A Circulating MicroRNA Profile in a Laser-Induced Mouse Model of Choroidal Neovascularization
Abstract
1. Introduction
2. Results
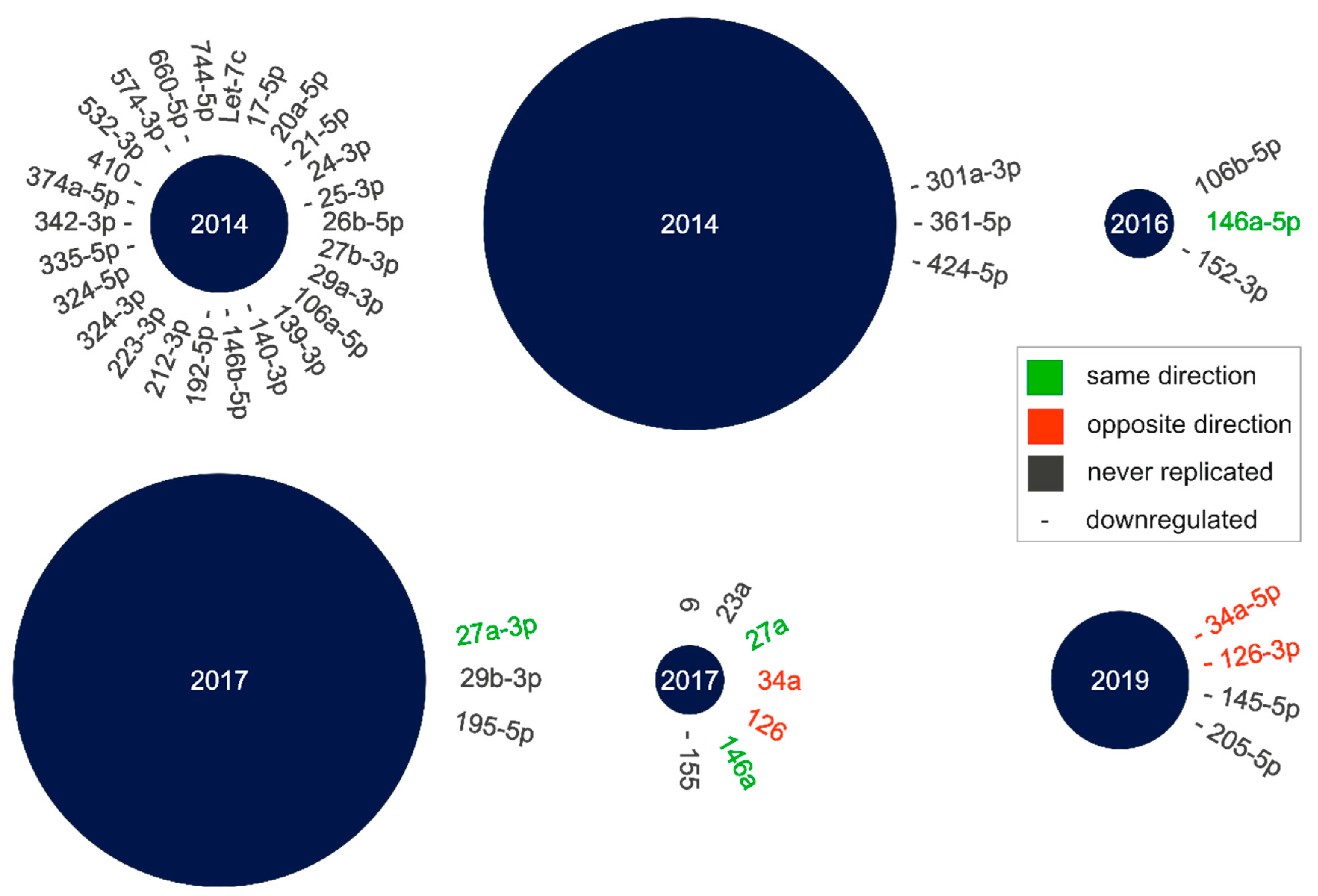
2.1. Literature Search for cmiRNA Dysregulation in nAMD Patients
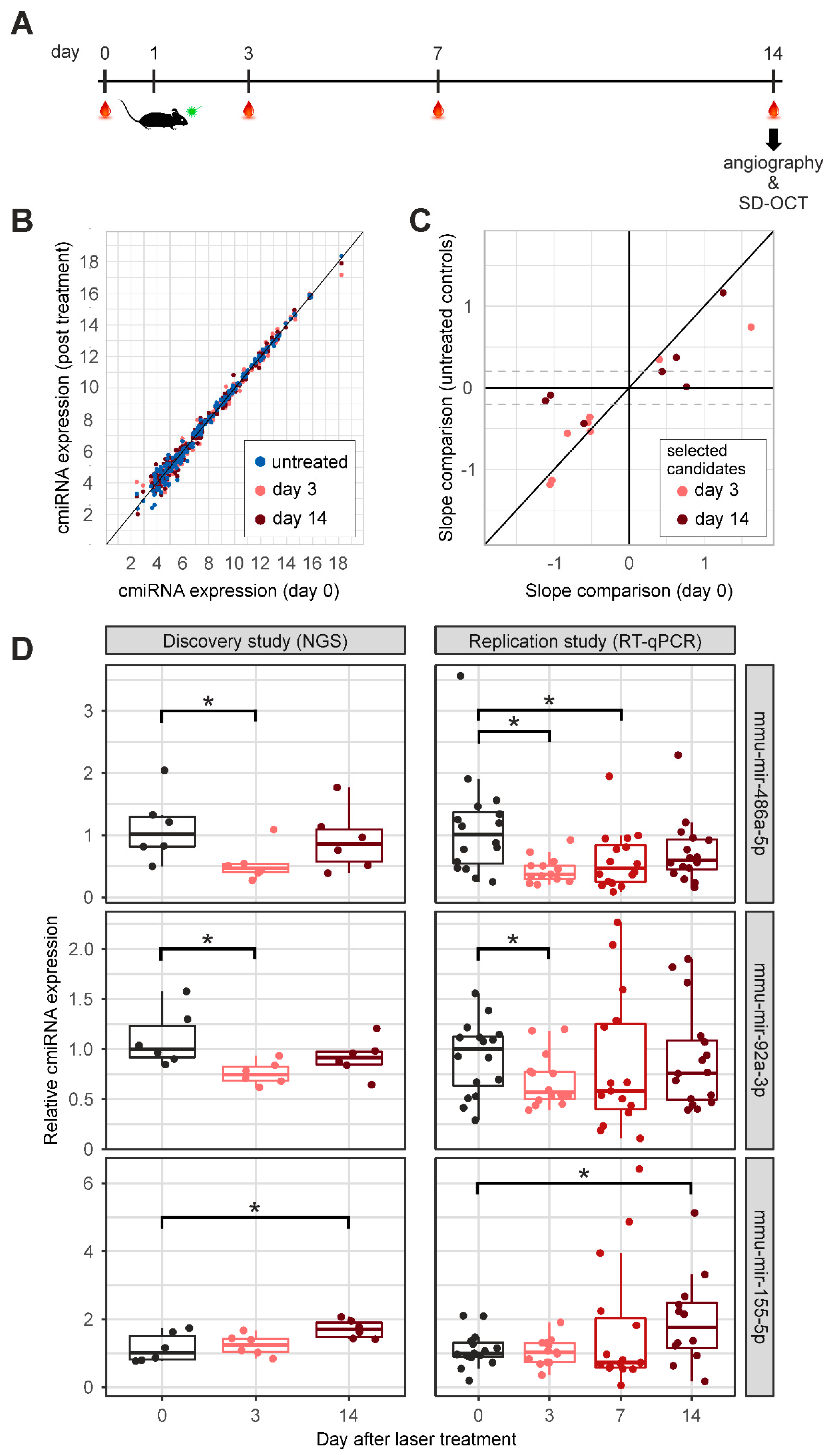
2.2. CmiRNA Expression after Laser-Induced CNV Formation in C57Bl/6 Mice
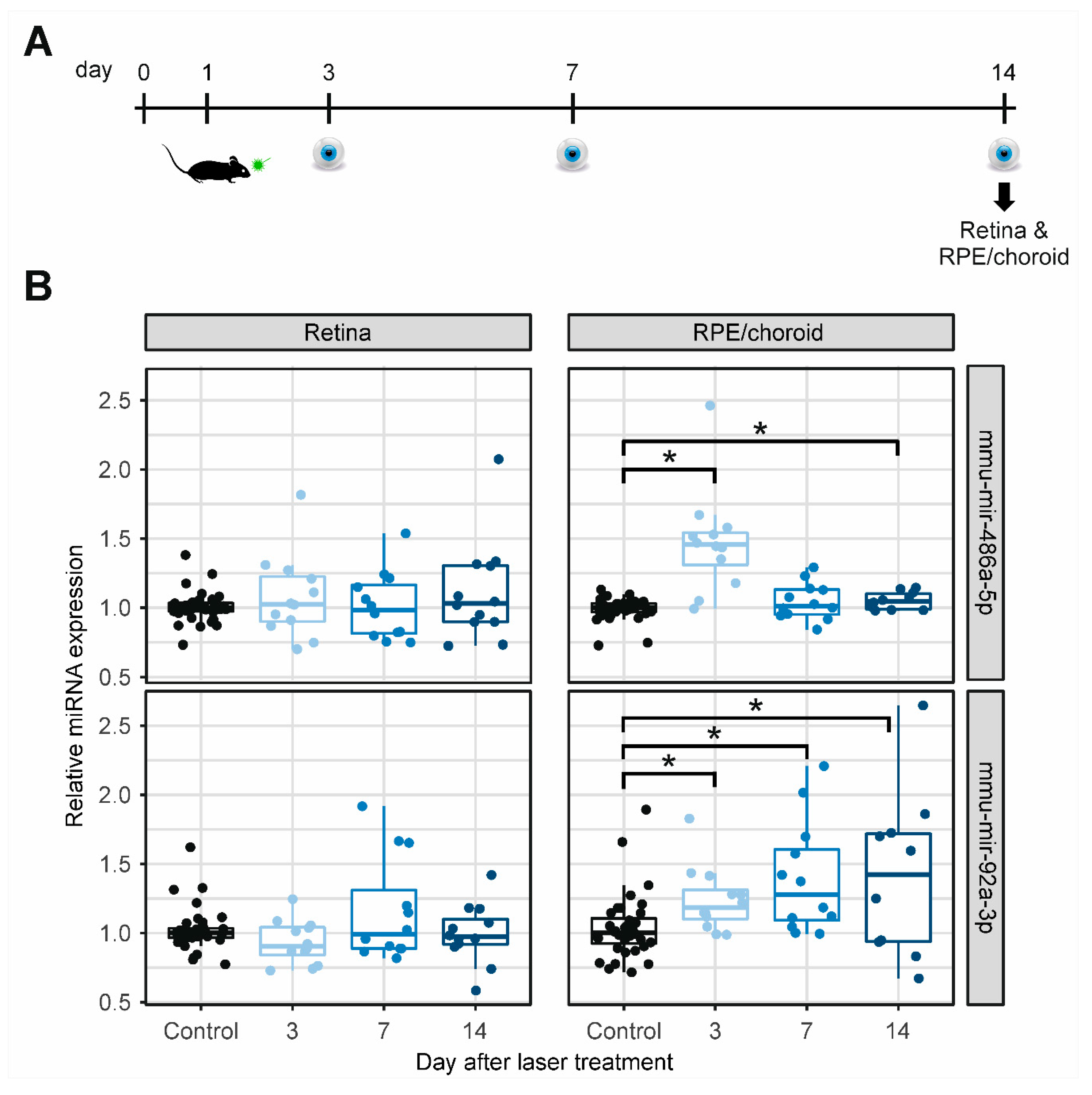
2.3. MiRNA Expression in Retinal and RPE/Choroidal Tissue
2.4. Functional Characterization of miRNAs in Murine Endothelial and Microglial Cells
3. Discussion
4. Materials and Methods
4.1. Ethical Statement on Animal Studies
4.2. Literture Analysis of Differentially Expressed cmiRNAs in nAMD Patients
4.3. Induction of CNV Lesions in the Mouse Model
4.4. Blood and Tissue Sample Preparation and cmiRNA Isolation
4.5. Sequencing of cmiRNAs and Data Analysis (Discovery Study)
4.6. RT-qPCR and Data Analysis (cmiRNA Replication Study and miRNA Expression in Tissue)
4.7. Cell Culture and miRNA Mimic Transfection
4.8. RT-qPCR to Detect Overexpression
4.9. Tube Formation Assay
4.10. Scratch Assay
4.11. Viability
4.12. NO Assay
4.13. Statistical Evaluation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AMD | age-related macular degeneration |
| ARPE-19 | human retinal pigment epithelium cell line |
| BV-2 | murine microglial cell line |
| C166 | murine endothelial yolk sac cell line |
| cmiRNA | circulating microRNA |
| CNV | choroidal neovascularization |
| DPBS | Dulbecco’s phosphate buffered saline |
| E-PAP | E. coli Poly (A) Polymerase I |
| FCS | fetal calf serum |
| FDR | false discovery rate |
| HUVEC | human umbilical vein endothelial cells |
| LPS | lipopolysaccharide |
| miRNA | microRNA |
| nAMD | neovascular age-related macular degeneration |
| NGS | next generation sequencing |
| NO | nitric oxide |
| RPE | retinal pigment epithelium cells |
| RT-qPCR | quantitative reverse transcription PCR |
| SD-OCT | spectral domain optical coherence tomography |
| SE | standard error |
| TMM | trimmed mean of M-values |
| VEGF | vascular endothelial growth factor |
References
- Shweiki, D.; Itin, A.; Soffer, D.; Keshet, E. Vascular endothelial growth factor induced by hypoxia may mediate hypoxia-initiated angiogenesis. Nature 1992, 359, 843–845. [Google Scholar] [CrossRef] [PubMed]
- Shima, D.T.; Adamis, A.P.; Ferrara, N.; Yeo, K.T.; Yeo, T.K.; Allende, R.; Folkman, J.; D’Amore, P.A. Hypoxic induction of endothelial cell growth factors in retinal cells: Identification and characterization of vascular endothelial growth factor (VEGF) as the mitogen. Mol. Med. 1995, 1, 182–193. [Google Scholar] [CrossRef] [PubMed]
