Dysregulation of Type II Transmembrane Serine Proteases and Ligand-Dependent Activation of MET in Urological Cancers
Abstract
1. Introduction
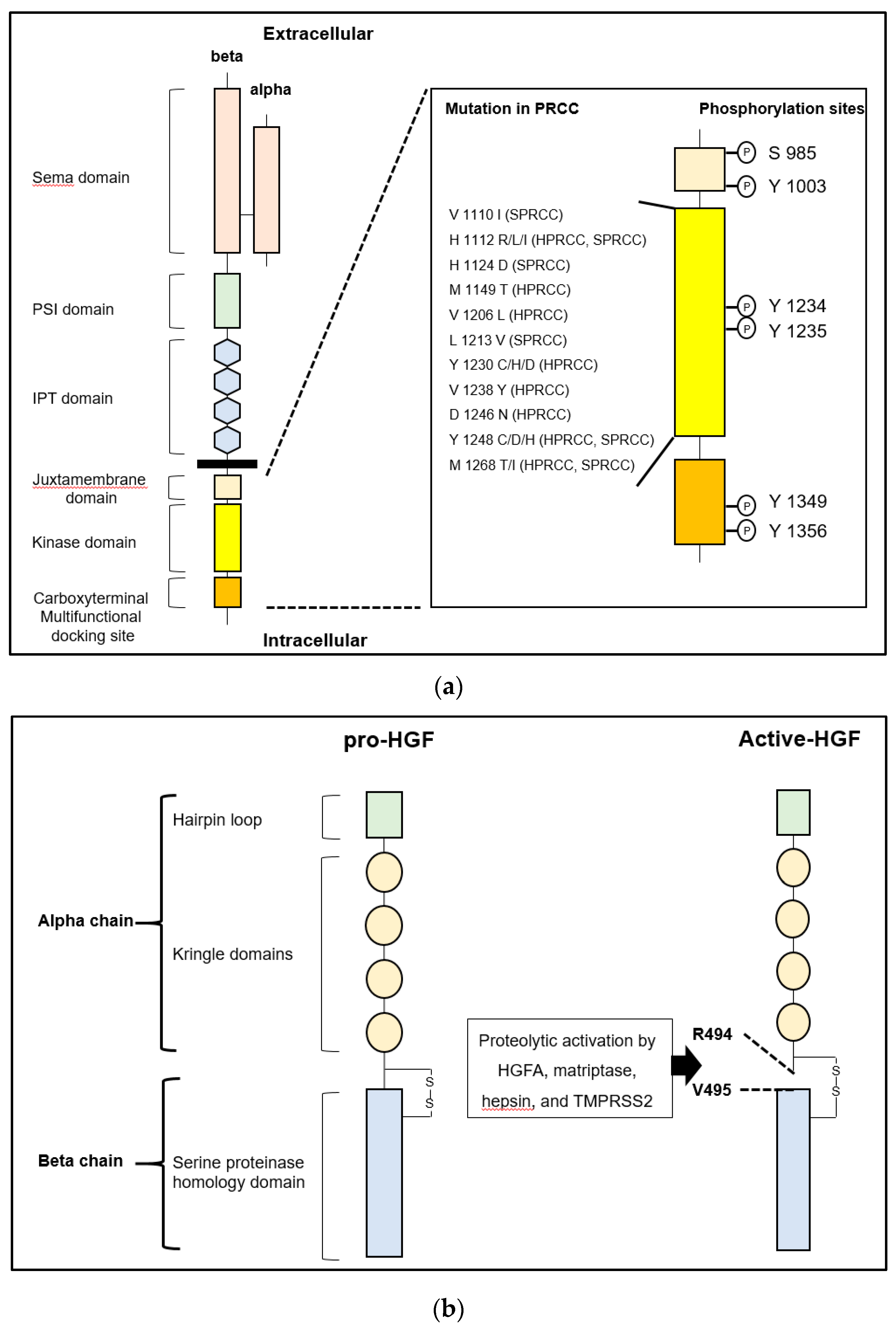
2. HGF/MET and the Related Molecules
2.1. HGF and MET in Cancer
2.1.1. HGF/MET Signaling Axis
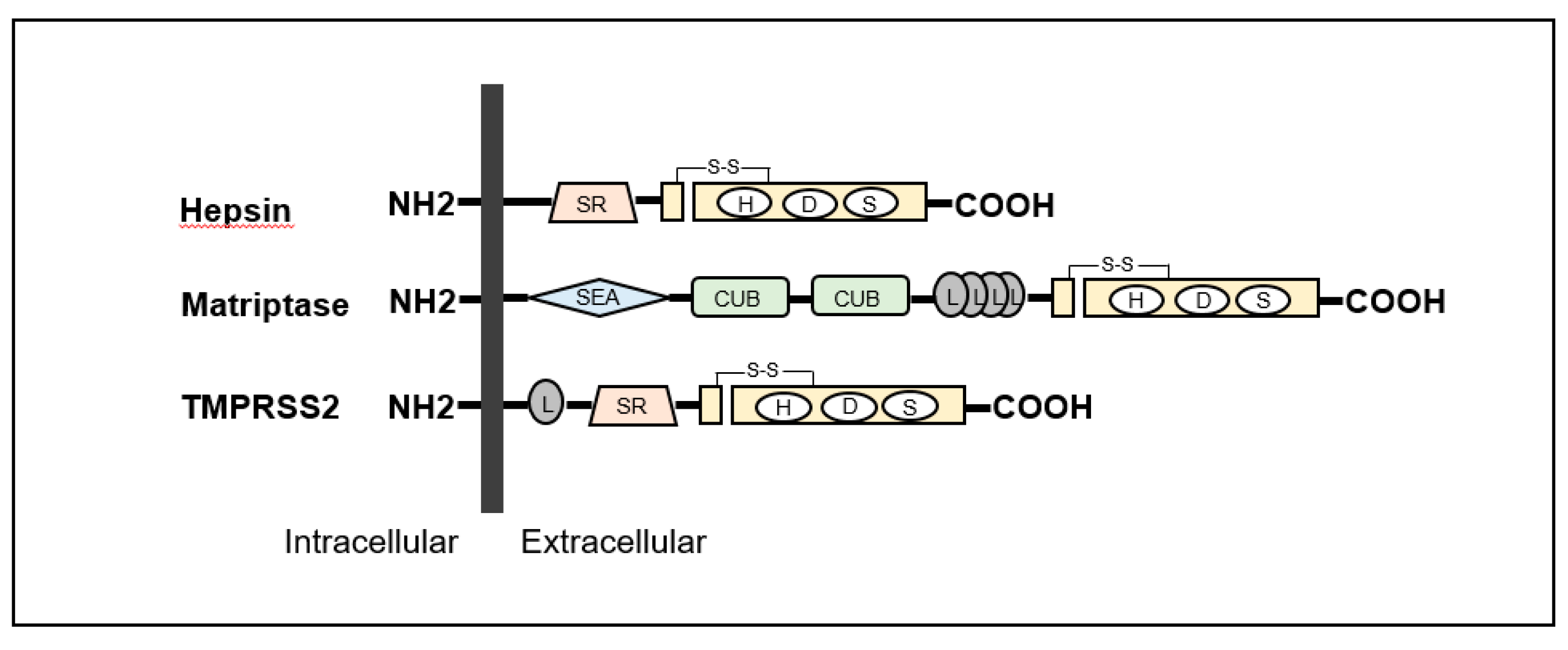
2.2. Cell Surface pro-HGF Activating Enzymes and the Regulators
2.2.1. Type-II Transmembrane Serine Proteases (TTSP) in Cancers
2.2.2. Matriptase
2.2.3. Hepsin
2.2.4. Regulators of TTSPs—HAIs
3. TTSPs and HAIs in Urological Cancers
3.1. Prostate Cancer
3.1.1. HGF/MET Signaling and Hepsin
3.1.2. Matriptase and HAIs
3.1.3. TMPRSS2 in PC
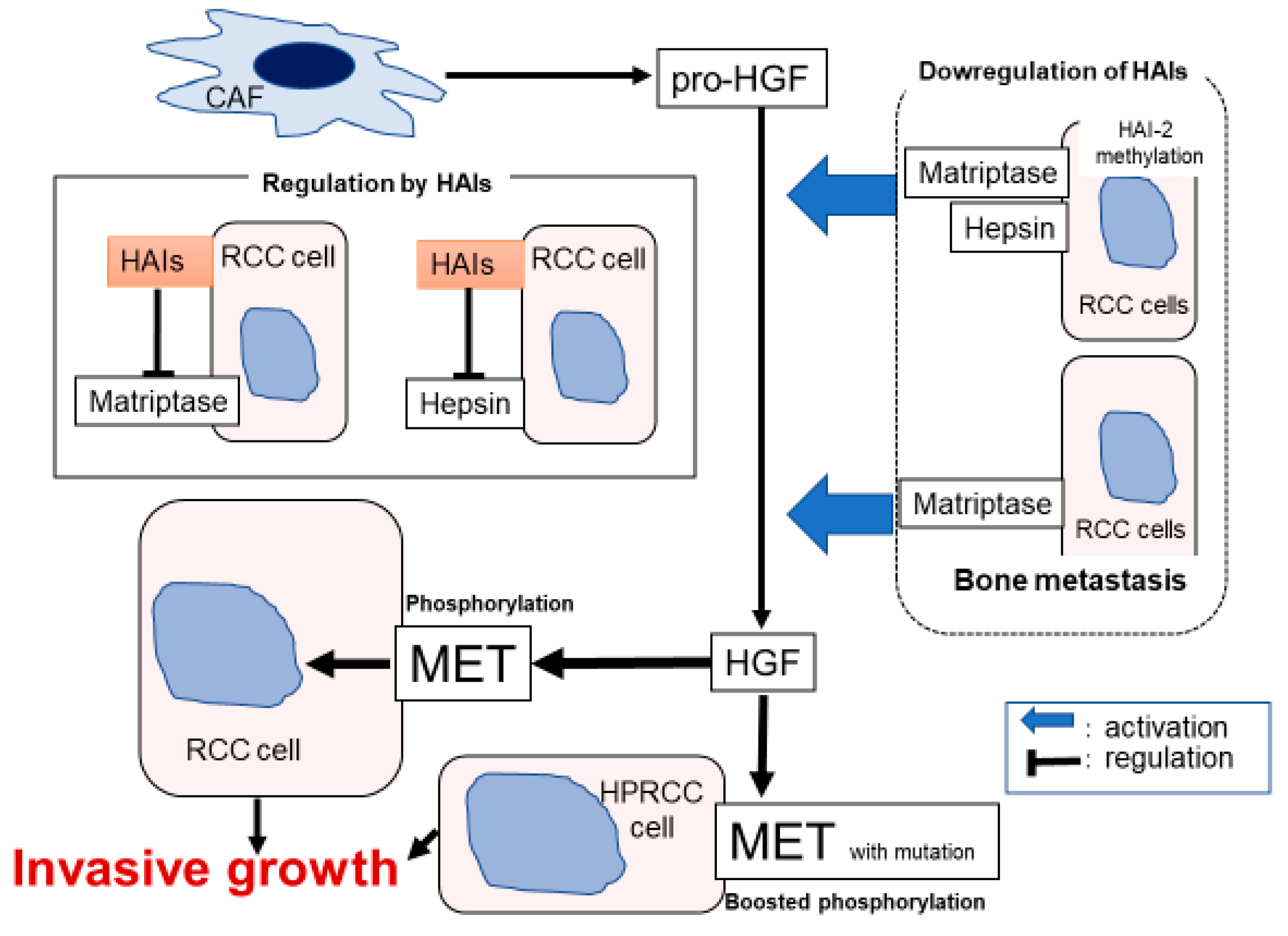
3.2. Renal Cell Carcinoma
3.2.1. HGF/MET Signaling
3.2.2. HAIs and TTSPs in RCC
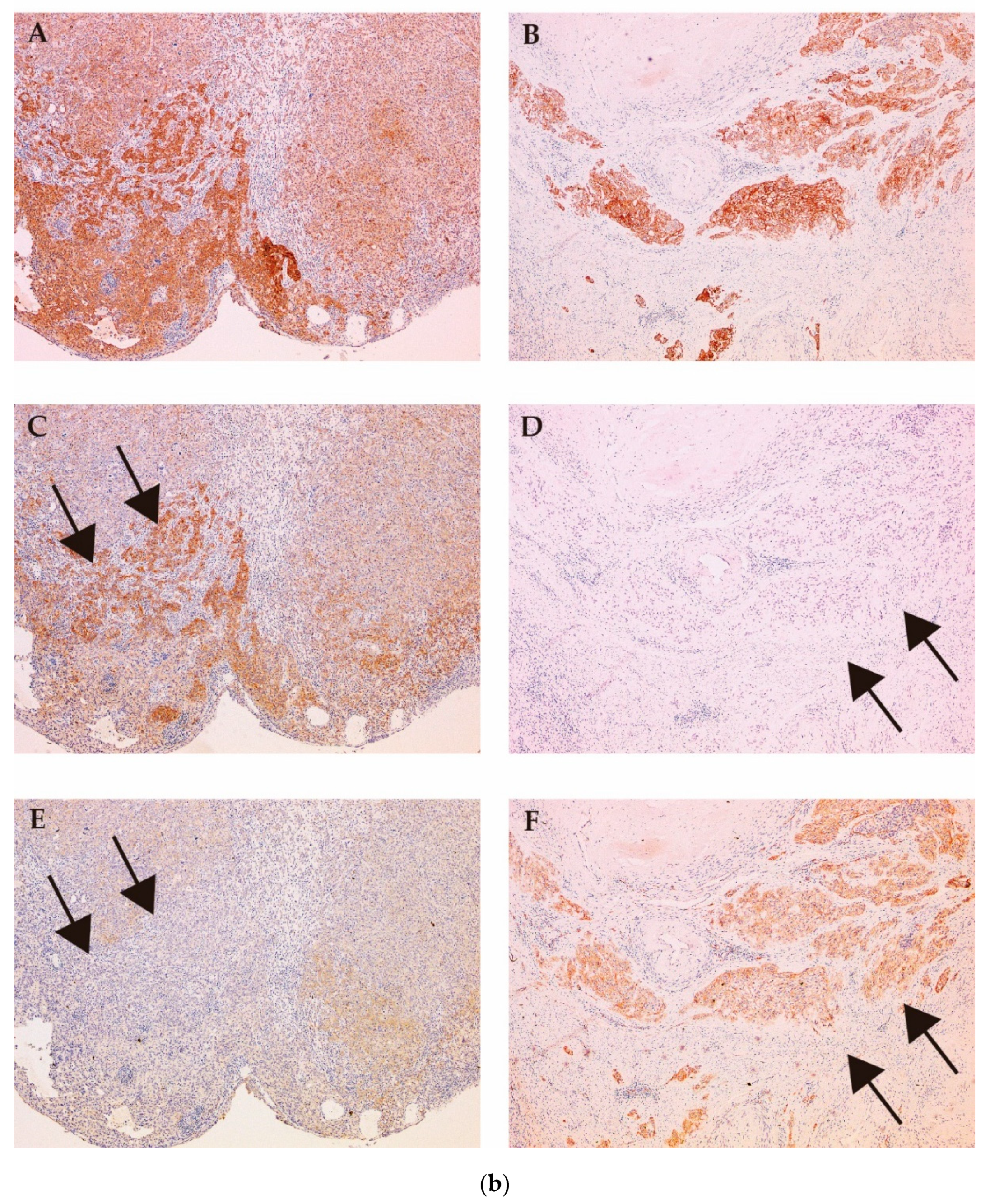
3.2.3. MET and Matriptase/HAI-2 in Bone Metastasis
3.3. Bladder Cancer
3.3.1. Significance of HGF-dependent MET Activation in Muscle Invasive Bladder Cancer
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| HGF | hepatocyte growth factor |
| HGFA | hepatocyte growth factor activator |
| HAI | hepatocyte growth factor activator inhibitor |
| PC | prostate cancer |
| RCC | renal cell carcinoma |
| EMT | epithelial-mesenchymal transition |
| TTSP | type II transmembrane serine protease |
| TMPRSS | transmembrane protease, serine |
| PRCC | papillary renal cell carcinoma |
| PDGF | platelet-derived growth factor |
| PAR | protease activated receptor |
| ENaC | epithelial sodium channel |
| RNA | ribonucleic acid |
| KD | Kunitz-type serine protease inhibitor domain |
| EPCAM | epithelial cell adhesion molecules |
| PIN | prostatic intraepithelial neoplasia |
| AR | androgen receptor |
| CRPC | castration resistant prostate cancer |
| TK | tyrosine kinase |
| VHL | von Hippel-Lindau |
| MMP | matrix metalloprotease |
| UC | urothelial carcinoma |
| SMURF | SMAD specific E3 ubiquitin protein ligase 2 |
| TGF | transforming growth factor |
References
- Turk, B.; Turk, D.; Turk, V. Protease signalling: the cutting edge. EMBO J. 2012, 4, 1630–1643. [Google Scholar] [CrossRef] [PubMed]
- Kataoka, H.; Tanaka, H.; Nagaike, K.; Uchiyama, S.; Itoh, H. Role of cancer cell-stroma interaction in invasive growth of cancer cells. Hum. Cell. 2003, 16, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Janetka, W.J.; Benson, M.R. Extracellular Targeting of Cell Signaling in Cancer; Strategies Directed at MET and RON Receptor Tyrosine Kinase Pathways; Wiley: Hoboken, NJ, USA, 2018; pp. 1–154. [Google Scholar]
- Kataoka, H.; Miyata, S.; Uchinokura, S.; Itoh, H. Roles of hepatocyte growth factor (HGF) activator and HGF activator inhibitor in the pericellular activation of HGF/scatter factor. Cancer Metastasis Rev. 2003, 22, 223–236. [Google Scholar] [CrossRef]
- Miyazawa, K.; Shimomura, T.; Kitamura, N. Activation of hepatocyte growth factor in the injured tissues is mediated by hepatocyte growth factor activator. J. Biol. Chem. 1996, 16, 3615–3618. [Google Scholar] [CrossRef] [PubMed]
- Shimomura, T.; Kondo, J.; Ochiai, M.; Naka, D.; Miyazawa, K.; Morimoto, Y.; Kitamura, N. Activation of the zymogen of hepatocyte growth factor activator by thrombin. J. Biol. Chem. 1993, 25, 22927–22932. [Google Scholar]
- Kataoka, H.; Kawaguchi, M. Hepatocyte growth factor activator (HGFA): pathophysiological functions in vivo. FEBS J. 2010, 277, 2230–2237. [Google Scholar] [CrossRef]
- Kirchhofer, D.; Peek, M.; Lipari, M.T.; Billeci, K.; Fan, B.; Moran, P. Hepsin activates pro-hepatocyte growth factor and is inhibited by hepatocyte growth factor activator inhibitor-1B (HAI-1B) and HAI-2. FEBS Lett. 2005, 28, 1945–1950. [Google Scholar] [CrossRef]
- Owen, K.A.; Qiu, D.; Alves, J.; Schumacher, A.M.; Kilpatrick, L.M.; Li, J.; Harris, J.L.; Ellis, V. Pericellular activation of hepatocyte growth factor by the transmembrane serine proteases matriptase and hepsin, but not by the membrane-associated protease uPA. Biochem. J. 2010, 426, 219–228. [Google Scholar] [CrossRef]
- Martin, C.E.; List, K. Cell surface-anchored serine proteases in cancer progression and metastasis. Cancer Metastasis Rev. 2019, 38, 357–387. [Google Scholar] [CrossRef]
