The Role of Erythroid Differentiation Regulator 1 (ERDR1) in the Control of Proliferation and Photodynamic Therapy (PDT) Response
Abstract
1. Introduction
2. Photodynamic Therapy (PDT) for Disease Treatment
3. Role of ERDR1 in PDT Response
3.1. ERDR1 Expression and Apoptosis Control
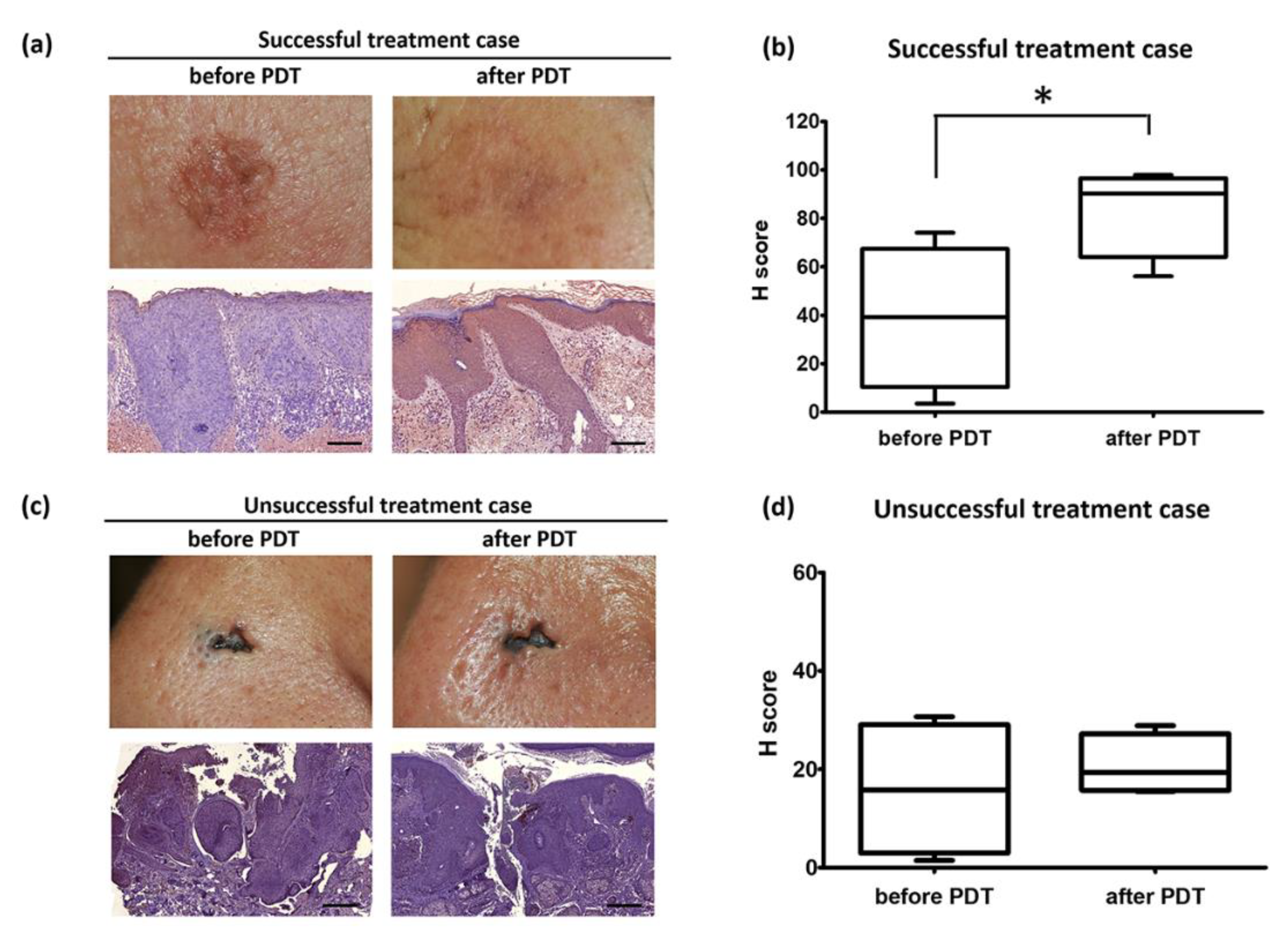
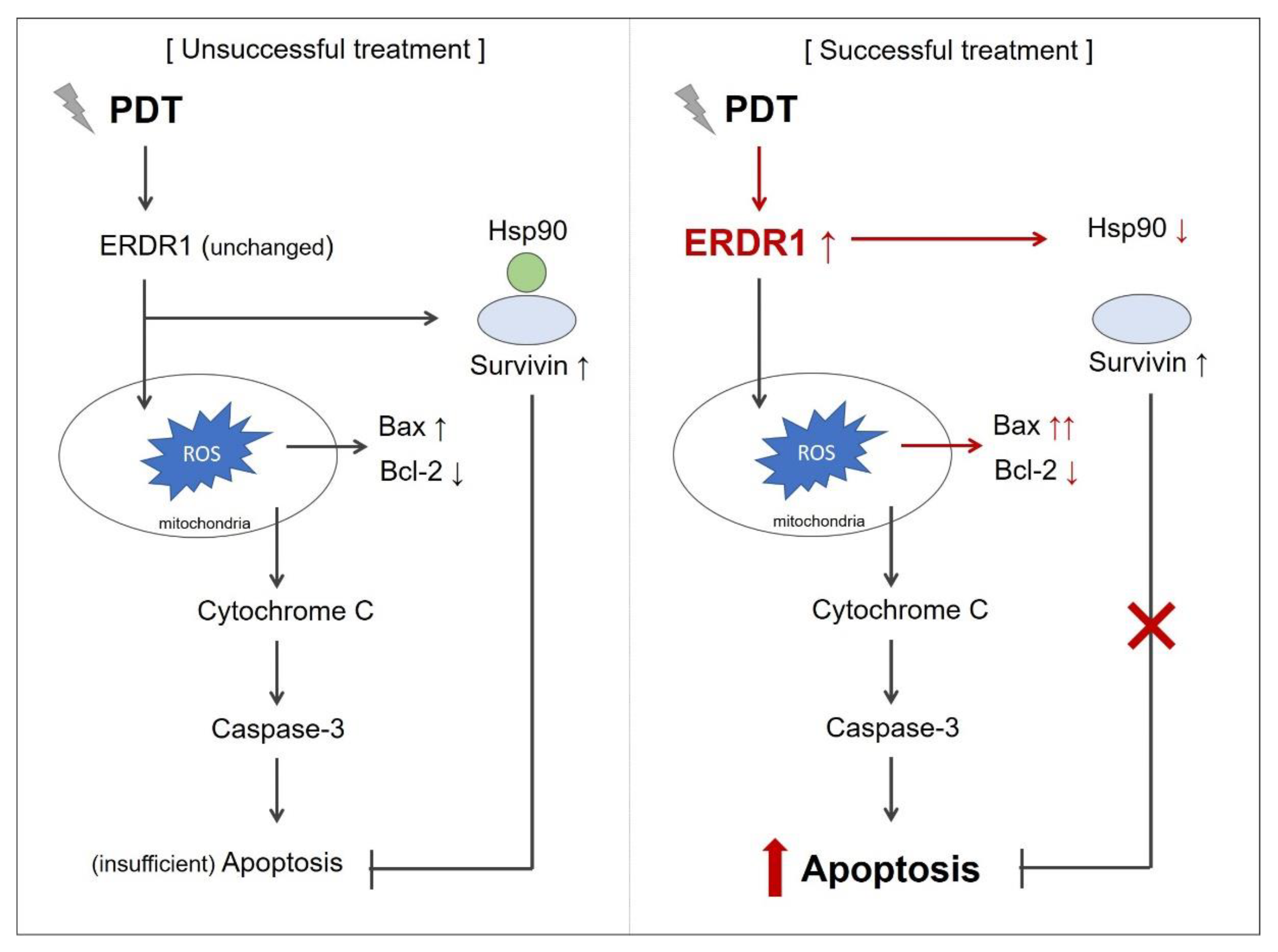
3.2. ERDR1 and Photodynamic Therapy
4. Materials and Methods
4.1. Skin Biopsy Specimens and Immunohistochemistry
4.2. Digital Images of Immunostained Tissues
4.3. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Dormer, P.; Spitzer, E.; Frankenberger, M.; Kremmer, E. Erythroid differentiation regulator (EDR), a novel, highly conserved factor I. Induction of haemoglobin synthesis in erythroleukaemic cells. Cytokine 2004, 26, 231–242. [Google Scholar] [CrossRef] [PubMed]
- Dormer, P.; Spitzer, E.; Moller, W. EDR is a stress-related survival factor from stroma and other tissues acting on early haematopoietic progenitors (E-Mix). Cytokine 2004, 27, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.O.; Ha, K.S. New insights into the mechanisms for photodynamic therapy-induced cancer cell death. Int. Rev. Cell Mol. Biol. 2012, 295, 139–174. [Google Scholar] [PubMed]
- Ericson, M.B.; Wennberg, A.M.; Larko, O. Review of photodynamic therapy in actinic keratosis and basal cell carcinoma. Ther. Clin. Risk Manag. 2008, 4, 1–9. [Google Scholar] [PubMed]
- Agostinis, P.; Berg, K.; Cengel, K.A.; Foster, T.H.; Girotti, A.W.; Gollnick, S.O.; Hahn, S.M.; Hamblin, M.R.; Juzeniene, A.; Kessel, D.; et al. Photodynamic therapy of cancer: An update. Ca A Cancer J. Clin. 2011, 61, 250–281. [Google Scholar] [CrossRef]
- Griffin, L.L.; Lear, J.T. Photodynamic Therapy and Non-Melanoma Skin Cancer. Cancers 2016, 8, 98. [Google Scholar] [CrossRef]
