Emerging Roles and Potential Applications of Non-Coding RNAs in Glioblastoma
Abstract
1. Introduction
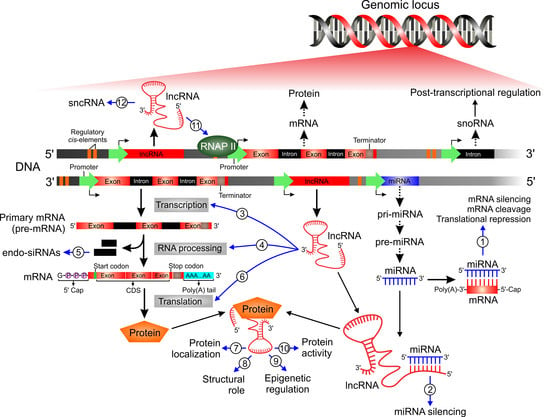
2. Classification and Biogenesis of ncRNAs
2.1. Classification
2.2. Biogenesis
3. Functional Roles and Mechanisms of Action of ncRNAs
3.1. Biological Function
- (i)
- Direct transcriptional regulation;
- (ii)
- Regulation of RNA processing events such as splicing, editing, subcellular localization, translation, and turnover/degradation;
- (iii)
- Chromatin modification;
- (iv)
- Regulation of genomic imprinting;
- (v)
- Post-translational regulation of protein activity;
- (vi)
- Facilitation of ribonucleoprotein (RNP) complex formation;
- (vii)
- Modulation of miRNA expression;
- (viii)
- Gene silencing through production of endogenous siRNA (endo-siRNA).
3.2. Mechanisms of Action
- (i)
- Signal: functions as a molecular signal inducing transcriptional activity. LncRNAs display tissue-specific expression and respond to different cellular stimuli, highlighting that their expression is highly controlled at the transcriptional level. They may act as molecular signals, since their transcription occurs in an orchestrated manner and depends on their subcellular location, allowing integration of responses to the different stimuli received;
- (ii)
- Decoy: binds to and titrates away other regulatory RNAs (e.g., miRNAs) or proteins (e.g., transcription factors). LncRNAs can act as a molecular sponge for RNA-binding proteins, such as chromatin remodelers, transcription factors or other regulatory factors. This mechanism plays a central role in both positive and negative transcription regulation by lncRNAs;
- (iii)
- Guide: directs the localization of ribonucleoprotein (RNP) complexes to specific targets (e.g., chromatin modification enzymes are recruited to promoter regions of the DNA). LncRNAs act as guides, directly binding to proteins and thus altering the location of RNPs to target regions, leading to changes in the pattern of gene expression. The regulatory components triggered by the lncRNAs include both repressive and activating complexes, as well as transcription factors;
- (iv)
- Scaffold: has a structural role as a platform upon which relevant molecular components (proteins and/or RNA) can be assembled into a complex. Molecular components can be assembled on lncRNAs, which can thus serve as a central platform, which will lead to transcriptional activation or repression. LncRNAs may bind to multiple effector partners forming complex scaffolding. In this network of interactions, these lncRNAs are responsible for addressing proteins to specific sites in the genome, thereby activating or repressing gene expression;
- (v)
- Enhancer: controls higher-order chromosomal looping in an enhancer-like model. In this functional archetype context, the levels of enhancer ncRNA (eRNA) positively correlate with the levels of messenger RNA synthesis, which are regulated by these lncRNAs, suggesting a ‘promoter-like’ role in gene expression control. The eRNAs act by recruiting the RNAP II to the promoter region.
4. Expression and Function of ncRNAs in Glioblastoma
4.1. Deregulated miRNAs in Glioblastoma Onset/Progression
4.1.1. Oncogenic miRNAs
hsa-miR-21
hsa-miR-10b
hsa-miR-221/222
4.1.2. Tumor Suppressor miRNAs
hsa-miR-128
hsa-miR-181b
hsa-miR-137
4.2. Deregulated LncRNAs in Glioblastoma Onset/Progression
4.2.1. Oncogenic LncRNAs
MALAT1
MEG3
HOTAIR
H19
NEAT1
XIST
4.2.2. Tumor Suppressor LncRNAs
RAMP2-AS1
CASC2
5. Innovative Clinical Applications of ncRNAs for Glioblastoma
6. Concluding Remarks
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| BAX | BCL2 associated X |
| BCL-2 | B-cell lymphoma 2 |
| Bcl-xL | B-cell lymphoma-extra large |
| BCNU | Carmustine |
| BMI-1 | BMI1 proto-oncogene, polycomb ring finger |
| BTB | Blood–tumor barrier |
| CASC2 | Cancer susceptibility candidate 2 |
| ceRNA | Competitor of endogenous RNA |
| CRNDE | Colorectal neoplasia differentially expressed |
| DNMT1 | DNA (cytosine-5)-methyltransferase 1 |
| EGFR | Epidermal growth factor receptor |
| ENCODE | Encyclopedia of DNA elements |
| eRNA | Enhancer RNA |
| exRNA | Extracellular RNA |
| EZH2 | Enhancer of zeste 2 polycomb repressive complex 2 subunit |
| FGF1 | Fibroblast growth factor 1 |
| FOXC1 | Forkhead box C1 |
| GSCs | Glioblastoma stem cells |
| GSK3B | Glycogen synthase kinase 3 beta |
| Gtl2 | Gene trap locus 2 |
| H19 | H19 imprinted maternally expressed transcript |
| HER2 | Human epidermal growth factor receptor 2 |
| HES1 | Hes family BHLH transcription factor 1 |
| Hif-1α | Hypoxia inducible factor 1 subunit alpha |
| HNPRK | Heterogeneous nuclear ribonucleoprotein K |
| HOTAIR | HOX transcript antisense RNA |
| HOXC | Homeobox C |
| HOXD10 | Homeobox D10 |
| IGF2 | Insulin-like growth factor 2 |
| lincRNA | Long intergenic RNA |
| lncRNA | Long non-coding RNA |
| LSD1 | Histone demethylase 1 |
| MALAT1 | Metastasis associated lung adenocarcinoma transcript 1 |
| MBNL | Muscleblind like splicing regulator |
| MDM2 | MDM2 proto-oncogene |
| MEG3 | Maternally expressed gene 3 |
| miRNA | MicroRNA |
| MMP | Matrix metalloprotease |
| mTOR | Mechanistic target of rapamycin kinase |
| NATs | Natural antisense transcripts |
| ncRNA | Non-coding RNA |
| NEAT1 | Nuclear paraspeckles assembly transcript 1 |
| NF-kB | Nuclear factor-kappa B subunit 1 |
| NKD1 | NKD inhibitor of WNT signaling pathway 1 |
| NONO/P54nrb | Non-POU domain containing octamer binding |
| NOTCH3 | Notch receptor 3 |
| NSCLC | Non-small cell lung cancer |
| ORF | Open reading frame |
| p70S6K1 | Ribosomal protein S6 kinase beta-1 |
| PDCD4 | Programmed cell death 4 |
| PIK3R1 | Phosphoinositide-3-kinase regulatory subunit 1 |
| piRNA | Piwi-interacting RNA |
| PRC2 | Polycomb repressive complex 2 |
| PTEN | Phosphatase and tensin homolog |
| PTPμ | Tyrosine phosphatase μ |
| PUMA | p53-upregulated modulator of apoptosis |
| RAC1 | Rac family small GTPase 1 |
| RECK | Reversion inducing cysteine rich protein with kazal motifs |
| RNAP II | RNA polymerase II |
| RNP | Ribonucleoprotein |
| rRNA | Ribosomal RNA |
| SART3 | Spliceosome associated factor 3, U4/U6 recycling protein |
| scaRNA | Cajal body-specific RNA |
| siRNA | Short interfering RNA |
| siRNA | Small interfering RNA |
| snoRNA | Small nucleolar RNA |
| snRNA | Small nuclear RNA |
| SOX2 | SRY-Box transcription factor 2 |
| SRSF2 | Serine and arginine rich splicing factor 2 |
| STAT3 | Signal transducer and activator of transcription 3 |
| SUZ12 | SUZ12 polycomb repressive complex 2 subunit |
| TAP63 | Tumor suppressor homolog of p53 |
| TCGA | The Cancer Genome Atlas |
| TIMP3 | TIMP metallopeptidase inhibitor 3 |
| TMZ | Temozolomide |
| tRNA | Transfer RNA |
| TS | Thymidylate synthase |
| UTR | Untranslated region |
| VEGF | Vascular endothelial growth factor A |
| WHO | World Health Organization |
| XIC | X inactivation center |
| XIST | X-inactive specific transcript |
| ZHX1 | Zinc fingers and homeoboxes 1 |
| ZO-2 | Zonula occludens 2 |
References
- Crick, F.H. On protein synthesis. Symp. Soc. Exp. Biol. 1958, 12, 138–163. [Google Scholar]
- Djebali, S.; Davis, C.A.; Merkel, A.; Dobin, A.; Lassmann, T.; Mortazavi, A.M.; Tanzer, A.; Lagarde, J.; Lin, W.; Schlesinger, F.; et al. Landscape of transcription in human cells. Nature 2012, 489, 101–108. [Google Scholar] [CrossRef]
- Forrest, A.R.R.; Kawaji, H.; Rehli, M.; Baillie, J.K.; De Hoon, M.J.L.; Haberle, V.; Lassmann, T.; Kulakovskiy, I.V. A promoter-level mammalian expression atlas. Nature 2014, 507, 462–470. [Google Scholar]
- Lonsdale, J.; Thomas, J.; Salvatore, M.; Phillips, R.; Lo, E.; Shad, S.; Hasz, R.; Walters, G.; García, F.; Young, N.; et al. The Genotype-Tissue Expression (GTEx) project. Nat. Genet. 2013, 45, 580–585. [Google Scholar] [CrossRef]
