Melatonin Non-Linearly Modulates Bull Spermatozoa Motility and Physiology in Capacitating and Non-Capacitating Conditions
Abstract
1. Introduction
2. Results
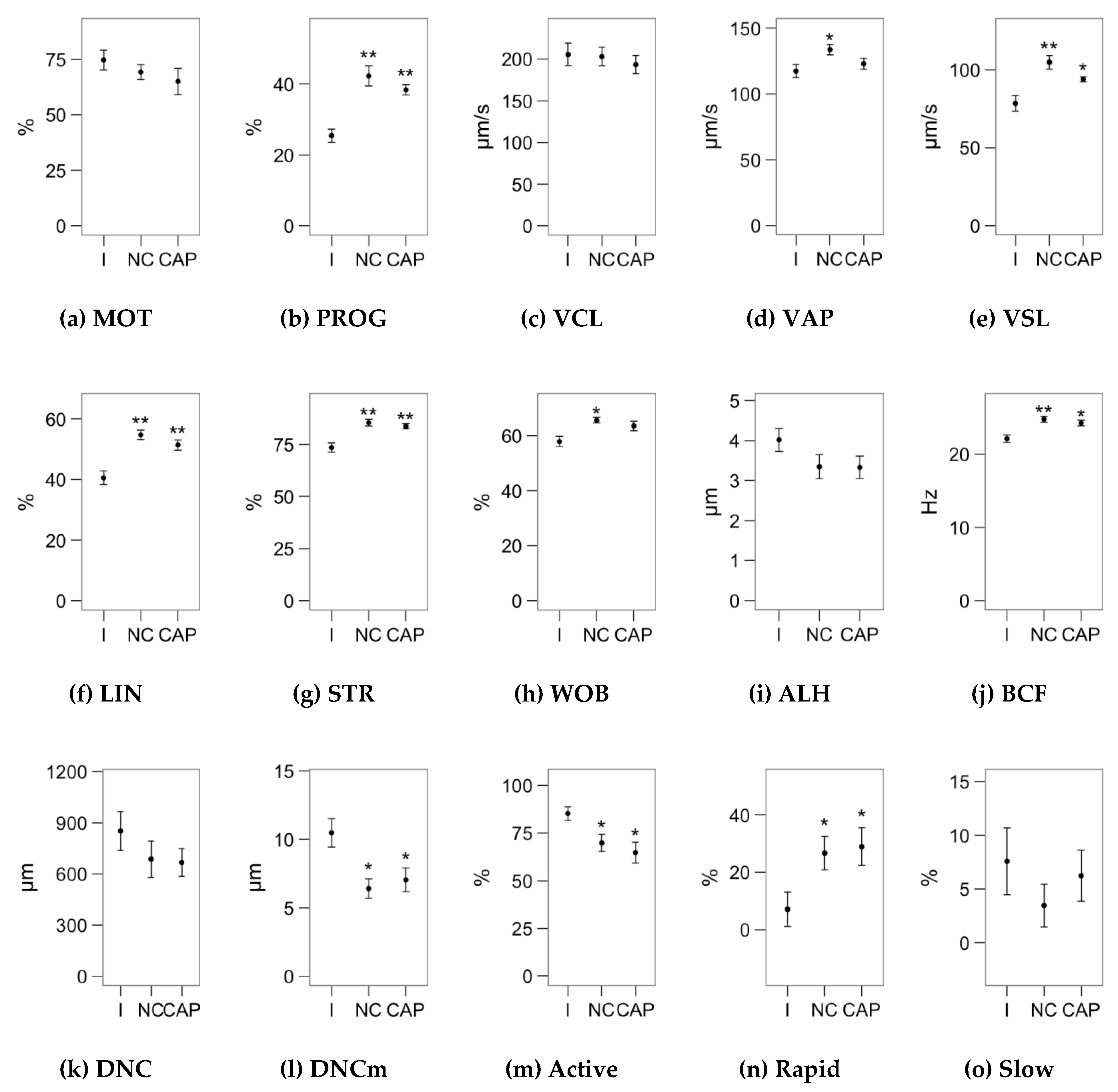
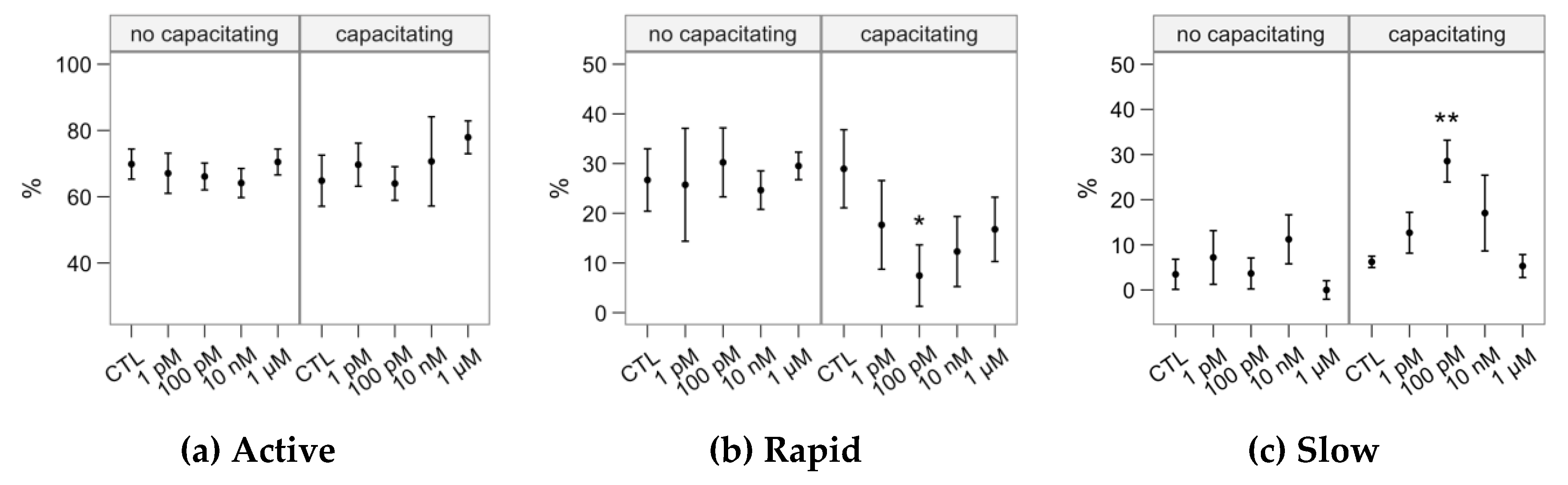
2.1. Effects of Melatonin Concentrations on Sperm Motility
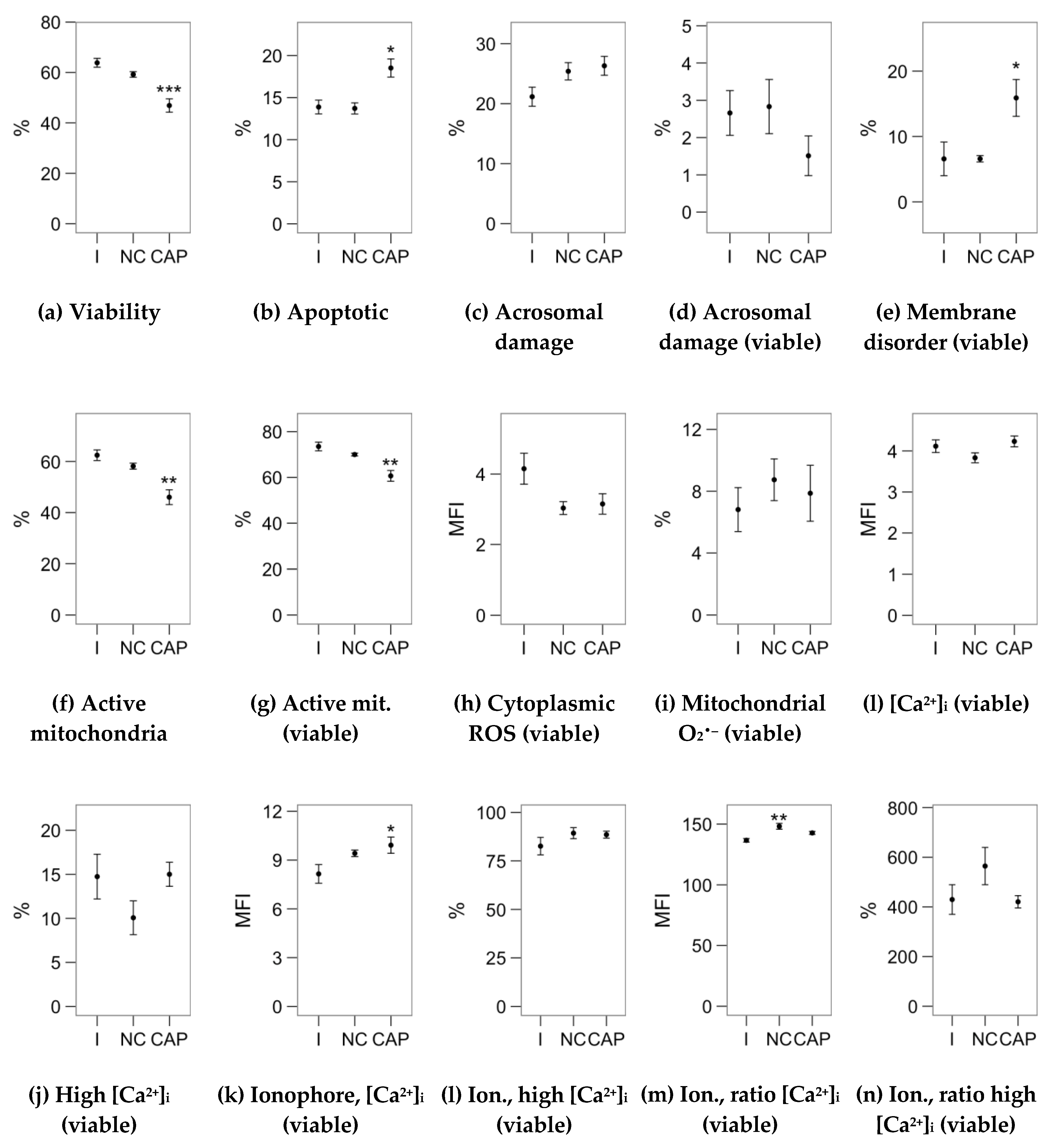
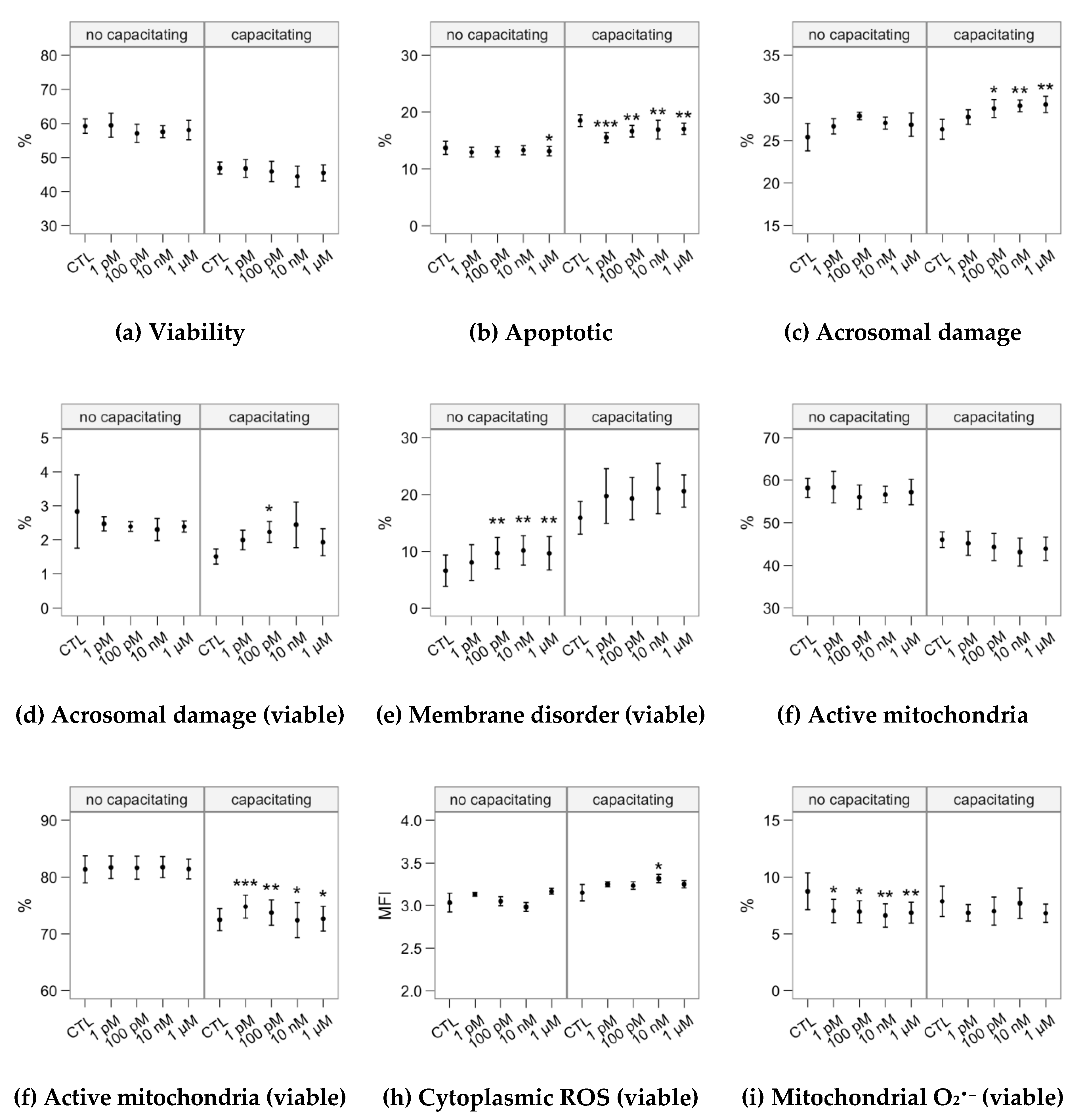
2.2. Effects of Melatonin Concentrations on Sperm Physiology (Flow Cytometry Analyses)
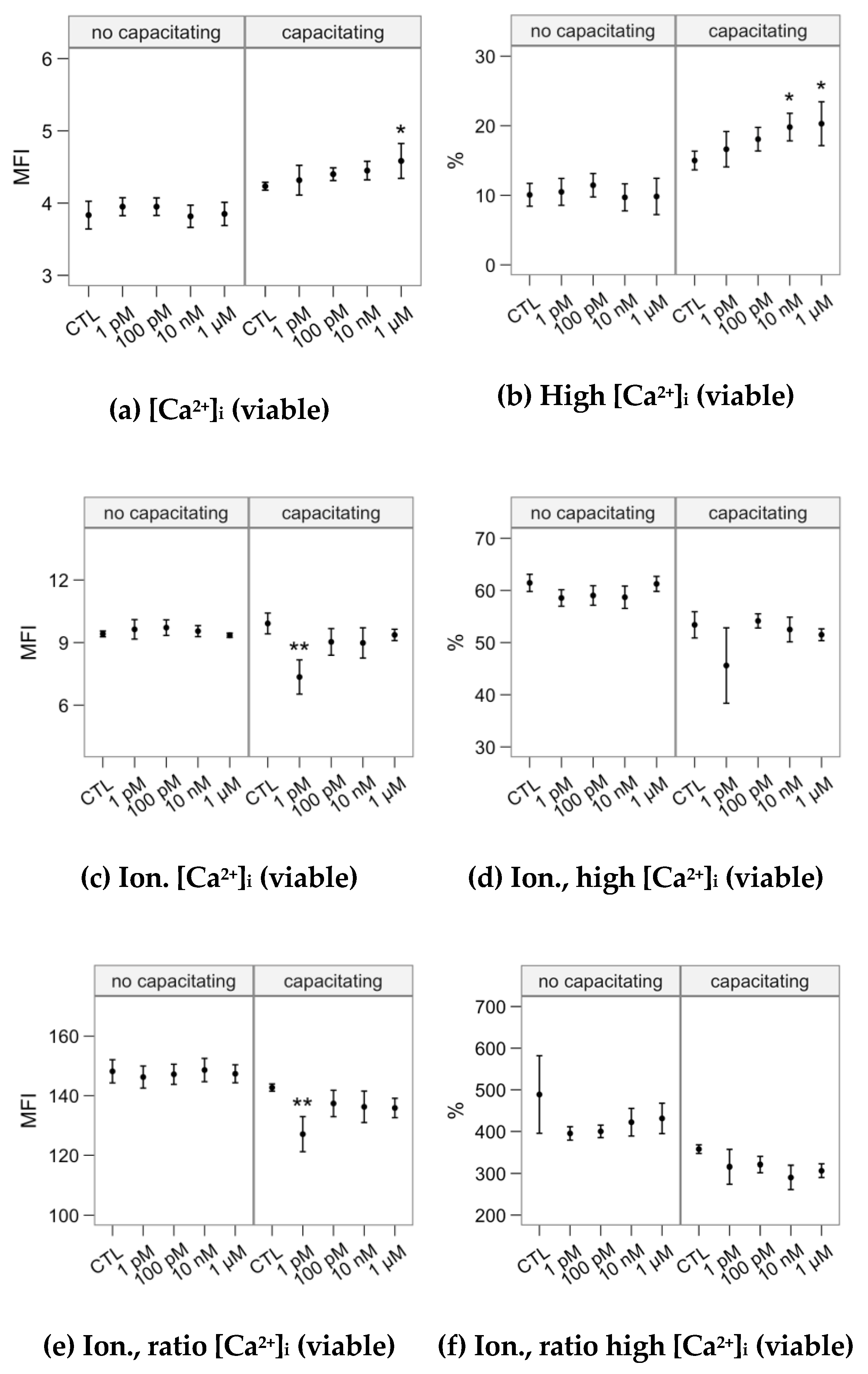
2.3. Effects of Melatonin Concentrations on Intracellular Calcium Concentration and the Response to the Ionophore Challenge (Flow Cytometry Analyses)
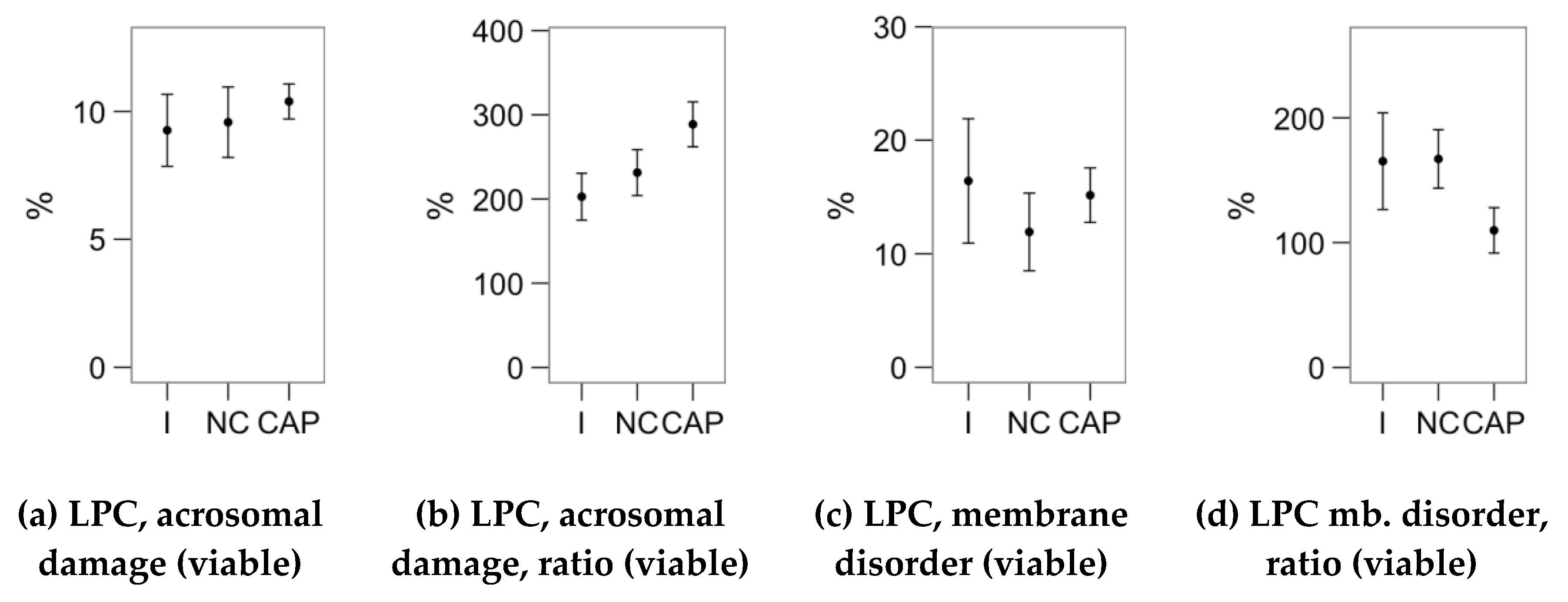
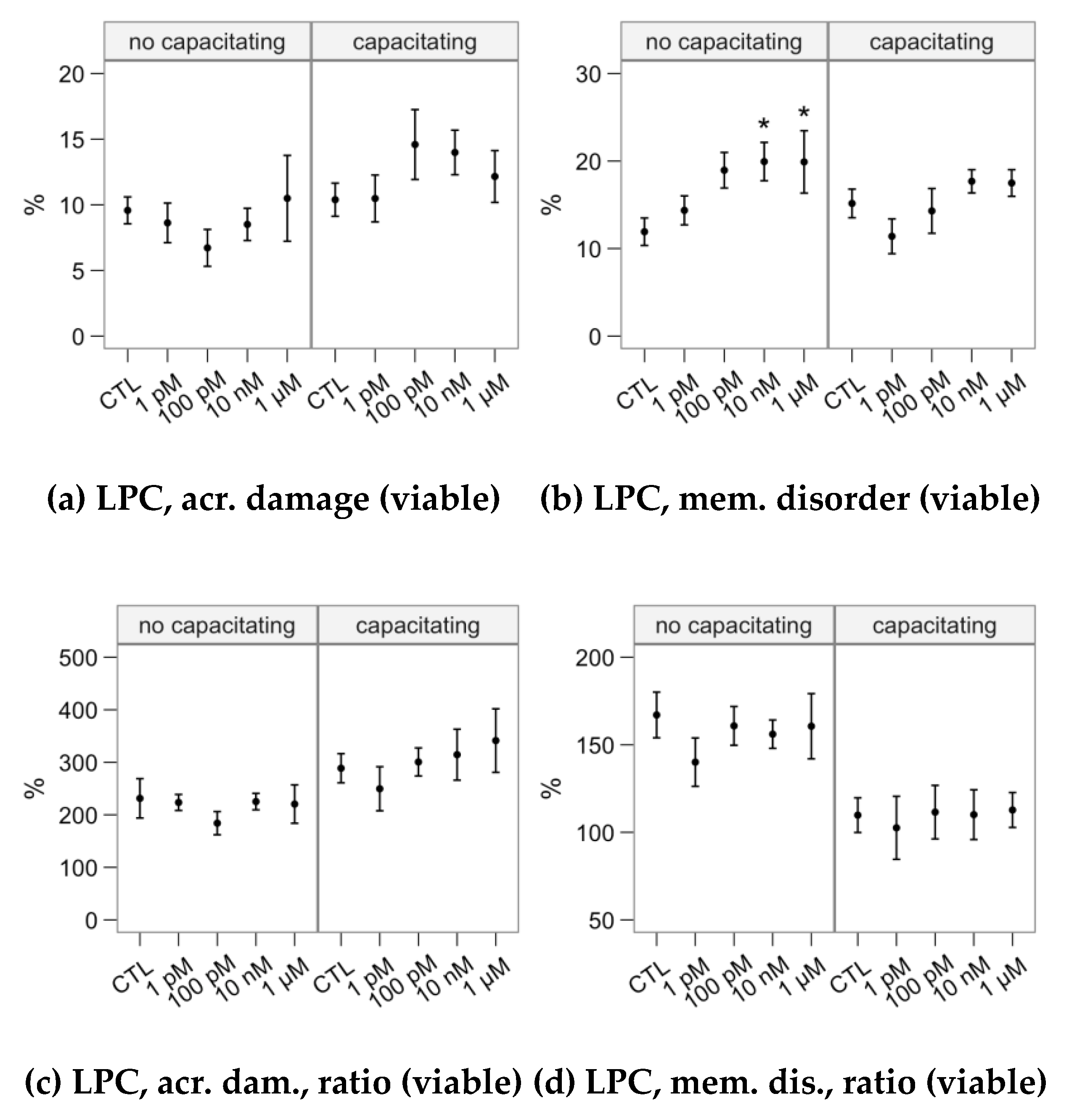
2.4. Effects of Melatonin Concentrations on the Response to Lysophosphatidylcholine Challenge (Flow Cytometry Analyses)
3. Discussion
4. Materials and Methods
4.1. Semen Samples and Experimental Design
4.2. Sperm Motility Analysis
4.3. Flow Cytometry Analysis of Sperm Physiology
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ALH | Amplitude of the lateral displacement of the sperm head (µm) |
| BCF | Frequency of the flagellar beat (Hz) |
| CM-H2DCFDA | Chloromethyl dihydroxy 2′,7′-di-chlorofluorescein acetate |
| DFI | DNA fragmentation index |
| %DFI | Proportion of spermatozoa with DFI above threshold (250) |
| DNC | Sperm dance (VCL × ALH; µm2/s) |
| DNCm | Sperm mean dance (VCL × ALH/VSL; µm) |
| DMSO | Dimethyl sulfoxide |
| %HDS | Proportion of spermatozoa with high DNA stainability |
| LIN | Linearity (VCL/VSL) |
| LPC | Lysophosphatidylcholine |
| MOT | Total motility |
| PROG | Progressive motility |
| PVP | Polyvinylpyrrolidone |
| VSL | Straight path velocity (µm/s) |
| SCSA | Sperm chromatin structure assay |
| STR | Straightness (VAP/VSL) |
| TALP | Tyrode-albumin-lactate-pyruvate medium |
| VAP | Average path velocity (µm/s) |
| VCL | Curvilinear velocity (µm/s) |
| WOB | Wobble (VAP/VCL) |
References
- Hardeland, R. Melatonin, hormone of darkness and more: Occurrence, control mechanisms, actions and bioactive metabolites. Cell. Mol. Life Sci. CMLS 2008, 65, 2001–2018. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Hidalgo, M.; de la Lastra, C.A.; Carrascosa-Salmoral, M.P.; Naranjo, M.C.; Gomez-Corvera, A.; Caballero, B.; Guerrero, J.M. Age-related changes in melatonin synthesis in rat extrapineal tissues. Exp. Gerontol. 2009, 44, 328–334. [Google Scholar] [CrossRef] [PubMed]
- Acuña-Castroviejo, D.; Escames, G.; Venegas, C.; Díaz-Casado, M.E.; Lima-Cabello, E.; López, L.C.; Rosales-Corral, S.; Tan, D.-X.; Reiter, R.J. Extrapineal melatonin: Sources, regulation, and potential functions. Cell. Mol. Life Sci. CMLS 2014, 71, 2997–3025. [Google Scholar] [CrossRef]