- Kawaguchi, M.; Kataoka, H. Mechanisms of hepatocyte growth factor activation in cancer tissues. Cancers (Basel) 2014, 29, 1890–1904. [Google Scholar] [CrossRef]
- Kataoka, H.; Kawaguchi, M.; Fukushima, T.; Shimomura, T. Hepatocyte growth factor activator inhibitors (HAI-1 and HAI-2): Emerging key players in epithelial integrity and cancer. Pathol. Int. 2018, 68, 145–158. [Google Scholar] [CrossRef] [PubMed]
- Gentile, A.; Trusolino, L.; Comoglio, P.M. The Met tyrosine kinase receptor in development and cancer. Cancer Metastasis Rev. 2008, 27, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Trusolino, L.; Bertotti, A.; Comoglio, P.M. MET signalling: Principles and functions in development, organ regeneration and cancer. Nat. Rev. Mol. Cell Biol. 2010, 11, 834–848. [Google Scholar] [CrossRef] [PubMed]
- Ma, P.C.; Maulik, G.; Christensen, J.; Salgia, R. c-Met: structure, functions and potential for therapeutic inhibition. Cancer Metastasis Rev. 2003, 22, 309–325. [Google Scholar] [CrossRef] [PubMed]
- Tovar, E.A.; Graveel, C.R. MET in human cancer: germline and somatic mutations. Ann. Transl. Med. 2017, 5, 205. [Google Scholar] [CrossRef]
- Tanabe, L.M.; List, K. The role of type II transmembrane serine protease-mediated signaling in cancer. FEBS J. 2017, 284, 1421–1436. [Google Scholar] [CrossRef]
- List, K.; Bugge, T.H.; Szabo, R. Matriptase: potent proteolysis on the cell surface. Mol. Med. 2006, 12, 1–7. [Google Scholar] [CrossRef]
- Wu, C.J.; Feng, X.; Lu, M.; Morimura, S.; Udey, M.C. Matriptase-mediated cleavage of EpCAM destabilizes claudins and dysregulates intestinal epithelial homeostasis. J. Clin. Investig. 2017, 127, 623–634. [Google Scholar] [CrossRef]
- Szabo, R.; Bugge, T.H. Membrane-anchored serine proteases in vertebrate cell and developmental biology. Annu. Rev. Cell Dev. Biol. 2011, 27, 213–235. [Google Scholar] [CrossRef]
- Rawlings, N.D.; Barrett, A.J.; Thomas, P.D.; Huang, X.; Bateman, A.; Finn, R.D. The MEROPS database of proteolytic enzymes, their substrates and inhibitors in 2017 and a comparison with peptidases in the PANTHER database. Nucleic Acids Res. 2018, 46, D624–D632. [Google Scholar] [CrossRef]
- Cheng, M.F.; Huang, M.S.; Lin, C.S.; Lin, L.H.; Lee, H.S.; Jiang, J.C.; Hsia, K.T. Expression of matriptase correlates with tumour progression and clinical prognosis in oral squamous cell carcinoma. Histopathology 2014, 65, 24–34. [Google Scholar] [PubMed]
- Tsuji, A.; Torres-Rosado, A.; Arai, T.; Le Beau, M.M.; Lemons, R.S.; Chou, S.H.; Kurachi, K. Hepsin, a cell membrane-associated protease. Characterization, tissue distribution, and gene localization. J. Biol. Chem. 1991, 5, 16948–16953. [Google Scholar]
- Tripathi, M.; Nandana, S.; Yamashita, H.; Ganesan, R.; Kirchhofer, D.; Quaranta, V. Laminin-332 is a substrate for hepsin, a protease associated with prostate cancer progression. J. Biol. Chem. 2008, 7, 30576–30584. [Google Scholar]
- Shimomura, T.; Denda, K.; Kitamura, A.; Kawaguchi, T.; Kito, M.; Kondo, J.; Kagaya, S.; Qin, L.; Takata, H.; Miyazawa, K.; et al. Hepatocyte growth factor activator inhibitor, a novel Kunitz-type serine protease inhibitor. J. Biol. Chem. 1997, 272, 6370–6376. [Google Scholar]
- Kawaguchi, T.; Qin, L.; Shimomura, T.; Kondo, J.; Matsumoto, K.; Denda, K.; Kitamura, N. Purification and cloning of hepatocyte growth factor activator inhibitor type 2, a Kunitz-type serine protease inhibitor. J. Biol. Chem. 1997, 272, 27558–27564. [Google Scholar] [PubMed]
- Marlor, C.W.; Delaria, K.A.; Davis, G.; Muller, D.K.; Greve, J.M.; Tamburini, P.P. Identification and cloning of human placental bikunin, a novel serine protease inhibitor containing two Kunitz domains. J. Biol. Chem. 1997, 272, 12202–12208. [Google Scholar]
- Kataoka, H.; Itoh, H.; Nuki, Y.; Hamasuna, R.; Naganuma, S.; Kitamura, N.; Shimomura, T. Mouse hepatocyte growth factor (HGF) activator inhibitor type 2 lacking the first Kunitz domain potently inhibits the HGF activator. Biochem. Biophy. Res. Commun. 2002, 290, 1096–1100. [Google Scholar]
- Kataoka, H.; Shimomura, T.; Kawaguchi, T.; Hamasuna, R.; Itoh, H.; Kitamura, N.; Miyazawa, K.; Koono, M. Hepatocyte growth factor activator inhibitor type 1 is a specific cell surface binding protein of hepatocyte growth factor activator (HGFA) and regulates HGFA activity in the pericellular microenvironment. J. Biol. Chem. 2000, 275, 40453–40462. [Google Scholar]