- Aiello, L.P.; Northrup, J.M.; Keyt, B.A.; Takagi, H.; Iwamoto, M.A. Hypoxic Regulation of Vascular Endothelial Growth Factor in Retinal Cells. Arch. Ophthalmol. 1995, 113, 1538–1544. [Google Scholar] [CrossRef] [PubMed]
- Chau, F.Y.; Wallace, D.; Vajaranant, T.; Herndon, L.; Lee, P.; Challa, P.; Allingham, R.; Maumenee, I. Osteogenesis Imperfecta and the Eye. Osteogenes. Imperfecta 2013, 289–303. [Google Scholar] [CrossRef]
- Avila, M.P.; Weiter, J.J.; Jalkh, A.E.; Trempe, C.L.; Pruett, R.C.; Schepens, C.L. Natural History of Choroidal Neovascularization in Degenerative Myopia. Ophthalmology 1984, 91, 1573–1581. [Google Scholar] [CrossRef]
- Sarks, S.H. New vessel formation beneath the retinal pigment epithelium in senile eyes. Br. J. Ophthalmol. 1973, 57, 951–965. [Google Scholar] [CrossRef]
- Pascolini, D.; Mariotti, S.P. Global estimates of visual impairment: 2010. Br. J. Ophthalmol. 2012, 96, 614–618. [Google Scholar] [CrossRef]
- Grossniklaus, H.E.; Green, W.R. Choroidal neovascularization. Am. J. Ophthalmol. 2004, 137, 496–503. [Google Scholar] [CrossRef]
- Keane, P.A.; de Salvo, G.; Sim, D.A.; Goverdhan, S.; Agrawal, R.; Tufail, A. Strategies for improving early detection and diagnosis of neovascular age-related macular degeneration. Clin. Ophthalmol. 2015, 9, 353–366. [Google Scholar] [CrossRef]
- Wong, T.; Chakravarthy, U.; Klein, R.; Mitchell, P.; Zlateva, G.; Buggage, R.; Fahrbach, K.; Probst, C.; Sledge, I. The Natural History and Prognosis of Neovascular Age-Related Macular Degeneration. Ophthalmology 2008, 115, 116–126.e111. [Google Scholar] [CrossRef]
- Fritsche, L.G.; Fariss, R.N.; Stambolian, D.; Abecasis, G.R.; Curcio, C.a.; Swaroop, A. Age-Related Macular Degeneration: Genetics and Biology Coming Together. Annu. Rev. Genomics Hum. Genet. 2014, 15, 151–171. [Google Scholar] [CrossRef] [PubMed]
- Grassmann, F.; Fauser, S.; Weber, B.H.F. The genetics of age-related macular degeneration (AMD)—Novel targets for designing treatment options? Eur. J. Pharm. Biopharm. 2015, 95, 194–202. [Google Scholar] [CrossRef] [PubMed]
- Grassmann, F.; Ach, T.; Brandl, C.; Heid, I.M.; Weber, B.H.F. What Does Genetics Tell Us About Age-Related Macular Degeneration? Annu. Rev. Vis. Sci. 2015, 1, 73–96. [Google Scholar] [CrossRef] [PubMed]
- Murad, N.; Kokkinaki, M.; Gunawardena, N.; Gunawan, M.S.; Hathout, Y.; Janczura, K.J.; Theos, A.C.; Golestaneh, N. MiR-184 regulates ezrin, LAMP-1 expression, affects phagocytosis in human retinal pigment epithelium and is downregulated in age-related macular degeneration. FEBS J. 2014, 281, 5251–5264. [Google Scholar] [CrossRef]