- Cebrián-Pérez, J.; Casao, A.; González-Arto, M.; Dos Santos Hamilton, T.; Pérez-Pé, R.; Muiño-Blanco, T. Melatonin in sperm biology: Breaking paradigms. Reprod. Domest. Anim. Zuchthyg. 2014, 49 (Suppl. S4), 11–21. [Google Scholar] [CrossRef]
- Li, C.; Zhou, X. Melatonin and male reproduction. Clin. Chim. Acta Int. J. Clin. Chem. 2015, 446, 175–180. [Google Scholar] [CrossRef] [PubMed]
- Gurer-Orhan, H.; Suzen, S. Melatonin, its metabolites and its synthetic analogs as multi-faceted compounds: Antioxidant, prooxidant and inhibitor of bioactivation reactions. Curr. Med. Chem. 2015, 22, 490–499. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Arto, M.; Hamilton, T.R.; Dos, S.; Gallego, M.; Gaspar-Torrubia, E.; Aguilar, D.; Serrano-Blesa, E.; Abecia, J.A.; Pérez-Pé, R.; Muiño-Blanco, T.; et al. Evidence of melatonin synthesis in the ram reproductive tract. Andrology 2016, 4, 163–171. [Google Scholar] [CrossRef] [PubMed]
- González-Arto, M.; Aguilar, D.; Gaspar-Torrubia, E.; Gallego, M.; Carvajal-Serna, M.; Herrera-Marcos, L.V.; Serrano-Blesa, E.; Hamilton, T.R.D.S.; Pérez-Pé, R.; Muiño-Blanco, T.; et al. Melatonin MT1 and MT2 Receptors in the Ram Reproductive Tract. Int. J. Mol. Sci. 2017, 18, 662. [Google Scholar] [CrossRef]
- Casao, A.; Cebrián, I.; Asumpção, M.E.; Pérez-Pé, R.; Abecia, J.A.; Forcada, F.; Cebrián-Pérez, J.A.; Muiño-Blanco, T. Seasonal variations of melatonin in ram seminal plasma are correlated to those of testosterone and antioxidant enzymes. Reprod. Biol. Endocrinol. 2010, 8, 59. [Google Scholar] [CrossRef]
- González-Arto, M.; Vicente-Carrillo, A.; Martínez-Pastor, F.; Fernández-Alegre, E.; Roca, J.; Miró, J.; Rigau, T.; Rodríguez-Gil, J.E.; Pérez-Pé, R.; Muiño-Blanco, T.; et al. Melatonin receptors MT1 and MT2 are expressed in spermatozoa from several seasonal and nonseasonal breeder species. Theriogenology 2016, 86, 1958–1968. [Google Scholar] [CrossRef]
- Barranco, I.; Casao, A.; Perez-Patiño, C.; Parrilla, I.; Muiño-Blanco, T.; Martinez, E.A.; Cebrian-Perez, J.A.; Roca, J. Profile and reproductive roles of seminal plasma melatonin of boar ejaculates used in artificial insemination programs. J. Anim. Sci. 2017, 95, 1660–1668. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tong, J.; Sheng, S.; Sun, Y.; Li, H.; Li, W.-P.; Zhang, C.; Chen, Z.-J. Melatonin levels in follicular fluid as markers for IVF outcomes and predicting ovarian reserve. Reprod. Camb. Engl. 2017, 153, 443–451. [Google Scholar] [CrossRef] [PubMed]
- Espino, J.; Ortiz, Á.; Bejarano, I.; Lozano, G.M.; Monllor, F.; García, J.F.; Rodríguez, A.B.; Pariente, J.A. Melatonin protects human spermatozoa from apoptosis via melatonin receptor- and extracellular signal-regulated kinase-mediated pathways. Fertil. Steril. 2011, 95, 2290–2296. [Google Scholar] [CrossRef] [PubMed]
