The Epigenetic Profile of Tumor Endothelial Cells. Effects of Combined Therapy with Antiangiogenic and Epigenetic Drugs on Cancer Progression
Abstract
1. Introduction
Literature Search
2. NECs versus TECs
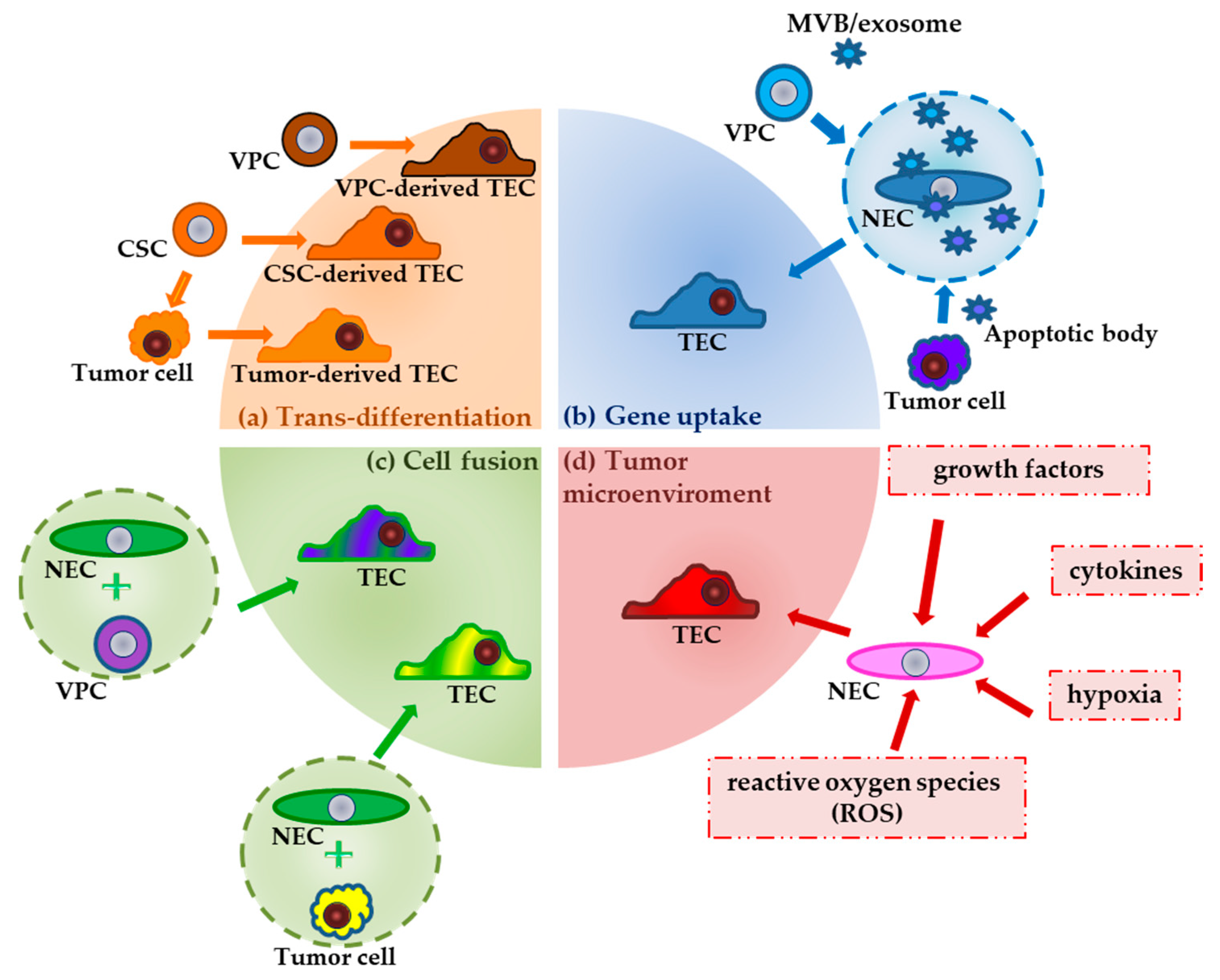
2.1. The Multiple Origins of TECs
2.2. Metabolic Reprogramming in NECs and TECs
3. Epigenetic Profiling of Tumor Endothelial Cells
3.1. The DNA Methylation Profile of TECs
3.2. Histone Posttranslational Modifications
3.3. miRNAs in Regulating NECs/TECs Metabolism
4. Role of TECs in Promoting the Metastatic Phenotype of Cancer Cells
TECs and Drug Resistance in Tumors
5. Combination of Anti-Angiogenic and Epigenetic Drugs-Based Therapy in Anticancer Treatment: Drug Candidates and Ongoing Clinical Trials
6. Conclusions and Future Directions
Funding
Conflicts of Interest
References
- Cines, D.B.; Pollak, E.S.; Buck, C.A.; Loscalzo, J.; Zimmerman, G.A.; McEver, R.P.; Pober, J.S.; Wick, T.M.; Konkle, B.A.; Schwartz, B.S.; et al. Endothelial cells in physiology and in the pathophysiology of vascular disorders. Blood 1998, 91, 3527–3561. [Google Scholar]
- Rohlenova, K.; Veys, K.; Miranda-Santos, I.; De Bock, K.; Carmeliet, P. Endothelial Cell Metabolism in Health and Disease. Trends Cell Biol. 2018, 28, 224–236. [Google Scholar] [CrossRef] [PubMed]
- Deanfield, J.E.; Halcox, J.P.; Rabelink, T.J. Endothelial Function and Dysfunction. Testing and Clinical Relevance. Circulation 2007, 115, 1285–1295. [Google Scholar] [CrossRef] [PubMed]
- Zecchin, A.; Borgers, G.; Carmeliet, P. Endothelial cells and cancer cells: Metabolic partners in crime? Curr. Opin. Hematol. 2015, 22, 234–242. [Google Scholar] [CrossRef] [PubMed]
- Furchgott, R.F.; Zawadzki, J.V. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature 1980, 299, 373–376. [Google Scholar] [CrossRef]
- Gryglewski, R.J.; Bunting, S.; Moncada, S.; Flower, R.J.; Vane, J.R. Arterial walls are protected against deposition of platelet thrombi by a substance (prostaglandin X) which they make from prostaglandin endoperoxides. Prostaglandins 1976, 12, 685–713. [Google Scholar] [CrossRef]
- Leung, D.W.; Cachianes, G.; Kuang, W.J.; Goeddel, D.V.; Ferrara, N. Vascular endothelial growth factor is a secreted angiogenic mitogen. Science 1989, 246, 1306–1309. [Google Scholar] [CrossRef]
- Dudley, A.C. Tumor endothelial cells. Cold Spring Harb. Perspect. Med. 2012, 2, a006536. [Google Scholar] [CrossRef]
- Xiao, L.; Kim, D.J.; Davis, C.L.; McCann, J.V.; Dunleavey, J.M.; Vanderlinden, A.K.; Xu, N.; Pattenden, S.G.; Frye, S.V.; Xu, X.; et al. Tumor Endothelial Cells with Distinct Patterns of TGFβ-Driven Endothelial-to-Mesenchymal Transition. Cancer Res. 2015, 75, 1244–1254. [Google Scholar] [CrossRef]
- Unterleuthner, D.; Neuhold, P.; Schwarz, K.; Janker, L.; Neuditschko, B.; Nivarthi, H.; Crncec, I.; Kramer, N.; Unger, C.; Hengstschläger, M.; et al. Cancer-associated fibroblast-derived WNT2 increases tumor angiogenesis in colon cancer. Angiogenesis 2019. [Google Scholar] [CrossRef]
- Aizawa, T.; Karasawa, H.; Funayama, R.; Shirota, M.; Suzuki, T.; Maeda, S.; Suzuki, H.; Yamamura, A.; Naitoh, T.; Nakayama, K.; et al. Cancer-associated fibroblasts secrete Wnt2 to promote cancer progression in colorectal cancer. Cancer Med. 2019, 8, 6370–6382. [Google Scholar] [CrossRef] [PubMed]
- Kramer, N.; Schmöllerl, J.; Unger, C.; Nivarthi, H.; Rudisch, A.; Unterleuthner, D.; Scherzer, M.; Riedl, A.; Artaker, M.; Crncec, I.; et al. Autocrine WNT2 signaling in fibroblasts promotes colorectal cancer progression. Oncogene 2017, 36, 5460–5472. [Google Scholar] [CrossRef] [PubMed]
- Sahai, E.; Astsaturov, I.; Cukierman, E.; DeNardo, D.G.; Egeblad, M.; Evans, R.M.; Fearon, D.; Greten, F.R.; Hingorani, S.R.; Hunter, T.; et al. A framework for advancing our understanding of cancer-associated fibroblasts. Nat. Rev. Cancer 2020, 20, 174–186. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Song, E. Turning foes to friends: Targeting cancer-associated fibroblasts. Nat. Rev. Drug Discov. 2019, 18, 9–115. [Google Scholar] [CrossRef] [PubMed]
- Félétou, M. The Endothelium, Part I: Multiple Functions of the Endothelial Cells—Focus on Endothelium-Derived Vasoactive Mediators. Colloq. Ser. Integr. Syst. Physiol. Mol. Funct. 2011, 3, 1–306. [Google Scholar] [CrossRef]
- Hida, K.; Maishi, N.; Torii, C.; Hida, Y. Tumor Angiogenesis—Characteristics of Tumor Endothelial Cells. Int. J. Clin. Oncol. 2016, 21, 206–212. [Google Scholar] [CrossRef]
- Sanctis, F.D.; Ugel, S.; Facciponte, J.; Facciabene, A. The dark side of tumor-associated endothelial cells. Semin. Immunol. 2018, 35, 35–47. [Google Scholar] [CrossRef]
- Hida, K.; Maishi, N. Abnormalities of tumor endothelial cells and cancer progression. Oral Sci. Int. 2018, 15, 1–6. [Google Scholar] [CrossRef]
- Matsuda, K.; Ohga, N.; Hida, Y.; Muraki, C.; Tsuchiya, K.; Kurosu, T.; Akino, T.; Shih, S.-C.; Totsuka, Y.; Klagsbrun, M.; et al. Isolated tumor endothelial cells maintain specific character during long-term culture. Biochem. Biophys. Res. Commun. 2010, 394, 947–954. [Google Scholar] [CrossRef]
- Ohmura-Kakutani, H.; Akiyama, K.; Maishi, N.; Ohga, N.; Hida, Y.; Kawamoto, T.; Iida, J.; Shindoh, M.; Tsuchiya, K.; Shinohara, N.; et al. Identification of Tumor Endothelial Cells with High Aldehyde Dehydrogenase Activity and a Highly Angiogenic Phenotype. PLoS ONE 2014, 9, e113910. [Google Scholar] [CrossRef]
- Fonsato, V.; Buttiglieri, S.; Deregibus, M.C.; Puntorieri, V.; Bussolati, B.; Camussi, G. Expression of Pax2 in Human Renal Tumor-Derived Endothelial Cells Sustains Apoptosis Resistance and Angiogenesis. Am. J. Pathol. 2006, 168, 706–713. [Google Scholar] [CrossRef]