- The ENCODE Project Consortium. An integrated encyclopedia of DNA elements in the human genome. Nature 2012, 489, 57–74. [Google Scholar] [CrossRef]
- Bolha, L.; Ravnik-Glavač, M.; Glavač, D. Long Noncoding RNAs as Biomarkers in Cancer. Dis. Markers 2017, 2017, 7243968. [Google Scholar] [CrossRef]
- Reis, E.; Verjovski-Almeida, S. Perspectives of Long Non-Coding RNAs in Cancer Diagnostics. Front. Genet. 2012, 3, 32. [Google Scholar] [CrossRef]
- Slack, F.J.; Chinnaiyan, A.M. The Role of Non-coding RNAs in Oncology. Cell 2019, 179, 1033–1055. [Google Scholar] [CrossRef]
- Thakkar, J.P.; Dolecek, T.A.; Horbinski, C.; Ostrom, Q.T.; Lightner, D.D.; Barnholtz-Sloan, J.S.; Villano, J.L. Epidemiologic and molecular prognostic review of glioblastoma. Cancer Epidemiol. Biomark. Prev. 2014, 23, 1985–1996. [Google Scholar] [CrossRef]
- Glaser, T.; Han, I.; Wu, L.; Zeng, X. Targeted Nanotechnology in Glioblastoma Multiforme. Front. Pharmacol. 2017, 8, 3–9. [Google Scholar] [CrossRef]
- Reifenberger, G.; Wirsching, H.-G.; Knobbe-Thomsen, C.B.; Weller, M. Advances in the molecular genetics of gliomas—implications for classification and therapy. Nat. Rev. Clin. Oncol. 2016, 14, 434–452. [Google Scholar] [CrossRef]
- Louis, D.N.; Perry, A.; Reifenberger, G.; Von Deimling, A.; Figarella-Branger, M.; Cavenee, W.K.; Ohgaki, H.; Wiestler, O.D.; Kleihues, P.; Ellison, D.W. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: A summary. Acta Neuropathol. 2016, 131, 803–820. [Google Scholar] [CrossRef] [PubMed]
- Weller, M.; Le Rhun, E.; Preusser, M.; Tonn, J.-C.; Weller, M. How we treat glioblastoma. ESMO Open 2019, 4, e000520. [Google Scholar] [CrossRef]
- Teng, K.-Y.; Ghoshal, K. Role of Noncoding RNAs as Biomarker and Therapeutic Targets for Liver Fibrosis. Gene Expr. 2015, 16, 155–162. [Google Scholar] [CrossRef]
- Viereck, J.; Thum, T. Circulating Noncoding RNAs as Biomarkers of Cardiovascular Disease and Injury. Circ. Res. 2017, 120, 381–399. [Google Scholar] [CrossRef]
- Zhang, G.; Pian, C.; Chen, Z.; Zhang, J.; Xu, M.; Zhang, L.-Y.; Chen, Y. Identification of cancer-related miRNA-lncRNA biomarkers using a basic miRNA-lncRNA network. PLoS ONE 2018, 13, e0196681. [Google Scholar] [CrossRef]
- Gibb, E.A.; Brown, C.J.; Lam, W.L. The functional role of long non-coding RNA in human carcinomas. Mol. Cancer 2011, 10, 38. [Google Scholar] [CrossRef]
- Kelemen, E.; Danis, J.; Göblös, A.; Bata-Csörgő, Z.; Széll, M. Exosomal long non-coding RNAs as biomarkers in human diseases. EJIFCC 2019, 30, 224–236. [Google Scholar]
- Deocesano-Pereira, C.; Machado, R.A.C.; De Jesus-Ferreira, H.C.; Marchini, T.; Pereira, T.F.; Carreira, A.C.O.; Sogayar, M.C. Functional impact of the long non-coding RNA MEG3 deletion by CRISPR/Cas9 in the human triple negative metastatic Hs578T cancer cell line. Oncol. Lett. 2019, 18, 5941–5951. [Google Scholar] [CrossRef]
- Arun, G.; Diermeier, S.; Spector, D.L. Therapeutic Targeting of Long Non-Coding RNAs in Cancer. Trends Mol. Med. 2018, 24, 257–277. [Google Scholar] [CrossRef]
- Slaby, O.; Laga, R.; Sedlacek, O. Therapeutic targeting of non-coding RNAs in cancer. Biochem. J. 2017, 474, 4219–4251. [Google Scholar] [CrossRef] [PubMed]
- Adams, B.D.; Parsons, C.; Walker, L.; Zhang, W.C.; Slack, F.J. Targeting noncoding RNAs in disease. J. Clin. Investig. 2017, 127, 761–771. [Google Scholar] [CrossRef] [PubMed]
- Matsui, M.; Corey, D.R. Non-coding RNAs as drug targets. Nat. Rev. Drug Discov. 2016, 16, 167–179. [Google Scholar] [CrossRef] [PubMed]
- Laurent, G.S.; Wahlestedt, C.; Kapranov, P. The Landscape of long noncoding RNA classification. Trends Genet. 2015, 31, 239–251. [Google Scholar] [CrossRef] [PubMed]
- Erhard, F.; Zimmer, R. Classification of ncRNAs using position and size information in deep sequencing data. Bioinformatics 2010, 26, i426–i432. [Google Scholar] [CrossRef]
- Kopp, F.; Mendell, J.T. Functional Classification and Experimental Dissection of Long Noncoding RNAs. Cell 2018, 172, 393–407. [Google Scholar] [CrossRef]
- Panwar, B.; Arora, A.; Raghava, G.P.S. Prediction and classification of ncRNAs using structural information. BMC Genom. 2014, 15, 127. [Google Scholar] [CrossRef]
- Derrien, T.; Johnson, R.; Bussotti, G.; Tanzer, A.; Djebali, S.; Tilgner, H.; Guernec, G.; Martín, D.; Merkel, A.; Knowles, D.G.; et al. The GENCODE v7 catalog of human long noncoding RNAs: Analysis of their gene structure, evolution, and expression. Genome Res. 2012, 22, 1775–1789. [Google Scholar] [CrossRef]
- Latgé, G.; Poulet, C.; Bours, V.; Josse, C.; Jérusalem, G. Natural Antisense Transcripts: Molecular Mechanisms and Implications in Breast Cancers. Int. J. Mol. Sci. 2018, 19, 123. [Google Scholar] [CrossRef]
- Ulitsky, I.; Bartel, B. lincRNAs: Genomics, evolution, and mechanisms. Cell 2013, 154, 26–46. [Google Scholar] [CrossRef]
- Faghihi, M.A.; Wahlestedt, C. Regulatory roles of natural antisense transcripts. Nat. Rev. Mol. Cell Boil. 2009, 10, 637–643. [Google Scholar] [CrossRef]
- Guttman, M.; Amit, I.; Garber, M.; French, C.; Lin, M.F.; Feldser, D.M.; Huarte, M.; Zuk, O.; Carey, B.W.; Cassady, J.P.; et al. Chromatin signature reveals over a thousand highly conserved large non-coding RNAs in mammals. Nature 2009, 458, 223–227. [Google Scholar] [CrossRef] [PubMed]
- Ayupe, A.C.; Tahira, A.C.; Camargo, L.; Beckedorff, F.C.; Verjovski-Almeida, S.; Reis, E. Global analysis of biogenesis, stability and sub-cellular localization of lncRNAs mapping to intragenic regions of the human genome. RNA Boil. 2015, 12, 877–892. [Google Scholar] [CrossRef] [PubMed]
- Rashid, F.; Shah, A.; Shan, G. Long Non-coding RNAs in the Cytoplasm. Genom. Proteom. Bioinform. 2016, 14, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Shi, Z.-M.; Chang, Y.-N.; Hu, Z.-M.; Qi, H.-X.; Hong, W. The ways of action of long non-coding RNAs in cytoplasm and nucleus. Gene 2014, 547, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Clark, M.; Choudhary, A.; Smith, M.A.; Taft, R.J.; Mattick, J. The dark matter rises: The expanding world of regulatory RNAs. Essays Biochem. 2013, 54, 1–16. [Google Scholar] [PubMed]
- Morris, K.V.; Mattick, J. The rise of regulatory RNA. Nat. Rev. Genet. 2014, 15, 423–437. [Google Scholar] [CrossRef]
- O’Brien, J.; Hayder, H.; Zayed, Y.; Peng, C. Overview of MicroRNA Biogenesis, Mechanisms of Actions, and Circulation. Front. Endocrinol. 2018, 9, 402. [Google Scholar] [CrossRef]
- Yin, S.; Yu, Y.; Reed, R. Primary microRNA processing is functionally coupled to RNAP II transcription in vitro. Sci. Rep. 2015, 5, 11992. [Google Scholar] [CrossRef]
- Lee, D.K.; Shin, C. Emerging roles of DROSHA beyond primary microRNA processing. RNA Boil. 2017, 15, 186–193. [Google Scholar] [CrossRef]
- Hu, S.; Wu, J.; Chen, L.; Shan, G. Signals from noncoding RNAs: Unconventional roles for conventional pol III transcripts. Int. J. Biochem. Cell Boil. 2012, 44, 1847–1851. [Google Scholar] [CrossRef] [PubMed]
- Batista, P.J.; Chang, H.Y. Long noncoding RNAs: Cellular address codes in development and disease. Cell 2013, 152, 1298–1307. [Google Scholar] [CrossRef] [PubMed]
- Moran, V.A.; Perera, R.J.; Khalil, A.M. Emerging functional and mechanistic paradigms of mammalian long non-coding RNAs. Nucleic Acids Res. 2012, 40, 6391–6400. [Google Scholar] [CrossRef] [PubMed]
- Wilusz, J.E.; Freier, S.M.; Spector, D.L. 3′ End Processing of a Long Nuclear-Retained Noncoding RNA Yields a tRNA-like Cytoplasmic RNA. Cell 2008, 135, 919–932. [Google Scholar] [CrossRef]
- Yang, L.; Duff, M.O.; Graveley, B.R.; Carmichael, G.G.; Chen, L.L. Genomewide characterization of non-polyadenylated RNAs. Genome Boil. 2011, 12, R16. [Google Scholar] [CrossRef]
- Mattioli, K.; Volders, P.-J.; Gerhardinger, C.; Lee, J.C.; Maass, P.G.; Melé, M.; Rinn, J.L. High-throughput functional analysis of lncRNA core promoters elucidates rules governing tissue specificity. Genome Res. 2019, 29, 344–355. [Google Scholar] [CrossRef] [PubMed]
