Mark4 Inhibited the Browning of White Adipose Tissue by Promoting Adipocytes Autophagy in Mice
Abstract
1. Introduction
2. Results
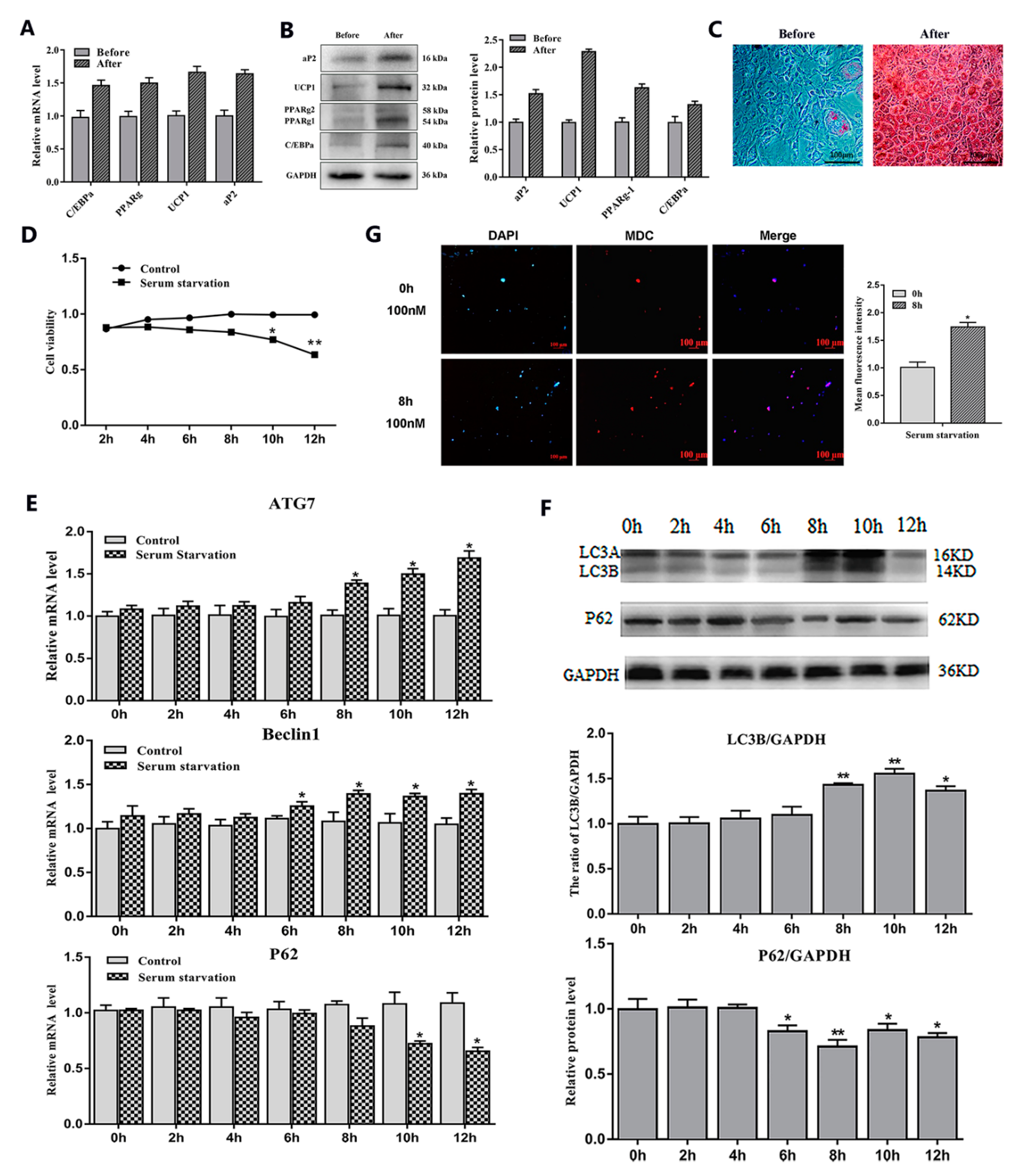
2.1. Serum Starvation and Rapamycin Treatment can Induce Adipocyte Autophagy
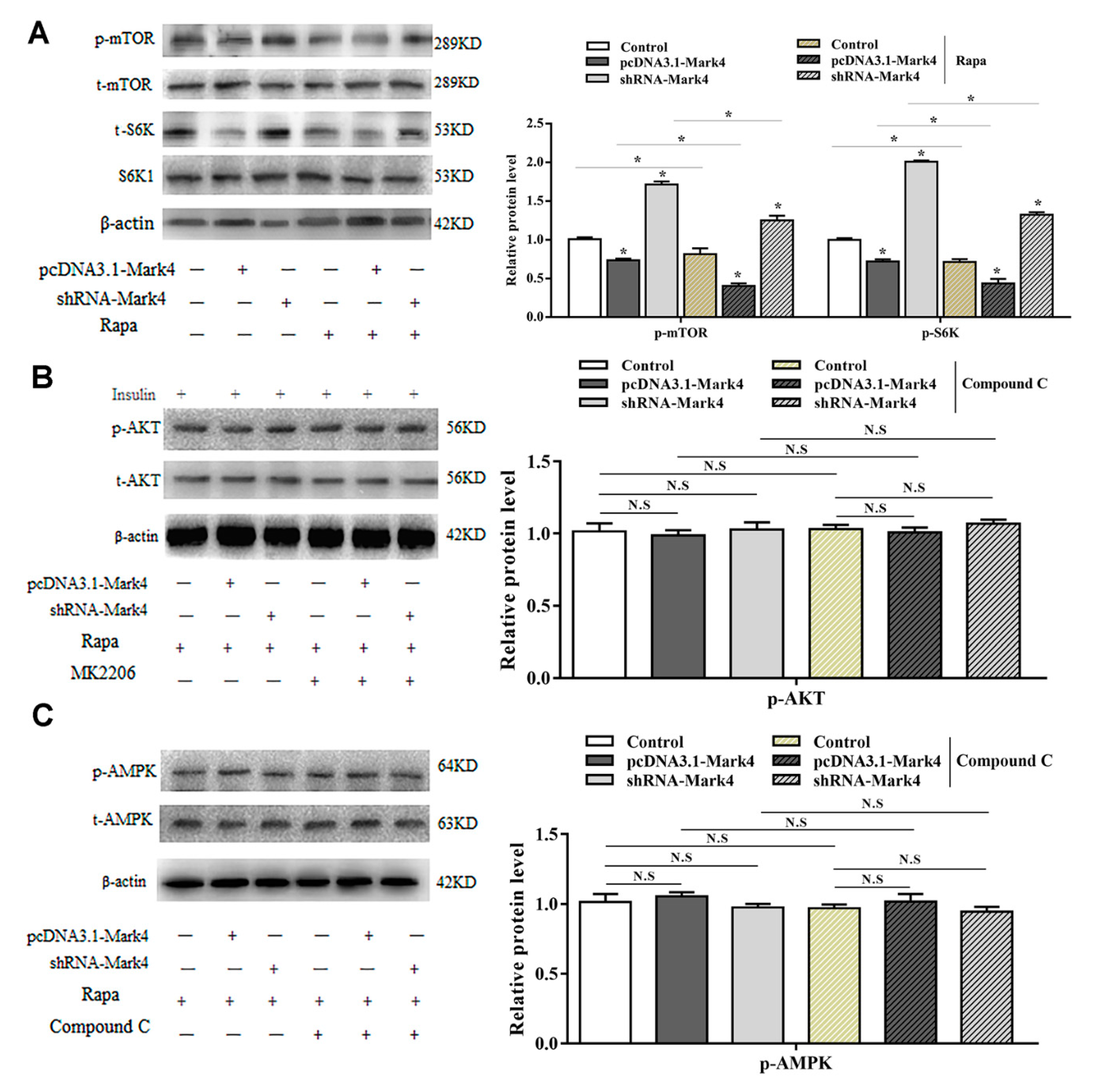
2.2. Mark4 Promoted Rapa-Induced Autophagy via Inhibiting the mTOR Signaling Pathway
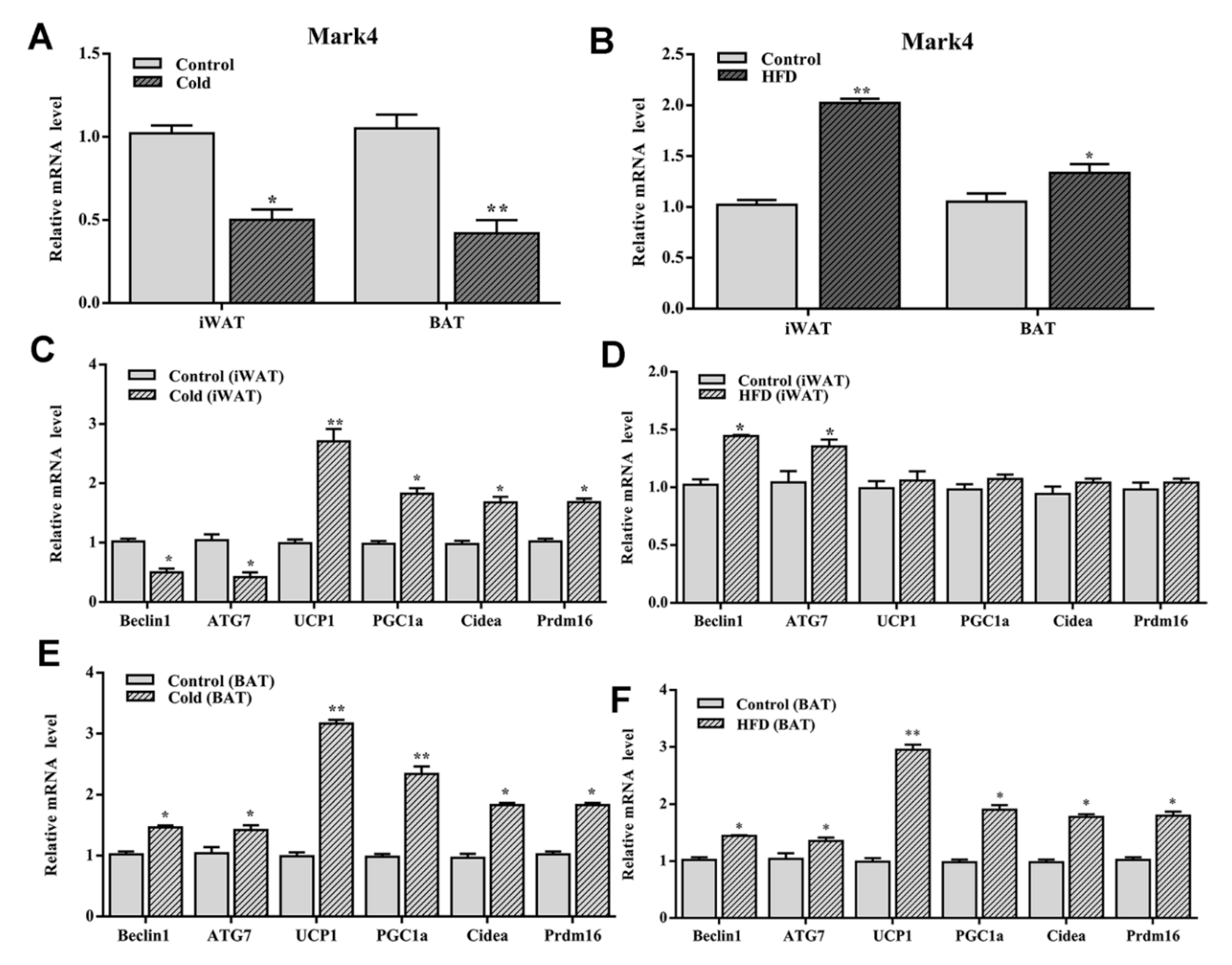
2.3. Mark4 Decreased Browning of White Adipose Tissue via Promoting Autophagy
3. Discussion
4. Materials and Methods
4.1. Animal Experiment
4.2. Cell Culture
4.3. Cell Viability Assay
4.4. GFP-LC3 Analysis and Subcellular Localization
4.5. Transfection of Adipocytes with Plasmids
4.6. MDC Staining Assessment
4.7. Oil Red O Staining
4.8. Real-Time Quantitative PCR Analysis
4.9. Protein Extraction and Western Blot Analysis
4.10. Drug Treatment in Adipocytes
4.11. Statistical Analysis
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AMPK | AMP-activated protein kinase |
| Atf4 | Activating transcription factor 4 |
| Atg5 | Autophagy-related gene 5 |
| Atg7 | Autophagy-related gene 7 |
| AV | Autophagic vacuoles |
| BAT | Brown adipose tissue |
| CCK8 | Cholecystokinin-8 |
| ER | Endoplasmic reticulum |
| Fgf21 | Fibroblast growth factor 21 |
| HFD | High-fat diet |
| JNK | Jun N-terminal kinase |
| LC3A | Microtubule-associated protein light chain 3 |
| LC3B | Microtubule associated protein 1 light chain 3β |
| MAPK | Mitogen-activated protein kinase |
| MAPs | Microtubule associated protein |
| MARKs | Microtubule associated protein serine/threonine kinase |
| Mark2 | Microtubules affinity regulated kinase 2 |
| Mark3 | Microtubules affinity regulated kinase 3 |
| Mark4 | Microtubules affinity regulated kinase 4 |
| MDC | Monodansylcadaverine |
| MR | Mineralocorticoid receptor |
| NF-κB | Nuclear factor-kappa B |
| PKB/AKT | Protein kinase B |
| PPARγ | Peroxisome proliferator-activated receptor gamma |