- Hida, K.; Hida, Y.; Amin, D.N.; Flint, A.F.; Panigrahy, D.; Morton, C.C.; Klagsbrun, M. Tumor-Associated Endothelial Cells with Cytogenetic Abnormalities. Cancer Res. 2004, 64, 8249–8255. [Google Scholar] [CrossRef] [PubMed]
- Akino, T.; Hida, K.; Hida, Y.; Tsuchiya, K.; Freedman, D.; Muraki, C.; Ohga, N.; Matsuda, K.; Akiyama, K.; Harabayashi, T.; et al. Cytogenetic Abnormalities of Tumor-Associated Endothelial Cells in Human Malignant Tumors. Am. J. Pathol. 2009, 175, 2657–2667. [Google Scholar] [CrossRef] [PubMed]
- Hida, K.; Maishi, N.; Annan, D.; Hida, Y. Contribution of Tumor Endothelial Cells in Cancer Progression. Int. J. Mol. Sci. 2018, 19, 1272. [Google Scholar] [CrossRef]
- Naito, H.; Wakabayashi, T.; Kidoya, H.; Muramatsu, F.; Takara, K.; Eino, D.; Yamane, K.; Iba, T.; Takakura, N. Endothelial Side Population Cells Contribute to Tumor Angiogenesis and Antiangiogenic Drug Resistance. Cancer Res. 2016, 76, 3200–3210. [Google Scholar] [CrossRef] [PubMed]
- Aird, W.C. Phenotypic Heterogeneity of the Endothelium. Circ. Res. 2007, 100, 158–173. [Google Scholar] [CrossRef]
- Chang, Y.S.; Tomaso, E.D.; Mcdonald, D.M.; Jones, R.; Jain, R.K.; Munn, L.L. Mosaic blood vessels in tumors: Frequency of cancer cells in contact with flowing blood. Proc. Natl. Acad. Sci. USA 2000, 97, 14608–14613. [Google Scholar] [CrossRef]
- Hashizume, H.; Baluk, P.; Morikawa, S.; Mclean, J.W.; Thurston, G.; Roberge, S.; Jain, R.K.; Mcdonald, D.M. Openings between Defective Endothelial Cells Explain Tumor Vessel Leakiness. Am. J. Pathol. 2000, 156, 1363–1380. [Google Scholar] [CrossRef]
- Denekamp, J.; Hobson, B. Endothelial-cell proliferation in experimental tumours. Br. J. Cancer 1982, 46, 711–720. [Google Scholar] [CrossRef]
- Maishi, N.; Kikuchi, H.; Sato, M.; Nagao-Kitamoto, H.; Annan, D.A.; Baba, S.; Hojo, T.; Yanagiya, M.; Ohba, Y.; Ishii, G.; et al. Development of Immortalized Human Tumor Endothelial Cells from Renal Cancer. Int. J. Mol. Sci. 2019, 20, 4595. [Google Scholar] [CrossRef]
- Amin, D.N.; Hida, K.; Bielenberg, D.R.; Klagsbrun, M. Tumor Endothelial Cells Express Epidermal Growth Factor Receptor (EGFR) but not ErbB3 and Are Responsive to EGF and to EGFR Kinase Inhibitors. Cancer Res. 2006, 66, 2173–2180. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y. Persistent vascular normalization as an alternative goal of anti-angiogenic cancer therapy. Cancer Sci. 2011, 102, 1253–1256. [Google Scholar] [CrossRef] [PubMed]
- Helfrich, I.; Scheffrahn, I.; Bartling, S.; Weis, J.; Felbert, V.V.; Middleton, M.; Kato, M.; Ergün, S.; Augustin, H.G.; Schadendorf, D. Resistance to antiangiogenic therapy is directed by vascular phenotype, vessel stabilization, and maturation in malignant melanoma. J. Exp. Med. 2010, 207, 491–503. [Google Scholar] [CrossRef] [PubMed]
- Taylor, S.M.; Nevis, K.R.; Park, H.L.; Rogers, G.C.; Rogers, S.L.; Cook, J.G.; Bautch, V.L. Angiogenic factor signaling regulates centrosome duplication in endothelial cells of developing blood vessels. Blood 2010, 116, 3108–3117. [Google Scholar] [CrossRef] [PubMed]
- Ohga, N.; Ishikawa, S.; Maishi, N.; Akiyama, K.; Hida, Y.; Kawamoto, T.; Sadamoto, Y.; Osawa, T.; Yamamoto, K.; Kondoh, M.; et al. Heterogeneity of Tumor Endothelial Cells. Am. J. Pathol. 2012, 180, 1294–1307. [Google Scholar] [CrossRef] [PubMed]
- Lyssiotis, C.A.; Kimmelman, A.C. Metabolic Interactions in the Tumor Microenvironment. Trends Cell Biol. 2017, 27, 863–875. [Google Scholar] [CrossRef]
- Zecchin, A.; Kalucka, J.; Dubois, C.; Carmeliet, P. How Endothelial Cells Adapt Their Metabolism to Form Vessels in Tumors. Front. Immunol. 2017, 8, 1750. [Google Scholar] [CrossRef]
- Cantelmo, A.R.; Conradi, L.C.; Brajic, A.; Goveia, J.; Kalucka, J.; Pircher, A.; Chaturvedi, P.; Hol, J.; Thienpont, B.; Teuwen, L.A.; et al. Inhibition of the Glycolytic Activator PFKFB3 in Endothelium Induces Tumor Vessel Normalization, Impairs Metastasis, and Improves Chemotherapy. Cancer Cell 2016, 30, 968–985. [Google Scholar] [CrossRef]
- Annan, D.A.; Maishi, N.; Soga, T.; Dawood, R.; Li, C.; Kikuchi, H.; Hojo, T.; Morimoto, M.; Kitamura, T.; Alam, M.T.; et al. Carbonic anhydrase 2 (CAII) supports tumor blood endothelial cell survival under lactic acidosis in the tumor microenvironment. Cell Commun. Signal. 2019, 17, 169. [Google Scholar] [CrossRef]
- De Bock, K.; Georgiadou, M.; Schoors, S.; Kuchnio, A.; Wong, B.W.; Cantelmo, A.R.; Quaegebeur, A.; Ghesquière, B.; Cauwenberghs, S.; Eelen, G.; et al. Role of PFKFB3-Driven Glycolysis in Vessel Sprouting. Cell 2013, 154, 651–663. [Google Scholar] [CrossRef]
- Fang, L.; Choi, S.-H.; Baek, J.S.; Liu, C.; Almazan, F.; Ulrich, F.; Wiesner, P.; Taleb, A.; Deer, E.; Pattison, J.; et al. Control of angiogenesis by AIBP-mediated cholesterol efflux. Nature 2013, 498, 118–122. [Google Scholar] [CrossRef] [PubMed]
- Elmasri, H.; Karaaslan, C.; Teper, Y.; Ghelfi, E.; Weng, M.; Ince, T.A.; Kozakewich, H.; Bischoff, J.; Cataltepe, S. Fatty acid binding protein 4 is a target of VEGF and a regulator of cell proliferation in endothelial cells. FASEB J. 2009, 23, 3865–3873. [Google Scholar] [CrossRef] [PubMed]
- Schoors, S.; Bruning, U.; Missiaen, R.; Queiroz, K.C.S.; Borgers, G.; Elia, I.; Zecchin, A.; Cantelmo, A.R.; Christen, S.; Goveia, J.; et al. Fatty acid carbon is essential for dNTP synthesis in endothelial cells. Nature 2015, 520, 192–197. [Google Scholar] [CrossRef] [PubMed]
- Krautkramer, K.A.; Kreznar, J.H.; Romano, K.A.; Vivas, E.I.; Barrett-Wilt, G.A.; Rabaglia, M.E.; Keller, M.P.; Attie, A.D.; Rey, F.E.; Denu, J.M. Diet-Microbiota Interactions Mediate Global Epigenetic Programming in Multiple Host Tissues. Mol. Cell 2016, 64, 982–992. [Google Scholar] [CrossRef]
- Alves, A.; Bassot, A.; Bulteau, A.-L.; Pirola, L.; Morio, B. Glycine Metabolism and Its Alterations in Obesity and Metabolic Diseases. Nutrients 2019, 11, 1356. [Google Scholar] [CrossRef]