- Kern, C.; Wang, Y.; Chitwood, J.; Korf, I.; Delany, M.E.; Cheng, H.G.; Medrano, J.F.; Van Eenennaam, A.L.; Ernst, C.; Ross, P.J.; et al. Genome-wide identification of tissue-specific long non-coding RNA in three farm animal species. BMC Genom. 2018, 19, 684. [Google Scholar] [CrossRef]
- Rinn, J.L.; Chang, H.Y. Genome regulation by long noncoding RNAs. Annu. Rev. Biochem. 2012, 81, 145–166. [Google Scholar] [CrossRef]
- Dhanoa, J.K.; Sethi, R.S.; Verma, R.; Arora, J.S.; Mukhopadhyay, C.S. Long non-coding RNA: Its evolutionary relics and biological implications in mammals: A review. J. Anim. Sci. Technol. 2018, 60, 25. [Google Scholar] [CrossRef]
- Tsai, M.-C.; Spitale, R.C.; Chang, H.Y. Long intergenic noncoding RNAs: New links in cancer progression. Cancer Res. 2011, 71, 3–7. [Google Scholar] [CrossRef]
- Fang, Y.; Fullwood, M.J. Roles, Functions, and Mechanisms of Long Non-coding RNAs in Cancer. Genom. Proteom. Bioinform. 2016, 14, 42–54. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.C.; Chang, H.Y. Molecular Mechanisms of Long Noncoding RNAs. Mol. Cell 2011, 43, 904–914. [Google Scholar] [CrossRef] [PubMed]
- Anastasiadou, E.; Jacob, L.S.; Slack, F.J. Non-coding RNA networks in cancer. Nat. Rev. Cancer 2017, 18, 5–18. [Google Scholar] [CrossRef] [PubMed]
- Møller, H.G.; Rasmussen, A.P.; Andersen, H.H.; Johnsen, K.B.; Henriksen, M.; Duroux, M. A Systematic Review of MicroRNA in Glioblastoma Multiforme: Micro-modulators in the Mesenchymal Mode of Migration and Invasion. Mol. Neurobiol. 2012, 47, 131–144. [Google Scholar] [CrossRef] [PubMed]
- Henriksen, M.; Johnsen, K.B.; Andersen, H.H.; Pilgaard, L.; Duroux, M. MicroRNA expression signatures determine prognosis and survival in glioblastoma multiforme—A systematic overview. Mol. Neurobiol. 2014, 50, 896–913. [Google Scholar] [CrossRef]
- Toraih, E.A.; El-Wazir, A.; Abdallah, H.Y.; Tantawy, M.A.; Fawzy, M.S. Deregulated MicroRNA Signature Following Glioblastoma Irradiation. Cancer Control. 2019, 26, 1073274819847226. [Google Scholar] [CrossRef]
- Sana, J.; Busek, P.; Fadrus, P.; Besse, A.; Radova, L.; Vecera, M.; Reguli, S.; Sromova, L.S.; Hilser, M.; Lipina, R.; et al. Identification of microRNAs differentially expressed in glioblastoma stem-like cells and their association with patient survival. Sci. Rep. 2018, 8, 2836. [Google Scholar] [CrossRef]
- Volinia, S.; Calin, G.A.; Liu, C.-G.; Ambs, S.; Cimmino, A.; Petrocca, F.; Visone, R.; Iorio, M.; Roldo, C.; Ferracin, M.; et al. A microRNA expression signature of human solid tumors defines cancer gene targets. Proc. Natl. Acad. Sci. USA 2006, 103, 2257–2261. [Google Scholar] [CrossRef]
- Chan, J.A. MicroRNA-21 Is an Antiapoptotic Factor in Human Glioblastoma Cells. Cancer Res. 2005, 65, 6029–6033. [Google Scholar] [CrossRef]
- Yang, C.H.; Yue, J.; Pfeffer, S.R.; Fan, M.; Paulus, E.; Hosni-Ahmed, A.; Sims, M.; Qayyum, S.; Davidoff, A.M.; Handorf, C.R.; et al. MicroRNA-21 Promotes Glioblastoma Tumorigenesis by Down-regulating Insulin-like Growth Factor-binding Protein-3 (IGFBP3). J. Boil. Chem. 2014, 289, 25079–25087. [Google Scholar] [CrossRef]
- Buscaglia, L.E.B.; Li, Y. Apoptosis and the target genes of microRNA-21. Chin. J. Cancer 2011, 30, 371–380. [Google Scholar] [CrossRef] [PubMed]
- Papagiannakopoulos, T.; Shapiro, A.; Kosik, K.S. MicroRNA-21 Targets a Network of Key Tumor-Suppressive Pathways in Glioblastoma Cells. Cancer Res. 2008, 68, 8164–8172. [Google Scholar] [CrossRef] [PubMed]
- Chao, T.-F.; Xiong, H.-H.; Liu, W.; Chen, Y.; Zhang, J.-X. MiR-21 mediates the radiation resistance of glioblastoma cells by regulating PDCD4 and hMSH2. Acta Acad. Med. Wuhan 2013, 33, 525–529. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Chen, J.; Yang, J.; Pan, T.; Zhang, S.; Wang, Z. MiR-21 protected human glioblastoma U87MG cells from chemotherapeutic drug temozolomide induced apoptosis by decreasing Bax/Bcl-2 ratio and caspase-3 activity. Brain Res. 2010, 1352, 255–264. [Google Scholar] [CrossRef]
- Da Costa, P.M.C.; Cardoso, A.L.; Nóbrega, C.; De Almeida, L.P.; Bruce, J.N.; Canoll, P.; De Lima, M.C.P. MicroRNA-21 silencing enhances the cytotoxic effect of the antiangiogenic drug sunitinib in glioblastoma. Hum. Mol. Genet. 2012, 22, 904–918. [Google Scholar] [CrossRef]
- Wang, G.B. MiR-21 enhanced glioma cells resistance to carmustine via decreasing Spry2 expression. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 5065–5071. [Google Scholar]
- Wong, S.T.S.; Zhang, X.-Q.; Zhuang, J.T.-F.; Chan, H.-L.; Li, C.-H.; Leung, G.K.K. MicroRNA-21 inhibition enhances in vitro chemosensitivity of temozolomide-resistant glioblastoma cells. Anticancer. Res. 2012, 32, 2835–2841. [Google Scholar]
- Gabriely, G.; Wurdinger, T.; Kesari, S.; Esau, C.C.; Burchard, J.; Linsley, P.S.; Krichevsky, A.M. MicroRNA 21 Promotes Glioma Invasion by Targeting Matrix Metalloproteinase Regulators. Mol. Cell. Boil. 2008, 28, 5369–5380. [Google Scholar] [CrossRef]
- Gabriely, G.; Yi, M.; Narayan, R.S.; Niers, J.M.; Wurdinger, T.; Imitola, J.; Ligon, K.L.; Kesari, S.; Esau, C.; Stephens, R.M.; et al. Human glioma growth is controlled by microRNA-10b. Cancer Res. 2011, 71, 3563–3572. [Google Scholar] [CrossRef]
- Guessous, F.; Alvarado-Velez, M.; Marcinkiewicz, L.; Zhang, Y.; Kim, J.; Heister, S.; Kefas, B.; Godlewski, J.; Schiff, D.; Purow, B.; et al. Oncogenic effects of miR-10b in glioblastoma stem cells. J. Neuro-Oncology 2013, 112, 153–163. [Google Scholar] [CrossRef]
- Sun, L.; Yan, W.; Wang, Y.; Sun, G.; Luo, H.; Zhang, J.; Wang, X.; You, Y.; Yang, Z.; Liu, N. MicroRNA-10b induces glioma cell invasion by modulating MMP-14 and uPAR expression via HOXD10. Brain Res. 2011, 1389, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Teo, S.; Lam, D.H.; Jeyaseelan, K.; Wang, S. MicroRNA-10b pleiotropically regulates invasion, angiogenicity and apoptosis of tumor cells resembling mesenchymal subtype of glioblastoma multiforme. Cell Death Dis. 2012, 3, e398. [Google Scholar] [CrossRef] [PubMed]
- Teplyuk, N.M.; Uhlmann, E.J.; Gabriely, G.; Volfovsky, N.; Wang, Y.; Teng, J.; Karmali, P.; Marcusson, E.; Peter, M.; Mohan, A.; et al. Therapeutic potential of targeting micro RNA -10b in established intracranial glioblastoma: First steps toward the clinic. EMBO Mol. Med. 2016, 8, 268–287. [Google Scholar] [CrossRef] [PubMed]
- El Fatimy, R.; Subramanian, S.; Uhlmann, E.J.; Krichevsky, A.M. Genome Editing Reveals Glioblastoma Addiction to MicroRNA-10b. Mol. Ther. 2017, 25, 368–378. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, J.; Hao, J.; Shi, Z.; Wang, Y.; Han, L.; Yu, S.; You, Y.; Jiang, T.; Wang, J.; et al. High level of miR-221/222 confers increased cell invasion and poor prognosis in glioma. J. Transl. Med. 2012, 10, 119. [Google Scholar] [CrossRef]
- Zhang, R.; Pang, B.; Xin, T.; Guo, H.; Xing, Y.; Xu, S.; Feng, B.; Liu, B.; Pang, Q. Plasma miR-221/222 Family as Novel Descriptive and Prognostic Biomarkers for Glioma. Mol. Neurobiol. 2016, 53, 1452–1460. [Google Scholar] [CrossRef]
- Hao, J.; Zhang, C.; Zhang, A.; Wang, K.; Jia, Z.; Wang, G.; Han, L.; Kang, C.; Pu, P. miR-221/222 is the regulator of Cx43 expression in human glioblastoma cells. Oncol. Rep. 2012, 27, 1504–1510. [Google Scholar]
- Medina, R.; Zaidi, S.K.; Liu, C.-G.; Stein, J.L.; Vanwijnen, A.J.; Croce, C.M.; Stein, G.S. MicroRNAs 221 and 222 bypass quiescence and compromise cell survival. Cancer Res. 2008, 68, 2773–2780. [Google Scholar] [CrossRef]
- Quintavalle, C.; Garofalo, M.; Zanca, C.; Romano, G.; Iaboni, M.; del Basso De Caro, M.; Martinez-Montero, J.C.; Incoronato, M.; Nuovo, G.; Croce, C.M.; et al. miR-221/222 overexpession in human glioblastoma increases invasiveness by targeting the protein phosphate PTPmu. Oncogene 2012, 31, 858–868. [Google Scholar] [CrossRef]