- Kawaguchi, M.; Takeda, N.; Hoshiko, S.; Yorita, K.; Baba, T.; Sawaguchi, A.; Nezu, Y.; Yoshikawa, T.; Fukushima, T.; Kataoka, H. Membrane-bound serine protease inhibitor HAI-1 is required for maintenance of intestinal epithelial integrity. Am. J. Pathol. 2011, 179, 1815–1826. [Google Scholar]
- Tanaka, H.; Nagaike, K.; Takeda, N.; Itoh, H.; Kohama, K.; Fukushima, T.; Miyata, S.; Uchiyama, S.; Uchinokura, S.; Shimomura, T.; et al. Hepatocyte growth factor activator inhibitor type 1 (HAI-1) is required for branching morphogenesis in the chorioallantoic placenta. Mol. Cell Biol. 2005, 25, 5687–5698. [Google Scholar]
- Hoshiko, S.; Kawaguchi, M.; Fukushima, T.; Haruyama, Y.; Yorita, K.; Tanaka, H.; Seiki, M.; Inatsu, H.; Kitamura, K.; Kataoka, H. Hepatocyte growth factor activator inhibitor type 1 is a suppressor of intestinal tumorigenesis. Cancer Res. 2013, 73, 2659–2670. [Google Scholar] [CrossRef] [PubMed]
- Baba, T.; Kawaguchi, M.; Fukushima, T.; Sato, Y.; Orikawa, H.; Yorita, K.; Tanaka, H.; Lin, C.Y.; Sakoda, S.; Kataoka, H. Loss of membrane-bound serine protease inhibitor HAI-1 induces oral squamous cell carcinoma cells’ invasiveness. J. Pathol. 2012, 228, 181–192. [Google Scholar] [PubMed]
- Ning, T.; Zhang, H.; Wang, X.; Li, S.; Zhang, L.; Deng, T.; Zhou, L.; Wang, X.; Liu, R.; Bai, M.; et al. miR-221 and miR-222 synergistically regulate hepatocyte growth factor activator inhibitor type 1 to promote cell proliferation and migration in gastric cancer. Tumour. Biol. 2017, 39, 1010428317701636. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Kawaguchi, M.; Haruyama, Y.; Kanemaru, A.; Fukushima, T.; Yamamoto, K.; Lin, C.Y.; Kataoka, H. Loss of hepatocyte growth factor activator inhibitor type 1 participates in metastatic spreading of human pancreatic cancer cells in a mouse orthotopic transplantation model. Cancer Sci. 2014, 105, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Oberst, M.D.; Johnson, M.D.; Dickson, R.B.; Lin, C.Y.; Singh, B.; Stewart, M.; Williams, A.; al-Nafussi, A.; Smyth, J.F.; Gabra, H.; et al. Expression of the serine protease matriptase and its inhibitor HAI-1 in epithelial ovarian cancer: Correlation with clinical outcome and tumor clinicopathological parameters. Clin. Cancer Res. 2002, 8, 1101–1107. [Google Scholar] [PubMed]
- Nakamura, K.; Abarzua, F.; Hongo, A.; Kodama, J.; Nasu, Y.; Kumon, H.; Hiramatsu, Y. The role of hepatocyte growth factor activator inhibitor-1 (HAI-1) as a prognostic indicator in cervical cancer. Int. J. Oncol. 2009, 35, 239–248. [Google Scholar] [CrossRef]
- Nakamura, K.; Hongo, A.; Kodama, J.; Hiramatsu, Y. The role of hepatocyte growth factor activator inhibitor (HAI)-1 and HAI-2 in endometrial cancer. Int. J. Cancer 2011, 128, 2613–2624. [Google Scholar]
- Hamasuna, R.; Kataoka, H.; Meng, J.Y.; Itoh, H.; Moriyama, T.; Wakisaka, S.; Koono, M. Reduced expression of hepatocyte growth factor activator inhibitor type-2/placental bikunin (HAI-2/PB) in human glioblastomas: Implication for anti-invasive role of HAI-2/PB in glioblastoma cells. Int. J. Cancer 2001, 93, 339–345. [Google Scholar] [CrossRef]
- Li, W.; Wang, B.E.; Moran, P.; Lipari, T.; Ganesan, R.; Corpuz, R.; Ludlam, M.J.; Gogineni, A.; Koeppen, H.; Bunting, S.; et al. Pegylated Kunitz domain inhibitor suppresses hepsin-mediated invasive tumor growth and metastasis. Cancer Res. 2009, 69, 8395–8402. [Google Scholar] [CrossRef]
- Fukushima, T.; Kawaguchi, M.; Yamasaki, M.; Tanaka, H.; Yorita, K.; Kataoka, H. Hepatocyte growth factor activator inhibitor type 1 suppresses metastatic pulmonary colonization of pancreatic carcinoma cells. Cancer Sci. 2011, 102, 407–413. [Google Scholar] [CrossRef]
- Betsunoh, H.; Mukai, S.; Akiyama, Y.; Fukushima, T.; Minamiguchi, N.; Hasui, Y.; Osada, Y.; Kataoka, H. Clinical relevance of hepsin and hepatocyte growth factor activator inhibitor type 2 expression in renal cell carcinoma. Cancer Sci. 2007, 98, 491–498. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Fukushima, T.; Takahashi, N.; Tanaka, H.; Kataoka, H. Hepatocyte growth factor activator inhibitor type 1 regulates epithelial to mesenchymal transition through membrane-bound serine proteinases. Cancer Res. 2009, 69, 1828–1835. [Google Scholar] [CrossRef] [PubMed]