- Loscalzo, J.; Handy, D.E. Epigenetic Modifications: Basic Mechanisms and Role in Cardiovascular Disease (2013 Grover Conference Series). Pulm. Circ. 2014, 4, 169–174. [Google Scholar] [CrossRef]
- Dupont, C.; Armant, D.; Brenner, C. Epigenetics: Definition, Mechanisms and Clinical Perspective. Semin. Reprod. Med. 2009, 27, 351–357. [Google Scholar] [CrossRef]
- Pirola, L.; Ciesielski, O.; Balcerczyk, A. The Methylation Status of the Epigenome: Its Emerging Role in the Regulation of Tumor Angiogenesis and Tumor Growth, and Potential for Drug Targeting. Cancers 2018, 10, 268. [Google Scholar] [CrossRef]
- Søreide, K. Cancer Epigenetics. In Handbook of Epigenetics; Elsevier: Amsterdam, The Netherlands, 2017; pp. 519–534. [Google Scholar]
- Mehta, A.; Dobersch, S.; Romero-Olmedo, A.J.; Barreto, G. Epigenetics in lung cancer diagnosis and therapy. Cancer Metastasis Rev. 2015, 34, 229–241. [Google Scholar] [CrossRef]
- Michalak, E.M.; Burr, M.L.; Bannister, A.J.; Dawson, M.A. The roles of DNA, RNA and histone methylation in ageing and cancer. Nat. Rev. Mol. Cell Biol. 2019, 20, 573–589. [Google Scholar] [CrossRef]
- Morgan, A.E.; Davies, J.T.; Mc Auley, T.M. The Role of DNA Methylation in Ageing and Cancer. Proc. Nutr. Soc. 2018, 77, 412–422. [Google Scholar] [CrossRef] [PubMed]
- Kurdistani, S.K. Histone Modifications in Cancer Biology and Prognosis. Epigenetics Dis. 2010, 91–106. [Google Scholar]
- Yan, M.S.; Marsden, P.A. Epigenetics in the Vascular Endothelium. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 2297–2306. [Google Scholar] [CrossRef] [PubMed]
- Majerski, A.A.; Quinton, A.C.; Marsden, P.A. Epigenetic Mechanisms of the Vascular Endothelium. Epigenet. Epigenom. 2014. [Google Scholar] [CrossRef]
- Dunn, J.; Thabet, S.; Jo, H. Flow-Dependent Epigenetic DNA Methylation in Endothelial Gene Expression and Atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 1562–1569. [Google Scholar] [CrossRef] [PubMed]
- Fish, J.E.; Matouk, C.C.; Rachlis, A.; Lin, S.; Tai, S.C.; Dabreo, C.; Marsden, P.A. The Expression of Endothelial Nitric-oxide Synthase Is Controlled by a Cell-specific Histone Code. J. Biol. Chem. 2005, 280, 24824–24838. [Google Scholar] [CrossRef]
- Costantino, S. Epigenetic mechanisms of vascular dysfunction in obesity and type 2 diabetes. Cardiovasc. Med. 2019, 22, w02066. [Google Scholar] [CrossRef]
- Lee, D.; Chiu, J. Atherosclerosis and Flow: Roles of Epigenetic Modulation in Vascular Endothelium. J. Biomed. Sci. 2019, 26, 56. [Google Scholar] [CrossRef]
- Jones, P.A. Functions of DNA methylation: Islands, start sites, gene bodies and beyond. Nat. Rev. Genet. 2012, 13, 484–492. [Google Scholar] [CrossRef]
- Chan, Y.; Fish, J.E.; Dabreo, C.; Lin, S.; Robb, G.B.; Teichert, A.-M.; Karantzoulis-Fegaras, F.; Keightley, A.; Steer, B.M.; Marsden, P.A. The Cell-specific Expression of Endothelial Nitric-oxide Synthase. J. Biol. Chem. 2004, 279, 35087–35100. [Google Scholar] [CrossRef]
- Shirodkar, A.V.; Bernard, R.S.; Gavryushova, A.; Kop, A.; Knight, B.J.; Yan, M.S.-C.; Man, H.-S.J.; Sud, M.; Hebbel, R.P.; Oettgen, P.; et al. A mechanistic role for DNA methylation in endothelial cell (EC)-enriched gene expression: Relationship with DNA replication timing. Blood 2013, 121, 3531–3540. [Google Scholar] [CrossRef] [PubMed]
- Hellebrekers, D.M.; Melotte, V.; Vire, E.; Langenkamp, E.; Molema, G.; Fuks, F.; Herman, J.G.; Criekinge, W.V.; Griffioen, A.W.; Engeland, M.V. Identification of Epigenetically Silenced Genes in Tumor Endothelial Cells. Cancer Res. 2007, 67, 4138–4148. [Google Scholar] [CrossRef] [PubMed]
- Hellebrekers, D.M.; Castermans, K.; Vire, E.; Dings, R.P.; Hoebers, N.T.; Mayo, K.H.; Egbrink, M.G.O.; Molema, G.; Fuks, F.; Engeland, M.V.; et al. Epigenetic Regulation of Tumor Endothelial Cell Anergy: Silencing of Intercellular Adhesion Molecule-1 by Histone Modifications. Cancer Res. 2006, 66, 10770–10777. [Google Scholar] [CrossRef] [PubMed]
- Luo, W.; Qiang, H.; Wang, D.; Deeb, K.K.; Ma, Y.; Morrison, C.D.; Liu, S.; Johnson, C.S.; Trump, D.L. Isolation and genome-wide expression and methylation characterization of CD31 cells from normal and malignant human prostate tissue. Oncotarget 2013, 4, 1472. [Google Scholar] [CrossRef]
- Deeb, K.K.; Luo, W.; Karpf, A.R.; Omilian, A.R.; Bshara, W.; Tian, L.; Tangrea, M.A.; Morrison, C.D.; Johnson, C.S.; Trump, D.L. Differential vitamin D 24-hydroxylase/CYP24A1gene promoter methylation in endothelium from benign and malignant human prostate. Epigenetics 2011, 6, 994–1000. [Google Scholar] [CrossRef]
- Croix, B.S. Genes Expressed in Human Tumor Endothelium. Science 2000, 289, 1197–1202. [Google Scholar] [CrossRef]
- Jones, D.T.; Lechertier, T.; Mitter, R.; Herbert, J.M.J.; Bicknell, R.; Jones, J.L.; Li, J.-L.; Buffa, F.; Harris, A.L.; Hodivala-Dilke, K. Gene Expression Analysis in Human Breast Cancer Associated Blood Vessels. PLoS ONE 2012, 7, e44294. [Google Scholar] [CrossRef]
- Chung, I.; Karpf, A.R.; Muindi, J.R.; Conroy, J.M.; Nowak, N.J.; Johnson, C.S.; Trump, D.L. Epigenetic silencing of CYP24 in tumor-derived endothelial cells contributes to selective growth inhibition by calcitriol. J. Biol. Chem. 2007, 282, 8704–8714. [Google Scholar] [CrossRef]
- Liu, B.; Xu, T.; Xu, X.; Cui, Y.; Xing, X. Biglycan promotes the chemotherapy resistance of colon cancer by activating NF-κB signal transduction. Mol. Cell. Biochem. 2018, 449, 285–294. [Google Scholar] [CrossRef]
- Maishi, N.; Ohba, Y.; Akiyama, K.; Ohga, N.; Hamada, J.-I.; Nagao-Kitamoto, H.; Alam, M.T.; Yamamoto, K.; Kawamoto, T.; Inoue, N.; et al. Tumour endothelial cells in high metastatic tumours promote metastasis via epigenetic dysregulation of biglycan. Sci. Rep. 2016, 6, 28039. [Google Scholar] [CrossRef]