- Bhattacharjee, S.; Zhao, Y.; Dua, P.; Rogaev, E.I.; Lukiw, W.J. MicroRNA-34α-mediated down-regulation of the microglial-enriched triggering receptor and phagocytosis-sensor TREM2 in age-related macular degeneration. PLoS ONE 2016, 11, e0150211. [Google Scholar] [CrossRef]
- Chim, S.S.C.; Shing, T.K.F.; Hung, E.C.W.; Leung, T.Y.; Lau, T.K.; Chiu, R.W.K.; Lo, Y.M.D. Detection and characterization of placental microRNAs in maternal plasma. Clin. Chem. 2008, 54, 482–490. [Google Scholar] [CrossRef]
- Berber, P.; Grassmann, F.; Kiel, C.; Weber, B.H.F. An Eye on Age-Related Macular Degeneration: The Role of MicroRNAs in Disease Pathology. Mol. Diagn. Ther. 2016, 21, 31–43. [Google Scholar] [CrossRef]
- Lambert, V.; Lecomte, J.; Hansen, S.; Blacher, S.; Gonzalez, M.-L.A.; Struman, I.; Sounni, N.E.; Rozet, E.; de Tullio, P.; Foidart, J.M.; et al. Laser-induced choroidal neovascularization model to study age-related macular degeneration in mice. Nat. Protoc. 2013, 8, 2197–2211. [Google Scholar] [CrossRef]
- Tobe, T.; Ortega, S.; Luna, J.D.; Ozaki, H.; Okamoto, N.; Derevjanik, N.L.; Vinores, S.A.; Basilico, C.; Campochiaro, P.A. Targeted disruption of the FGF2 gene does not prevent choroidal neovascularization in a murine model. Am. J. Pathol. 1998, 153, 1641–1646. [Google Scholar] [CrossRef]
- Lückoff, A.; Caramoy, A.; Scholz, R.; Prinz, M.; Kalinke, U.; Langmann, T. Interferon-beta signaling in retinal mononuclear phagocytes attenuates pathological neovascularization. EMBO Mol. Med. 2016, 8, 670–678. [Google Scholar] [CrossRef]
- Ertekin, S.; Yildirim, Ö.; Dinç, E.; Ayaz, L.; Balci Fidanci, Ş.; Tamer, L. Evaluation of circulating miRNAs in wet age-related macular degeneration. Mol. Vis. 2014, 20, 1057–1066. [Google Scholar] [PubMed]
- Grassmann, F.; Schoenberger, P.G.A.; Brandl, C.; Schick, T.; Hasler, D.; Meister, G.; Fleckenstein, M.; Lindner, M.; Helbig, H.; Fauser, S.; et al. A circulating MicroRNA profile is associated with late- stage neovascular age-related macular degeneration. PLoS ONE 2014, 9, e107461. [Google Scholar] [CrossRef] [PubMed]
- Ménard, C.; Rezende, F.A.; Miloudi, K.; Wilson, A.; Hardy, P.; Sangiovanni, J.P.; Guire, V.D.; Sapieha, P. MicroRNA signatures in vitreous humour and plasma of patients with exudative AMD. Oncotarget 2016, 7, 19171–19184. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ren, C.; Liu, Q.; Wei, Q.; Cai, W.; He, M.; Du, Y.; Xu, D.; Wu, Y.; Yu, J. Circulating miRNAs as potential biomarkers of age-related macular degeneration. Cell. Physiol. Biochem. 2017, 41, 1413–1423. [Google Scholar] [CrossRef]