- Kawaguchi, M.; Yamamoto, K.; Takeda, N.; Fukushima, T.; Yamashita, F.; Sato, K.; Kitamura, K.; Hippo, Y.; Janetka, J.W.; Kataoka, H. Hepatocyte growth factor activator inhibitor-2 stabilizes Epcam and maintains epithelial organization in the mouse intestine. Commun. Biol. 2019, 2, 11. [Google Scholar] [CrossRef] [PubMed]
- Morris, M.R.; Gentle, D.; Abdulrahman, M.; Maina, E.N.; Gupta, K.; Banks, R.E.; Wiesener, M.S.; Kishida, T.; Yao, M.; The, B.; et al. Tumor suppressor activity and epigenetic inactivation of hepatocyte growth factor activator inhibitor type 2/SPINT2 in papillary and clear cell renal cell carcinoma. Cancer Res. 2005, 65, 4598–4606. [Google Scholar] [CrossRef]
- Hwang, S.; Kim, H.E.; Min, M.; Raghunathan, R.; Panova, I.P.; Munshi, R.; Ryu, B. Epigenetic silencing of SPINT2 promotes cancer cell motility via HGF-MET pathway activation in Melanoma. J. Investig. Dermatol. 2015, 135, 2283–2291. [Google Scholar] [CrossRef]
- Yue, D.; Fan, Q.; Chen, X.; Li, F.; Wang, L.; Huang, L.; Dong, W.; Chen, X.; Zhang, Z.; Liu, J.; et al. Epigenetic inactivation of SPINT2 is associated with tumor suppressive function in esophageal squamous cell carcinoma. Exp. Cell Res. 2014, 322, 149–158. [Google Scholar] [CrossRef]
- Dong, W.; Chen, X.; Xie, J.; Sun, P.; Wu, Y. Epigenetic inactivation and tumor suppressor activity of HAI-2/SPINT2 in gastric cancer. Int. J. Cancer 2010, 127, 1526–1534. [Google Scholar] [CrossRef]
- Tsai, C.H.; Teng, C.H.; Tu, Y.T.; Cheng, T.S.; Wu, S.R.; Ko, C.J.; Shyu, H.Y.; Lan, S.W.; Huang, H.P.; Tzeng, S.F.; et al. HAI-2 suppresses the invasive growth and metastasis of prostate cancer through regulation of matriptase. Oncogene 2014, 18, 4643–4652. [Google Scholar] [CrossRef]
- Roversi, F.M.; Olalla Saad, S.T.; Machado-Neto, J.A. Serine peptidase inhibitor Kunitz type 2 (SPINT2) in cancer development and progression. Biomed. Pharmacother. 2018, 101, 278–286. [Google Scholar] [CrossRef]
- Yamamoto, K.; Kawaguchi, M.; Shimomura, T.; Izumi, A.; Konari, K.; Honda, A.; Lin, C.Y.; Johnson, M.D.; Yamashita, Y.; Fukushima, T.; et al. Hepatocyte growth factor activator inhibitor-2 (HAI-2)/SPINT2 contributes to invasive growth of oral squamous cell carcinoma cells. Oncotarget 2018, 8, 11691–11706. [Google Scholar] [CrossRef]
- Van Leenders, G.J.; Schalken, J.A. Epithelial cell differentiation in the human prostate epithelium: implications for the pathogenesis and therapy of prostate cancer. Crit. Rev. Oncol. Hematol. 2003, 46, 3–10. [Google Scholar] [CrossRef]
- Mukai, S.; Yorita, K.; Yamasaki, K.; Nagai, T.; Kamibeppu, T.; Sugie, S.; Kida, K.; Onizuka, C.; Tsukino, H.; Kamimura, T.; et al. Expression of human kallikrein 1-related peptidase 4 (KLK4) and MET phosphorylation in prostate cancer tissue: immunohistochemical analysis. Hum. Cell. 2015, 28, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Mi, J.; Hooker, E.; Balog, S.; Zeng, H.; Johnson, D.T.; He, Y.; Yu, E.J.; Wu, H.; Le, V.; Lee, D.H.; et al. Activation of hepatocyte growth factor/MET signaling initiates oncogenic transformation and enhances tumor aggressiveness in the murine prostate. J. Biol. Chem. 2018, 28, 20123–20136. [Google Scholar] [CrossRef] [PubMed]
- Klezovitch, O.; Chevillet, J.; Mirosevich, J.; Roberts, R.L.; Matusik, R.J.; Vasioukhin, V. Hepsin promotes prostate cancer progression and metastasis. Cancer Cell 2004, 6, 185–195. [Google Scholar] [CrossRef]
- Nandana, S.; Ellwood-Yen, K.; Sawyers, C.; Wills, M.; Weidow, B.; Case, T.; Vasioukhin, V.; Matusik, R. Hepsin cooperates with MYC in the progression of adenocarcinoma in a prostate cancer mouse model. Prostate 2010, 1, 591–600. [Google Scholar] [CrossRef]
- Magee, J.A.; Araki, T.; Patil, S.; Ehrig, T.; True, L.; Humphrey, P.A.; Catalona, W.J.; Watson, M.A.; Milbrandt, J. Expression profiling reveals hepsin overexpression in prostate cancer. Cancer Res. 2001, 61, 5692–5696. [Google Scholar]
- Tang, X.; Mahajan, S.S.; Nguyen, L.T.; Béliveau, F.; Leduc, R.; Simon, J.A.; Vasioukhin, V. Targeted inhibition of cell-surface serine protease Hepsin blocks prostate cancer bone metastasis. Oncotarget 2014, 5, 1352–1362. [Google Scholar] [CrossRef]