- Zhang, C.-Z.; Kang, C.; Pu, P.; Wang, G.-X.; Jia, Z.; Zhang, A.-L.; Han, L.; Xu, P. Inhibitory effect of knocking down microRNA-221 and microRNA-222 on glioma cell growth in vitro and in vivo. Zhonghua zhong liu za zhi Chinese J. Oncol. 2009, 31, 721–726. [Google Scholar]
- Zhang, C.; Zhang, J.; Zhang, A.-L.; Shi, Z.; Han, L.; Jia, Z.; Yang, W.-D.; Wang, G.-X.; Jiang, T.; You, Y.; et al. MiR-221 and miR-222 target PUMA to induce cell survival in glioblastoma. Mol. Cancer 2010, 9, 229. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zhang, J.; Han, L.; Zhang, A.; Zhang, C.; Zheng, Y.; Jiang, T.; Pu, P.; Jiang, C.; Kang, C. Downregulation of miR-221/222 sensitizes glioma cells to temozolomide by regulating apoptosis independently of p53 status. Oncol. Rep. 2012, 27, 854–860. [Google Scholar]
- Quintavalle, C.; Mangani, D.; Roscigno, G.; Romano, G.; Diaz-Lagares, A.; Iaboni, M.; Donnarumma, E.; Fiore, D.; De Marinis, P.; Soini, Y.; et al. MiR-221/222 target the DNA methyltransferase MGMT in glioma cells. PLoS ONE 2013, 8, e74466. [Google Scholar]
- Li, W.; Guo, F.; Wang, P.; Hong, S.; Zhang, C. miR-221/222 confers radioresistance in glioblastoma cells through activating Akt independent of PTEN status. Curr. Mol. Med. 2014, 14, 185–195. [Google Scholar] [CrossRef] [PubMed]
- Huse, J.T.; Brennan, C.; Hambardzumyan, L.; Wee, B.; Pena, J.; Rouhanifard, S.; Sohn-Lee, C.; Le Sage, C.; Agami, R.; Tuschl, T.; et al. The PTEN-regulating microRNA miR-26a is amplified in high-grade glioma and facilitates gliomagenesis in vivo. Genome Res. 2009, 23, 1327–1337. [Google Scholar] [CrossRef] [PubMed]
- Ernst, A.; Campos, B.; Meier, J.; Devens, F.; Liesenberg, F.; Wolter, M.; Reifenberger, G.; Herold-Mende, C.; Lichter, P.; Radlwimmer, B. De-repression of CTGF via the miR-17-92 cluster upon differentiation of human glioblastoma spheroid cultures. Oncogene 2010, 29, 3411–3422. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Jia, Z.; Li, B.; Zhang, A.; Wang, G.; Pu, P.; Chen, Z.; Wang, Z.; Yang, W. MiR-19 regulates the proliferation and invasion of glioma by RUNX3 via beta-catenin/Tcf-4 signaling. Oncotarget 2017, 8, 110785–110796. [Google Scholar] [CrossRef]
- Chen, R.; Liu, H.; Cheng, Q.; Jiang, B.; Peng, R.; Zou, Q.; Yang, W.; Yang, X.; Wu, X.; Chen, Z. MicroRNA-93 promotes the malignant phenotypes of human glioma cells and induces their chemoresistance to temozolomide. Boil. Open 2016, 5, 669–677. [Google Scholar] [CrossRef]
- Zhao, W.-Y.; Wang, Y.; An, Z.-J.; Shi, C.-G.; Zhu, G.-A.; Wang, B.; Lu, M.-Y.; Pan, C.-K.; Chen, P. Downregulation of miR-497 promotes tumor growth and angiogenesis by targeting HDGF in non-small cell lung cancer. Biochem. Biophys. Res. Commun. 2013, 435, 466–471. [Google Scholar] [CrossRef]
- Fang, L.; Deng, Z.; Shatseva, T.; Yang, J.; Peng, C.; Du, W.W.; Yee, A.J.; Ang, L.C.; He, C.; Shan, S.W.; et al. MicroRNA miR-93 promotes tumor growth and angiogenesis by targeting integrin-beta8. Oncogene 2011, 30, 806–821. [Google Scholar] [CrossRef] [PubMed]
- Dews, M.; Fox, J.L.; Hultine, S.; Sundaram, P.; Wang, W.; Liu, Y.Y.; Furth, E.; Enders, G.H.; El-Deiry, W.; Schelter, J.M.; et al. The myc-miR-17~92 axis blunts TGF{beta} signaling and production of multiple TGF{beta}-dependent antiangiogenic factors. Cancer Res. 2010, 70, 8233–8246. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.; Chen, W.; Wang, J. MicroRNA-20a Regulates Glioma Cell Proliferation, Invasion, and Apoptosis by Targeting CUGBP Elav-Like Family Member 2. World Neurosurg. 2019, 121, e519–e527. [Google Scholar] [CrossRef] [PubMed]
- Peng, G.; Yuan, X.; Yuan, J.; Liu, Q.; Dai, M.; Shen, C.; Ma, J.; Liao, Y.; Jiang, W. miR-25 promotes glioblastoma cell proliferation and invasion by directly targeting NEFL. Mol. Cell. Biochem. 2015, 409, 103–111. [Google Scholar] [CrossRef]
- Zhang, J.; Gong, X.; Tian, K.; Chen, D.; Sun, J.; Wang, G.; Guo, M. miR-25 promotes glioma cell proliferation by targeting CDKN1C. Biomed. Pharmacother. 2015, 71, 7–14. [Google Scholar] [CrossRef]
- Tong, L.; Chu, M.; Yan, B.; Zhao, W.; Liu, S.; Wei, W.; Lou, H.; Zhang, S.; Ma, S.; Xu, J.; et al. MTDH promotes glioma invasion through regulating miR-130b-ceRNAs. Oncotarget 2017, 8, 17738–17749. [Google Scholar] [CrossRef]
- Xiao, Z.-Q.; Yin, T.-K.; Li, Y.-X.; Zhang, J.-H.; Gu, J.-J. miR-130b regulates the proliferation, invasion and apoptosis of glioma cells via targeting of CYLD. Oncol. Rep. 2017, 38, 167–174. [Google Scholar] [CrossRef]
- Agrawal, R.; Pandey, P.; Jha, P.; Dwivedi, V.; Sarkar, C.; Kulshreshtha, R. Hypoxic signature of microRNAs in glioblastoma: Insights from small RNA deep sequencing. BMC Genom. 2014, 15, 686. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.-X.; Shang, C.; Hong, Y.; Guo, Y.; Liu, Y.-H. MiR-210 Up-Regulation Inhibits Proliferation and Induces Apoptosis in Glioma Cells by Targeting SIN3A. Med. Sci. Monit. 2014, 20, 2571–2577. [Google Scholar] [CrossRef]
- Ling, N.; Gu, J.; Lei, Z.; Li, M.; Zhao, J.; Zhang, H.-T.; Li, X. microRNA-155 regulates cell proliferation and invasion by targeting FOXO3a in glioma. Oncol. Rep. 2013, 30, 2111–2118. [Google Scholar] [CrossRef]
- Marsigliante, S.; D’Urso, P.I.; Storelli, C.; Mallardo, M.; Gianfreda, C.D.; Montinaro, A.; Cimmino, A.; Pietro, C. miR-155 is up-regulated in primary and secondary glioblastoma and promotes tumour growth by inhibiting GABA receptors. Int. J. Oncol. 2012, 41, 228–234. [Google Scholar] [CrossRef]
- Zhou, J.; Wang, W.; Gao, Z.; Peng, X.; Chen, X.; Chen, W.; Xu, W.; Xu, H.; Lin, M.C.; Jiang, S. MicroRNA-155 Promotes Glioma Cell Proliferation via the Regulation of MXI1. PLoS ONE 2013, 8, e83055. [Google Scholar] [CrossRef]
- Zhen, L.; Li, J.; Zhang, M.; Yang, K. MiR-10b decreases sensitivity of glioblastoma cells to radiation by targeting AKT. J. Biol. Res. (Thessalon) 2016, 23, 14. [Google Scholar] [CrossRef]
- Li, J.; Li, Q.; Lin, L.; Wang, R.; Chen, L.; Du, W.; Jiang, C.; Li, R. Targeting the Notch1 oncogene by miR-139-5p inhibits glioma metastasis and epithelial-mesenchymal transition (EMT). BMC Neurol. 2018, 18, 133. [Google Scholar] [CrossRef] [PubMed]
- Dai, S.; Wang, X.; Li, X.; Cao, Y. MicroRNA-139-5p acts as a tumor suppressor by targeting ELTD1 and regulating cell cycle in glioblastoma multiforme. Biochem. Biophys. Res. Commun. 2015, 467, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Godlewski, J.; Nowicki, M.O.; Bronisz, A.; Williams, S.; Otsuki, A.; Nuovo, G.; Raychaudhury, A.; Newton, H.B.; Chiocca, E.A.; Lawler, S. Targeting of the Bmi-1 Oncogene/Stem Cell Renewal Factor by MicroRNA-128 Inhibits Glioma Proliferation and Self-Renewal. Cancer Res. 2008, 68, 9125–9130. [Google Scholar] [CrossRef]
- Ciafre’, S.A.; Galardi, S.; Mangiola, A.; Ferracin, M.; Liu, C.-G.; Sabatino, G.; Negrini, M.; Maira, G.; Croce, C.; Farace, M. Extensive modulation of a set of microRNAs in primary glioblastoma. Biochem. Biophys. Res. Commun. 2005, 334, 1351–1358. [Google Scholar] [CrossRef]
- Zhang, Y.; Chao, T.; Li, R.; Chen, Y.; Yan, X.; Gong, Y.; Yin, B.; Liu, W.; Qiang, B.; Zhao, J.; et al. MicroRNA-128 inhibits glioma cells proliferation by targeting transcription factor E2F3a. J. Mol. Med. 2008, 87, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.-H.; Cheng, C.-H.; Shih, C.-M.; Ho, K.-H.; Lin, C.-W.; Lee, C.-C.; Liu, A.-J.; Chang, C.-K.; Chen, K.-C. The Inhibition of microRNA-128 on IGF-1-Activating mTOR Signaling Involves in Temozolomide-Induced Glioma Cell Apoptotic Death. PLoS ONE 2016, 11, e0167096. [Google Scholar] [CrossRef]
- Wang, Z.; Bao, Z.; Yan, W.; You, G.; Wang, Y.; Li, X.-J.; Zhang, W. Isocitrate dehydrogenase 1 (IDH1) mutation-specific microRNA signature predicts favorable prognosis in glioblastoma patients with IDH1 wild type. J. Exp. Clin. Cancer Res. 2013, 32, 59. [Google Scholar] [CrossRef]