- Romano, G.L.; Platania, C.B.M.; Drago, F.; Salomone, S.; Ragusa, M.; Barbagallo, C.; Pietro, C.D.; Purrello, M.; Reibaldi, M.; Avitabile, T.; et al. Retinal and circulating miRNAs in age-related macular degeneration: An in vivo animal and human study. Front. Pharmacol. 2017, 8, 1–10. [Google Scholar] [CrossRef]
- Blasiak, J.; Watala, C.; Tuuminen, R.; Kivinen, N.; Koskela, A.; Uusitalo-Järvinen, H.; Tuulonen, A.; Winiarczyk, M.; Mackiewicz, J.; Zmorzyński, S.; et al. Expression of VEGFA-regulating miRNAs and mortality in wet AMD. J. Cell. Mol. Med. 2019, 23, 8464–8471. [Google Scholar] [CrossRef]
- O’Brien, J.; Hayder, H.; Zayed, Y.; Peng, C. Overview of microRNA biogenesis, mechanisms of actions, and circulation. Front. Endocrinol. 2018, 9, 1–12. [Google Scholar] [CrossRef]
- Denli, A.M.; Tops, B.B.J.; Plasterk, R.H.A.; Ketting, R.F.; Hannon, G.J. Processing of primary microRNAs by the Microprocessor complex. Nature 2004, 432, 231–235. [Google Scholar] [CrossRef]
- Zhang, H.; Kolb, F.A.; Jaskiewicz, L.; Westhof, E.; Filipowicz, W. Single processing center models for human Dicer and bacterial RNase III. Cell 2004, 118, 57–68. [Google Scholar] [CrossRef]
- Ro, S.; Park, C.; Young, D.; Sanders, K.M.; Yan, W. Tissue-dependent paired expression of miRNAs. Nucleic Acids Res. 2007, 35, 5944–5953. [Google Scholar] [CrossRef]
- Meijer, H.A.; Smith, E.M.; Bushell, M. Regulation of miRNA strand selection: Follow the leader? Biochem. Soc. Trans. 2014, 42, 1135–1140. [Google Scholar] [CrossRef]
- Svensson, C.; Fernaeus, S.Z.; Part, K.; Reis, K.; Land, T. LPS-induced iNOS expression in Bv-2 cells is suppressed by an oxidative mechanism acting on the JNK pathway--a potential role for neuroprotection. Brain Res. 2010, 1322, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Leung, A.K.L.; Sharp, P.A. MicroRNA Functions in Stress Responses. Mol. Cell 2010, 40, 205–215. [Google Scholar] [CrossRef] [PubMed]
- Cremer, T.J.; Ravneberg, D.H.; Clay, C.D.; Piper-Hunter, M.G.; Marsh, C.B.; Elton, T.S.; Gunn, J.S.; Amer, A.; Kanneganti, T.D.; Schlesinger, L.S.; et al. MiR-155 induction by F. novicida but not the virulent F. tularensis results in SHIP down-regulation and enhanced pro-inflammatory cytokine response. PLoS ONE 2009, 4, e8508. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Wu, M.; Li, M.; Li, D.; Peng, A.; Nie, X.; Sun, M.; Wang, J.; Wu, Y.; Deng, Q.; et al. MiR-155 suppresses bacterial clearance in Pseudomonas aeruginosa-induced keratitis by targeting Rheb. J. Infect. Dis. 2014, 210, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Alexandrov, P.N.; Dua, P.; Hill, J.M.; Bhattacharjee, S.; Zhao, Y.; Lukiw, W.J. MicroRNA (miRNA) speciation in Alzheimer’s disease (AD) cerebrospinal fluid (CSF) and extracellular fluid (ECF). Int. J. Biochem. Mol. Biol. 2012, 3, 365–373. [Google Scholar]