| Rapa | Rapamycin |
| WAT | White adipose tissue |
| β3-AR | β3-adrenergic receptor |
| 3-MA | 3-methyl adenine |
References
- Hurov, J.; Piwnica-Worms, H. The Par-1/MARK family of protein kinases: From polarity to metabolism. Cell Cycle 2007, 6, 1966–1969. [Google Scholar] [CrossRef] [PubMed]
- Kato, T.; Satoh, S.; Okabe, H.; Kitahara, O.; Ono, K.; Kihara, C.; Tanaka, T.; Tsunoda, T.; Yamaoka, Y.; Nakamura, Y.; et al. Isolation of a novel human gene, MARKL1, homologous to MARK3 and its involvement in hepatocellular carcinogenesis. Neoplasia 2001, 3, 4–9. [Google Scholar] [CrossRef] [PubMed]
- Magnani, I.; Novielli, C.; Bellini, M.; Roversi, G.; Bello, L.; Larizza, L. Multiple localization of endogenous MARK4L protein in human glioma. Anal. Cell. Pathol. 2009, 31, 357–370. [Google Scholar]
- Magnani, I.; Novielli, C.; Fontana, L.; Tabano, S.; Rovina, D.; Moroni, R.F.; Bauer, D.; Mazzoleni, S.; Colombo, E.A.; Tedeschi, G.; et al. Differential signature of the centrosomal MARK4 isoforms in glioma. Anal. Cell. Pathol. 2011, 34, 319–338. [Google Scholar] [CrossRef]
- DiBona, V.L.; Zhu, W.; Shah, M.K.; Rafalia, A.; Ben, C.H.; Crockett, D.P.; Zhang, H. Loss of Par1b/MARK2 primes microglia during brain development and enhances their sensitivity to injury. J. Neuroinflammation. 2019, 16, 11. [Google Scholar] [CrossRef]
- Hart, M.; Zulkipli, I.; Shrestha, R.L.; Dang, D.; Conti, D.; Gul, P.; Kujawiak, I.; Draviam, V.M. MARK2/Par1b kinase present at centrosomes and retraction fibres corrects spindle off-centring induced by actin disassembly. Open Biol. 2019, 9, 180263. [Google Scholar] [CrossRef]
- Li, L.; Guan, K.L. Microtubule-associated Protein/Microtubule Affinity-regulating Kinase 4 (MARK4) Is a Negative Regulator of the Mammalian Target of Rapamycin Complex 1 (mTORC1). J. Biol. Chem. 2013, 288, 703–708. [Google Scholar] [CrossRef]