- Braathen, L.R.; Szeimies, R.M.; Basset-Seguin, N.; Bissonnette, R.; Foley, P.; Pariser, D.; Roelandts, R.; Wennberg, A.M.; Morton, C.A. International Society for Photodynamic Therapy in Dermatology. Guidelines on the use of photodynamic therapy for nonmelanoma skin cancer: An international consensus. J. Am. Acad. Dermatol. 2007, 56, 125–143. [Google Scholar] [CrossRef]
- Morton, C.A. A synthesis of the world’s guidelines on photodynamic therapy for non-melanoma skin cancer. G Ital. Derm. Venereol. 2018, 153, 783–792. [Google Scholar] [CrossRef]
- Buytaert, E.; Dewaele, M.; Agostinis, P. Molecular effectors of multiple cell death pathways initiated by photodynamic therapy. Biochim. Biophys. Acta 2007, 1776, 86–107. [Google Scholar] [CrossRef]
- Robertson, C.A.; Evans, D.H.; Abrahamse, H. Photodynamic therapy (PDT): A short review on cellular mechanisms and cancer research applications for PDT. J. Photochem. Photobiol. B Biol. 2009, 96, 1–8. [Google Scholar] [CrossRef]
- Lam, M.; Oleinick, N.L.; Nieminen, A.L. Photodynamic therapy-induced apoptosis in epidermoid carcinoma cells. Reactive oxygen species and mitochondrial inner membrane permeabilization. J. Biol. Chem. 2001, 276, 47379–47386. [Google Scholar] [CrossRef] [PubMed]
- Nakaseko, H.; Kobayashi, M.; Akita, Y.; Tamada, Y.; Matsumoto, Y. Histological changes and involvement of apoptosis after photodynamic therapy for actinic keratoses. Br. J. Dermatol. 2003, 148, 122–127. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.M.; Chee, S.K.; Yuen, G.Y.; Olivo, M. Photodynamic therapy induced Fas-mediated apoptosis in human carcinoma cells. Int. J. Mol. Med. 2002, 9, 257–270. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, M.; Ahmad, N.; Gupta, S.; Mukhtar, H. Involvement of Bcl-2 and Bax in photodynamic therapy-mediated apoptosis. Antisense Bcl-2 oligonucleotide sensitizes RIF 1 cells to photodynamic therapy apoptosis. J. Biol. Chem. 2001, 276, 15481–15488. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Ye, X.; Niu, Q.; Wang, C.; Li, Y. Time course of apoptosis induced by photodynamic therapy with PsD007 in LT12 acute myeloid leukemia cells. Lasers Med. Sci. 2016, 31, 817–824. [Google Scholar] [CrossRef] [PubMed]
