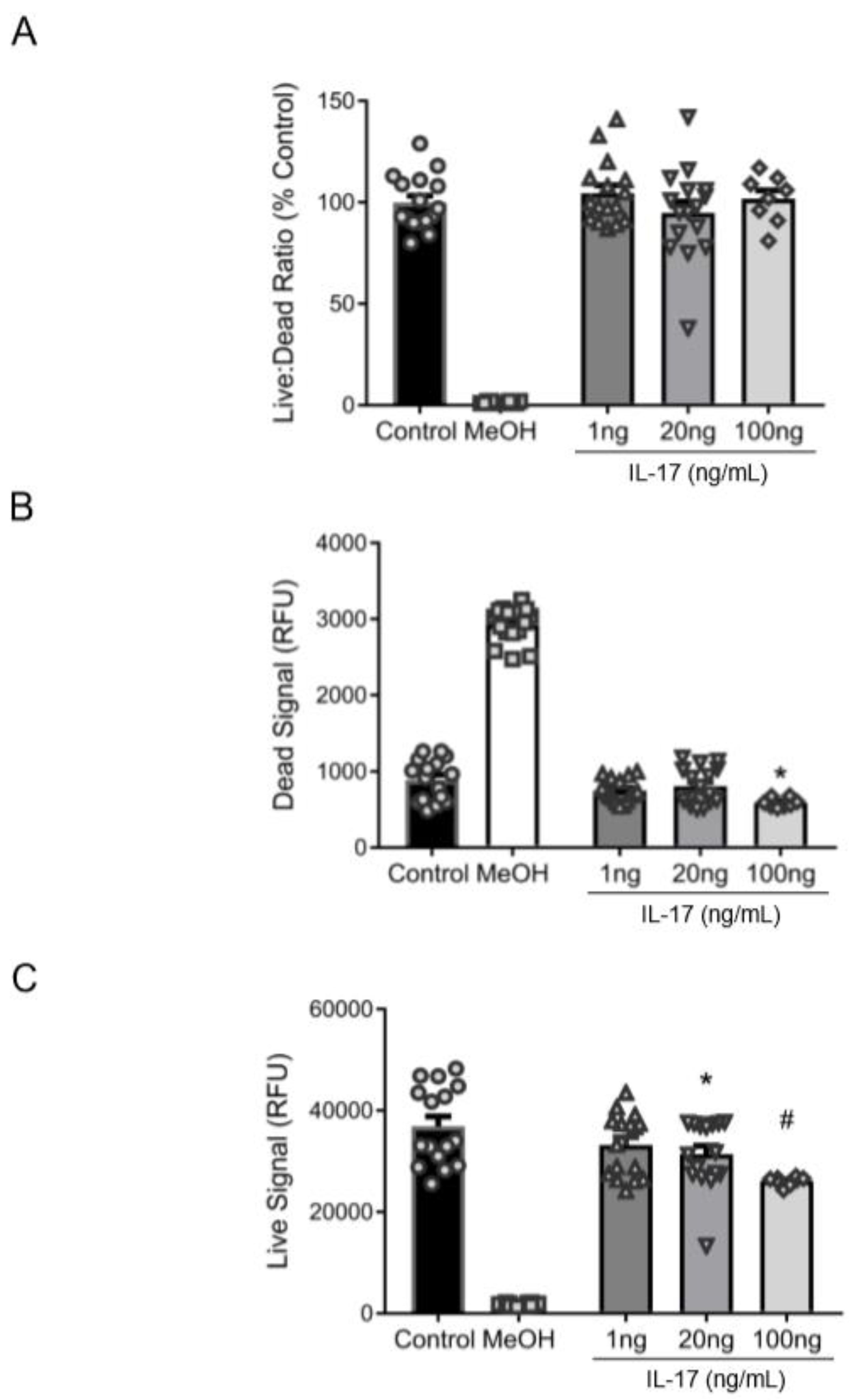
3.2. Reduction in βENaC by IL-17 Is Not Associated with Cell Death
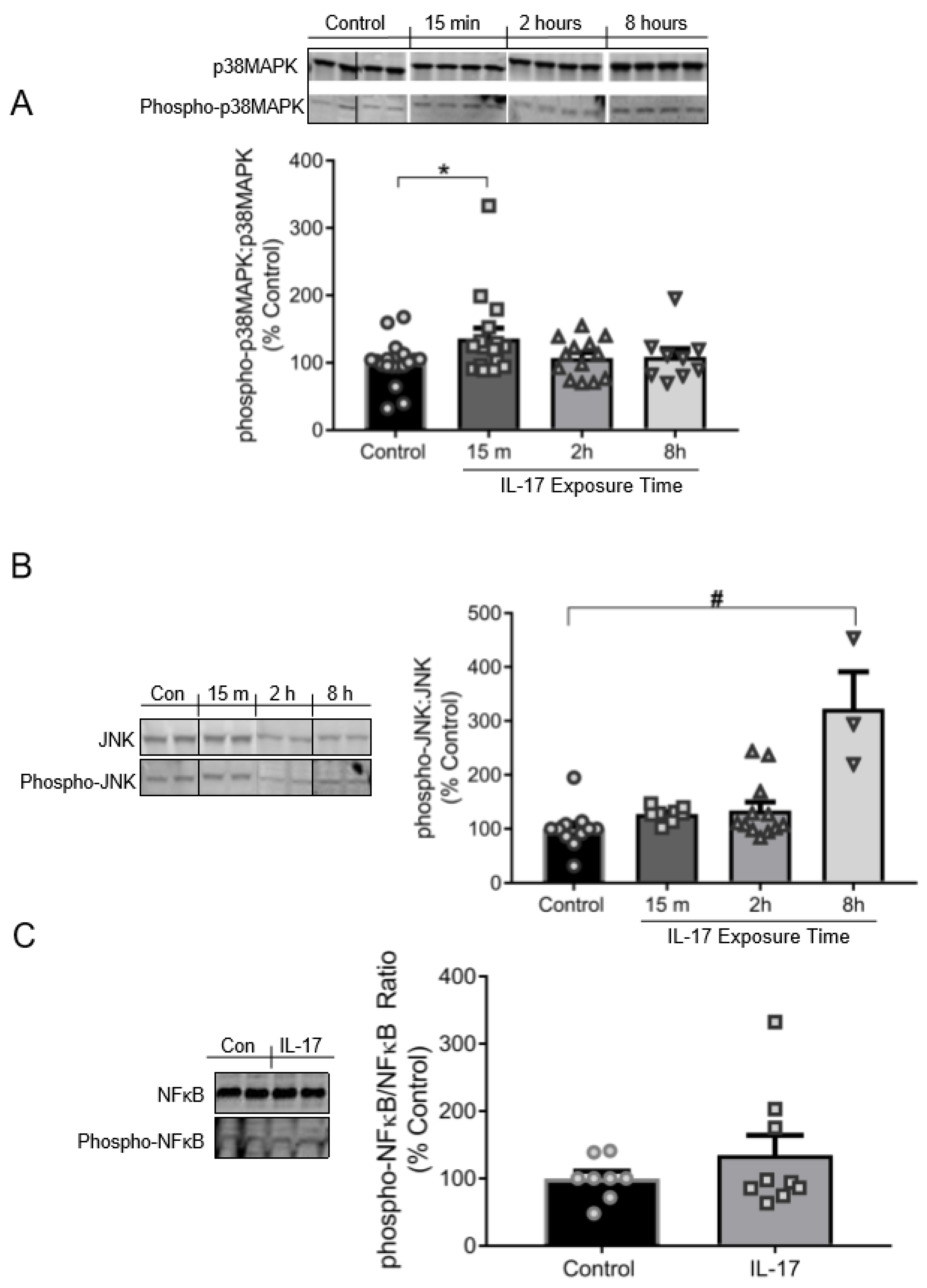
3.3. IL-17 Induces the Phosphorylation of p38MAPK and JNK, but Not NFκB
3.4. Role of p38MAPK and JNK in the Reduction of βENaC by IL-17
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Drummond, H.A.; Grifoni, S.C.; Jernigan, N.L. A new trick for an old dogma: Enac proteins as mechanotransducers in vascular smooth muscle. Physiology (Bethesda) 2008, 23, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Kimes, B.W.; Brandt, B.L. Characterization of two putative smooth muscle cell lines from rat thoracic aorta. Exp. Cell Res. 1976, 98, 349–366. [Google Scholar] [CrossRef]
- Ben-Shahar, Y. Sensory functions for degenerin/epithelial sodium channels (deg/enac). Adv. Genet. 2011, 76, 1–26. [Google Scholar] [PubMed]
- Drummond, H.A.; Gebremedhin, D.; Harder, D.R. Degenerin/epithelial Na+ channel proteins: Components of a vascular mechanosensor. Hypertension 2004, 44, 643–648. [Google Scholar] [CrossRef] [PubMed]
- Jernigan, N.L.; Drummond, H.A. Vascular enac proteins are required for renal myogenic constriction. Am. J. Physiol. Renal Physiol. 2005, 289, F891–F901. [Google Scholar] [CrossRef] [PubMed]
- Drummond, H.A. Betaenac is a molecular component of a vsmc mechanotransducer that contributes to renal blood flow regulation, protection from renal injury, and hypertension. Front. Physiol. 2012, 3, 341. [Google Scholar] [CrossRef]
- Drummond, H.A.; Stec, D.E. Betaenac acts as a mechanosensor in renal vascular smooth muscle cells that contributes to renal myogenic blood flow regulation, protection from renal injury and hypertension. J. Nephrol. Res. 2015, 1, 1–9. [Google Scholar] [CrossRef][Green Version]
- Ge, Y.; Gannon, K.; Gousset, M.; Liu, R.; Murphey, B.; Drummond, H.A. Impaired myogenic constriction of the renal afferent arteriole in a mouse model of reduced betaenac expression. Am. J. Physiol. Renal Physiol. 2012, 302, F1486–F1493. [Google Scholar] [CrossRef][Green Version]
- Grifoni, S.C.; Chiposi, R.; McKey, S.E.; Ryan, M.J.; Drummond, H.A. Altered whole kidney blood flow autoregulation in a mouse model of reduced beta-enac. Am. J. Physiol. Renal Physiol. 2010, 298, F285–F292. [Google Scholar] [CrossRef]
- Guan, Z.; Pollock, J.S.; Cook, A.K.; Hobbs, J.L.; Inscho, E.W. Effect of epithelial sodium channel blockade on the myogenic response of rat juxtamedullary afferent arterioles. Hypertension 2009, 54, 1062–1069. [Google Scholar] [CrossRef]