- Casao, A.; Mendoza, N.; Pérez-Pé, R.; Grasa, P.; Abecia, J.-A.; Forcada, F.; Cebrián-Pérez, J.A.; Muino-Blanco, T. Melatonin prevents capacitation and apoptotic-like changes of ram spermatozoa and increases fertility rate. J. Pineal Res. 2010, 48, 39–46. [Google Scholar] [CrossRef]
- Fujinoki, M. Melatonin-enhanced hyperactivation of hamster sperm. Reproduction 2008, 136, 533–541. [Google Scholar] [CrossRef]
- Li, C.-Y.; Hao, H.-S.; Zhao, Y.-H.; Zhang, P.-P.; Wang, H.-Y.; Pang, Y.-W.; Du, W.-H.; Zhao, S.-J.; Liu, Y.; Huang, J.-M.; et al. Melatonin Improves the Fertilization Capacity of Sex-Sorted Bull Sperm by Inhibiting Apoptosis and Increasing Fertilization Capacitation via MT1. Int. J. Mol. Sci. 2019, 20, 3921. [Google Scholar] [CrossRef]
- Gimeno-Martos, S.; Casao, A.; Yeste, M.; Cebrián-Pérez, J.A.; Muiño-Blanco, T.; Pérez-Pé, R. Melatonin reduces cAMP-stimulated capacitation of ram spermatozoa. Reprod. Fertil. Dev. 2019, 31, 420–431. [Google Scholar] [CrossRef]
- O’Flaherty, C. Redox regulation of mammalian sperm capacitation. Asian J. Androl. 2015, 17, 583–590. [Google Scholar] [CrossRef]
- Gervasi, M.G.; Visconti, P.E. Chang’s meaning of capacitation: A molecular perspective. Mol. Reprod. Dev. 2016, 83, 860–874. [Google Scholar] [CrossRef]
- Casao, A.; Gallego, M.; Abecia, J.A.; Forcada, F.; Pérez-Pé, R.; Muiño-Blanco, T.; Cebrián-Pérez, J.Á. Identification and immunolocalisation of melatonin MT(1) and MT(2) receptors in Rasa Aragonesa ram spermatozoa. Reprod. Fertil. Dev. 2012, 24, 953–961. [Google Scholar] [CrossRef]
- Oishi, A.; Cecon, E.; Jockers, R. Melatonin Receptor Signaling: Impact of Receptor Oligomerization on Receptor Function. Int. Rev. Cell Mol. Biol. 2018, 338, 59–77. [Google Scholar] [PubMed]
- Hardeland, R. Melatonin: Signaling mechanisms of a pleiotropic agent. BioFactors 2009, 35, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Jockers, R.; Maurice, P.; Boutin, J.A.; Delagrange, P. Melatonin receptors, heterodimerization, signal transduction and binding sites: what’s new? Br. J. Pharmacol. 2008, 154, 1182–1195. [Google Scholar] [CrossRef] [PubMed]
- Luchetti, F.; Canonico, B.; Betti, M.; Arcangeletti, M.; Pilolli, F.; Piroddi, M.; Canesi, L.; Papa, S.; Galli, F. Melatonin signaling and cell protection function. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2010, 24, 3603–3624. [Google Scholar] [CrossRef] [PubMed]
- Wertheimer, E.; Krapf, D.; de la Vega-Beltran, J.L.; Sánchez-Cárdenas, C.; Navarrete, F.; Haddad, D.; Escoffier, J.; Salicioni, A.M.; Levin, L.R.; Buck, J.; et al. Compartmentalization of distinct cAMP signaling pathways in mammalian sperm. J. Biol. Chem. 2013, 288, 35307–35320. [Google Scholar] [CrossRef] [PubMed]
- Harayama, H. Roles of intracellular cyclic AMP signal transduction in the capacitation and subsequent hyperactivation of mouse and boar spermatozoa. J. Reprod. Dev. 2013, 59, 421–430. [Google Scholar] [CrossRef]