- Bannister, A.J.; Kouzarides, T. Regulation of chromatin by histone modifications. Cell Res. 2011, 21, 381–395. [Google Scholar] [CrossRef] [PubMed]
- Eberharter, A.; Becker, P.B. Histone acetylation: A switch between repressive and permissive chromatin. EMBO Rep. 2002, 3, 224–229. [Google Scholar] [CrossRef] [PubMed]
- Gregory, P.D.; Wagner, K.; Hörz, W. Histone Acetylation and Chromatin Remodeling. Exp. Cell Res. 2001, 265, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Bannister, A.J.; Schneider, R.; Kouzarides, T. Histone Methylation. Cell 2002, 109, 801–806. [Google Scholar] [CrossRef]
- Howe, F.S.; Fischl, H.; Murray, S.C.; Mellor, J. Is H3K4me3 instructive for transcription activation? BioEssays 2016, 39, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Bacanamwo, M.; Harrison, D.G. Activation of p300 Histone Acetyltransferase Activity Is an Early Endothelial Response to Laminar Shear Stress and Is Essential for Stimulation of Endothelial Nitric-oxide Synthase mRNA Transcription. J. Biol. Chem. 2008, 283, 16293–16298. [Google Scholar] [CrossRef]
- Urbich, C.; Rössig, L.; Kaluza, D.; Potente, M.; Boeckel, J.-N.; Knau, A.; Diehl, F.; Geng, J.-G.; Hofmann, W.-K.; Zeiher, A.M.; et al. HDAC5 is a repressor of angiogenesis and determines the angiogenic gene expression pattern of endothelial cells. Blood 2009, 113, 5669–5679. [Google Scholar] [CrossRef]
- Park, D.; Park, H.; Kim, Y.; Kim, H.; Jeoung, D. HDAC3 acts as a negative regulator of angiogenesis. BMB Rep. 2014, 47, 227–232. [Google Scholar] [CrossRef]
- Thambyrajah, R.; Fadlullah, M.Z.; Proffitt, M.; Patel, R.; Cowley, S.M.; Kouskoff, V.; Lacaud, G. HDAC1 and HDAC2 Modulate TGF-β Signaling during Endothelial-to-Hematopoietic Transition. Stem Cell Rep. 2018, 10, 1369–1383. [Google Scholar] [CrossRef]
- Margariti, A.; Zampetaki, A.; Xiao, Q.; Zhou, B.; Karamariti, E.; Martin, D.; Yin, X.; Mayr, M.; Li, H.; Zhang, Z.; et al. Histone Deacetylase 7 Controls Endothelial Cell Growth Through Modulation of β-Catenin. Circ. Res. 2010, 106, 1202–1211. [Google Scholar] [CrossRef]
- Barroso, M.; Kao, D.; Blom, H.J.; Almeida, I.T.D.; Castro, R.; Loscalzo, J.; Handy, D.E. S-adenosylhomocysteine induces inflammation through NFkB: A possible role for EZH2 in endothelial cell activation. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2016, 1862, 82–92. [Google Scholar] [CrossRef] [PubMed]
- Balcerczyk, A.; Rybaczek, D.; Wojtala, M.; Pirola, L.; Okabe, J.; El-Osta, A. Pharmacological inhibition of arginine and lysine methyltransferases induces nuclear abnormalities and suppresses angiogenesis in human endothelial cells. Biochem. Pharmacol. 2016, 121, 18–32. [Google Scholar] [CrossRef] [PubMed]
- Wojtala, M.; Macierzyńska-Piotrowska, E.; Rybaczek, D.; Pirola, L.; Balcerczyk, A. Pharmacological and transcriptional inhibition of the G9a histone methyltransferase suppresses proliferation and modulates redox homeostasis in human microvascular endothelial cells. Pharmacol. Res. 2018, 128, 252–263. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.; Wu, X.; Zhao, Q.; Gao, J.; Huo, D.; Liu, X.; Ye, Z.; Dong, X.; Fu, Z.; Shang, Y.; et al. DOT1L promotes angiogenesis through cooperative regulation of VEGFR2 with ETS-1. Oncotarget 2016, 7, 69674–69687. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.-N.; Xu, Q.-Q.; Thakur, A.; Alfred, M.O.; Chakraborty, M.; Ghosh, A.; Yu, X.-B. Endothelial dysfunction in diabetes and hypertension: Role of microRNAs and long non-coding RNAs. Life Sci. 2018, 213, 258–268. [Google Scholar] [CrossRef]
- Orso, F.; Quirico, L.; Dettori, D.; Coppo, R.; Virga, F.; Ferreira, L.C.; Paoletti, C.; Baruffaldi, D.; Penna, E.; Taverna, D. Role of miRNAs in tumor and endothelial cell interactions during tumor progression. Semin. Cancer Biol. 2020, 60, 214–224. [Google Scholar] [CrossRef]
- Wang, S.; Aurora, A.B.; Johnson, B.A.; Qi, X.; Mcanally, J.; Hill, J.A.; Richardson, J.A.; Bassel-Duby, R.; Olson, E.N. The Endothelial-Specific MicroRNA miR-126 Governs Vascular Integrity and Angiogenesis. Dev. Cell 2008, 15, 261–271. [Google Scholar] [CrossRef]
- Fish, J.E.; Santoro, M.M.; Morton, S.U.; Yu, S.; Yeh, R.-F.; Wythe, J.D.; Ivey, K.N.; Bruneau, B.G.; Stainier, D.Y.; Srivastava, D. miR-126 Regulates Angiogenic Signaling and Vascular Integrity. Dev. Cell 2008, 15, 272–284. [Google Scholar] [CrossRef]
- Sessa, R.; Seano, G.; Blasio, L.D.; Gagliardi, P.A.; Isella, C.; Medico, E.; Cotelli, F.; Bussolino, F.; Primo, L. The miR-126 regulates Angiopoietin-1 signaling and vessel maturation by targeting p85β. Biochim. Biophys. Acta (BBA) Mol. Cell Res. 2012, 1823, 1925–1935. [Google Scholar] [CrossRef]
- Solingen, C.V.; Seghers, L.; Bijkerk, R.; Duijs, J.M.; Roeten, M.K.; Oeveren-Rietdijk, A.M.V.; Baelde, H.J.; Monge, M.; Vos, J.B.; Boer, H.C.D.; et al. Antagomir-mediated silencing of endothelial cell specific microRNA-126 impairs ischemia-induced angiogenesis. J. Cell. Mol. Med. 2008, 13, 1577–1585. [Google Scholar] [CrossRef]
- Sun, H.-X.; Zeng, D.-Y.; Li, R.-T.; Pang, R.-P.; Yang, H.; Hu, Y.-L.; Zhang, Q.; Jiang, Y.; Huang, L.-Y.; Tang, Y.-B.; et al. Essential Role of MicroRNA-155 in Regulating Endothelium-Dependent Vasorelaxation by Targeting Endothelial Nitric Oxide Synthase. Hypertension 2012, 60, 1407–1414. [Google Scholar] [CrossRef] [PubMed]
- Muramatsu, F.; Kidoya, H.; Naito, H.; Sakimoto, S.; Takakura, N. microRNA-125b inhibits tube formation of blood vessels through translational suppression of VE-cadherin. Oncogene 2012, 32, 414–421. [Google Scholar] [CrossRef] [PubMed]
- Hung, J.; Miscianinov, V.; Sluimer, J.C.; Newby, D.E.; Baker, A.H. Targeting Non-coding RNA in Vascular Biology and Disease. Front. Physiol. 2018, 9, 1655. [Google Scholar] [CrossRef] [PubMed]