- Kim, E.C.; Ahn, D.S.; Yeon, S.I.; Lim, M.; Lee, Y.H. Epithelial Na+ channel proteins are mechanotransducers of myogenic constriction in rat posterior cerebral arteries. Exp. Physiol. 2012, 97, 544–555. [Google Scholar] [CrossRef]
- Kim, E.C.; Choi, S.K.; Lim, M.; Yeon, S.I.; Lee, Y.H. Role of endogenous Enac and TRP channels in the myogenic response of rat posterior cerebral arteries. PLoS ONE 2013, 8, e84194. [Google Scholar] [CrossRef] [PubMed]
- Nagasawa, T.; Imig, J.D. Afferent arteriolar responses to beta, gamma-methylene atp and 20-hete are not blocked by enac inhibition. Physiol. Rep. 2013, 1, e00082. [Google Scholar] [CrossRef]
- Syntichaki, P.; Tavernarakis, N. Genetic models of mechanotransduction: The nematode Caenorhabditis elegans. Physiol. Rev. 2004, 84, 1097–1153. [Google Scholar] [CrossRef] [PubMed]
- VanLandingham, L.G.; Gannon, K.P.; Drummond, H.A. Pressure-induced constriction is inhibited in a mouse model of reduced betaenac. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2009, 297, R723–R728. [Google Scholar] [CrossRef][Green Version]
- Wang, X.; Takeya, K.; Aaronson, P.I.; Loutzenhiser, K.; Loutzenhiser, R. Effects of amiloride, benzamil, and alterations in extracellular Na+ on the rat afferent arteriole and its myogenic response. Am. J. Physiol. Renal Physiol. 2008, 295, F272–F282. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Grifoni, S.C.; Gannon, K.P.; Stec, D.E.; Drummond, H.A. Enac proteins contribute to VSMC migration. Am. J. Physiol. Heart Circ. Physiol. 2006, 291, H3076–H3086. [Google Scholar] [CrossRef]
- Benos, D.J.; Stanton, B.A. Functional domains within the degenerin/epithelial sodium channel (deg/enac) superfamily of ion channels. J. Physiol 1999, 520 Pt 3, 631–644. [Google Scholar] [CrossRef]
- Canessa, C.M.; Schild, L.; Buell, G.; Thorens, B.; Gautschi, I.; Horisberger, J.D.; Rossier, B.C. Amiloride-sensitive epithelial na+ channel is made of three homologous subunits. Nature 1994, 367, 463–467. [Google Scholar] [CrossRef]
- Kellenberger, S.; Schild, L. Epithelial sodium channel/degenerin family of ion channels: A variety of functions for a shared structure. Physiol. Rev. 2002, 82, 735–767. [Google Scholar] [CrossRef]
- Bonny, O.; Chraibi, A.; Loffing, J.; Jaeger, N.F.; Grunder, S.; Horisberger, J.D.; Rossier, B.C. Functional expression of a pseudohypoaldosteronism type i mutated epithelial Na+ channel lacking the pore-forming region of its alpha subunit. J. Clin. Invest. 1999, 104, 967–974. [Google Scholar] [CrossRef]