- Buffone, M.G.; Wertheimer, E.V.; Visconti, P.E.; Krapf, D. Central role of soluble adenylyl cyclase and cAMP in sperm physiology. Biochim. Biophys. Acta 2014, 1842, 2610–2620. [Google Scholar] [CrossRef]
- Cheuquemán, C.; Arias, M.E.; Risopatrón, J.; Felmer, R.; Álvarez, J.; Mogas, T.; Sánchez, R. Supplementation of IVF medium with melatonin: Effect on sperm functionality and in vitro produced bovine embryos. Andrologia 2015, 47, 604–615. [Google Scholar] [CrossRef]
- Tomás-Zapico, C.; Coto-Montes, A. A proposed mechanism to explain the stimulatory effect of melatonin on antioxidative enzymes. J. Pineal Res. 2005, 39, 99–104. [Google Scholar] [CrossRef]
- Slominski, R.M.; Reiter, R.J.; Schlabritz-Loutsevitch, N.; Ostrom, R.S.; Slominski, A.T. Melatonin membrane receptors in peripheral tissues: Distribution and functions. Mol. Cell. Endocrinol. 2012, 351, 152–166. [Google Scholar] [CrossRef]
- Ortiz, A.; Espino, J.; Bejarano, I.; Lozano, G.M.; Monllor, F.; García, J.F.; Pariente, J.A.; Rodríguez, A.B. High endogenous melatonin concentrations enhance sperm quality and short-term in vitro exposure to melatonin improves aspects of sperm motility. J. Pineal Res. 2011, 50, 132–139. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Arto, M.; Luna, C.; Pérez-Pé, R.; Muiño-Blanco, T.; Cebrián-Pérez, J.A.; Casao, A. New evidence of melatonin receptor contribution to ram sperm functionality. Reprod. Fertil. Dev. 2016, 28, 924–935. [Google Scholar] [CrossRef] [PubMed]
- Loren, P.; Sánchez, R.; Arias, M.-E.; Felmer, R.; Risopatrón, J.; Cheuquemán, C. Melatonin Scavenger Properties against Oxidative and Nitrosative Stress: Impact on Gamete Handling and In Vitro Embryo Production in Humans and Other Mammals. Int. J. Mol. Sci. 2017, 18, 1119. [Google Scholar] [CrossRef] [PubMed]
- da Silva, C.M.B.; Macías-García, B.; Miró-Morán, A.; González-Fernández, L.; Morillo-Rodriguez, A.; Ortega-Ferrusola, C.; Gallardo-Bolaños, J.M.; Stilwell, G.; Tapia, J.A.; Peña, F.J. Melatonin reduces lipid peroxidation and apoptotic-like changes in stallion spermatozoa. J. Pineal Res. 2011, 51, 172–179. [Google Scholar] [CrossRef]
- Succu, S.; Berlinguer, F.; Pasciu, V.; Satta, V.; Leoni, G.G.; Naitana, S. Melatonin protects ram spermatozoa from cryopreservation injuries in a dose-dependent manner. J. Pineal Res. 2011, 50, 310–318. [Google Scholar] [CrossRef]
- Ferrusola, C.O.; Fernández, L.G.; Sandoval, C.S.; García, B.M.; Martínez, H.R.; Tapia, J.A.; Peña, F.J. Inhibition of the mitochondrial permeability transition pore reduces “apoptosis like” changes during cryopreservation of stallion spermatozoa. Theriogenology 2010, 74, 458–465. [Google Scholar] [CrossRef]