- Rovina, D.; Fontana, L.; Monti, L.; Novielli, C.; Panini, N.; Sirchia, S.M.; Erba, E.; Magnani, I.; Larizza, L. Microtubule-associated protein/microtubule affinity-regulating kinase 4 (MARK4) plays a role in cell cycle progression and cytoskeletal dynamics. Eur. J. Cell Biol. 2019, 93, 355–365. [Google Scholar] [CrossRef]
- Sun, C.; Tian, L.; Nie, J.; Zhang, H.; Han, X.; Shi, Y. Inactivation of MARK4, an AMP-activated protein kinase (AMPK)-related kinase, leads to insulin hypersensitivity and resistance to diet-induced obesity. J. Biol. Chem. 2018, 287, 38305–38315. [Google Scholar] [CrossRef]
- Feng, M.; Tian, L.; Gan, L.; Liu, Z.; Sun, C. Mark4 promotes adipogenesis and triggers apoptosis in 3T3-L1 adipocytes by activating JNK1 and inhibiting p38MAPK pathways. Biol. Cell 2014, 106, 294–307. [Google Scholar] [CrossRef]
- Liu, Z.; Gan, L.; Chen, Y.; Luo, D.; Zhang, Z.; Cao, W.; Zhou, Z.; Lin, X.; Sun, C. Mark4 promotes oxidative stress and inflammation via binding to PPARγ and activating NF-κB pathway in mice adipocytes. Sci. Rep. 2016, 6, 21382. [Google Scholar] [CrossRef] [PubMed]
- Ouimet, M. Autophagy in obesity and atherosclerosis: Interrelationships between cholesterol homeostasis, lipoprotein metabolism and autophagy in macrophages and other systems. Biochim. Et Biophys. Acta Mol. Cell Biol. Lipids 2013, 1831, 1124–1133. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Klionsky, D.J. Eaten alive: A history of macroautophagy. Nat. Cell Biol. 2012, 12, 814. [Google Scholar] [CrossRef] [PubMed]
- Armani, A.; Cinti, F.; Marzolla, V.; Morgan, J.; Cranston, G.A.; Antelmi, A.; Carpinelli, G.; Canese, R.; Pagotto, U.; Quarta, C.; et al. Mineralocorticoid receptor antagonism induces browning of white adipose tissue through impairment of autophagy and prevents adipocyte dysfunction in high-fat-diet-fed mice. Faseb J. 2014, 28, 3745–3757. [Google Scholar] [CrossRef]
- Singh, R.; Xiang, Y.; Wang, Y.; Baikati, K.; Cuervo, A.M.; Luu, Y.K.; Tang, Y.; Pessin, J.E.; Schwartz, G.J.; Czaja, M.J. Autophagy regulates adipose mass and differentiation in mice. J. Clin. Investig. 2009, 119, 3329–3339. [Google Scholar] [CrossRef] [PubMed]