- Pal, P.; Xi, H.; Kaushal, R.; Sun, G.; Jin, C.H.; Jin, L.; Suarez, B.K.; Catalona, W.J.; Deka, R. Variants in the HEPSIN gene are associated with prostate cancer in men of European origin. Hum. Genet. 2006, 120, 187–192. [Google Scholar] [CrossRef]
- Knudsen, B.S.; Gmyrek, G.A.; Inra, J.; Scherr, D.S.; Vaughan, E.D.; Nanus, D.M.; Kattan, M.W.; Gerald, W.L.; Vande Woude, G.F. High expression of the Met receptor in prostate cancer metastasis to bone. Urology 2002, 60, 1113–1117. [Google Scholar] [CrossRef]
- Verhoef, E.I.; Kolijn, K.; De Herdt, M.J.; van der Steen, B.; Hoogland, A.M.; Sleddens, H.F.; Looijenga, L.H.; van Leenders, G.J. MET expression during prostate cancer progression. Oncotarget 2016, 24, 31029–31036. [Google Scholar] [CrossRef]
- Nakashiro, K.; Hara, S.; Shinohara, Y.; Oyasu, M.; Kawamata, H.; Shintani, S.; Hamakawa, H.; Oyasu, R. Phenotypic switch from paracrine to autocrine role of hepatocyte growth factor in an androgen-independent human prostatic carcinoma cell line, CWR22R. Am. J. Pathol. 2004, 165, 533–540. [Google Scholar] [CrossRef]
- Maeda, A.; Nakashiro, K.; Hara, S.; Sasaki, T.; Miwa, Y.; Tanji, N.; Yokoyama, M.; Hamakawa, H.; Oyasu, R. Inactivation of AR activates HGF/c-Met system in human prostatic carcinoma cells. Biochem. Biophys. Res. Commun. 2006, 8, 1158–1165. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.; De Bono, J.; Sternberg, C.; Le Moulec, S.; Oudard, S.; De Giorgi, U.; Krainer, M.; Bergman, A.; Hoelzer, W.; De Wit, R.; et al. Phase III Study of Cabozantinib in Previously Treated Metastatic Castration-Resistant Prostate Cancer: COMET-1. J. Clin. Oncol. 2016, 1, 3005–3013. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Y.; Feng, F.Y.; Wang, Y.; Cao, X.; Han, S.; Wilder-Romans, K.; Navone, N.M.; Logothetis, C.; Taichman, R.S.; Keller, E.T.; et al. Mechanistic Support for Combined MET and AR Blockade in Castration-Resistant Prostate Cancer. Neoplasia 2016, 18, 1–9. [Google Scholar] [CrossRef]
- Sanders, A.J.; Parr, C.; Davies, G.; Martin, T.A.; Lane, J.; Mason, M.D.; Jiang, W.G. Genetic reduction of matriptase-1 expression is associated with a reduction in the aggressive phenotype of prostate cancer cells in vitro and in vivo. J. Exp. Ther. Oncol. 2006, 6, 39–48. [Google Scholar]
- Warren, M.; Twohig, M.; Pier, T.; Eickhoff, J.; Lin, C.Y.; Jarrard, D.; Huang, W. Protein expression of matriptase and its cognate inhibitor HAI-1 in human prostate cancer: a tissue microarray and automated quantitative analysis. Appl. Immunohistochem. Mol. Morphol. 2009, 17, 23–30. [Google Scholar] [CrossRef]
- Wu, S.R.; Teng, C.H.; Tu, Y.T.; Ko, C.J.; Cheng, T.S.; Lan, S.W.; Lin, H.Y.; Lin, H.H.; Tu, H.F.; Hsiao, P.W.; et al. The Kunitz Domain I of Hepatocyte Growth Factor Activator Inhibitor-2 Inhibits Matriptase Activity and Invasive Ability of Human Prostate Cancer Cells. Sci. Rep. 2017, 8, 15101. [Google Scholar] [CrossRef]
- Pereira, M.S.; de Almeida, G.C.; Pinto, F.; Viana-Pereira, M.; Reis, R.M. SPINT2 deregulation in prostate carcinoma. J. Histochem. Cytochem. 2016, 64, 32–41. [Google Scholar] [CrossRef]
- Bergum, C.; List, K. Loss of the matriptase inhibitor HAI-2 during prostate cancer progression. Prostate 2010, 15, 1422–1428. [Google Scholar] [CrossRef]
- Wilson, S.; Greer, B.; Hooper, J.; Zijlstra, A.; Walker, B.; Quigley, J.; Hawthorne, S. The membrane-anchored serine protease, TMPRSS2, activates PAR-2 in prostate cancer cells. Biochem. J. 2005, 15 Pt 3, 967–972. [Google Scholar] [CrossRef]
- Matsuyama, S.; Nao, N.; Shirato, K.; Kawase, M.; Saito, S.; Takayama, I.; Nagata, N.; Sekizuka, T.; Katoh, H.; Kato, F.; et al. Enhanced isolation of SARS-CoV-2 by TMPRSS2-expressing cells. Proc. Natl. Acad. Sci. USA 2020, 12. [Google Scholar] [CrossRef] [PubMed]
- Uhlén, M.; Fagerberg, L.; Hallström, B.M.; Lindskog, C.; Oksvold, P.; Mardinoglu, A.; Sivertsson, Å.; Kampf, C.; Sjöstedt, E.; Asplund, A.; et al. The Human Protein Atlas, Tissue-based map of the human proteome. Science 2015, 347, 1260419. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.W.; Mao, H.J.; Wu, Y.L.; Tanaka, Y.; Zhang, W. TMPRSS2: A potential target for treatment of influenza virus and coronavirus infections. Biochimie 2017, 142, 1–10. [Google Scholar] [CrossRef] [PubMed]