- Ashrafi, I.; Kohram, H.; Ardabili, F.F. Antioxidative effects of melatonin on kinetics, microscopic and oxidative parameters of cryopreserved bull spermatozoa. Anim. Reprod. Sci. 2013, 139, 25–30. [Google Scholar] [CrossRef]
- Zhu, Z.; Li, R.; Lv, Y.; Zeng, W. Melatonin protects rabbit spermatozoa from cryo-damage via decreasing oxidative stress. Cryobiology 2019, 88, 1–8. [Google Scholar] [CrossRef]
- Domínguez-Rebolledo, A.E.; Fernández-Santos, M.R.; Bisbal, A.; Ros-Santaella, J.L.; Ramón, M.; Carmona, M.; Martínez-Pastor, F.; Garde, J.J. Improving the effect of incubation and oxidative stress on thawed spermatozoa from red deer by using different antioxidant treatments. Reprod. Fertil. Dev. 2010, 22, 856–870. [Google Scholar] [CrossRef]
- Mata-Campuzano, M.; Alvarez-Rodríguez, M.; Alvarez, M.; Anel, L.; de Paz, P.; Garde, J.J.; Martínez-Pastor, F. Effect of several antioxidants on thawed ram spermatozoa submitted to 37 °C up to four hours. Reprod. Domest. Anim. Zuchthyg. 2012, 47, 907–914. [Google Scholar] [CrossRef]
- Tosti, E.; Ménézo, Y. Gamete activation: Basic knowledge and clinical applications. Hum. Reprod. Update 2016, 22, 420–439. [Google Scholar] [CrossRef] [PubMed]
- Mortimer, S.T.; van der Horst, G.; Mortimer, D. The future of computer-aided sperm analysis. Asian J. Androl. 2015, 17, 545–553. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Pastor, F.; Mata-Campuzano, M.; Alvarez-Rodríguez, M.; Alvarez, M.; Anel, L.; de Paz, P. Probes and techniques for sperm evaluation by flow cytometry. Reprod. Domest. Anim. Zuchthyg. 2010, 45 (Suppl. S2), 67–78. [Google Scholar] [CrossRef]
- Ramón, M.; Martínez-Pastor, F.; García-Álvarez, O.; Maroto-Morales, A.; Soler, A.J.; Jiménez-Rabadán, P.; Fernández-Santos, M.R.; Bernabéu, R.; Garde, J.J. Taking advantage of the use of supervised learning methods for characterization of sperm population structure related with freezability in the Iberian red deer. Theriogenology 2012, 77, 1661–1672. [Google Scholar] [CrossRef]
- Ramón, M.; Martínez-Pastor, F. Implementation of novel statistical procedures and other advanced approaches to improve analysis of CASA data. Reprod. Fertil. Dev. 2018, 30, 860–866. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Pastor, F.; Tizado, E.J.; Garde, J.J.; Anel, L.; de Paz, P. Statistical Series: Opportunities and challenges of sperm motility subpopulation analysis. Theriogenology 2011, 75, 783–795. [Google Scholar] [CrossRef]
- Tamura, H.; Takasaki, A.; Taketani, T.; Tanabe, M.; Kizuka, F.; Lee, L.; Tamura, I.; Maekawa, R.; Asada, H.; Yamagata, Y.; et al. Melatonin as a free radical scavenger in the ovarian follicle. Endocr. J. 2013, 60, 1–13. [Google Scholar] [CrossRef]
- Rocco, M.; Betarelli, R.; Placci, A.; Fernández-Novell, J.M.; Spinaci, M.; Casao, A.; Muiño-Blanco, T.; Cebrián-Pérez, J.A.; Peña, A.; Rigau, T.; et al. Melatonin affects the motility and adhesiveness of in vitro capacitated boar spermatozoa via a mechanism that does not depend on intracellular ROS levels. Andrology 2018, 6, 720–736. [Google Scholar] [CrossRef]