- Skop, V.; Cahova, M.; Dankova, H.; Papackova, Z.; Palenickova, E.; Svoboda, P.; Zidkova, J.; Kazdova, L. Autophagy inhibition in early but not in later stages prevents 3T3-L1 differentiation: Effect on mitochondrial remodeling. Differentiation 2014, 87, 220–229. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Kaushik, S.; Wang, Y.; Xiang, Y.; Novak, I.; Komatsu, M.; Tanaka, K.; Cuervo, A.M.; Czaja, M.J. Autophagy regulates lipid metabolism. Nature 2009, 458, 1131. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Xu, J.; Zhang, X.; Yang, J.; Zhang, D.; Huang, J.; Lv, P.; Shen, W.; Yang, Y. Berberine attenuates autophagy in adipocytes by targeting BECN1. Autophagy 2014, 10, 1776–1786. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.H.; Jeong, Y.T.; Oh, H.; Kim, S.H.; Cho, J.M.; Kim, Y.N.; Kim, S.S.; Kim, D.H.; Hur, K.Y.; Kim, H.K.; et al. Autophagy deficiency leads to protection from obesity and insulin resistance by inducing Fgf21 as a mitokine. Nat. Med. 2013, 19, 83–92. [Google Scholar] [CrossRef]
- Gan, L.; Liu, Z.; Luo, D.; Ren, Q.; Wu, H.; Li, C.; Sun, C. Reduced endoplasmic reticulum stress-mediated autophagy is required for leptin alleviating inflammation in adipose tissue. Front. Immunol. 2017, 8, 1507. [Google Scholar] [CrossRef]
- Giampieri, F.; Afrin, S.; Forbes-Hernandez, T.Y.; Gasparrini, M.; Cianciosi, D.; Reboredo-Rodriguez, P.; Varela-Lopez, A.; Quiles, J.L.; Battino, M. Autophagy in Human Health and Disease: Novel Therapeutic Opportunities. Antioxid. Redox Signal. 2018, 2017, 7234. [Google Scholar] [CrossRef] [PubMed]
- Klionsky, D.J.; Abdelmohsen, K.; Abe, A.; Abedin, M.J.; Abeliovich, H.; Acevedo Arozena, A.; Adachi, H.; Adams, C.M.; Adams, P.D.; Adeli, K.; et al. Guidelines for the use and interpretation of assays for monitoring autophagy. Autophagy 2012, 8, 445–544. [Google Scholar] [CrossRef] [PubMed]