- Zhang, J.; Cheng, Y.; Cui, W.; Li, M.; Li, B.; Guo, L. MicroRNA-155 modulates Th1 and Th17 cell differentiation and is associated with multiple sclerosis and experimental autoimmune encephalomyelitis. J. Neuroimmunol. 2014, 266, 56–63. [Google Scholar] [CrossRef]
- Kluiver, J.; Poppema, S.; de Jong, D.; Blokzijl, T.; Harms, G.; Jacobs, S.; Kroesen, B.J.; van den Berg, A. BIC and miR-155 are highly expressed in Hodgkin, primary mediastinal and diffuse large B cell lymphomas. J. Pathol. 2005, 207, 243–249. [Google Scholar] [CrossRef]
- Ryu, J.K.; Hong, S.M.; Karikari, C.A.; Hruban, R.H.; Goggins, M.G.; Maitra, A. Aberrant microRNA-155 expression is an early event in the multistep progression of pancreatic adenocarcinoma. Pancreatology 2010, 10, 66–73. [Google Scholar] [CrossRef]
- Qin, W.; Ren, Q.; Liu, T.; Huang, Y.; Wang, J. MicroRNA-155 is a novel suppressor of ovarian cancer-initiating cells that targets CLDN1. FEBS Lett. 2013, 587, 1434–1439. [Google Scholar] [CrossRef]
- Nowakowski, T.J.; Rani, N.; Golkaram, M.; Zhou, H.R.; Alvarado, B.; Huch, K.; West, J.A.; Leyrat, A.; Pollen, A.A.; Kriegstein, A.R.; et al. Regulation of cell-type-specific transcriptomes by microRNA networks during human brain development. Nat. Neurosci. 2018, 21, 1784–1792. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Zheng, J.; Chen, Z.; Liu, Y.; Dura, B.; Kwak, M.; Xavier-Ferrucio, J.; Lu, Y.C.; Zhang, M.; Roden, C.; et al. Single-cell microRNA-mRNA co-sequencing reveals non-genetic heterogeneity and mechanisms of microRNA regulation. Nat. Commun. 2019, 10, 95. [Google Scholar] [CrossRef] [PubMed]
- Kohram, F.; Fallah, P.; Shamsara, M.; Bolandi, Z.; Rassoulzadegan, M.; Soleimani, M.; Ghanbarian, H. Cell type-dependent functions of microRNA-92a. J. Cell. Biochem. 2018, 119, 5798–5804. [Google Scholar] [CrossRef] [PubMed]
- Karlstetter, M.; Scholz, R.; Rutar, M.; Wong, W.T.; Provis, J.M.; Langmann, T. Retinal microglia: Just bystander or target for therapy? Prog. Retin. Eye Res. 2015, 45, 30–57. [Google Scholar] [CrossRef]
- Fernández-Messina, L.; Gutiérrez-Vázquez, C.; Rivas-García, E.; Sánchez-Madrid, F.; de la Fuente, H. Immunomodulatory role of microRNAs transferred by extracellular vesicles. Biol. Cell 2015, 107, 61–77. [Google Scholar] [CrossRef]