- Jung, M.K.; Park, Y.; Song, S.B.; Cheon, S.Y.; Park, S.; Houh, Y.; Ha, S.; Kim, H.J.; Park, J.M.; Kim, T.S.; et al. Erythroid differentiation regulator 1, an interleukin 18-regulated gene, acts as a metastasis suppressor in melanoma. J. Investig. Dermatol. 2011, 131, 2096–2104. [Google Scholar] [CrossRef]
- Lee, Y.B.; Kim, H.J.; Jung, H.Y.; Park, Y.G.; Kim, S.Y.; Cho, B.K.; Cho, D.; Park, H.J. Downregulation of erythroid differentiation regulator 1 as a novel marker of skin tumors. Int. J. Dermatol. 2014, 53, 723–730. [Google Scholar] [CrossRef]
- Kim, K.E.; Houh, Y.; Lee, J.; Kim, S.; Cho, D.; Park, H.J. Downregulation of erythroid differentiation regulator 1 (Erdr1) plays a critical role in psoriasis pathogenesis. Exp. Dermatol. 2016, 25, 570–572. [Google Scholar] [CrossRef]
- Kim, H.J.; Song, S.B.; Yang, Y.; Eun, Y.S.; Cho, B.K.; Park, H.J.; Cho, D.H. Erythroid differentiation regulator 1 (Erdr1) is a proapototic factor in human keratinocytes. Exp. Dermatol. 2011, 20, 920–925. [Google Scholar] [CrossRef]
- Soto, R.; Petersen, C.; Novis, C.L.; Kubinak, J.L.; Bell, R.; Stephens, W.Z.; Lane, T.E.; Fujinami, R.S.; Bosque, A.; O’Connell, R.M.; et al. Microbiota promotes systemic T-cell survival through suppression of an apoptotic factor. Proc. Natl. Acad. Sci. USA 2017, 114, 5497–5502. [Google Scholar] [CrossRef]
- Soengas, M.S.; Lowe, S.W. Apoptosis and melanoma chemoresistance. Oncogene 2003, 22, 3138–3151. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Jung, M.K.; Park, H.J.; Kim, K.E.; Cho, D. Erdr1 Suppresses Murine Melanoma Growth via Regulation of Apoptosis. Int. J. Mol. Sci. 2016, 17, 107. [Google Scholar] [CrossRef] [PubMed]
- Shin, M.K.; Jeong, K.H.; Choi, H.; Ahn, H.J.; Lee, M.H. Heat shock protein 90 inhibitor enhances apoptosis by inhibiting the AKT pathway in thermal-stimulated SK-MEL-2 human melanoma cell line. J. Dermatol. Sci. 2018, 90, 357–360. [Google Scholar] [CrossRef]