- Richard, J.E.; López-Ferreras, L.; Chanclón, B.; Eerola, K.; Micallef, P.; Skibicka, K.P.; Wernstedt Asterholm, I. CNS β-adrenergic receptor activation regulates feeding behavior, white fat browning, and body weight. Am. J. Physiol. Endocrinol. Metab. 2017, 313, E344–E358. [Google Scholar] [CrossRef] [PubMed]
- Kuhns, S.; Schmidt, K.N.; Reymann, J.; Gilbert, D.F.; Neuner, A.; Hub, B.; Carvalho, R.; Wiedemann, P.; Zentgraf, H.; Erfle, H.; et al. The microtubule affinity regulating kinase MARK4 promotes axoneme extension during early ciliogenesis. J. Cell Biol. 2013, 200, 505–522. [Google Scholar] [CrossRef]
- Dower, C.M.; Wills, C.A.; Frisch, S.M.; Wang, H.G. Mechanisms and context underlying the role of autophagy in cancer metastasis. Autophagy 2018, 14, 1110–1128. [Google Scholar] [CrossRef]
- Racanelli, A.C.; Kikkers, S.A.; Choi, A.M.K.; Cloonan, S.M. Autophagy and inflammation in chronic respiratory disease. Autophagy 2018, 14, 221–232. [Google Scholar] [CrossRef]
- Hosokawa, N.; Hara, T.; Kaizuka, T.; Kishi, C.; Takamura, A.; Miura, Y.; Iemura, S.; Natsume, T.; Takehana, K.; Yamada, N.; et al. Nutrient-dependent mTORC1 association with the ULK1–Atg13–FIP200 complex required for autophagy. Mol. Biol. Cell 2009, 20, 1981–1991. [Google Scholar] [CrossRef]
- Jung, C.H.; Jun, C.B.; Ro, S.H.; Kim, Y.M.; Otto, N.M.; Cao, J.; Kundu, M.; Kim, D.H. ULK-Atg13-FIP200 complexes mediate mTOR signaling to the autophagy machinery. Mol. Biol. Cell 2009, 20, 1992–2003. [Google Scholar] [CrossRef]
- Kim, J.; Kundu, M.; Viollet, B.; Guan, K.L. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat. Cell Biol. 2011, 13, 132. [Google Scholar] [CrossRef]
- Wong, P.M.; Feng, Y.; Wang, J.; Shi, R.; Jiang, X. Regulation of autophagy by coordinated action of mTORC1 and protein phosphatase 2A. Nat. Commun. 2015, 6, 8048. [Google Scholar] [CrossRef]