- Salybekov, A.A.; Salybekova, A.K.; Pola, R.; Asahara, T. Sonic Hedgehog Signaling Pathway in Endothelial Progenitor Cell Biology for Vascular Medicine. Int. J. Mol. Sci. 2018, 19, 3040. [Google Scholar] [CrossRef]
- Pan, Z.; Tian, Y.; Niu, G.; Cao, C. Role of microRNAs in remodeling the tumor microenvironment (Review). Int. J. Oncol. 2019. [Google Scholar] [CrossRef]
- Zhu, K.; Pan, Q.; Zhang, X.; Kong, L.-Q.; Fan, J.; Dai, Z.; Wang, L.; Yang, X.-R.; Hu, J.; Wan, J.-L.; et al. MiR-146a enhances angiogenic activity of endothelial cells in hepatocellular carcinoma by promoting PDGFRA expression. Carcinogenesis 2013, 34, 2071–2079. [Google Scholar] [CrossRef]
- Würdinger, T.; Tannous, B.A.; Saydam, O.; Skog, J.; Grau, S.; Soutschek, J.; Weissleder, R.; Breakefield, X.O.; Krichevsky, A.M. miR-296 Regulates Growth Factor Receptor Overexpression in Angiogenic Endothelial Cells. Cancer Cell 2008, 14, 382–393. [Google Scholar] [CrossRef]
- Thuringer, D.; Boucher, J.; Jego, G.; Pernet, N.; Cronier, L.; Hammann, A.; Solary, E.; Garrido, C. Transfer of functional microRNAs between glioblastoma and microvascular endothelial cells through gap junctions. Oncotarget 2016, 7, 73925–73934. [Google Scholar] [CrossRef]
- Zeng, Z.; Li, Y.; Pan, Y.; Lan, X.; Song, F.; Sun, J.; Zhou, K.; Liu, X.; Ren, X.; Wang, F.; et al. Cancer-derived exosomal miR-25-3p promotes pre-metastatic niche formation by inducing vascular permeability and angiogenesis. Nat. Commun. 2018, 9, 5395. [Google Scholar] [CrossRef]
- Kosaka, N.; Iguchi, H.; Hagiwara, K.; Yoshioka, Y.; Takeshita, F.; Ochiya, T. Neutral Sphingomyelinase 2 (nSMase2)-dependent Exosomal Transfer of Angiogenic MicroRNAs Regulate Cancer Cell Metastasis. J. Biol. Chem. 2013, 288, 10849–10859. [Google Scholar] [CrossRef]
- Fan, Y.-C.; Mei, P.-J.; Chen, C.; Miao, F.-A.; Zhang, H.; Li, Z.-L. MiR-29c inhibits glioma cell proliferation, migration, invasion and angiogenesis. J. Neuro-Oncol. 2013, 115, 179–188. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.K.; Park, S.R.; Jung, B.K.; Jeon, Y.K.; Lee, Y.S.; Kim, M.K.; Kim, Y.G.; Jang, J.Y.; Kim, C.W. Exosomes derived from mesenchymal stem cells suppress angiogenesis by down-regulating VEGF expression in breast cancer cells. PLoS ONE 2013, 8, e84256. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, G.; Wu, X.; Jiang, Z.; Kasman, I.; Yao, J.; Guan, Y.; Oeh, J.; Modrusan, Z.; Bais, C.; Sampath, D.; et al. Tumour-secreted miR-9 promotes endothelial cell migration and angiogenesis by activating the JAK-STAT pathway. EMBO J. 2012, 31, 3513–3523. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Yang, F.; Zhang, T.; Wang, W.; Xi, W.; Li, Y.; Zhang, D.; Huo, Y.; Zhang, J.; Yang, A.; et al. MiR-9 promotes tumorigenesis and angiogenesis and is activated by MYC and OCT4 in human glioma. J. Exp. Clin. Cancer Res. 2019, 38, 99. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Zhang, H.; Ge, S.; Ning, T.; Bai, M.; Li, J.; Li, S.; Sun, W.; Deng, T.; Zhang, L.; et al. Exosome-Derived miR-130a Activates Angiogenesis in Gastric Cancer by Targeting C-MYB in Vascular Endothelial Cells. Mol. Ther. 2018, 26, 2466–2475. [Google Scholar] [CrossRef]
- Liang, L.; Zhao, L.; Zan, Y.; Zhu, Q.; Ren, J.; Zhao, X. MiR-93-5p enhances growth and angiogenesis capacity of HUVECs by down-regulating EPLIN. Oncotarget 2017, 8, 107033–107043. [Google Scholar] [CrossRef]
- Fang, L.; Deng, Z.; Shatseva, T.; Yang, J.; Peng, C.; Du, W.W.; Yee, A.J.; Ang, L.C.; He, C.; Shan, S.W.; et al. MicroRNA miR-93 promotes tumor growth and angiogenesis by targeting integrin-β8. Oncogene 2010, 30, 806–821. [Google Scholar] [CrossRef]
- Kong, W.; He, L.; Richards, E.J.; Challa, S.; Xu, C.-X.; Permuth-Wey, J.; Lancaster, J.M.; Coppola, D.; Sellers, T.A.; Djeu, J.Y.; et al. Upregulation of miRNA-155 promotes tumour angiogenesis by targeting VHL and is associated with poor prognosis and triple-negative breast cancer. Oncogene 2013, 33, 679–689. [Google Scholar] [CrossRef]
- Mao, G.; Liu, Y.; Fang, X.; Liu, Y.; Fang, L.; Lin, L.; Liu, X.; Wang, N. Tumor-derived microRNA-494 promotes angiogenesis in non-small cell lung cancer. Angiogenesis 2015, 18, 373–382. [Google Scholar] [CrossRef]
- Liu, X.; Gao, X.; Zhang, W.; Zhu, T.; Bi, W.; Zhang, Y. MicroRNA-204 deregulation in lung adenocarcinoma controls the biological behaviors of endothelial cells potentially by modulating Janus kinase 2-signal transducer and activator of transcription 3 pathway. IUBMB Life 2017, 70, 81–91. [Google Scholar] [CrossRef]
- Zhang, L.; Lv, Z.; Xu, J.; Chen, C.; Ge, Q.; Li, P.; Wei, D.; Wu, Z.; Sun, X. Micro RNA -134 inhibits osteosarcoma angiogenesis and proliferation by targeting the VEGFA / VEGFR 1 pathway. FEBS J. 2018, 285, 1359–1371. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.-H.; Tsai, H.-C.; Cheng, Y.-C.; Lin, C.-Y.; Huang, Y.-L.; Tsai, C.-H.; Xu, G.-H.; Wang, S.-W.; Fong, Y.-C.; Tang, C.-H. CTGF promotes osteosarcoma angiogenesis by regulating miR-543/angiopoietin 2 signaling. Cancer Lett. 2017, 391, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Yamakuchi, M.; Lotterman, C.D.; Bao, C.; Hruban, R.H.; Karim, B.; Mendell, J.T.; Huso, D.; Lowenstein, C.J. P53-induced microRNA-107 inhibits HIF-1 and tumor angiogenesis. Proc. Natl. Acad. Sci. USA 2010, 107, 6334–6339. [Google Scholar] [CrossRef]
- Yan, J.-J.; Zhang, Y.-N.; Liao, J.-Z.; Ke, K.-P.; Chang, Y.; Li, P.-Y.; Wang, M.; Lin, J.-S.; He, X.-X. MiR-497 suppresses angiogenesis and metastasis of hepatocellular carcinoma by inhibiting VEGFA and AEG-1. Oncotarget 2015, 6, 29527–29542. [Google Scholar] [CrossRef] [PubMed]