- Wesch, D.; Althaus, M.; Miranda, P.; Cruz-Muros, I.; Fronius, M.; Gonzalez-Hernandez, T.; Clauss, W.G.; Alvarez de la Rosa, D.; Giraldez, T. Differential n termini in epithelial Na+ channel delta-subunit isoforms modulate channel trafficking to the membrane. Am. J. Physiol. Cell Physiol. 2012, 302, C868–C879. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.Y.; Choi, Y.S.; Kim, S.J.; Son, E.J.; Choi, H.S.; Yoon, J.H. Interleukin-1beta suppresses epithelial sodium channel beta-subunit expression and enac-dependent fluid absorption in human middle ear epithelial cells. Eur. J. Pharmacol. 2007, 567, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Dames, P.; Bergann, T.; Fromm, A.; Bucker, R.; Barmeyer, C.; Krug, S.M.; Fromm, M.; Schulzke, J.D. Interleukin-13 affects the epithelial sodium channel in the intestine by coordinated modulation of stat6 and p38 mapk activity. J. Physiol. 2015, 593, 5269–5282. [Google Scholar] [CrossRef]
- Wynne, B.M.; Zou, L.; Linck, V.; Hoover, R.S.; Ma, H.P.; Eaton, D.C. Regulation of lung epithelial sodium channels by cytokines and chemokines. Front. Immunol. 2017, 8, 766. [Google Scholar] [CrossRef]
- Dagenais, A.; Frechette, R.; Yamagata, Y.; Yamagata, T.; Carmel, J.F.; Clermont, M.E.; Brochiero, E.; Masse, C.; Berthiaume, Y. Downregulation of enac activity and expression by TNF-alpha in alveolar epithelial cells. Am. J. Physiol. Lung Cell Mol. Physiol. 2004, 286, L301–L311. [Google Scholar] [CrossRef] [PubMed]
- Galietta, L.J.; Pagesy, P.; Folli, C.; Caci, E.; Romio, L.; Costes, B.; Nicolis, E.; Cabrini, G.; Goossens, M.; Ravazzolo, R.; et al. IL-4 is a potent modulator of ion transport in the human bronchial epithelium in vitro. J. Immunol. 2002, 168, 839–845. [Google Scholar] [CrossRef] [PubMed]
- Roux, J.; Kawakatsu, H.; Gartland, B.; Pespeni, M.; Sheppard, D.; Matthay, M.A.; Canessa, C.M.; Pittet, J.F. Interleukin-1beta decreases expression of the epithelial sodium channel alpha-subunit in alveolar epithelial cells via a p38 mapk-dependent signaling pathway. J. Biol. Chem. 2005, 280, 18579–18589. [Google Scholar] [CrossRef]
- Amatya, N.; Garg, A.V.; Gaffen, S.L. IL-17 signaling: The yin and the yang. Trends Immunol. 2017, 38, 310–322. [Google Scholar] [CrossRef]
- Gaffen, S.L. An overview of IL-17 function and signaling. Cytokine 2008, 43, 402–407. [Google Scholar] [CrossRef]