- Scherer, P.E. Adipose tissue: From lipid storage compartment to endocrine organ. Diabetes 2006, 55, 1537–1545. [Google Scholar] [CrossRef] [PubMed]
- Cinti, S. The adipose organ: Morphological perspectives of adipose tissues. Proc. Nutr. Soc. 2001, 60, 319–328. [Google Scholar] [CrossRef] [PubMed]
- Stanford, K.I.; Middelbeek, R.J.; Townsend, K.L.; An, D.; Nygaard, E.B.; Hitchcox, K.M.; Markan, K.R.; Nakano, K.; Hirshman, M.F.; Tseng, Y.H.; et al. Brown adipose tissue regulates glucose homeostasis and insulin sensitivity. J. Clin. Investig. 2012, 123, 1. [Google Scholar] [CrossRef] [PubMed]
- Vitali, A.; Murano, I.; Zingaretti, M.C.; Frontini, A.; Ricquier, D.; Cinti, S. The adipose organ of obesity-prone C57BL/6J mice is composed of mixed white and brown adipocytes. J. Lipid Res. 2012, 53, 619–629. [Google Scholar] [CrossRef] [PubMed]
- Murano, I.; Barbatelli, G.; Giordano, A.; Cinti, S. Noradrenergic parenchymal nerve fiber branching after cold acclimatisation correlates with brown adipocyte density in mouse adipose organ. J. Anat. 2009, 214, 171–178. [Google Scholar] [CrossRef]
- Rosenwald, M.; Perdikari, A.; Rülicke, T.; Wolfrum, C. Bi-directional interconversion of brite and white adipocytes. Nat. Cell Biol. 2013, 15, 659. [Google Scholar] [CrossRef]
- Cypess, A.M.; Lehman, S.; Williams, G.; Tal, I.; Rodman, D.; Goldfine, A.B.; Kuo, F.C.; Palmer, E.L.; Tseng, Y.H.; Doria, A.; et al. Identification and importance of brown adipose tissue in adult humans. New Engl. J. Med. 2009, 360, 1509–1517. [Google Scholar] [CrossRef] [PubMed]
- Gavaldà-Navarro, A.; Moreno-Navarrete, J.M.; Quesada-López, T.; Cairó, M.; Giralt, M.; Fernández-Real, J.M.; Villarroya, F. Lipopolysaccharide-binding protein is a negative regulator of adipose tissue browning in mice and humans. Diabetologia 2016, 59, 2208–2218. [Google Scholar] [CrossRef]
- Seale, P.; Conroe, H.M.; Estall, J.; Kajimura, S.; Frontini, A.; Ishibashi, J.; Cohen, P.; Cinti, S.; Spiegelman, B.M. Prdm16 determines the thermogenic program of subcutaneous white adipose tissue in mice. J. Clin. Investig. 2011, 121, 96–105. [Google Scholar] [CrossRef]