- Tu, Y.; Liu, L.; Zhao, D.; Liu, Y.; Ma, X.; Fan, Y.; Wan, L.; Huang, T.; Cheng, Z.; Shen, B. Overexpression of miRNA-497 inhibits tumor angiogenesis by targeting VEGFR2. Sci. Rep. 2015, 5, 13827. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.-H.; Zhou, H.-C.; Zeng, C.; Yang, J.; Liu, Y.; Huang, X.; Zhang, J.-P.; Guan, X.-Y.; Zhuang, S.-M. MicroRNA-29b suppresses tumor angiogenesis, invasion, and metastasis by regulating matrix metalloproteinase 2 expression. Hepatology 2011, 54, 1729–1740. [Google Scholar] [CrossRef]
- Chou, J.; Lin, J.H.; Brenot, A.; Kim, J.-W.; Provot, S.; Werb, Z. GATA3 suppresses metastasis and modulates the tumour microenvironment by regulating microRNA-29b expression. Nat. Cell Biol. 2013, 15, 201–213. [Google Scholar] [CrossRef]
- Pecot, C.V.; Rupaimoole, R.; Yang, D.; Akbani, R.; Ivan, C.; Lu, C.; Wu, S.; Han, H.-D.; Shah, M.Y.; Rodriguez-Aguayo, C.; et al. Tumour angiogenesis regulation by the miR-200 family. Nat. Commun. 2013, 4, 2427. [Google Scholar] [CrossRef]
- Shih, T.-C.; Tien, Y.-J.; Wen, C.-J.; Yeh, T.-S.; Yu, M.-C.; Huang, C.-H.; Lee, Y.-S.; Yen, T.-C.; Hsieh, S.-Y. MicroRNA-214 downregulation contributes to tumor angiogenesis by inducing secretion of the hepatoma-derived growth factor in human hepatoma. J. Hepatol. 2012, 57, 584–591. [Google Scholar] [CrossRef]
- Du, C.; Lv, Z.; Cao, L.; Ding, C.; Gyabaah, O.-A.K.; Xie, H.; Zhou, L.; Wu, J.; Zheng, S. MiR-126-3p suppresses tumor metastasis and angiogenesis of hepatocellular carcinoma by targeting LRP6 and PIK3R2. J. Transl. Med. 2014, 12, 259. [Google Scholar] [CrossRef]
- Chen, H.; Li, L.; Wang, S.; Lei, Y.; Ge, Q.; Lv, N.; Zhou, X.; Chen, C. Reduced miR-126 expression facilitates angiogenesis of gastric cancer through its regulation on VEGF-A. Oncotarget 2014, 5, 11873–11885. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Sun, B.; Wang, J.; Wang, Y. miR-622 inhibits angiogenesis by suppressing the CXCR4–VEGFA axis in colorectal cancer. Gene 2019, 699, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Yin, Q.; Wang, P.-P.; Peng, R.; Zhou, H. MiR-19a enhances cell proliferation, migration, and invasiveness through enhancing lymphangiogenesis by targeting thrombospondin-1 in colorectal cancer. Biochem. Cell Biol. 2019, 97, 731–739. [Google Scholar] [CrossRef] [PubMed]
- Yamada, N.; Nakagawa, Y.; Tsujimura, N.; Kumazaki, M.; Noguchi, S.; Mori, T.; Hirata, I.; Maruo, K.; Akao, Y. Role of Intracellular and Extracellular MicroRNA-92a in Colorectal Cancer. Transl. Oncol. 2013, 6, 482–492. [Google Scholar] [CrossRef]
- Kochan-Jamrozy, K.; Króliczewski, J.; Moszyńska, A.; Collawn, J.F.; Bartoszewski, R. miRNA networks modulate human endothelial cell adaptation to cyclic hypoxia. Cell. Signal. 2019, 54, 150–160. [Google Scholar] [CrossRef]
- Zhao, D.; Tu, Y.; Wan, L.; Bu, L.; Huang, T.; Sun, X.; Wang, K.; Shen, B. In Vivo Monitoring of Angiogenesis Inhibition via Down-Regulation of Mir-21 in a VEGFR2-Luc Murine Breast Cancer Model Using Bioluminescent Imaging. PLoS ONE 2013, 8, e71472. [Google Scholar] [CrossRef]
- Sabatel, C.; Malvaux, L.; Bovy, N.; Deroanne, C.; Lambert, V.; Gonzalez, M.-L.A.; Colige, A.; Rakic, J.-M.; Noël, A.; Martial, J.A.; et al. MicroRNA-21 Exhibits Antiangiogenic Function by Targeting RhoB Expression in Endothelial Cells. PLoS ONE 2011, 6, e16979. [Google Scholar] [CrossRef]
- Veliceasa, D.; Biyashev, D.; Qin, G.; Misener, S.; Mackie, A.R.; Kishore, R.; Volpert, O.V. Therapeutic manipulation of angiogenesis with miR-27b. Vasc. Cell 2015, 7, 6. [Google Scholar] [CrossRef]
- Liu, H.-T.; Xing, A.-Y.; Chen, X.; Ma, R.-R.; Wang, Y.-W.; Shi, D.-B.; Zhang, H.; Li, P.; Chen, H.-F.; Li, Y.-H.; et al. MicroRNA-27b, microRNA-101 and microRNA-128 inhibit angiogenesis by down-regulating vascular endothelial growth factor C expression in gastric cancers. Oncotarget 2015, 6, 37458–37470. [Google Scholar] [CrossRef]
- Maishi, N.; Annan, D.A.; Kikuchi, H.; Hida, Y.; Hida, K. Tumor Endothelial Heterogeneity in Cancer Progression. Cancers 2019, 11, 1511. [Google Scholar] [CrossRef]
- Gavard, J.; Gutkind, J.S. VEGF controls endothelial-cell permeability by promoting the β-arrestin-dependent endocytosis of VE-cadherin. Nat. Cell Biol. 2006, 8, 1223–1234. [Google Scholar] [CrossRef]
- Butler, J.M.; Kobayashi, H.; Rafii, S. Instructive role of the vascular niche in promoting tumour growth and tissue repair by angiocrine factors. Nat. Rev. Cancer 2010, 10, 138–146. [Google Scholar] [CrossRef]
- Pirtskhalaishvili, G.; Nelson, J.B. Endothelium-derived factors as paracrine mediators of prostate cancer progression. Prostate 2000, 44, 77–87. [Google Scholar] [CrossRef]
- Brantley-Sieders, D.M.; Dunaway, C.M.; Rao, M.; Short, S.; Hwang, Y.; Gao, Y.; Li, D.; Jiang, A.; Shyr, Y.; Wu, J.Y.; et al. Angiocrine Factors Modulate Tumor Proliferation and Motility through EphA2 Repression of Slit2 Tumor Suppressor Function in Endothelium. Cancer Res. 2010, 71, 976–987. [Google Scholar] [CrossRef] [PubMed]
- Wieland, E.; Rodriguez-Vita, J.; Liebler, S.S.; Mogler, C.; Moll, I.; Herberich, S.E.; Espinet, E.; Herpel, E.; Menuchin, A.; Chang-Claude, J.; et al. Endothelial Notch1 Activity Facilitates Metastasis. Cancer Cell 2017, 31, 355–367. [Google Scholar] [CrossRef] [PubMed]
- Maishi, N.; Ohga, N.; Hida, Y.; Akiyama, K.; Kitayama, K.; Osawa, T.; Onodera, Y.; Shinohara, N.; Nonomura, K.; Shindoh, M.; et al. CXCR7: A novel tumor endothelial marker in renal cell carcinoma. Pathol. Int. 2012, 62, 309–317. [Google Scholar] [CrossRef]
- Berahovich, R.D.; Zabel, B.A.; Lewén, S.; Walters, M.J.; Ebsworth, K.; Wang, Y.; Jaen, J.C.; Schall, T.J. Endothelial expression of CXCR7 and the regulation of systemic CXCL12 levels. Immunology 2013, 141, 111–122. [Google Scholar] [CrossRef]
- Stacer, A.C.; Fenner, J.; Cavnar, S.P.; Xiao, A.; Zhao, S.; Chang, S.L.; Salomonnson, A.; Luker, K.E.; Luker, G.D. Endothelial CXCR7 regulates breast cancer metastasis. Oncogene 2015, 35, 1716–1724. [Google Scholar] [CrossRef]