- Peruzzi, P.; Bronisz, A.; Nowicki, M.O.; Wang, Y.; Ogawa, D.; Price, R.; Nakano, I.; Kwon, C.-H.; Hayes, J.; Lawler, S.E.; et al. MicroRNA-128 coordinately targets Polycomb Repressor Complexes in glioma stem cells. Neuro-Oncology 2013, 15, 1212–1224. [Google Scholar] [CrossRef]
- Ye, L.; Yu, G.; Wang, C.; Du, B.; Sun, D.; Liu, J.; Qi, T.; Yu, X.; Wei, W.; Cheng, J.; et al. MicroRNA-128a, BMI1 polycomb ring finger oncogene, and reactive oxygen species inhibit the growth of U-87 MG glioblastoma cells following exposure to X-ray radiation. Mol. Med. Rep. 2015, 12, 6247–6254. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Shi, Z.-M.; Wang, J.; Yan, Z.; You, Y.-P.; Li, C.-Y.; Qian, X.; Yin, Y.; Zhao, P.; Wang, Y.-Y.; Wang, X.-F.; et al. MiR-128 Inhibits Tumor Growth and Angiogenesis by Targeting p70S6K1. PLoS ONE 2012, 7, e32709. [Google Scholar] [CrossRef] [PubMed]
- Conti, A.; Aguennouz, M.; La Torre, D.; Tomasello, C.; Cardali, S.M.; Angileri, F.; Maio, F.; Cama, A.; Germanò, A.; Vita, G.; et al. miR-21 and 221 upregulation and miR-181b downregulation in human grade II–IV astrocytic tumors. J. Neuro-Oncology 2009, 93, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.-M.; Wang, X.-F.; Qian, X.; Tao, T.; Wang, L.; Chen, Q.-D.; Wang, X.-R.; Cao, L.; Wang, Y.-Y.; Zhang, J.-X.; et al. MiRNA-181b suppresses IGF-1R and functions as a tumor suppressor gene in gliomas. RNA 2013, 19, 552–560. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Cao, Y.; Dong, W.; Lin, Y.; Wang, Q.; Wu, W.; Hua, X.; Ling, Y.; Xie, X.; Hu, S.; et al. The clinical characteristics and prognostic significance of AID, miR-181b, and miR-155 expression in adult patients with de novo B-cell acute lymphoblastic leukemia. Leuk. Lymphoma 2017, 58, 1–12. [Google Scholar] [CrossRef]
- Zhai, F.; Chen, X.; He, Q.; Zhang, H.; Hu, Y.; Wang, D.; Liu, S.; Zhang, Y. MicroRNA-181 inhibits glioblastoma cell growth by directly targeting CCL8. Oncol. Lett. 2019, 18, 1922–1930. [Google Scholar] [CrossRef]
- Zhou, Y.; Peng, Y.; Liu, M.; Jiang, Y. MicroRNA-181b Inhibits Cellular Proliferation and Invasion of Glioma Cells via Targeting Sal-Like Protein 4. Oncol. Res. Featur. Preclin. Clin. Cancer Ther. 2017, 25, 947–957. [Google Scholar] [CrossRef]
- Li, P.; Lu, X.; Wang, Y.; Sun, L.; Qian, C.; Yan, W.; Liu, N.; You, Y.; Fu, Z. MiR-181b suppresses proliferation of and reduces chemoresistance to temozolomide in U87 glioma stem cells. J. Biomed. Res. 2010, 24, 436–443. [Google Scholar] [CrossRef]
- Sun, Y.-C.; Wang, J.; Guo, C.; Sai, K.; Wang, J.; Chen, F.-R.; Yang, Q.-Y.; Chen, Y.; Wang, J.; Tony, T.; et al. MiR-181b sensitizes glioma cells to teniposide by targeting MDM2. BMC Cancer 2014, 14, 611. [Google Scholar] [CrossRef]
- Bier, A.; Giladi, N.; Kronfeld, N.; Lee, H.K.; Cazacu, S.; Finniss, S.; Xiang, C.; Poisson, L.; Decarvalho, A.C.; Slavin, S.; et al. MicroRNA-137 is downregulated in glioblastoma and inhibits the stemness of glioma stem cells by targeting RTVP-1. Oncotarget 2013, 4, 665–676. [Google Scholar] [CrossRef]
- Silber, J.; Lim, D.A.; Petritsch, C.K.; Persson, A.; Maunakea, A.K.; Yu, M.; Vandenberg, S.R.; Ginzinger, D.G.; James, C.; Costello, J.F.; et al. miR-124 and miR-137 inhibit proliferation of glioblastoma multiforme cells and induce differentiation of brain tumor stem cells. BMC Med. 2008, 6, 14. [Google Scholar] [CrossRef] [PubMed]
- Li, H.-Y.; Li, Y.-M.; Shi, X.-W.; Chen, H. Circulating microRNA-137 is a potential biomarker for human glioblastoma. Eur. Rev. Med Pharmacol. Sci. 2016, 20, 3599–3604. [Google Scholar] [PubMed]
- Sun, G.; Cao, Y.; Shi, L.; Sun, L.; Wang, Y.; Chen, C.; Wan, Z.; Fu, L.; You, Y. Overexpressed miRNA-137 Inhibits Human Glioma Cells Growth by Targeting Rac1. Cancer Biotherapy Radiopharm. 2013, 28, 327–334. [Google Scholar] [CrossRef]
- Zhang, Z.; Song, X.; Tian, H.; Miao, Y.; Feng, X.; Li, Y.; Wang, H. MicroRNA-137 inhibits growth of glioblastoma through EGFR suppression. Am. J. Transl. Res. 2017, 9, 1492–1499. [Google Scholar]
- Guessous, F.; Zhang, Y.; Kofman, A.; Catania, A.; Li, Y.; Schiff, D.; Purow, B.; Abounader, R.; Kofman, A. microRNA-34a is tumor suppressive in brain tumors and glioma stem cells. Cell Cycle 2010, 9, 1031–1036. [Google Scholar] [CrossRef]
- Li, Y.; Guessous, F.; Zhang, Y.; Dipierro, C.; Kefas, B.; Johnson, E.; Marcinkiewicz, L.; Jiang, J.; Yang, Y.; Schmittgen, T.D.; et al. MicroRNA-34a inhibits glioblastoma growth by targeting multiple oncogenes. Cancer Res. 2009, 69, 7569–7576. [Google Scholar] [CrossRef]
- Cui, J.G.; Zhao, Y.; Sethi, P.; Li, Y.Y.; Mahta, A.; Culicchia, F.; Lukiw, W.J. Micro-RNA-128 (miRNA-128) down-regulation in glioblastoma targets ARP5 (ANGPTL6), Bmi-1 and E2F-3a, key regulators of brain cell proliferation. J. Neuro-Oncology 2009, 98, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Zheng, G.; Gu, Z.; Guo, Z. MiR-137 inhibits proliferation and angiogenesis of human glioblastoma cells by targeting EZH2. J. Neuro-Oncology 2015, 122, 481–489. [Google Scholar] [CrossRef] [PubMed]
- Lv, Z.; Yang, L. miR-124 inhibits the growth of glioblastoma through the downregulation of SOS1. Mol. Med. Rep. 2013, 8, 345–349. [Google Scholar] [CrossRef]
- Qiao, W.; Guo, B.; Zhou, H.; Xu, W.; Chen, Y.; Liang, Y.; Dong, B. miR-124 suppresses glioblastoma growth and potentiates chemosensitivity by inhibiting AURKA. Biochem. Biophys. Res. Commun. 2017, 486, 43–48. [Google Scholar] [CrossRef]
- Wu, H.; Liu, Q.; Cai, T.; Chen, Y.-D.; Liao, F.; Wang, Z.-F. MiR-136 modulates glioma cell sensitivity to temozolomide by targeting astrocyte elevated gene-1. Diagn. Pathol. 2014, 9, 173. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Wu, J.; Guan, H.; Cai, J.; Fang, L.; Li, J.; Li, M. MiR-136 promotes apoptosis of glioma cells by targeting AEG-1 and Bcl-2. FEBS Lett. 2012, 586, 3608–3612. [Google Scholar] [CrossRef] [PubMed]
- Tao, T.; Wang, Y.; Luo, H.; Yao, L.; Wang, L.; Wang, J.; Yan, W.; Zhang, J.; Wang, H.; Shi, Y.; et al. Involvement of FOS-mediated miR-181b/miR-21 signalling in the progression of malignant gliomas. Eur. J. Cancer 2013, 49, 3055–3063. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Sai, K.; Chen, F.-R.; Chen, Z.-P. miR-181b modulates glioma cell sensitivity to temozolomide by targeting MEK1. Cancer Chemother. Pharmacol. 2013, 72, 147–158. [Google Scholar] [CrossRef]
- Hui, W.; Yuntao, L.; Lu, Y.; WenSheng, L.; ChaoFeng, L.; Haiyong, H.; Yueyang, B. MicroRNA-195 Inhibits the Proliferation of Human Glioma Cells by Directly Targeting Cyclin D1 and Cyclin E1. PLoS ONE 2013, 8, 54932. [Google Scholar] [CrossRef]
- Zhang, Q.-Q.; Xu, H.; Huang, M.-B.; Ma, L.-M.; Huang, Q.-J.; Yao, Q.; Zhou, H.; Qu, L.-H. MicroRNA-195 plays a tumor-suppressor role in human glioblastoma cells by targeting signaling pathways involved in cellular proliferation and invasion. Neuro-Oncology 2012, 14, 278–287. [Google Scholar] [CrossRef]
- Poliseno, L.; Salmena, L.; Zhang, J.; Carver, B.; Haveman, W.J.; Pandolfi, P.P. A coding-independent function of gene and pseudogene mRNAs regulates tumour biology. Nature 2010, 465, 1033–1038. [Google Scholar] [CrossRef]
- Mazor, G.; Levin, L.; Picard, D.; Ahmadov, U.; Carén, H.; Borkhardt, A.; Reifenberger, G.; Leprivier, G.; Remke, M.; Rotblat, B. The lncRNA TP73-AS1 is linked to aggressiveness in glioblastoma and promotes temozolomide resistance in glioblastoma cancer stem cells. Cell Death Dis. 2019, 10, 246. [Google Scholar] [CrossRef]
- Sathipati, S.Y.; Sahu, D.; Huang, H.-C.; Lin, Y.; Ho, S.-Y. Identification and characterization of the lncRNA signature associated with overall survival in patients with neuroblastoma. Sci. Rep. 2019, 9, 5125. [Google Scholar] [CrossRef]
- Liu, S.; Mitra, R.; Zhao, M.-M.; Fan, W.; Eischen, C.M.; Yin, F.; Zhao, Z. The Potential Roles of Long Noncoding RNAs (lncRNA) in Glioblastoma Development. Mol. Cancer Ther. 2016, 15, 2977–2986. [Google Scholar] [CrossRef]