- De Candia, P.; De Rosa, V.; Casiraghi, M.; Matarese, G. Extracellular RNAs: A secret arm of immune system regulation. J. Biol. Chem. 2016, 291, 7221–7228. [Google Scholar] [CrossRef]
- Mittelbrunn, M.; Gutiérrez-Vázquez, C.; Villarroya-Beltri, C.; González, S.; Sánchez-Cabo, F.; González, M.Á.; Bernad, A.; Sánchez-Madrid, F. Unidirectional transfer of microRNA-loaded exosomes from T cells to antigen-presenting cells. Nat. Commun. 2011, 2, 282. [Google Scholar] [CrossRef]
- Ayaz, L.; Dinç, E. Evaluation of microRNA responses in ARPE-19 cells against the oxidative stress. Cutan. Ocul. Toxicol. 2018, 37, 121–126. [Google Scholar] [CrossRef]
- Bellezza, I. Oxidative stress in age-related macular degeneration: NRF2 as therapeutic target. Front. Pharmacol. 2018, 9, 1–7. [Google Scholar] [CrossRef]
- Bonauer, A.; Carmona, G.; Iwasaki, M.; Mione, M.; Koyanagi, M.; Fischer, A.; Burchfield, J.; Fox, H.; Doebele, C.; Ohtani, K.; et al. MicroRNA-92a Controls Angiogenesis and Functional Recovery of Ischemic Tissues in Mice. Science 2009, 324, 1710–1713. [Google Scholar] [CrossRef]
- Zhang, L.; Zhou, M.; Qin, G.; Weintraub, N.L.; and Tang, Y. MiR-92a regulates viability and angiogenesis of endothelial cells under oxidative stress. Biochem. Biophys. Res. Commun. 2014, 23, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Fujita, M.; Otani, H.; Iwasaki, M.; Yoshioka, K.; Shimazu, T.; Shiojima, I.; Tabata, Y. Antagomir-92a impregnated gelatin hydrogel microsphere sheet enhances cardiac regeneration after myocardial infarction in rats. Regen. Ther. 2016, 5, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Gallant-Behm, C.L.; Piper, J.; Dickinson, B.A.; Dalby, C.M.; Pestano, L.A.; Jackson, A.L. A synthetic microRNA-92a inhibitor (MRG-110) accelerates angiogenesis and wound healing in diabetic and nondiabetic wounds. Wound Rep. Regen. 2018, 26, 311–323. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.B.; Moolani, H.V.; Sene, A.; Sidhu, R.; Kell, P.; Lin, J.B.; Dong, Z.; Ban, N.; Ory, D.S.; Apte, R.S. Macrophage microRNA-150 promotes pathological angiogenesis as seen in age-related macular degeneration. JCI Insight 2018, 3, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Litwińska, Z.; Sobuś, A.; Łuczkowska, K.; Grabowicz, A.; Mozolewska-Piotrowska, K.; Safranow, K.; Kawa, M.P.; Machaliński, B.; Machalińska, A. The Interplay Between Systemic Inflammatory Factors and MicroRNAs in Age-Related Macular Degeneration. Front. Aging Neurosci. 2019, 11, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Elbay, A.; Ercan, Ç.; Akbaş, F.; Bulut, H.; Ozdemir, H. Three new circulating microRNAs may be associated with wet age-related macular degeneration. Scand. J. Clin. Lab. Investig. 2019, 79, 388–394. [Google Scholar] [CrossRef]