- Yadav, A.; Kumar, B.; Yu, J.-G.; Old, M.; Teknos, T.N.; Kumar, P. Tumor-Associated Endothelial Cells Promote Tumor Metastasis by Chaperoning Circulating Tumor Cells and Protecting Them from Anoikis. PLoS ONE 2015, 10, e0141602. [Google Scholar] [CrossRef]
- Leone, P.; Di Lernia, G.; Solimando, A.G.; Cicco, S.; Saltarella, I.; Lamanuzzi, A.; Ria, R.; Frassanito, M.A.; Ponzoni, M.; Ditonno, P.; et al. Bone marrow endothelial cells sustain a tumor-specific CD8+ T cell subset with suppressive function in myeloma patients. Oncoimmunology 2018, 8, e1486949. [Google Scholar] [CrossRef]
- Aleksakhina, S.N.; Kashyap, A.; Imyanitov, E.N. Mechanisms of acquired tumor drug resistance. Biochim. Biophys. Acta (BBA) Rev. Cancer 2019, 1872, 188310. [Google Scholar] [CrossRef] [PubMed]
- Quintanal-Villalonga, Á.; Chan, J.M.; Yu, H.A.; Pe’Er, D.; Sawyers, C.L.; Triparna, S.; Rudin, C.M. Lineage plasticity in cancer: A shared pathway of therapeutic resistance. Nat. Rev. Clin. Oncol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Raffaghello, L.; Longo, V.D. Starvation, detoxification, and multidrug resistance in cancer therapy. Drug Resist. Updates 2012, 15, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Hida, K.; Akiyama, K.; Ohga, N.; Maishi, N.; Hida, Y. Tumour endothelial cells acquire drug resistance in a tumour microenvironment. J. Biochem. 2013, 153, 243–249. [Google Scholar] [CrossRef]
- Akiyama, K.; Ohga, N.; Hida, Y.; Kawamoto, T.; Sadamoto, Y.; Ishikawa, S.; Maishi, N.; Akino, T.; Kondoh, M.; Matsuda, A.; et al. Tumor Endothelial Cells Acquire Drug Resistance by MDR1 Up-Regulation via VEGF Signaling in Tumor Microenvironment. Am. J. Pathol. 2012, 180, 1283–1293. [Google Scholar] [CrossRef]
- Bussolati, B.; Deambrosis, I.; Russo, S.; Deregibus, M.C.; Camussi, G. Altered angiogenesis and survival in human tumor-derived endothelial cells. FASEB J. 2003, 17, 1159–1161. [Google Scholar] [CrossRef]
- Xiong, Y.-Q.; Sun, H.-C.; Zhang, W.; Zhu, X.-D.; Zhuang, P.-Y.; Zhang, J.-B.; Wang, L.; Wu, W.-Z.; Qin, L.-X.; Tang, Z.-Y. Human Hepatocellular Carcinoma Tumor-derived Endothelial Cells Manifest Increased Angiogenesis Capability and Drug Resistance Compared with Normal Endothelial Cells. Clin. Cancer Res. 2009, 15, 4838–4846. [Google Scholar] [CrossRef]
- Cao, Z.; Scandura, J.M.; Inghirami, G.G.; Shido, K.; Ding, B.-S.; Rafii, S. Molecular Checkpoint Decisions Made by Subverted Vascular Niche Transform Indolent Tumor Cells into Chemoresistant Cancer Stem Cells. Cancer Cell 2017, 31, 110–126. [Google Scholar] [CrossRef]
- Ribeiro, M.L.; Reyes-Garau, D.; Armengol, M.; Fernández-Serrano, M.; Roué, G. Recent Advances in the Targeting of Epigenetic Regulators in B-Cell Non-Hodgkin Lymphoma. Front. Genet. 2019, 10, 986. [Google Scholar] [CrossRef]
- Richart, L.; Margueron, R. Drugging histone methyltransferases in cancer. Curr. Opin. Chem. Biol. 2020, 56, 51–62. [Google Scholar] [CrossRef]
- Daver, N.; Garcia-Manero, G.; Basu, S.; Boddu, P.C.; Alfayez, M.; Cortes, J.E.; Konopleva, M.; Ravandi-Kashani, F.; Jabbour, E.; Kadia, T.; et al. Efficacy, Safety, and Biomarkers of Response to Azacitidine and Nivolumab in Relapsed/Refractory Acute Myeloid Leukemia: A Nonrandomized, Open-Label, Phase II Study. Cancer Discov. 2019, 9, 370–383. [Google Scholar] [CrossRef] [PubMed]
- Levy, B.P.; Giaccone, G.; Besse, B.; Felip, E.; Garassino, M.C.; Domine Gomez, M.; Garrido, P.; Piperdi, B.; Ponce-Aix, S.; Menezes, D.; et al. Randomised phase 2 study of pembrolizumab plus CC-486 versus pembrolizumab plus placebo in patients with previously treated advanced non-small cell lung cancer. Eur. J. Cancer. 2019, 108, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Strickler, J.H.; Starodub, A.N.; Jia, J.; Meadows, K.L.; Nixon, A.B.; Dellinger, A.; Morse, M.A.; Uronis, H.E.; Marcom, P.K.; Zafar, S.Y.; et al. Phase I study of bevacizumab, everolimus, and panobinostat (LBH-589) in advanced solid tumors. Cancer Chemother. Pharmacol. 2012, 70, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Yeh, T.L.; Leissing, T.M.; Abboud, M.I.; Thinnes, C.C.; Atasoylu, O.; Holt-Martyn, J.P.; Zhang, D.; Tumber, A.; Lippl, K.; Lohans, C.T.; et al. Molecular and cellular mechanisms of HIF prolyl hydroxylase inhibitors in clinical trials. Chem. Sci. 2017, 8, 7651–7668. [Google Scholar] [CrossRef] [PubMed]
- Kachamakova-Trojanowska, N.; Podkalicka, P.; Bogacz, T.; Barwacz, S.; Józkowicz, A.; Dulak, J.; Łoboda, A. HIF-1 stabilization exerts anticancer effects in breast cancer cells in vitro and in vivo. Biochem. Pharmacol. 2020, 20, 113922. [Google Scholar] [CrossRef] [PubMed]
- Semenza, G.L.; Wang, G.L. A nuclear factor induced by hypoxia via de novo protein synthesis binds to the human erythropoietin gene enhancer at a site required for transcriptional activation. Mol. Cell Biol. 1992, 12, 5447–5454. [Google Scholar] [CrossRef]
- Choudhry, H.; Harris, A.L. Advances in Hypoxia-Inducible Factor Biology. Cell Metab. 2018, 27, 281–298. [Google Scholar] [CrossRef]
- Lv, X.; Li, J.; Zhang, C.; Hu, T.; Li, S.; He, S.; Yan, H.; Tan, Y.; Lei, M.; Wen, M.; et al. The role of hypoxia-inducible factors in tumor angiogenesis and cell metabolism. Genes Dis. 2016, 4, 19–24. [Google Scholar] [CrossRef]


| ECs | TECs | NECs | References | |
|---|---|---|---|---|
| Feature | ||||
| Phenotype | Proangiogenic, antiapoptotic | Anti-inflammatory, anticoagulative, antifibrotic | [19,20,25,26] | |
| Morphology | Irregular | Regular | [27,28] | |
| Monolayer structure | Defective with disorganized, overlapping, branches and loosely attached cells | Uniform | [28] | |
| Ploidy | Aneuploid | Diploid | [22,23] | |
| Proliferation time | Fast | Slow | [29,30] | |