- Senaud, J.; Vendrely, R.; Tronche, P. On the nature of the toxic substance of the sarcosporidic cysts of the sheep (Toxoplasmea), active on rabbits. C. R. Acad. Hebd. Seances Acad. Sci. D 1968, 266, 1137–1138. [Google Scholar] [PubMed]
- Matsuda, N.; Hattori, Y. Vascular biology in sepsis: Pathophysiological and therapeutic significance of vascular dysfunction. J. Smooth Muscle Res. 2007, 43, 117–137. [Google Scholar] [CrossRef]
- Sprague, A.H.; Khalil, R.A. Inflammatory cytokines in vascular dysfunction and vascular disease. Biochem. Pharmacol. 2009, 78, 539–552. [Google Scholar] [CrossRef]
- Patel, R.V.; Shelling, M.L.; Prodanovich, S.; Federman, D.G.; Kirsner, R.S. Psoriasis and vascular disease-risk factors and outcomes: A systematic review of the literature. J. Gen. Intern. Med. 2011, 26, 1036–1049. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, S.P.; Handa, R.; Gulati, G.S.; Sharma, S.; Pandey, R.M.; Aggarwal, P.; Ramakrishnan, L.; Shankar, S. Peripheral vascular disease in systemic lupus erythematosus. Lupus 2007, 16, 720–723. [Google Scholar] [CrossRef] [PubMed]
- De Miguel, C.; Rudemiller, N.P.; Abais, J.M.; Mattson, D.L. Inflammation and hypertension: New understandings and potential therapeutic targets. Curr. Hypertens. Rep. 2015, 17, 507. [Google Scholar] [CrossRef]
- Raines, E.W.; Ferri, N. Thematic review series: The immune system and atherogenesis. Cytokines affecting endothelial and smooth muscle cells in vascular disease. J. Lipid Res. 2005, 46, 1081–1092. [Google Scholar] [CrossRef]
- D’Amico, R.; Fusco, R.; Gugliandolo, E.; Cordaro, M.; Siracusa, R.; Impellizzeri, D.; Peritore, A.F.; Crupi, R.; Cuzzocrea, S.; Di Paola, R. Effects of a new compound containing Palmitoylethanolamide and baicalein in myocardial ischaemia/reperfusion injury in vivo. Phytomedicine 2019, 54, 27–42. [Google Scholar] [CrossRef]
- Impellizzeri, D.; Siracusa, R.; Cordaro, M.; Crupi, R.; Peritore, A.F.; Gugliandolo, E.; D’Amico, R.; Petrosino, S.; Evangelista, M.; Di Paola, R.; et al. N-palmitoylethanolamine-oxazoline (Pea-oxa): A new therapeutic strategy to reduce neuroinflammation, oxidative stress associated to vascular dementia in an experimental model of repeated bilateral common carotid arteries occlusion. Neurobiol. Dis. 2019, 125, 77–91. [Google Scholar] [CrossRef]
- Karbach, S.; Croxford, A.L.; Oelze, M.; Schuler, R.; Minwegen, D.; Wegner, J.; Koukes, L.; Yogev, N.; Nikolaev, A.; Reissig, S.; et al. Interleukin 17 drives vascular inflammation, endothelial dysfunction, and arterial hypertension in psoriasis-like skin disease. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 2658–2668. [Google Scholar] [CrossRef]