- Ulańczyk, Z.; Sobuś, A.; Łuczkowska, K.; Grabowicz, A.; Mozolewska-Piotrowska, K.; Safranow, K.; Kawa, M.P.; Pałucha, A.; Krawczyk, M.; Sikora, P.; et al. Associations of microRNAs, angiogenesis-regulating factors and CFH Y402H polymorphism—An attempt to search for systemic biomarkers in age-related macular degeneration. Int. J. Mol. Sci. 2019, 20, 5750. [Google Scholar] [CrossRef]
- Szemraj, M.; Bielecka-Kowalska, A.; Oszajca, K.; Krajewska, M.; Goś, R.; Jurowski, P.; Kowalski, M.; Szemraj, J. Serum MicroRNAs as Potential Biomarkers of AMD. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2015, 21, 2734–2742. [Google Scholar] [CrossRef]
- Szemraj, M.; Oszajca, K.; Szemraj, J.; Jurowski, P. MicroRNA expression analysis in serum of patients with congenital hemochromatosis and age-related macular degeneration (AMD). Med. Sci. Monit. 2017, 23, 4050–4060. [Google Scholar] [CrossRef]
- Karlstetter, M.; Kopatz, J.; Aslanidis, A.; Shahraz, A.; Caramoy, A.; Linnartz-Gerlach, B.; Lin, Y.; Lückoff, A.; Fauser, S.; Düker, K.; et al. Polysialic acid blocks mononuclear phagocyte reactivity, inhibits complement activation, and protects from vascular damage in the retina. EMBO Mol. Med. 2017, 9, 154–166. [Google Scholar] [CrossRef]
- Kosaka, N.; Izumi, H.; Sekine, K.; Ochiya, T. microRNA as a new immune-regulatory agent in breast milk. Silence 2010, 1, 7. [Google Scholar] [CrossRef] [PubMed]
- Friedländer, M.R.; Chen, W.; Adamidi, C.; Maaskola, J.; Einspanier, R.; Knespel, S.; Rajewsky, N. Discovering microRNAs from deep sequencing data using miRDeep. Nat. Biotechnol. 2008, 26, 407–415. [Google Scholar] [CrossRef] [PubMed]
- Langmead, B.; Salzberg, S.L. Fast gapped-read alignment with Bowtie 2. Nat. Methods 2012, 9, 357–359. [Google Scholar] [CrossRef] [PubMed]
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2010. [Google Scholar]
- Robinson, M.D.; Oshlack, A. A scaling normalization method for differential expression analysis of RNA-seq data. Genome Biol. 2010, 11, R25. [Google Scholar] [CrossRef]
- Johnson, W.E.; Li, C.; Rabinovic, A. Adjusting batch effects in microarray expression data using empirical Bayes methods. Biostatistics 2007, 8, 118–127. [Google Scholar] [CrossRef]
- Pinheiro, J.; Bates, D.; Saikat, D.; Sarkar, D.; Team, R.D.C. nlme: Linear and Nonlinear Mixed Effects Models. R package version 3.1-145. Available online: https://CRAN.R-project.org/package=nlme (accessed on 1 April 2020).