- Bauters, D.; Cobbaut, M.; Geys, L.; Van, L.J.; Hemmeryckx, B.; Lijnen, H.R. Loss of ADAMTS5 enhances brown adipose tissue mass and promotes browning of white adipose tissue via CREB signaling. Mol. Metab. 2017, 6, 715–724. [Google Scholar] [CrossRef]
- Jiang, C.; Cano-Vega, M.A.; Yue, F.; Kuang, L.; Narayanan, N.; Uzunalli, G.; Merkel, M.P.; Kuang, S.; Deng, M. Dibenzazepine-Loaded Nanoparticles Induce Local Browning of White Adipose Tissue to Counteract Obesity. Mol. Ther. 2017, 25, 1718–1729. [Google Scholar] [CrossRef] [PubMed]
- Gan, L.; Liu, Z.; Jin, W.; Zhou, Z.; Sun, C. Foxc2 enhances proliferation and inhibits apoptosis through activating Akt/mTORC1 signaling pathway in mouse preadipocytes. J. Lipid Res. 2015, 56, 1471–1480. [Google Scholar] [CrossRef] [PubMed]






© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, K.; Cai, J.; Pan, M.; Sun, Q.; Sun, C. Mark4 Inhibited the Browning of White Adipose Tissue by Promoting Adipocytes Autophagy in Mice. Int. J. Mol. Sci. 2020, 21, 2752. https://doi.org/10.3390/ijms21082752
Yang K, Cai J, Pan M, Sun Q, Sun C. Mark4 Inhibited the Browning of White Adipose Tissue by Promoting Adipocytes Autophagy in Mice. International Journal of Molecular Sciences. 2020; 21(8):2752. https://doi.org/10.3390/ijms21082752
Chicago/Turabian StyleYang, Kun, Jiarui Cai, Miao Pan, Qian Sun, and Chao Sun. 2020. "Mark4 Inhibited the Browning of White Adipose Tissue by Promoting Adipocytes Autophagy in Mice" International Journal of Molecular Sciences 21, no. 8: 2752. https://doi.org/10.3390/ijms21082752
APA StyleYang, K., Cai, J., Pan, M., Sun, Q., & Sun, C. (2020). Mark4 Inhibited the Browning of White Adipose Tissue by Promoting Adipocytes Autophagy in Mice. International Journal of Molecular Sciences, 21(8), 2752. https://doi.org/10.3390/ijms21082752