- Li, J.; Zhu, Y.; Wang, H.; Ji, X. Targeting Long Noncoding RNA in Glioma: A Pathway Perspective. Mol. Ther. Nucleic Acids 2018, 13, 431–441. [Google Scholar] [CrossRef] [PubMed]
- Hutchinson, J.N.; Ensminger, A.W.; Clemson, C.M.; Lynch, C.R.; Lawrence, J.B.; Chess, A. A screen for nuclear transcripts identifies two linked noncoding RNAs associated with SC35 splicing domains. BMC Genom. 2007, 8, 39. [Google Scholar] [CrossRef] [PubMed]
- Ji, P.; Diederichs, S.; Wang, W.; Boing, S.; Metzger, R.; Schneider, P.M.; Tidow, N.; Brandt, B.; Buerger, H.; Bulk, E.; et al. MALAT-1, a novel noncoding RNA, and thymosin beta4 predict metastasis and survival in early-stage non-small cell lung cancer. Oncogene 2003, 22, 8031–8041. [Google Scholar] [CrossRef] [PubMed]
- Liao, K.; Lin, Y.; Gao, W.; Xiao, Z.; Medina, R.; Dmitriev, P.; Cui, J.; Zhuang, Z.; Zhao, X.; Qiu, Y.; et al. Blocking lncRNA MALAT1/miR-199a/ZHX1 Axis Inhibits Glioblastoma Proliferation and Progression. Mol. Ther. Nucleic Acids 2019, 18, 388–399. [Google Scholar] [CrossRef]
- Tripathi, V.; Ellis, J.D.; Shen, Z.; Song, D.Y.; Pan, Q.; Watt, A.T.; Freier, S.M.; Bennett, C.F.; Sharma, A.; Bubulya, P.A.; et al. The Nuclear-Retained Noncoding RNA MALAT1 Regulates Alternative Splicing by Modulating SR Splicing Factor Phosphorylation. Mol. Cell 2010, 39, 925–938. [Google Scholar] [CrossRef]
- Bond, C.S.; Fox, A. Paraspeckles: Nuclear bodies built on long noncoding RNA. J. Cell Boil. 2009, 186, 637–644. [Google Scholar] [CrossRef]
- Zhuang, M.; Zhao, S.; Jiang, Z.; Wang, S.; Sun, P.; Quan, J.; Yan, D.; Wang, X.-S. MALAT1 sponges miR-106b-5p to promote the invasion and metastasis of colorectal cancer via SLAIN2 enhanced microtubules mobility. EBioMedicine 2019, 41, 286–298. [Google Scholar] [CrossRef]
- Cao, S.; Wang, Y.; Li, J.; Lv, M.; Niu, H.; Tian, Y. Tumor-suppressive function of long noncoding RNA MALAT1 in glioma cells by suppressing miR-155 expression and activating FBXW7 function. Am. J. Cancer Res. 2016, 6, 2561–2574. [Google Scholar]
- Wang, Y.; Zhang, Y.; Yang, T.; Zhao, W.; Wang, N.; Li, P.; Zeng, X.; Zhang, W. Long non-coding RNA MALAT1 for promoting metastasis and proliferation by acting as a ceRNA of miR-144-3p in osteosarcoma cells. Oncotarget 2017, 8, 59417–59434. [Google Scholar] [CrossRef]
- Tao, F.; Tian, X.; Ruan, S.; Shen, M.; Zhang, Z. miR-211 sponges lncRNA MALAT1 to suppress tumor growth and progression through inhibiting PHF19 in ovarian carcinoma. FASEB J. 2018, 32, 6330–6343. [Google Scholar] [CrossRef]
- Wang, S.; Yu, W.; Chen, J.; Yao, T.; Deng, F. LncRNA MALAT1 sponges miR-203 to promote inflammation in myocardial ischemia-reperfusion injury. Int. J. Cardiol. 2018, 268, 245. [Google Scholar] [CrossRef]
- Chen, W.; Xu, X.-K.; Li, J.-L.; Kong, K.-K.; Li, H.; Chen, C.; He, J.; Wang, F.; Li, P.; Ge, X.-S.; et al. MALAT1 is a prognostic factor in glioblastoma multiforme and induces chemoresistance to temozolomide through suppressing miR-203 and promoting thymidylate synthase expression. Oncotarget 2017, 8, 22783–22799. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, X.; Klibanski, A. MEG3 noncoding RNA: A tumor suppressor. J. Mol. Endocrinol. 2012, 48, R45–R53. [Google Scholar] [CrossRef]
- Da Rocha, S.T.; Edwards, C.; Ito, M.; Ogata, T.; Ferguson-Smith, A. Genomic imprinting at the mammalian Dlk1-Dio3 domain. Trends Genet. 2008, 24, 306–316. [Google Scholar] [CrossRef]
- Schuster-Gossler, K.; Bilinski, P.; Sado, T.; Ferguson-Smith, A.; Gossler, A. The mouseGtl2 gene is differentially expressed during embryonic development, encodes multiple alternatively spliced transcripts, and may act as an RNA. Dev. Dyn. 1998, 212, 214–228. [Google Scholar] [CrossRef]
- Al-Rugeebah, A.; Alanazi, M.; Parine, N.R. MEG3: An Oncogenic Long Non-coding RNA in Different Cancers. Pathol. Oncol. Res. 2019, 25, 859–874. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhong, Y.; Wang, Y.; Zhang, X.; Batista, D.L.; Gejman, R.; Ansell, P.J.; Zhao, J.; Weng, C.; Klibanski, A. Activation of p53 by MEG3 Non-coding RNA. J. Boil. Chem. 2007, 282, 24731–24742. [Google Scholar] [CrossRef]
- Zhao, H.; Wang, X.; Feng, X.; Li, X.; Pan, L.; Liu, J.; Wang, F.; Yuan, Z.; Yang, L.; Yu, J.; et al. Long non-coding RNA MEG3 regulates proliferation, apoptosis, and autophagy and is associated with prognosis in glioma. J. Neuro-Oncology 2018, 140, 281–288. [Google Scholar] [CrossRef]
- Wang, P.; Ren, Z.; Sun, P. Overexpression of the long non-coding RNA MEG3 impairs in vitro glioma cell proliferation. J. Cell. Biochem. 2012, 113, 1868–1874. [Google Scholar] [CrossRef]
- Zhang, S.; Guo, W. Long non-coding RNA MEG3 suppresses the growth of glioma cells by regulating the miR-96-5p/MTSS1 signaling pathway. Mol. Med. Rep. 2019, 20, 4215–4225. [Google Scholar] [CrossRef]
- Li, J.; Bian, E.-B.; He, X.-J.; Ma, C.-C.; Zong, G.; Wang, H.-L.; Zhao, B. Epigenetic repression of long non-coding RNA MEG3 mediated by DNMT1 represses the p53 pathway in gliomas. Int. J. Oncol. 2015, 48, 723–733. [Google Scholar] [CrossRef] [PubMed]
- Qin, N.; Tong, G.-F.; Sun, L.-W.; Xu, X.-L. Long Noncoding RNA MEG3 Suppresses Glioma Cell Proliferation, Migration, and Invasion by Acting as a Competing Endogenous RNA of miR-19a. Oncol. Res. Featur. Preclin. Clin. Cancer Ther. 2017, 25, 1471–1478. [Google Scholar] [CrossRef] [PubMed]
- Hajjari, M.; Salavaty, A. HOTAIR: An oncogenic long non-coding RNA in different cancers. Cancer Boil. Med. 2015, 12, 1–9. [Google Scholar]
- Rinn, J.L.; Kertesz, M.; Wang, J.; Squazzo, S.L.; Xu, X.; Brugmann, S.A.; Goodnough, L.H.; Helms, J.A.; Farnham, P.J.; Segal, E.; et al. Functional Demarcation of Active and Silent Chromatin Domains in Human HOX Loci by Noncoding RNAs. Cell 2007, 129, 1311–1323. [Google Scholar] [CrossRef]
- Liu, X.-H.; Sun, M.; Nie, F.; Ge, Y.; Zhang, E.-B.; Yin, D.-D.; Kong, R.; Chen, J.; Lu, K.-H.; Li, J.-H.; et al. Lnc RNA HOTAIR functions as a competing endogenous RNA to regulate HER2 expression by sponging miR-331-3p in gastric cancer. Mol. Cancer 2014, 13, 92. [Google Scholar] [CrossRef]
- Ma, M.; Li, C.-X.; Zhang, Y.; Weng, M.-Z.; Zhang, M.-D.; Qin, Y.-Y.; Gong, W.; Quan, Z.-W. Long non-coding RNA HOTAIR, a c-Myc activated driver of malignancy, negatively regulates miRNA-130a in gallbladder cancer. Mol. Cancer 2014, 13, 156. [Google Scholar] [CrossRef]
- Gupta, R.A.; Shah, N.; Wang, K.C.; Kim, J.; Horlings, H.M.; Wong, D.J.; Tsai, M.-C.; Hung, T.; Argani, P.; Rinn, J.L.; et al. Long non-coding RNA HOTAIR reprograms chromatin state to promote cancer metastasis. Nature 2010, 464, 1071–1106. [Google Scholar] [CrossRef]
- Tan, S.K.; Pastori, C.; Penas, C.; Komotar, R.J.; Ivan, M.E.; Wahlestedt, C.; Ayad, N.G. Serum long noncoding RNA HOTAIR as a novel diagnostic and prognostic biomarker in glioblastoma multiforme. Mol. Cancer 2018, 17, 74. [Google Scholar] [CrossRef]
- Zhang, J.-X.; Han, L.; Bao, Z.-S.; Wang, Y.-Y.; Chen, L.-Y.; Yan, W.; Yu, S.-Z.; Pu, P.-Y.; Liu, N.; You, Y.-P.; et al. HOTAIR, a cell cycle–associated long noncoding RNA and a strong predictor of survival, is preferentially expressed in classical and mesenchymal glioma. Neuro-Oncology 2013, 15, 1595–1603. [Google Scholar] [CrossRef]
- Ke, J.; Yao, Y.-L.; Zheng, J.; Wang, P.; Liu, Y.-H.; Ma, J.; Li, Z.; Liu, X.-B.; Li, Z.-Q.; Wang, Z.-H.; et al. Knockdown of long non-coding RNA HOTAIR inhibits malignant biological behaviors of human glioma cells via modulation of miR-326. Oncotarget 2015, 6, 21934–21949. [Google Scholar] [CrossRef]
- Zhou, X.; Ren, Y.; Zhang, J.; Zhang, C.; Zhang, K.; Han, L.; Kong, L.; Wei, J.; Chen, L.; Yang, J.; et al. HOTAIR is a therapeutic target in glioblastoma. Oncotarget 2015, 6, 8353–8365. [Google Scholar] [CrossRef] [PubMed]