- Heinze, G.; Ploner, M.; Dunkler, D.; Southworth, H. Logistf: Firth’s Bias Reduced Logistic Regression, 2013.
- Hurteau, G.J.; Spivack, S.D.; Brock, G.J. Potential mRNA degradation targets of hsa-miR-200c, identified using informatics and qRT-PCR. Cell Cycle 2006, 5, 1951–1956. [Google Scholar] [CrossRef]
- Yoon, H.; Belmonte, K.C.; Kasten, T.; Bateman, R.; Kim, J. Intra- and Inter-individual Variability of microRNA Levels in Human Cerebrospinal Fluid: Critical Implications for Biomarker Discovery. Sci. Rep. 2017, 7, 12720. [Google Scholar] [CrossRef]
- Benjamini, Y.; Hochberg, Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Soc. Ser. B 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Pollard, K.S.; Dudoit, S.; van der Laan, M.J. Multiple Testing Procedures: The multtest Package and Applications to Genomics. In Bioinformatics and Computational Biology Solutions Using R and Bioconductor; Springer-Verlag: Berlin/Heidelberg, Germany, 2005. [Google Scholar] [CrossRef]
- Ponce, M.L. Tube formation: An in vitro matrigel angiogenesis assay. Methods Mol. Biol. 2009, 467, 183–188. [Google Scholar] [CrossRef]
- Carpentier, G. Angiogenesis Analyzer for ImageJ. ImageJ News, 5 October 2012. [Google Scholar]
- Liang, C.C.; Park, A.Y.; Guan, J.L. In vitro scratch assay: A convenient and inexpensive method for analysis of cell migration in vitro. Nat. Protoc. 2007, 2, 329–333. [Google Scholar] [CrossRef]
- Ogle, D.H.; Wheeler, P.; Dinno, A. FSA: Fisheries Stock Analysis. R Package Version 0.8.25, 2019.

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kiel, C.; Berber, P.; Karlstetter, M.; Aslanidis, A.; Strunz, T.; Langmann, T.; Grassmann, F.; Weber, B.H.F. A Circulating MicroRNA Profile in a Laser-Induced Mouse Model of Choroidal Neovascularization. Int. J. Mol. Sci. 2020, 21, 2689. https://doi.org/10.3390/ijms21082689
Kiel C, Berber P, Karlstetter M, Aslanidis A, Strunz T, Langmann T, Grassmann F, Weber BHF. A Circulating MicroRNA Profile in a Laser-Induced Mouse Model of Choroidal Neovascularization. International Journal of Molecular Sciences. 2020; 21(8):2689. https://doi.org/10.3390/ijms21082689
Chicago/Turabian StyleKiel, Christina, Patricia Berber, Marcus Karlstetter, Alexander Aslanidis, Tobias Strunz, Thomas Langmann, Felix Grassmann, and Bernhard H.F. Weber. 2020. "A Circulating MicroRNA Profile in a Laser-Induced Mouse Model of Choroidal Neovascularization" International Journal of Molecular Sciences 21, no. 8: 2689. https://doi.org/10.3390/ijms21082689
APA StyleKiel, C., Berber, P., Karlstetter, M., Aslanidis, A., Strunz, T., Langmann, T., Grassmann, F., & Weber, B. H. F. (2020). A Circulating MicroRNA Profile in a Laser-Induced Mouse Model of Choroidal Neovascularization. International Journal of Molecular Sciences, 21(8), 2689. https://doi.org/10.3390/ijms21082689