- Liu, K.S.; Liu, H.; Qi, J.H.; Liu, Q.Y.; Liu, Z.; Xia, M.; Xing, G.W.; Wang, S.X.; Wang, Y.F. SNX-2112, an Hsp90 inhibitor, induces apoptosis and autophagy via degradation of Hsp90 client proteins in human melanoma A-375 cells. Cancer Lett. 2012, 318, 180–188. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.E.; Houh, Y.; Park, H.J.; Cho, D. Therapeutic Effects of Erythroid Differentiation Regulator 1 on Imiquimod-Induced Psoriasis-Like Skin Inflammation. Int. J. Mol. Sci. 2016, 17, 244. [Google Scholar] [CrossRef] [PubMed]
- Woo, Y.R.; Lim, J.H.; Jeong, S.W.; Cho, D.H.; Park, H.J. Analysis of apoptosis-associated molecules Erythroid differentiation regulator 1, bcl-2 and p53 in actinic keratosis after treatment with ingenol mebutate. Exp. Dermatol. 2017, 26, 1012–1017. [Google Scholar] [CrossRef]
- Grigalavicius, M.; Juraleviciute, M.; Kwitniewski, M.; Juzeniene, A. The influence of photodynamic therapy with 5-aminolevulinic acid on senescent skin cancer cells. Photodiagn. Photodyn. 2017, 17, 29–34. [Google Scholar] [CrossRef]
- Broekgaarden, M.; Weijer, R.; van Gulik, T.M.; Hamblin, M.R.; Heger, M. Tumor cell survival pathways activated by photodynamic therapy: A molecular basis for pharmacological inhibition strategies. Cancer Metastasis Rev. 2015, 34, 643–690. [Google Scholar] [CrossRef]
- Legat, F.J.; Wolf, P. Daylight photodynamic therapy: Where and when is it possible? Br. J. Dermatol. 2017, 176, 1440–1441. [Google Scholar] [CrossRef]
- Tomas-Velazquez, A.; Redondo, P. Switching From Conventional Photodynamic Therapy to Daylight Photodynamic Therapy For Actinic Keratoses: Systematic Review and Meta-analysis. Actas Dermosifiliogr. 2017, 108, 282–292. [Google Scholar] [CrossRef]
- Morton, C.A.; Braathen, L.R. Daylight Photodynamic Therapy for Actinic Keratoses. Am. J. Clin. Dermatol. 2018, 19, 647–656. [Google Scholar] [CrossRef] [PubMed]