- Madhur, M.S.; Funt, S.A.; Li, L.; Vinh, A.; Chen, W.; Lob, H.E.; Iwakura, Y.; Blinder, Y.; Rahman, A.; Quyyumi, A.A.; et al. Role of interleukin 17 in inflammation, atherosclerosis, and vascular function in apolipoprotein e-deficient mice. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 1565–1572. [Google Scholar] [CrossRef] [PubMed]
- Schuler, R.; Efentakis, P.; Wild, J.; Lagrange, J.; Garlapati, V.; Molitor, M.; Kossmann, S.; Oelze, M.; Stamm, P.; Li, H.; et al. T cell-derived il-17a induces vascular dysfunction via perivascular fibrosis formation and dysregulation of (.)no/cgmp signaling. Oxid Med. Cell Longev. 2019, 2019, 6721531. [Google Scholar] [CrossRef] [PubMed]
- Garty, H.; Palmer, L.G. Epithelial sodium channels: Function, structure, and regulation. Physiol. Rev. 1997, 77, 359–396. [Google Scholar] [CrossRef] [PubMed]
- Kusche-Vihrog, K.; Tarjus, A.; Fels, J.; Jaisser, F. The epithelial Na+ channel: A new player in the vasculature. Curr. Opin. Nephrol. Hypertens. 2014, 23, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Chung, W.S.; Weissman, J.L.; Farley, J.; Drummond, H.A. Betaenac is required for whole cell mechanically gated currents in renal vascular smooth muscle cells. Am. J. Physiol. Renal Physiol. 2013, 304, F1428–F1437. [Google Scholar] [CrossRef] [PubMed]
- Drummond, H.A.; Grifoni, S.C.; Abu-Zaid, A.; Gousset, M.; Chiposi, R.; Barnard, J.M.; Murphey, B.; Stec, D.E. Renal inflammation and elevated blood pressure in a mouse model of reduced {beta}-Enac. Am. J. Physiol. Renal Physiol. 2011, 301, F443–F449. [Google Scholar] [CrossRef]
- Schramm, P.; Klein, K.U.; Falkenberg, L.; Berres, M.; Closhen, D.; Werhahn, K.J.; David, M.; Werner, C.; Engelhard, K. Impaired cerebrovascular autoregulation in patients with severe sepsis and sepsis-associated delirium. Crit. Care 2012, 16, R181. [Google Scholar] [CrossRef]
- Duncan, J.; Younes, S.T.; Hildebrandt, E.; Ryan, M.J.; Granger, J.P.; Drummond, H.A. Tumor necrosis factor-alpha impairs cerebral blood flow in pregnant rats: Role of vascular beta-epithelial Na(+) channel. Am. J. Physiol. Heart Circ. Physiol. 2020. [Google Scholar] [CrossRef]
- Cheng, G.; Wei, L.; Xiurong, W.; Xiangzhen, L.; Shiguang, Z.; Songbin, F. Il-17 stimulates migration of carotid artery vascular smooth muscle cells in an mmp-9 dependent manner via p38 mapk and erk1/2-dependent nf-kappab and ap-1 activation. Cell. Mol. Neurobiol. 2009, 29, 1161–1168. [Google Scholar] [CrossRef]