- St John, J.; Powell, K.; Conley-Lacomb, M.K.; Chinni, S.R. TMPRSS2-ERG Fusion Gene Expression in Prostate Tumor Cells and Its Clinical and Biological Significance in Prostate Cancer Progression. J. Cancer Sci. Ther. 2012, 26, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Krumbholz, M.; Agaimy, A.; Stoehr, R.; Burger, M.; Wach, S.; Taubert, H.; Wullich, B.; Hartmann, A.; Metzler, M. Molecular Composition of Genomic TMPRSS2-ERG Rearrangements in Prostate Cancer. Dis. Markers. 2019, 5085373. [Google Scholar] [CrossRef]
- Lucas, J.M.; True, L.; Hawley, S.; Matsumura, M.; Morrissey, C.; Vessella, R.; Nelson, P.S. The androgen-regulated type II serine protease TMPRSS2 is differentially expressed and mislocalized in prostate adenocarcinoma. J. Pathol. 2008, 215, 118–125. [Google Scholar] [CrossRef]
- Lucas, J.M.; Heinlein, C.; Kim, T.; Hernandez, S.A.; Malik, M.S.; True, L.D.; Morrissey, C.; Corey, E.; Montgomery, B.; Mostaghel, E.; et al. The androgen-regulated protease TMPRSS2 activates a proteolytic cascade involving components of the tumor microenvironment and promotes prostate cancer metastasis. Cancer Discov. 2014, 4, 1310–1325. [Google Scholar] [CrossRef]
- Ko, C.J.; Huang, C.C.; Lin, H.Y.; Juan, C.P.; Lan, S.W.; Shyu, H.Y.; Wu, S.R.; Hsiao, P.W.; Huang, H.P.; Shun, C.T.; et al. Androgen-Induced TMPRSS2 Activates Matriptase and Promotes Extracellular Matrix Degradation, Prostate Cancer Cell Invasion, Tumor Growth, and Metastasis. Cancer Res. 2015, 15, 2949–2960. [Google Scholar] [CrossRef]
- Chen, S.C.; Kuo, P.L. Bone metastasis from renal cell carcinoma. Int. J. Mol. Sci. 2016, 17, E987. [Google Scholar] [CrossRef]
- Rhoades Smith, K.E.; Bilen, M.A. A Review of Papillary Renal Cell Carcinoma and MET Inhibitors. Kidney Cancer. 2019, 1, 151–161. [Google Scholar] [CrossRef]
- Joffre, C.; Barrow, R.; Ménard, L.; Calleja, V.; Hart, I.R.; Kermorgant, S. A direct role for Met endocytosis in tumorigenesis. Nat. Cell Biol. 2011, 5, 827–837. [Google Scholar] [CrossRef] [PubMed]
- Maher, E.R. Hereditary renal cell carcinoma syndromes: diagnosis, surveillance and management. World J. Urol. 2018, 36, 1891–1898. [Google Scholar] [CrossRef] [PubMed]
- Harshman, L.C.; Choueiri, T.K. Targeting the hepatocyte growth factor/c-Met signaling pathway in renal cell carcinoma. Cancer J. 2013, 19, 316–323. [Google Scholar] [CrossRef] [PubMed]
- Miyata, Y.; Kanetake, H.; Kanda, S. Presence of phosphorylated hepatocyte growth factor receptor/c-Met is associated with tumor progression and survival in patients with conventional renal cell carcinoma. Clin. Cancer Res. 2006, 15, 4876–4881. [Google Scholar] [CrossRef]
- Nandagopal, L.; Sonpavde, G.P.; Agarwal, N. Investigational MET inhibitors to treat Renal cell carcinoma. Expert Opin. Investig. Drugs 2019, 28, 851–860. [Google Scholar] [CrossRef]
- Mukai, S.; Yorita, K.; Kawagoe, Y.; Katayama, Y.; Nakahara, K.; Kamibeppu, T.; Sugie, S.; Tukino, H.; Kamoto, T.; Kataoka, H. Matriptase and MET are prominently expressed at the site of bone metastasis in renal cell carcinoma: immunohistochemical analysis. Hum. Cell 2015, 28, 44–50. [Google Scholar] [CrossRef]
- Yamasaki, K.; Mukai, S.; Sugie, S.; Nagai, T.; Nakahara, K.; Kamibeppu, T.; Sakamoto, H.; Shibasaki, N.; Terada, N.; Toda, Y.; et al. Dysregulated HAI-2 Plays an Important Role in Renal Cell Carcinoma Bone Metastasis through Ligand-Dependent MET Phosphorylation. Cancers (Basel) 2018, 8, 10. [Google Scholar] [CrossRef]
- D’Amico, L.; Belisario, D.; Migliardi, G.; Grange, C.; Bussolati, B.; D’Amelio, P.; Perera, T.; Dalmasso, E.; Dalle Carbonare, L.; Godio, L.; et al. C-met inhibition blocks bone metastasis development induced by renal cancer stem cells. Oncotarget 2016, 19, 45525–45537. [Google Scholar] [CrossRef]
- Choueiri, T.K.; Escudier, B.; Powles, T.; Tannir, N.M.; Mainwaring, P.N.; Rini, B.I.; Hammers, H.J.; Donskov, F.; Roth, B.J.; Peltola, K.; et al. Cabozantinib versus everolimus in advanced renal cell carcinoma (meteor): Final results from a randomised, open-label, phase 3 trial. Lancet Oncol 2016, 17, 917–927. [Google Scholar] [CrossRef]