- Fujinoki, M.; Takei, G.L. Estrogen suppresses melatonin-enhanced hyperactivation of hamster spermatozoa. J. Reprod. Dev. 2015, 61, 287–295. [Google Scholar] [CrossRef]
- Najafi, A.; Adutwum, E.; Yari, A.; Salehi, E.; Mikaeili, S.; Dashtestani, F.; Abolhassani, F.; Rashki, L.; Shiasi, S.; Asadi, E. Melatonin affects membrane integrity, intracellular reactive oxygen species, caspase3 activity and AKT phosphorylation in frozen thawed human sperm. Cell Tissue Res. 2018, 372, 149–159. [Google Scholar] [CrossRef]
- Cruz, M.H.C.; Leal, C.L.V.; da Cruz, J.F.; Tan, D.-X.; Reiter, R.J. Role of melatonin on production and preservation of gametes and embryos: A brief review. Anim. Reprod. Sci. 2014, 145, 150–160. [Google Scholar] [CrossRef] [PubMed]
- Bejarano, I.; Monllor, F.; Marchena, A.M.; Ortiz, A.; Lozano, G.; Jiménez, M.I.; Gaspar, P.; García, J.F.; Pariente, J.A.; Rodríguez, A.B.; et al. Exogenous melatonin supplementation prevents oxidative stress-evoked DNA damage in human spermatozoa. J. Pineal Res. 2014, 57, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Colas, C.; James, P.; Howes, L.; Jones, R.; Cebrian-Perez, J.A.; Muiño-Blanco, T. Cyclic-AMP initiates protein tyrosine phosphorylation independent of cholesterol efflux during ram sperm capacitation. Reprod. Fertil. Dev. 2008, 20, 649–658. [Google Scholar] [CrossRef] [PubMed]
- Blottner, S.; Nehring, H.; Torner, H. Individual differences in capacitation of bull spermatozoa by heparin in vitro: Relationship to fertility. Theriogenology 1990, 34, 619–628. [Google Scholar] [CrossRef]
- Martín-Hidalgo, D.; Barón, F.J.; Bragado, M.J.; Carmona, P.; Robina, A.; García-Marín, L.J.; Gil, M.C. The effect of melatonin on the quality of extended boar semen after long-term storage at 17 °C. Theriogenology 2011, 75, 1550–1560. [Google Scholar] [CrossRef]
- Chung, J.-J.; Shim, S.-H.; Everley, R.A.; Gygi, S.P.; Zhuang, X.; Clapham, D.E. Structurally distinct Ca(2+) signaling domains of sperm flagella orchestrate tyrosine phosphorylation and motility. Cell 2014, 157, 808–822. [Google Scholar] [CrossRef]
- Ramis, M.R.; Esteban, S.; Miralles, A.; Tan, D.-X.; Reiter, R.J. Protective Effects of Melatonin and Mitochondria-targeted Antioxidants Against Oxidative Stress: A Review. Curr. Med. Chem. 2015, 22, 2690–2711. [Google Scholar] [CrossRef]
- Naresh, S.; Atreja, S.K. The protein tyrosine phosphorylation during in vitro capacitation and cryopreservation of mammalian spermatozoa. Cryobiology 2015, 70, 211–216. [Google Scholar] [CrossRef]
- Gadella, B.M.; Luna, C. Cell biology and functional dynamics of the mammalian sperm surface. Theriogenology 2014, 81, 74–84. [Google Scholar] [CrossRef]