- De Oliveira, P.S.; Cardoso, P.R.; Lima, E.V.; Pereira, M.C.; Duarte, A.L.; Pitta Ida, R.; Rego, M.J.; Pitta, M.G. Il-17a, il-22, il-6, and il-21 serum levels in plaque-type psoriasis in brazilian patients. Mediat. Inflamm. 2015, 2015, 819149. [Google Scholar] [CrossRef]
- Cornelius, D.C.; Hogg, J.P.; Scott, J.; Wallace, K.; Herse, F.; Moseley, J.; Wallukat, G.; Dechend, R.; LaMarca, B. Administration of interleukin-17 soluble receptor c suppresses th17 cells, oxidative stress, and hypertension in response to placental ischemia during pregnancy. Hypertension 2013, 62, 1068–1073. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Sommerville, L.J.; Cuneo, A.; Kelemen, S.E.; Autieri, M.V. Expression and suppressive effects of interleukin-19 on vascular smooth muscle cell pathophysiology and development of intimal hyperplasia. Am. J. Pathol. 2008, 173, 901–909. [Google Scholar] [CrossRef] [PubMed]
- Mazighi, M.; Pelle, A.; Gonzalez, W.; el Mtairag, M.; Philippe, M.; Henin, D.; Michel, J.B.; Feldman, L.J. Il-10 inhibits vascular smooth muscle cell activation in vitro and in vivo. Am. J. Physiol. Heart Circ. Physiol. 2004, 287, H866–H871. [Google Scholar] [CrossRef] [PubMed]
- Cuneo, A.A.; Herrick, D.; Autieri, M.V. Il-19 reduces vsmc activation by regulation of mRNA regulatory factor hur and reduction of mRNA stability. J. Mol. Cell. Cardiol. 2010, 49, 647–654. [Google Scholar] [CrossRef] [PubMed]
- Gu, C.; Wu, L.; Li, X. Il-17 family: Cytokines, receptors and signaling. Cytokine 2013, 64, 477–485. [Google Scholar] [CrossRef] [PubMed]
- Noubade, R.; Krementsov, D.N.; Del Rio, R.; Thornton, T.; Nagaleekar, V.; Saligrama, N.; Spitzack, A.; Spach, K.; Sabio, G.; Davis, R.J.; et al. Activation of p38 mapk in cd4 t cells controls il-17 production and autoimmune Encephalomyelitis. Blood 2011, 118, 3290–3300. [Google Scholar] [CrossRef] [PubMed]
- Li, J.K.; Nie, L.; Zhao, Y.P.; Zhang, Y.Q.; Wang, X.; Wang, S.S.; Liu, Y.; Zhao, H.; Cheng, L. IL-17 mediates inflammatory reactions via p38/c-Fos and JNK/c-Jun activation in an Ap-1-dependent manner in human nucleus pulposus cells. J. Transl. Med. 2016, 14, 77. [Google Scholar]
- Iyoda, M.; Shibata, T.; Kawaguchi, M.; Hizawa, N.; Yamaoka, T.; Kokubu, F.; Akizawa, T. IL-17a and IL-17f stimulate chemokines via mapk pathways (erk1/2 and p38 but not JNK) in mouse cultured mesangial cells: Synergy with TNF-alpha and IL-1beta. Am. J. Physiol. Renal Physiol. 2010, 298, F779–F787. [Google Scholar] [CrossRef]