- Gabory, A.; Ripoche, M.-A.; Le Digarcher, A.; Watrin, F.; Ziyyat, A.; Forné, T.; Jammes, H.; Ainscough, J.F.X.; Surani, A.; Journot, L.; et al. H19 acts as a trans regulator of the imprinted gene network controlling growth in mice. Development 2009, 136, 3413–3421. [Google Scholar] [CrossRef] [PubMed]
- Brannan, C.I.; Dees, E.C.; Ingram, R.S.; Tilghman, S.M. The product of the H19 gene may function as an RNA. Mol. Cell. Boil. 1990, 10, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Leighton, P.A.; Saam, J.R.; Ingram, R.S.; Stewart, C.L.; Tilghman, S.M. An enhancer deletion affects both H19 and Igf2 expression. Genome Res. 1995, 9, 2079–2089. [Google Scholar] [CrossRef]
- Hibi, K.; Nakamura, H.; Hirai, A.; Fujikake, Y.; Kasai, Y.; Akiyama, S.; Ito, K.; Takagi, H. Loss of H19 imprinting in esophageal cancer. Cancer Res. 1996, 56, 480–482. [Google Scholar]
- Lottin, S.; Adriaenssens, E.; Dupressoir, T.; Berteaux, N.; Montpellier, C.; Coll, J.; Dugimont, T.; Curgy, J.J. Overexpression of an ectopic H19 gene enhances the tumorigenic properties of breast cancer cells. Carcinogenesis 2002, 23, 1885–1895. [Google Scholar] [CrossRef]
- Wu, W.; Hu, Q.; Nie, E.; Yu, T.; Wu, Y.; Zhi, T.; Jiang, K.; Shen, F.; Wang, Y.; Zhang, J.; et al. Hypoxia induces H19 expression through direct and indirect Hif-1α activity, promoting oncogenic effects in glioblastoma. Sci. Rep. 2017, 7, 45029. [Google Scholar] [CrossRef]
- Hu, Q.; Yin, J.; Zeng, A.; Jin, X.; Zhang, Z.; Yan, W.; You, Y. H19 Functions as a Competing Endogenous RNA to Regulate EMT by Sponging miR-130a-3p in Glioma. Cell. Physiol. Biochem. 2018, 50, 233–245. [Google Scholar] [CrossRef]
- Shi, Y.; Wang, Y.; Luan, W.; Wang, P.; Tao, T.; Zhang, J.; Qian, J.; Liu, N.; You, Y. Long Non-Coding RNA H19 Promotes Glioma Cell Invasion by Deriving miR-675. PLoS ONE 2014, 9, e86295. [Google Scholar] [CrossRef]
- Jiang, X.; Yan, Y.; Hu, M.; Chen, X.; Wang, Y.; Dai, Y.; Wu, D.; Zhuang, Z.; Xia, H. Increased level of H19 long noncoding RNA promotes invasion, angiogenesis, and stemness of glioblastoma cells. J. Neurosurg 2016, 124, 129–136. [Google Scholar] [CrossRef]
- Fazi, B.; Garbo, S.; Toschi, N.; Mangiola, A.; Lombari, M.; Sicari, D.; Battistelli, C.; Galardi, S.; Michienzi, A.; Trevisi, G.; et al. The lncRNA H19 positively affects the tumorigenic properties of glioblastoma cells and contributes to NKD1 repression through the recruitment of EZH2 on its promoter. Oncotarget 2018, 9, 15512–15525. [Google Scholar] [CrossRef] [PubMed]
- Saha, S.; Murthy, S.; Rangarajan, P. Identification and characterization of a virus-inducible non-coding RNA in mouse brain. J. Gen. Virol. 2006, 87, 1991–1995. [Google Scholar] [CrossRef] [PubMed]
- Clemson, C.M.; Hutchinson, J.N.; Sara, S.A.; Ensminger, A.W.; Fox, A.; Chess, A.; Lawrence, J.B. An Architectural Role for a Nuclear Noncoding RNA: NEAT1 RNA Is Essential for the Structure of Paraspeckles. Mol. Cell 2009, 33, 717–726. [Google Scholar] [CrossRef] [PubMed]
- Dong, P.; Xiong, Y.; Yue, J.; Hanley, S.J.B.; Kobayashi, N.; Todo, Y.; Watari, H. Long Non-coding RNA NEAT1: A Novel Target for Diagnosis and Therapy in Human Tumors. Front. Genet. 2018, 9, 471. [Google Scholar] [CrossRef] [PubMed]
- Zhou, K.; Zhang, C.; Yao, H.; Zhang, X.; Zhou, Y.; Che, Y.; Huang, Y. Knockdown of long non-coding RNA NEAT1 inhibits glioma cell migration and invasion via modulation of SOX2 targeted by miR-132. Mol. Cancer 2018, 17, 105. [Google Scholar] [CrossRef] [PubMed]
- Gong, W.; Zheng, J.; Liu, X.; Ma, J.; Liu, Y.; Xue, Y. Knockdown of NEAT1 restrained the malignant progression of glioma stem cells by activating microRNA let-7e. Oncotarget 2016, 7, 62208–62223. [Google Scholar] [CrossRef]
- Chen, Q.; Cai, J.; Wang, Q.; Wang, Y.; Liu, M.; Yang, J.; Zhou, J.; Kang, C.; Li, M.; Jiang, C. Long Noncoding RNA NEAT1, Regulated by the EGFR Pathway, Contributes to Glioblastoma Progression Through the WNT/beta-Catenin Pathway by Scaffolding EZH2. Clin. Cancer Res. 2018, 24, 684–695. [Google Scholar] [CrossRef]
- Yu, H.; Xu, Q.; Liu, F.; Ye, X.; Wang, J.; Meng, X. Identification and Validation of Long Noncoding RNA Biomarkers in Human Non–Small-Cell Lung Carcinomas. J. Thorac. Oncol. 2015, 10, 645–654. [Google Scholar] [CrossRef]
- Chow, J.C.; Yen, Z.; Ziesche, S.M.; Brown, C.J. Silencing of the mammalian X chromosome. Annu. Rev. Genom. Hum. Genet. 2005, 6, 69–92. [Google Scholar] [CrossRef]
- Heard, E. Dosage compensation in mammals: Fine-tuning the expression of the X chromosome. Genome Res. 2006, 20, 1848–1867. [Google Scholar] [CrossRef]
- Ng, K.; Pullirsch, D.; Leeb, M.; Wutz, A. Xist and the order of silencing. EMBO Rep. 2007, 8, 34–39. [Google Scholar] [CrossRef]
- Yu, H.; Xue, Y.; Wang, P.; Liu, X.; Ma, J.; Zheng, J.; Li, Z.; Cai, H.; Liu, Y. Knockdown of long non-coding RNA XIST increases blood-tumor barrier permeability and inhibits glioma angiogenesis by targeting miR-137. Oncogenesis 2017, 6, e303. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Li, Z.; Ma, K.; Li, X.; Tian, N.; Duan, J.; Xiao, X.; Wang, Y. Long Non-coding RNA XIST Promotes Glioma Tumorigenicity and Angiogenesis by Acting as a Molecular Sponge of miR-429. J. Cancer 2017, 8, 4106–4116. [Google Scholar] [CrossRef]
- Yao, Y.; Ma, J.; Xue, Y.-X.; Wang, P.; Li, Z.; Liu, J.; Chen, L.; Xi, Z.; Teng, H.; Wang, Z.; et al. Knockdown of long non-coding RNA XIST exerts tumor-suppressive functions in human glioblastoma stem cells by up-regulating miR-152. Cancer Lett. 2015, 359, 75–86. [Google Scholar] [CrossRef] [PubMed]
- Jing, S.Y.; Lu, Y.Y.; Yang, J.K.; Deng, W.Y.; Zhou, Q.; Jiao, B.H. Expression of long non-coding RNA CRNDE in glioma and its correlation with tumor progression and patient survival. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 3992–3996. [Google Scholar] [PubMed]
- Zheng, J.; Li, X.-D.; Wang, P.; Liu, X.-B.; Xue, Y.-X.; Hu, Y.; Li, Z.; Li, Z.-Q.; Wang, Z.-H.; Liu, Y.-H. CRNDE affects the malignant biological characteristics of human glioma stem cells by negatively regulating miR-186. Oncotarget 2015, 6, 25339–25355. [Google Scholar] [CrossRef] [PubMed]
- Li, D.-X.; Fei, X.-R.; Dong, Y.-F.; Cheng, C.-D.; Yang, Y.; Deng, X.-F.; Huang, H.-L.; Niu, W.-X.; Zhou, C.-X.; Xia, C.-Y.; et al. The long non-coding RNA CRNDE acts as a ceRNA and promotes glioma malignancy by preventing miR-136-5p-mediated downregulation of Bcl-2 and Wnt2. Oncotarget 2017, 8, 88163–88178. [Google Scholar] [CrossRef]
- Baldinu, P.; Cossu, A.G.M.; Manca, A.; Satta, M.P.; Sini, M.C.; Rozzo, C.; Cherchi, P.; Gianfrancesco, F.; Pintus, A.; Carboni, A.; et al. Identification of a novel candidate gene, CASC2, in a region of common allelic loss at chromosome 10q26 in human endometrial cancer. Hum. Mutat. 2004, 23, 318–326. [Google Scholar] [CrossRef]
- Wang, P.; Liu, Y.-H.; Yao, Y.-L.; Li, Z.; Li, Z.-Q.; Ma, J.; Xue, Y.-X. Long non-coding RNA CASC2 suppresses malignancy in human gliomas by miR-21. Cell. Signal. 2015, 27, 275–282. [Google Scholar] [CrossRef]
- Wang, R.; Li, Y.; Zhu, G.; Tian, B.; Zeng, W.; Yang, Y.; Li, Z. Long noncoding RNA CASC2 predicts the prognosis of glioma patients and functions as a suppressor for gliomas by suppressing Wnt/beta-catenin signaling pathway. Neuropsychiatr Dis. Treat 2017, 13, 1805–1813. [Google Scholar] [CrossRef]