- Hess, K.C.; Jones, B.H.; Marquez, B.; Chen, Y.; Ord, T.S.; Kamenetsky, M.; Miyamoto, C.; Zippin, J.H.; Kopf, G.S.; Suarez, S.S.; et al. The soluble adenylyl cyclase in sperm mediates multiple signaling events required for fertilization. Dev. Cell 2005, 9, 249–259. [Google Scholar] [CrossRef]
- Chan, A.S.L.; Lai, F.P.L.; Lo, R.K.H.; Voyno-Yasenetskaya, T.A.; Stanbridge, E.J.; Wong, Y.H. Melatonin mt1 and MT2 receptors stimulate c-Jun N-terminal kinase via pertussis toxin-sensitive and -insensitive G proteins. Cell Signal. 2002, 14, 249–257. [Google Scholar] [CrossRef]
- Breitbart, H.; Rotman, T.; Rubinstein, S.; Etkovitz, N. Role and regulation of PI3K in sperm capacitation and the acrosome reaction. Mol. Cell. Endocrinol. 2010, 314, 234–238. [Google Scholar] [CrossRef] [PubMed]
- Ickowicz, D.; Finkelstein, M.; Breitbart, H. Mechanism of sperm capacitation and the acrosome reaction: Role of protein kinases. Asian J. Androl. 2012, 14, 816–821. [Google Scholar] [CrossRef]
- Battistone, M.A.; Da Ros, V.G.; Salicioni, A.M.; Navarrete, F.A.; Krapf, D.; Visconti, P.E.; Cuasnicú, P.S. Functional human sperm capacitation requires both bicarbonate-dependent PKA activation and down-regulation of Ser/Thr phosphatases by Src family kinases. Mol. Hum. Reprod. 2013, 19, 570–580. [Google Scholar] [CrossRef] [PubMed]
- Branham, M.T.; Mayorga, L.S.; Tomes, C.N. Calcium-induced acrosomal exocytosis requires cAMP acting through a protein kinase A-independent, Epac-mediated pathway. J. Biol. Chem. 2006, 281, 8656–8666. [Google Scholar] [CrossRef]
- Benítez-King, G. Melatonin as a cytoskeletal modulator: Implications for cell physiology and disease. J. Pineal Res. 2006, 40, 1–9. [Google Scholar] [CrossRef]
- Nesci, S.; Spinaci, M.; Galeati, G.; Nerozzi, C.; Pagliarani, A.; Algieri, C.; Tamanini, C.; Bucci, D. Sperm function and mitochondrial activity: An insight on boar sperm metabolism. Theriogenology 2020, 144, 82–88. [Google Scholar] [CrossRef]
- Verstegen, J.; Iguer-Ouada, M.; Onclin, K. Computer assisted semen analyzers in andrology research and veterinary practice. Theriogenology 2002, 57, 149–179. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: https://www.R-project.org/ (accessed on 15 July 2019).
- Fernández-Gago, R.; Álvarez-Rodríguez, M.; Alonso, M.E.; González, J.R.; Alegre, B.; Domínguez, J.C.; Martínez-Pastor, F. Thawing boar semen in the presence of seminal plasma improves motility, modifies subpopulation patterns and reduces chromatin alterations. Reprod. Fertil. Dev. 2017, 29, 1576–1584. [Google Scholar] [CrossRef]
- Fernández-Gago, R.; Domínguez, J.C.; Martínez-Pastor, F. Seminal plasma applied post-thawing affects boar sperm physiology: A flow cytometry study. Theriogenology 2013, 80, 400–410. [Google Scholar] [CrossRef]