- Applegarth, A. On structures. J. Am. Psychoanal Assoc. 1989, 37, 1097–1107. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Xie, S.; Li, J.; Wang, J.H.; Wu, Q.; Yang, P.; Hsu, H.C.; Smythies, L.E.; Mountz, J.D. IL-17 activates the canonical NF-kappab signaling pathway in autoimmune b cells of bxd2 mice to upregulate the expression of regulators of g-protein signaling 16. J. Immunol. 2010, 184, 2289–2296. [Google Scholar] [CrossRef] [PubMed]
- Shen, F.; Hu, Z.; Goswami, J.; Gaffen, S.L. Identification of common transcriptional regulatory elements in interleukin-17 target genes. J. Biol. Chem. 2006, 281, 24138–24148. [Google Scholar] [CrossRef] [PubMed]
- Sonder, S.U.; Saret, S.; Tang, W.; Sturdevant, D.E.; Porcella, S.F.; Siebenlist, U. IL-17-induced NF-kappab activation via CIKS/Act1: Physiologic significance and signaling mechanisms. J. Biol. Chem. 2011, 286, 12881–12890. [Google Scholar] [CrossRef] [PubMed]
- Fey, D.; Croucher, D.R.; Kolch, W.; Kholodenko, B.N. Crosstalk and signaling switches in mitogen-activated protein kinase cascades. Front. Physiol. 2012, 3, 355. [Google Scholar] [CrossRef] [PubMed]
- Pietrowski, E.; Bender, B.; Huppert, J.; White, R.; Luhmann, H.J.; Kuhlmann, C.R. Pro-inflammatory effects of interleukin-17A on vascular smooth muscle cells involve NAD(P)H- oxidase derived reactive oxygen species. J. Vasc. Res. 2011, 48, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Marino, G.I.; Kotsias, B.A. Expression of the epithelial sodium channel sensitive to amiloride (Enac) in normal and preeclamptic human placenta. Placenta 2013, 34, 197–200. [Google Scholar] [CrossRef] [PubMed]
- Ryan, M.J.; Gilbert, E.L.; Glover, P.H.; George, E.M.; Masterson, C.W.; McLemore, G.R., Jr.; LaMarca, B.; Granger, J.P.; Drummond, H.A. Placental ischemia impairs middle cerebral artery myogenic responses in the pregnant rat. Hypertension 2011, 58, 1126–1131. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; He, G.; Yang, Y.; Liu, Y.; Diao, R.; Sheng, K.; Liu, X.; Xu, W. Reduced expression of Enac in placenta tissues of patients with severe preeclampsia is related to compromised trophoblastic cell migration and invasion during pregnancy. PLoS ONE 2013, 8, e72153. [Google Scholar] [CrossRef]
- Warrington, J.P.; Fan, F.; Murphy, S.R.; Roman, R.J.; Drummond, H.A.; Granger, J.P.; Ryan, M.J. Placental ischemia in pregnant rats impairs cerebral blood flow autoregulation and increases blood-brain barrier permeability. Physiol. Rep. 2014, 2, e12134. [Google Scholar] [CrossRef]