- Escudier, B.; Powles, T.; Motzer, R.J.; Olencki, T.; Aren Frontera, O.; Oudard, S.; Rolland, F.; Tomczak, P.; Castellano, D.; Appleman, L.J.; et al. Cabozantinib, a new standard of care for patients with advanced renal cell carcinoma and bone metastases? Subgroup analysis of the meteor trial. J. Clin. Oncol. 2018, 36, 765–772. [Google Scholar] [CrossRef]
- Roemer, A.; Schwettmann, L.; Jung, M.; Stephan, C.; Roigas, J.; Kristiansen, G.; Loening, S.A.; Lichtinghagen, R.; Jung, K. The membrane proteases adams and hepsin are differentially expressed in renal cell carcinoma. Are they potential tumor markers? J. Urol. 2004, 172 Pt 6, 2162–2166. [Google Scholar] [CrossRef]
- Yamauchi, M.; Kataoka, H.; Itoh, H.; Seguchi, T.; Hasui, Y.; Osada, Y. Hepatocyte growth factor activator inhibitor types 1 and 2 are expressed by tubular epithelium in kidney and down-regulated in renal cell carcinoma. J. Urol. 2004, 171, 890–896. [Google Scholar] [CrossRef] [PubMed]
- Weber, K.; Doucet, M.; Kominsky, S. Renal cell carcinoma bone metastasis—Elucidating the molecular targets. Cancer Metastasis Rev. 2007, 26, 691–704. [Google Scholar] [CrossRef] [PubMed]
- Abol-Enein, H.; Kava, B.R.; Carmack, A.J. Nonurothelial cancer of the bladder. Urology 2007, 69, 93–104. [Google Scholar] [CrossRef] [PubMed]
- Parr, C.; Jiang, W.G. Expression of hepatocyte growth factor/scatter factor, its activator, inhibitors and the c-Met receptor in human cancer cells. Int. J. Oncol. 2001, 19, 857–863. [Google Scholar] [CrossRef] [PubMed]
- Miyata, Y.; Sagara, Y.; Kanda, S.; Hayashi, T.; Kanetake, H. Phosphorylated hepatocyte growth factor receptor/c-Met is associated with tumor growth and prognosis in patients with bladder cancer: correlation with matrix metalloproteinase-2 and -7 and E-cadherin. Hum. Pathol. 2009, 40, 496–504. [Google Scholar] [CrossRef]
- Yamasaki, K.; Mukai, S.; Nagai, T.; Nakahara, K.; Fujii, M.; Terada, N.; Ohno, A.; Sato, Y.; Toda, Y.; Kataoka, H.; et al. Matriptase-Induced Phosphorylation of MET is Significantly Associated with Poor Prognosis in Invasive Bladder Cancer; an Immunohistochemical Analysis. Int. J. Mol. Sci. 2018, 22, 19. [Google Scholar] [CrossRef]
- Tamatani, T.; Hattori, K.; Iyer, A.; Tamatani, K.; Oyasu, R. Hepatocyte growth factor is an invasion/migration factor of rat urothelial carcinoma cells in vitro. Carcinogenesis 1999, 20, 957–962. [Google Scholar] [CrossRef]
- Nakahara, K.; Yamasaki, K.; Nagai, T.; Fujii, M.; Akioka, T.; Takamori, H.; Terada, N.; Mukai, S.; Sato, Y.; Kamoto, T. Expression of protease activating receptor-2 (PAR-2) is positively correlated with the recurrence of non-muscle invasive bladder cancer: an immunohistochemical analysis. Res. Rep. Urol. 2019, 11, 97–104. [Google Scholar] [CrossRef]
- McNeil, B.K.; Sorbellini, M.; Grubb, R.L., 3rd; Apolo, A.; Cecchi, F.; Athauda, G.; Cohen, B.; Giubellino, A.; Simpson, H.; Agarwal, P.K.; et al. Preliminary evaluation of urinary soluble Met as a biomarker for urothelial carcinoma of the bladder. J. Transl. Med. 2014, 21, 199. [Google Scholar] [CrossRef]
- Matsumoto, K.; Umitsu, M.; De Silva, D.M.; Roy, A.; Bottaro, D.P. Hepatocyte growth factor/MET in cancer progression and biomarker discovery. Cancer Sci. 2017, 108, 296–307. [Google Scholar] [CrossRef] [PubMed]
- Shintani, T.; Kusuhara, Y.; Daizumoto, K.; Dondoo, T.O.; Yamamoto, H.; Mori, H.; Fukawa, T.; Nakatsuji, H.; Fukumori, T.; Takahashi, M.; et al. The Involvement of Hepatocyte Growth Factor-MET-Matrix Metalloproteinase 1 Signaling in Bladder Cancer Invasiveness and Proliferation. Effect of the MET Inhibitor, Cabozantinib (XL184), on Bladder Cancer Cells. Urology 2017, 101, 169–169.e7. [Google Scholar] [CrossRef] [PubMed]
- Sim, W.J.; Iyengar, P.V.; Lama, D.; Lui, S.K.L.; Ng, H.C.; Haviv-Shapira, L.; Domany, E.; Kappei, D.; Tan, T.Z.; Saei, A.; et al. c-Met activation leads to the establishment of a TGFβ-receptor regulatory network in bladder cancer progression. Nat. Commun. 2019, 25, 4349. [Google Scholar] [CrossRef] [PubMed]
- Owusu, B.Y.; Thomas, S.; Venukadasula, P.; Han, Z.; Janetka, J.W.; Galemmo, R.A., Jr.; Klampfer, L. Targeting the tumor-promoting microenvironment in MET-amplified NSCLC cells with a novel inhibitor of pro-HGF activation. Oncotarget 2017, 8, 63014–63025. [Google Scholar] [CrossRef] [PubMed]