- Buccarelli, M.; Marconi, M.; Pacioni, S.; De Pascalis, I.; D’Alessandris, Q.G.; Martini, M.; Ascione, B.; Malorni, W.; Larocca, L.M.; Pallini, R.; et al. Inhibition of autophagy increases susceptibility of glioblastoma stem cells to temozolomide by igniting ferroptosis. Cell Death Dis. 2018, 9, 841. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Shen, F.; Du, J.; Fang, X.; Li, X.; Su, J.; Wang, X.; Huang, X.; Liu, Z. Upregulation of CASC2 sensitized glioma to temozolomide cytotoxicity through autophagy inhibition by sponging miR-193a-5p and regulating mTOR expression. Biomed. Pharmacother. 2018, 97, 844–850. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Hodges, T.R.; Song, R.; Gong, Y.; Calin, G.A.; Heimberger, A.B.; Zhao, H. Serum HOTAIR and GAS5 levels as predictors of survival in patients with glioblastoma. Mol. Carcinog 2018, 57, 137–141. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Wang, P.; Liu, J.; Zheng, J.; Liu, Y.; Chen, J.; Xue, Y. Gas5 Exerts Tumor-suppressive Functions in Human Glioma Cells by Targeting miR-222. Mol. Ther. 2015, 23, 1899–1911. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Yu, W.; Zhu, S.; Cheng, K.; Xu, H.; Lv, Y.; Long, X.; Ma, L.; Huang, J.; Sun, S.; et al. Long noncoding RNA GAS5 regulates the proliferation, migration, and invasion of glioma cells by negatively regulating miR-18a-5p. J. Cell Physiol. 2018, 234, 757–768. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, Y.; Zheng, J.; Liu, X.; Chen, J.; Liu, L.; Wang, P.; Xue, Y. GAS5 suppresses malignancy of human glioma stem cells via a miR-196a-5p/FOXO1 feedback loop. Biochim. Biophys. Acta Mol. Cell Res. 2017, 1864, 1605–1617. [Google Scholar] [CrossRef] [PubMed]
| Major Oncogenic miRNAs in Glioblastoma | |||
|---|---|---|---|
| MicroRNA | Validated Targets | Functional Effects in Glioblastoma | References |
| hsa-miR-21 | IGFBP3, RECK, TIMP3, ANP32A, Bcl-2, PTEN, HNRPK, TAP63, MSH2 LRRFIP1, PDCD4 (…) | Promotes cell proliferation, invasion, chemoresistance and tumor growth in vivo | [60,61,63] |
| hsa-miR-26a | PTEN | Enhances tumor formation in vivo | [85] |
| hsa-miR-19a/b | RUNX3, CTGF | Promotes cell proliferation and G1 cell cycle progression; modulates cell apoptosis and invasion | [86,87] |
| hsa-miR-93 | Integrin-β8, P21 | Promotes cell proliferation, cell cycle progression, migration, invasion, and chemoresistance; induces angiogenesis and enhances tumor growth in vivo | [88,89,90] |
| hsa-miR-221/222 | P27, AKT, PUMA, P57, PTPμ, Cx43, TIMP3, MGMT | Promotes cell proliferation, invasion, and chemoresistance; modulates cell apoptosis and tumor growth in vivo | [75,77,78,79,80,81,82,83] |
| hsa-miR-20a | TGFβ-RII, CTGF, CELF2, LRIG1 | Promotes cell proliferation, invasion and inhibits apoptosis | [86,91,92] |
| hsa-miR-25 | Mdm2, TSC1, P57, NEFL | Promotes cell proliferation, invasion and cell cycle progression | [93,94] |
| hsa-miR-130b | CYLD | Promotes cell proliferation, invasion and inhibits apoptosis | [95,96] |
| hsa-miR-210 | HIF3α, SIN3A | Promotes cell proliferation and inhibits cell apoptosis; mediates hypoxic survival and enhances chemoresistance | [97,98] |
| hsa-miR-155 | GABRA-1, FOXO3a, MXI1, MAPK13/14 | Promotes cell proliferation and invasion and inhibits apoptosis | [94,99,100,101] |
| hsa-miR-10b | PTEN, BIM, P21, P16, TFAP2C, MBNL2, MBNL3, SART3, HOXD10 | Promotes cell proliferation, cell cycle progression, migration, invasion, and inhibits apoptosis; modulates tumor growth in vivo | [69,70,71,73,102,103,104] |
| Major Tumor Suppressor miRNAs in Glioblastoma | |||
|---|---|---|---|
| MicroRNA | Validated Targets | Functional Effects in Glioblastoma | References |
| hsa-mir-34a | SIRT1, c-Met, Notch1/2, PDGFRA, Msi1 | Inhibits cell proliferation, cell cycle progression, cell survival, invasion, and tumor growth in vivo | [125,126] |
| hsa-miR-128 | WEE1, p70S6K1, Msi1, E2F3a, SUZ12, Bmi-1, EGFR, PDGFRα, ANGPTL6 | Decreases radioresistance, attenuates cell proliferation, tumor growth and angiogenesis | [105,108,110,111,112,127] |
| hsa-miR-137 | RTVP-1, COX-2, EGFR, CDK6, RTVP-1 Rac1 | Inhibits proliferation and invasion and reduces stemness; increases apoptosis and promotes cell cycle arrest | [120,121,124,128] |
| hsa-miR-124 | AURKA, SOS1 | Inhibits proliferation, reduces stemness, promotes cell cycle arrest and increases chemosensitivity. | [121,129,130] |
| hsa-miR136 | AEG-1, Bcl-2 | Promotes apoptosis and increases chemosensitivity | [131,132] |
| hsa-miR-181b | FOS, MEK1, IGF-1R, CCL8, MDM2 | Inhibits proliferation, migration, and invasion; promotes cell cycle arrest; suppresses angiogenesis and tumor growth in vivo | [114,116,117,119,133,134] |
| hsa-miR-195 | E2F3, CCND3, Cyclin D1, Cyclin E1 | Inhibits proliferation, migration, and invasion; promotes cell cycle arrest and reduces tumor growth in vivo | [135,136] |
| hsa-miR-139-5p | ELTD1, Notch1 | Inhibits proliferation and invasion; promotes apoptosis; reduces tumor growth and prolongs survival in vivo | [103,104] |
| Major Oncogenic LncRNAs in Glioblastoma | ||||
|---|---|---|---|---|
| LncRNA | Target miRNA | Mechanism of Action | Functional Effects in Glioblastoma | References |
| MALAT1 | miR-199a miR-203 | Acts as a molecular sponge for miRNAs | Promotes cell proliferation and tumorigenesis; leads to resistance to TMZ-treatment | [144,152] |
| MEG3 | miR-19a | Acts as a ceRNA for miRNAs, represses PTEN expression and controls the expression of p53-target genes | Increases cell proliferation, migration, and invasion | [162] |
| HOTAIR | miR-326 | Binds to EZH2 and regulates FGF1-dependent pathway by acting as a sponge for miRNA | Promotes cell proliferation and glioblastoma cells growth | [171] |
| H19 | miR-675 miR-130a-3p | Binds to EZH2 and acts as a molecular sponge for miRNAs | Promotes invasion, angiogenesis, stemness and increased glioblastoma cells growth | [179,180] |
| NEAT1 | miR-132 let-7e | Binds to EZH2, functions as a scaffold RNA by interacting with target genes and triggers β-catenin translocation | Promotes tumor progression, regulates invasiveness of glioblastoma cells and promotes GSCs migration and invasion | [186,187] |
| XIST | miR-137 miR-429 miR-152 | Acts as a ceRNA for miRNAs and promotes transcriptional inactivation of ZO-2 and FOXC1 | Promotes angiogenesis and has a potential role in GSCs | [192,193,194] |
| TP73-AS1 | - | Is linked to reduced ALDH1A1 expression | Promotes tumor aggressiveness and TMZ resistance in GSCs; prognostic biomarker | [138] |
| CRNDE | miR-186 miR-136-5p | Negatively regulates miRNAs | Promotes cell growth and GSCs proliferation; is a prognostic factor for glioblastoma patients | [195,196,197] |
| Major Tumor Suppressor LncRNAs in Glioblastoma | ||||
|---|---|---|---|---|
| LncRNA | Target miRNA | Mechanism of Action | Functional Effects in Glioblastoma | References |
| RAMP2-AS1 | - | Interacts with DHC10/NOTCH3/HES1-signaling pathway | Reduces tumor growth | [140] |
| CASC2 | miR-21 miR-193a-5p | Binds directly to miRNA, suppresses the Wnt/β-catenin signaling pathway and regulates mTOR expression | Inhibits autophagy and malignancy in glioblastoma cells, sensitizes GSCs to TMZ-treatment leading to ferroptosis | [200,203] |
| GAS5 | miR-222 miR-196a-5p miR-18a-5p | Acts as a molecular sponge for miRNAs | Promotes proliferation in glioblastoma cells and GSCs; prognostic predictor of survival | [204,205,206] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
DeOcesano-Pereira, C.; Machado, R.A.C.; Chudzinski-Tavassi, A.M.; Sogayar, M.C. Emerging Roles and Potential Applications of Non-Coding RNAs in Glioblastoma. Int. J. Mol. Sci. 2020, 21, 2611. https://doi.org/10.3390/ijms21072611
DeOcesano-Pereira C, Machado RAC, Chudzinski-Tavassi AM, Sogayar MC. Emerging Roles and Potential Applications of Non-Coding RNAs in Glioblastoma. International Journal of Molecular Sciences. 2020; 21(7):2611. https://doi.org/10.3390/ijms21072611
Chicago/Turabian StyleDeOcesano-Pereira, Carlos, Raquel A. C. Machado, Ana Marisa Chudzinski-Tavassi, and Mari Cleide Sogayar. 2020. "Emerging Roles and Potential Applications of Non-Coding RNAs in Glioblastoma" International Journal of Molecular Sciences 21, no. 7: 2611. https://doi.org/10.3390/ijms21072611
APA StyleDeOcesano-Pereira, C., Machado, R. A. C., Chudzinski-Tavassi, A. M., & Sogayar, M. C. (2020). Emerging Roles and Potential Applications of Non-Coding RNAs in Glioblastoma. International Journal of Molecular Sciences, 21(7), 2611. https://doi.org/10.3390/ijms21072611