- Kalantar, F.; Rajaei, S.; Heidari, A.B.; Mansouri, R.; Rashidi, N.; Izad, M.H.; Mirahmadian, M. Serum levels of tumor necrosis factor-alpha, interleukin-15 and interleukin-10 in patients with pre-eclampsia in comparison with normotensive pregnant women. Iran. J. Nurs. Midwifery Res. 2013, 18, 463–466. [Google Scholar]
- LaMarca, B.; Speed, J.; Fournier, L.; Babcock, S.A.; Berry, H.; Cockrell, K.; Granger, J.P. Hypertension in response to chronic reductions in uterine perfusion in pregnant rats: Effect of tumor necrosis factor-alpha blockade. Hypertension 2008, 52, 1161–1167. [Google Scholar] [CrossRef]
- Molvarec, A.; Czegle, I.; Szijarto, J.; Rigo, J., Jr. Increased circulating interleukin-17 levels in preeclampsia. J. Reprod. Immunol. 2015, 112, 53–57. [Google Scholar] [CrossRef] [PubMed]
- Cornelius, D.C.; Amaral, L.M.; Harmon, A.; Wallace, K.; Thomas, A.J.; Campbell, N.; Scott, J.; Herse, F.; Haase, N.; Moseley, J.; et al. An increased population of regulatory T cells improves the pathophysiology of placental ischemia in a rat model of preeclampsia. Am. J. Physiol. Regul. Integr Comp. Physiol. 2015, 309, R884–R891. [Google Scholar] [CrossRef] [PubMed]
- Chakravarty, A.; Chakrabarti, S.D. The neurology of eclampsia: Some observations. Neurol. India 2002, 50, 128–135. [Google Scholar] [PubMed]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duncan, J.W.; Granger, J.P.; Ryan, M.J.; Drummond, H.A. Interleukin-17 Reduces βENaC via MAPK Signaling in Vascular Smooth Muscle Cells. Int. J. Mol. Sci. 2020, 21, 2953. https://doi.org/10.3390/ijms21082953
Duncan JW, Granger JP, Ryan MJ, Drummond HA. Interleukin-17 Reduces βENaC via MAPK Signaling in Vascular Smooth Muscle Cells. International Journal of Molecular Sciences. 2020; 21(8):2953. https://doi.org/10.3390/ijms21082953
Chicago/Turabian StyleDuncan, Jeremy W., Joey P. Granger, Michael J. Ryan, and Heather A. Drummond. 2020. "Interleukin-17 Reduces βENaC via MAPK Signaling in Vascular Smooth Muscle Cells" International Journal of Molecular Sciences 21, no. 8: 2953. https://doi.org/10.3390/ijms21082953
APA StyleDuncan, J. W., Granger, J. P., Ryan, M. J., & Drummond, H. A. (2020). Interleukin-17 Reduces βENaC via MAPK Signaling in Vascular Smooth Muscle Cells. International Journal of Molecular Sciences, 21(8), 2953. https://doi.org/10.3390/ijms21082953