- Ledesma, A.; Fernández-Alegre, E.; Cano, A.; Hozbor, F.; Martínez-Pastor, F.; Cesari, A. Seminal plasma proteins interacting with sperm surface revert capacitation indicators in frozen-thawed ram sperm. Anim. Reprod. Sci. 2016, 173, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Crespo-Félez, I.; Castañeda-Sampedro, A.; Sánchez, D.I.; Fernández-Alegre, E.; Álvarez-Rodríguez, M.; Domínguez, J.C.; Morrell, J.M.; Martínez-Pastor, F. Effect of Single Layer Centrifugation Porcicoll (70%, 80% and 90%) or supplementation with reduced glutathione, seminal plasma and bovine serum albumin on frozen-thawed boar sperm. Anim. Reprod. Sci. 2017, 187, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Kuznetsova, A.; Brockhoff, P.B.; Christensen, R.H.B. lmerTest Package: Tests in Linear Mixed Effects Models. J. Stat. Softw. 2017, 82, 1–26. [Google Scholar] [CrossRef]

| Cluster | VCL | VSL | VAP | LIN | STR | WOB | ALH | BCF | DNC | DNCm | % |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Active | 218.2 ± 55.1 | 97.9 ± 43.6 | 131.8 ± 29.8 | 48.0 ± 17.0 | 81.0 ± 16.1 | 61.5 ± 10.6 | 4.1 ± 1.5 | 25.5 ± 6.8 | 885.8 ± 523.5 | 8.9 ± 5.8 | 71.2 |
| Rapid | 94.4 ± 38.1 | 42.2 ± 34.0 | 61.5 ± 31.1 | 46.2 ± 24.8 | 74.8 ± 22.5 | 64.4 ± 15.4 | 1.9 ± 0.7 | 14.9 ± 10.8 | 175.5 ± 114.4 | 4.3 ± 3.0 | 20.5 |
| Slow | 50.3 ± 21.0 | 11.2 ± 8.0 | 26.2 ± 11.1 | 24.7 ± 16.8 | 49.7 ± 25.6 | 52.8 ± 14.7 | 1.5 ± 0.5 | 7.0 ± 4.9 | 74.5 ± 51.4 | 6.2 ± 5.0 | 8.3 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernández-Alegre, E.; Álvarez-Fernández, I.; Domínguez, J.C.; Casao, A.; Martínez-Pastor, F. Melatonin Non-Linearly Modulates Bull Spermatozoa Motility and Physiology in Capacitating and Non-Capacitating Conditions. Int. J. Mol. Sci. 2020, 21, 2701. https://doi.org/10.3390/ijms21082701
Fernández-Alegre E, Álvarez-Fernández I, Domínguez JC, Casao A, Martínez-Pastor F. Melatonin Non-Linearly Modulates Bull Spermatozoa Motility and Physiology in Capacitating and Non-Capacitating Conditions. International Journal of Molecular Sciences. 2020; 21(8):2701. https://doi.org/10.3390/ijms21082701
Chicago/Turabian StyleFernández-Alegre, Estela, Indira Álvarez-Fernández, Juan Carlos Domínguez, Adriana Casao, and Felipe Martínez-Pastor. 2020. "Melatonin Non-Linearly Modulates Bull Spermatozoa Motility and Physiology in Capacitating and Non-Capacitating Conditions" International Journal of Molecular Sciences 21, no. 8: 2701. https://doi.org/10.3390/ijms21082701
APA StyleFernández-Alegre, E., Álvarez-Fernández, I., Domínguez, J. C., Casao, A., & Martínez-Pastor, F. (2020). Melatonin Non-Linearly Modulates Bull Spermatozoa Motility and Physiology in Capacitating and Non-Capacitating Conditions. International Journal of Molecular Sciences, 21(8), 2701. https://doi.org/10.3390/ijms21082701