| Lifespan | Short | Long | [30] | |
| Responsiveness to angiogenic growth factors | High | Low | [20,31] | |
| miRNA Released by Cancer Cells | Effect on Angiogenesis Progress | Endothelial Target | Reference |
|---|---|---|---|
| miR-9 | Pro-angiogenic | SOCS5, JAK-STAT pathway, COL18A1, THBS2, PTCH1, PHD3 | [104,105] |
| miR-494 | Pro-angiogenic | PTEN | [96] |
| miR-146a | Pro-angiogenic | PDGFR, VEGF | [97] |
| miR-296 | Pro-angiogenic | HGS | [98] |
| miR-130a | Pro-angiogenic | c-MYB | [106] |
| miR-93 | Pro-angiogenic | ITGB8, EPLIN, LATS2 | [107,108] |
| miR-155 | Pro-angiogenic | VHL | [109] |
| miR-132 | Pro-angiogenic | P120RasGAP | [110] |
| miR-204 | Anti-angiogenic | VEGF, PDGF, JAK2, HIF-1A | [111] |
| miR-134 | Anti-angiogenic | VEGF-A, VEGFR1 | [112] |
| miR-543 | Anti-angiogenic | ANGPT2 | [113] |
| miR-107 | Anti-angiogenic | HIF-1B, VEGF-A | [114] |
| miR-497 | Anti-angiogenic | VEGF-A, VEGFR | [115,116] |
| miR-29b | Anti-angiogenic | MMP2, VEGF-A, PDGF, MMP9, ITGA6, ITGB1, TGFB | [117,118] |
| miR-200 | Anti-angiogenic | IL6, CSCL1 | [119] |
| miR-214 | Anti-angiogenic | HDGF | [120] |
| miR-126 | Anti-angiogenic | LRP6, PIK3R2, VEGF-A | [121,122] |
| miR-622 | Anti-angiogenic | VEGFA | [123] |
| miR-17-92 | Pro-angiogenic | TSP1, DKK3 | [124,125] |
| Anti-angiogenic | EDNRB | [126] | |
| miR-21 | Pro-angiogenic | VEGF, VEGFR, HIF-1A | [127] |
| Anti-angiogenic | RhoB | [128] | |
| miR-27B | Pro-angiogenic | DLL4/NOTCH | [129] |
| Anti-angiogenic | VEGF-C | [130] |
| Epi-Drug | Epi-Target | Cancer Type | Clinical Trial Registration Number | Phase of the Study | ||
|---|---|---|---|---|---|---|
| Angio-Drug | Angio-Target | |||||
| 5-Azacitidine | DNTM1 | Renal cell carcinoma | NCT00934440 | II | ||
| Bevacizumab | VEGF | |||||
| 5-Azacitidine | DNTM1 | Metastatic colorectal cancer; | NCT02260440 | II | ||
| Pembrolizumab | VEGF | |||||
| Advanced solid tumors; | NCT02959437 | I/II | ||||
| Melanoma and other malignant neoplasms of skin metastatic melanoma; | NCT02816021 | II | ||||
| carcinoma, non-small lung cancer | NCT02546986 | II | ||||
| 5-Azacitidine | DNTM1 | AML childhood | NCT03825367 | I/II | ||
| Nivolumab | VEGF | Acute myeloid leukemia | NCT02397720 | II | ||
| Decitabine | DNTM1 | Childhood solid tumor, childhood lymphoma, relapsed cancer refractory, cancer adult solid tumor, adult lymphoma; | NCT03445858 | Early phase I | ||
| Pembrolizumab | VEGF | |||||
| relapsed acute myeloid leukemia; | NCT02996474 | I/II | ||||
| acute myeloid leukemia, high-risk myelodysplastic syndrome; | NCT03969446 | I | ||||
| Carcinoma, non-small-cell lung cancer | NCT03233724 | I/II | ||||
| Decitabine | DNTM1 | Lung cancer, non-small cell lung cancer | NCT02664181 | II | ||
| Nivolumab | VEGF | |||||
| Decitabine, 5-Azacitidine | DNTM1 | Acute myeloid leukemia, myelodysplastic syndrome, myelodysplastic syndrome with excess blasts-2 | NCT03092674 | II, phase III-suspended | ||
| Nivolumab | VEGF | |||||
| Abexinostat | HDACs: I, II, IV | Metastatic solid tumors | NCT01543763 | I | ||
| Pazopanib | VEGF | |||||
| Vorinostat | HDACs: I, II, IV | Leukemia/myelodysplastic syndromes; | NCT00278330 | I | ||
| Alvocidib | CDK1, CDK2, CDK4 | |||||
| Adult solid tumors | NCT01645514 | I | ||||
| Vorinostat | HDACs: I, II, IV | Lung cancer | NCT02151721 | I | ||
| Gefitinib | EGFR | |||||
| Vorinostat | HDACs: I, II, IV | Advanced cancers | NCT01087554 | I | ||
| Sirolimus | mTOR | |||||
| Panobinostat | HDACs: I, II, IV | Breast cancer | NCT00567879 | I | ||
| Terastuzumab | Heregulinβ1 | |||||
| Panobinostat | HDACs: I, II, IV | Advanced solid tumors | NCT01055795 | I | ||
| Bevacizumab, Everolimus | VEGF mTOR | |||||
| Vorinostat | HDACs: I, II, IV | Multiple myeloma; | NCT00858234 | I | ||
| Leukemia, Myelodysplastic syndromes; | NCT00818649 | II | ||||
| Bortezomib | VEGF, IL-6, Ang1/2 | |||||
| Lymphoma | NCT00810576 | II | ||||
| Tazemetostat | EZH2 HMT | Metastatic bladder urothelial carcinoma, metastatic urothelial carcinoma | NCT03854474 | I/II | ||
| Pembrolizumab | PD-1 | |||||
| Tazemetostat | EZH2 HMT | Follicular lymphoma | NCT02220842 | I | ||
| Atezolizumab (MPDL3280A) | PD-L1, VEGF, Semaphorin4D | |||||
| INCB059872 | LSD1 KDM | Solid Tumors and Hematologic Malignancy | NCT02712905 | I/II | ||
| Nivolumab Pembrolizumab | VEGF VEGF | |||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ciesielski, O.; Biesiekierska, M.; Panthu, B.; Vialichka, V.; Pirola, L.; Balcerczyk, A. The Epigenetic Profile of Tumor Endothelial Cells. Effects of Combined Therapy with Antiangiogenic and Epigenetic Drugs on Cancer Progression. Int. J. Mol. Sci. 2020, 21, 2606. https://doi.org/10.3390/ijms21072606
Ciesielski O, Biesiekierska M, Panthu B, Vialichka V, Pirola L, Balcerczyk A. The Epigenetic Profile of Tumor Endothelial Cells. Effects of Combined Therapy with Antiangiogenic and Epigenetic Drugs on Cancer Progression. International Journal of Molecular Sciences. 2020; 21(7):2606. https://doi.org/10.3390/ijms21072606
Chicago/Turabian StyleCiesielski, Oskar, Marta Biesiekierska, Baptiste Panthu, Varvara Vialichka, Luciano Pirola, and Aneta Balcerczyk. 2020. "The Epigenetic Profile of Tumor Endothelial Cells. Effects of Combined Therapy with Antiangiogenic and Epigenetic Drugs on Cancer Progression" International Journal of Molecular Sciences 21, no. 7: 2606. https://doi.org/10.3390/ijms21072606
APA StyleCiesielski, O., Biesiekierska, M., Panthu, B., Vialichka, V., Pirola, L., & Balcerczyk, A. (2020). The Epigenetic Profile of Tumor Endothelial Cells. Effects of Combined Therapy with Antiangiogenic and Epigenetic Drugs on Cancer Progression. International Journal of Molecular Sciences, 21(7), 2606. https://doi.org/10.3390/ijms21072606