- Pennacchietti, S.; Cazzanti, M.; Bertotti, A.; Rideout, W.M., 3rd; Han, M.; Gyuris, J.; Perera, T.; Comoglio, P.M.; Trusolino, L.; Michieli, P. Microenvironment-derived HGF overcomes genetically determined sensitivity to anti-MET drugs. Cancer Res. 2014, 15, 6598–6609. [Google Scholar] [CrossRef]
- Suda, K.; Mizuuchi, H.; Maehara, Y.; Mitsudomi, T. Acquired resistance mechanisms to tyrosine kinase inhibitors in lung cancer with activating epidermal growth factor receptor mutation--diversity, ductility, and destiny. Cancer Metastasis Rev. 2012, 31, 807–814. [Google Scholar] [CrossRef]
- Lee, Y.H.; Apolo, A.B.; Agarwal, P.K.; Bottaro, D.P. Characterization of HGF/Met Signaling in Cell Lines Derived From Urothelial Carcinoma of the Bladder. Cancers (Basel) 2014, 25, 2313–2329. [Google Scholar] [CrossRef]
- Miyata, Y.; Asai, A.; Mitsunari, K.; Matsuo, T.; Ohba, K.; Mochizuki, Y.; Sakai, H. Met in urological cancers. Cancers (Basel) 2014, 16, 2387–4203. [Google Scholar] [CrossRef]
- Han, Z.; Harris, P.K.; Jones, D.E.; Chugani, R.; Kim, T.; Agarwal, M.; Shen, W.; Wildman, S.A.; Janetka, J.W. Inhibitors of HGFA, matriptase, and hepsin serine proteases: A nonkinase strategy to block cell signaling in Cancer. ACS Med. Chem. Lett. 2014, 5, 1219–2124. [Google Scholar] [CrossRef]
- Franco, F.M.; Jones, D.E.; Harris, P.K.; Han, Z.; Wildman, S.A.; Jarvis, C.M.; Janetka, J.W. Structure-based discovery of small molecule hepsin and HGFA protease inhibitors: Evaluation of potency and selectivity derived from distinct binding pockets. Bioorg. Med. Chem. 2015, 23, 2328–2343. [Google Scholar] [CrossRef]
- Han, Z.; Harris, P.K.; Karmakar, P.; Kim, T.; Owusu, B.Y.; Wildman, S.A.; Klampfer, L.; Janetka, J.W. α-Ketobenzothiazole serine protease inhibitors of aberrant HGF/c-MET and MSP/RON kinase pathway signaling in cancer. ChemMedChem 2016, 11, 585–599. [Google Scholar] [CrossRef] [PubMed]
- Forbs, D.; Thiel, S.; Stella, M.C.; Sturzebecher, A.; Schweinitz, A.; Steinmetzer, T.; Stürzebecher, J.; Uhland, K. In vitro inhibition of matriptase prevents invasive growth of cell lines of prostate and colon carcinoma. Int. J. Oncol. 2005, 27, 1061–1070. [Google Scholar] [CrossRef] [PubMed]
- Ustach, C.V.; Huang, W.; Conley-LaComb, M.K.; Lin, C.Y.; Che, M.; Abrams, J.; Kim, H.R. A novel signaling axis of matriptase/PDGF-D/ss-PDGFR in human prostate cancer. Cancer Res 2010, 70, 9631–9640. [Google Scholar] [CrossRef] [PubMed]



| Subfamily | Protease |
|---|---|
| HAT/DESC | HAT |
| DESC1 | |
| TMPRSS 11A | |
| HAT-like 4 | |
| HAT-like 5 | |
| Hepsin/TMPRSS | Hepsin (TMPRSS1) |
| TMPRSS 2 | |
| TMPRSS 3 | |
| TMPRSS 4 | |
| TMPRSS 13 | |
| Enteropeptidase | |
| Spinesin | |
| Matriptase | Matriptase |
| Matriptase 2 | |
| Matriptase 3 | |
| Polyserase | |
| Corin | Corin |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mukai, S.; Yamasaki, K.; Fujii, M.; Nagai, T.; Terada, N.; Kataoka, H.; Kamoto, T. Dysregulation of Type II Transmembrane Serine Proteases and Ligand-Dependent Activation of MET in Urological Cancers. Int. J. Mol. Sci. 2020, 21, 2663. https://doi.org/10.3390/ijms21082663
Mukai S, Yamasaki K, Fujii M, Nagai T, Terada N, Kataoka H, Kamoto T. Dysregulation of Type II Transmembrane Serine Proteases and Ligand-Dependent Activation of MET in Urological Cancers. International Journal of Molecular Sciences. 2020; 21(8):2663. https://doi.org/10.3390/ijms21082663
Chicago/Turabian StyleMukai, Shoichiro, Koji Yamasaki, Masato Fujii, Takahiro Nagai, Naoki Terada, Hiroaki Kataoka, and Toshiyuki Kamoto. 2020. "Dysregulation of Type II Transmembrane Serine Proteases and Ligand-Dependent Activation of MET in Urological Cancers" International Journal of Molecular Sciences 21, no. 8: 2663. https://doi.org/10.3390/ijms21082663
APA StyleMukai, S., Yamasaki, K., Fujii, M., Nagai, T., Terada, N., Kataoka, H., & Kamoto, T. (2020). Dysregulation of Type II Transmembrane Serine Proteases and Ligand-Dependent Activation of MET in Urological Cancers. International Journal of Molecular Sciences, 21(8), 2663. https://doi.org/10.3390/ijms21082663