- Ferrario, A.; Rucker, N.; Wong, S.; Luna, M.; Gomer, C.J. Survivin, a member of the inhibitor of apoptosis family, is induced by photodynamic therapy and is a target for improving treatment response. Cancer Res. 2007, 67, 4989–4995. [Google Scholar] [CrossRef] [PubMed]
- Ferrario, A.; Gomer, C.J. Targeting the 90 kDa heat shock protein improves photodynamic therapy. Cancer Lett. 2010, 289, 188–194. [Google Scholar] [CrossRef] [PubMed]
- Thomas, A.P.; Lee, A.J.; Palanikumar, L.; Jana, B.; Kim, K.; Kim, S.; Ok, H.; Seol, J.; Kim, D.; Kang, B.H.; et al. Mitochondrial heat shock protein-guided photodynamic therapy. Chem. Commun. 2019, 55, 12631–12634. [Google Scholar] [CrossRef] [PubMed]
- Bugaj, A.M. Targeted photodynamic therapy—A promising strategy of tumor treatment. Photochem. Photobiol. Sci. 2011, 10, 1097–1109. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, S.; Kim, K.E.; Park, H.J.; Cho, D. The Role of Erythroid Differentiation Regulator 1 (ERDR1) in the Control of Proliferation and Photodynamic Therapy (PDT) Response. Int. J. Mol. Sci. 2020, 21, 2603. https://doi.org/10.3390/ijms21072603
Park S, Kim KE, Park HJ, Cho D. The Role of Erythroid Differentiation Regulator 1 (ERDR1) in the Control of Proliferation and Photodynamic Therapy (PDT) Response. International Journal of Molecular Sciences. 2020; 21(7):2603. https://doi.org/10.3390/ijms21072603
Chicago/Turabian StylePark, Sunyoung, Kyung Eun Kim, Hyun Jeong Park, and Daeho Cho. 2020. "The Role of Erythroid Differentiation Regulator 1 (ERDR1) in the Control of Proliferation and Photodynamic Therapy (PDT) Response" International Journal of Molecular Sciences 21, no. 7: 2603. https://doi.org/10.3390/ijms21072603
APA StylePark, S., Kim, K. E., Park, H. J., & Cho, D. (2020). The Role of Erythroid Differentiation Regulator 1 (ERDR1) in the Control of Proliferation and Photodynamic Therapy (PDT) Response. International Journal of Molecular Sciences, 21(7), 2603. https://doi.org/10.3390/ijms21072603




