Oral Bioavailability Enhancement and Anti-Fatigue Assessment of the Andrographolide Loaded Solid Dispersion
Abstract
1. Introduction
2. Results and Discussion
2.1. Formulation Screening by Dissolution
2.2. Differential Scanning Calorimetric Analysis
2.3. Powder X-Ray Diffraction Analysis
2.4. Fourier Transform Infrared Spectroscopy
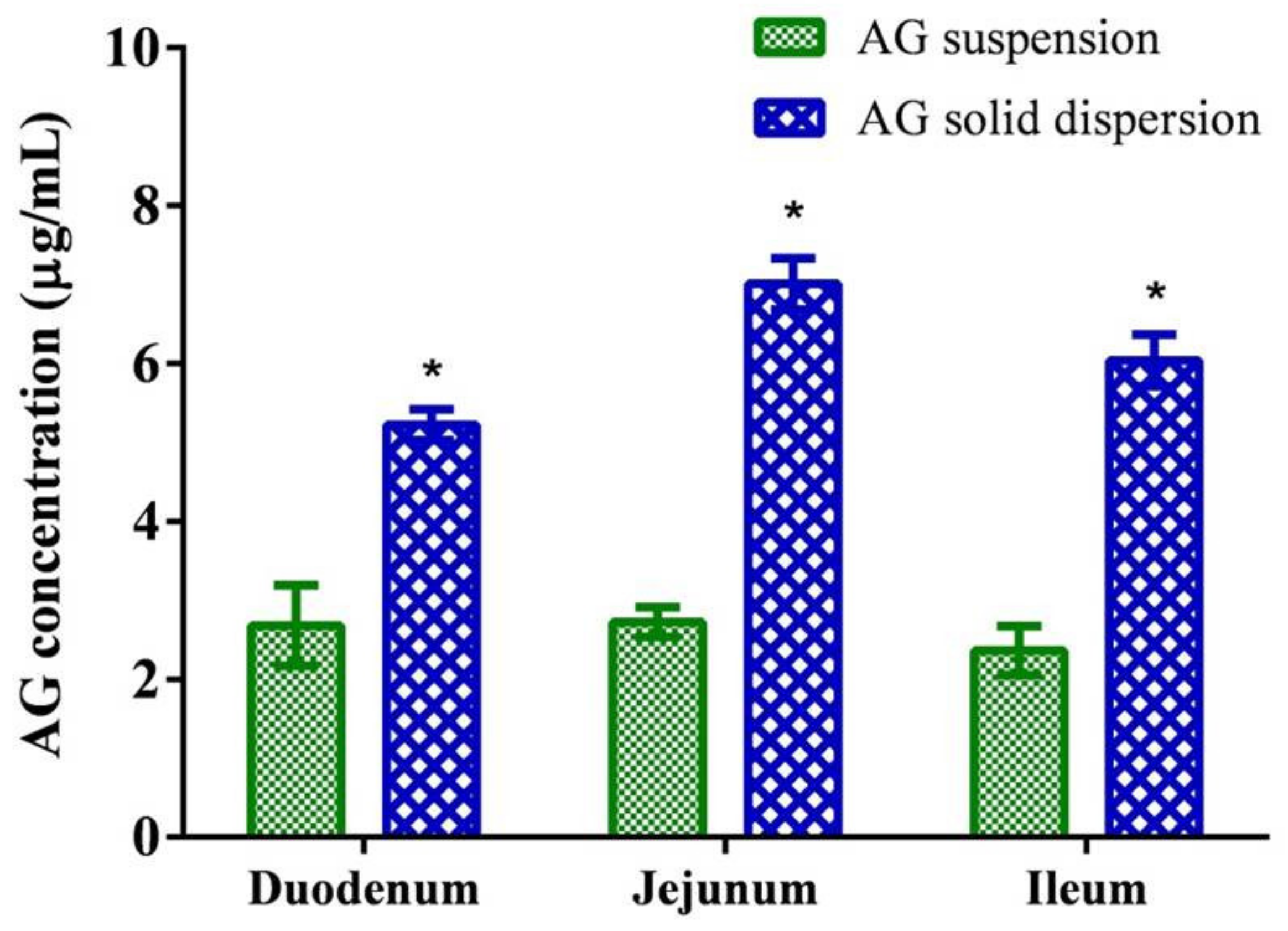
2.5. Ex vivo Permeability
2.6. Oral Bioavailability Assessment
2.7. Anti-Fatigue Effects
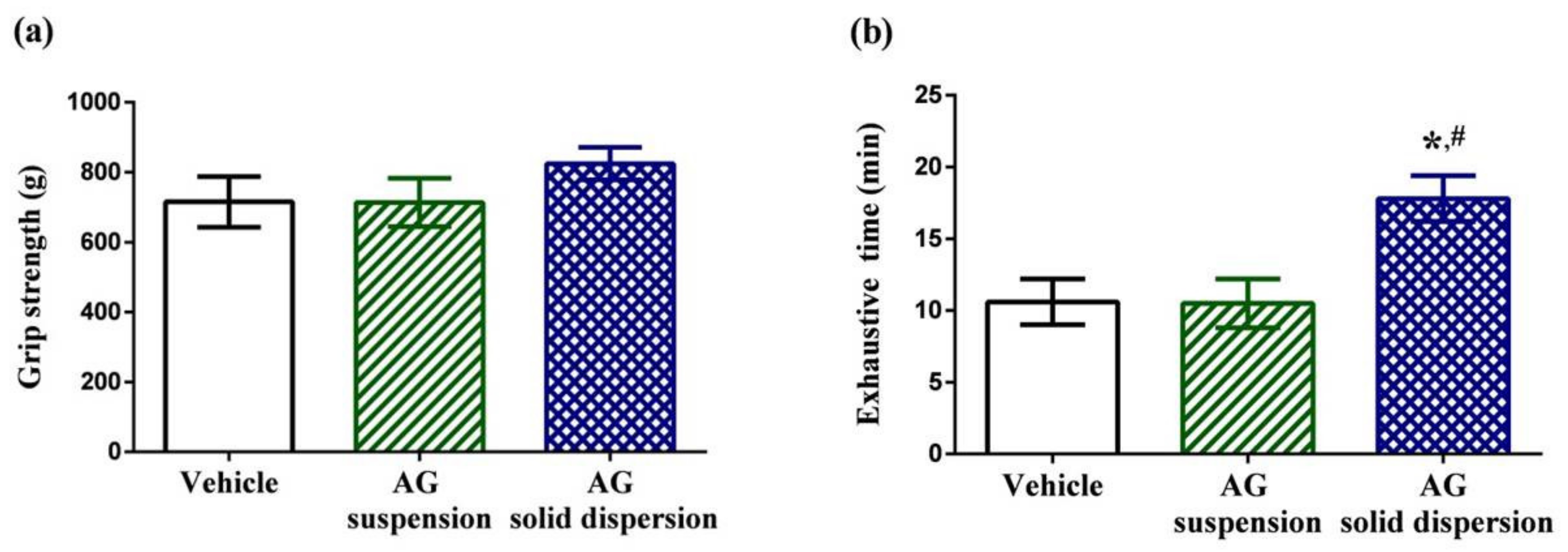
2.7.1. Physical Performance Test
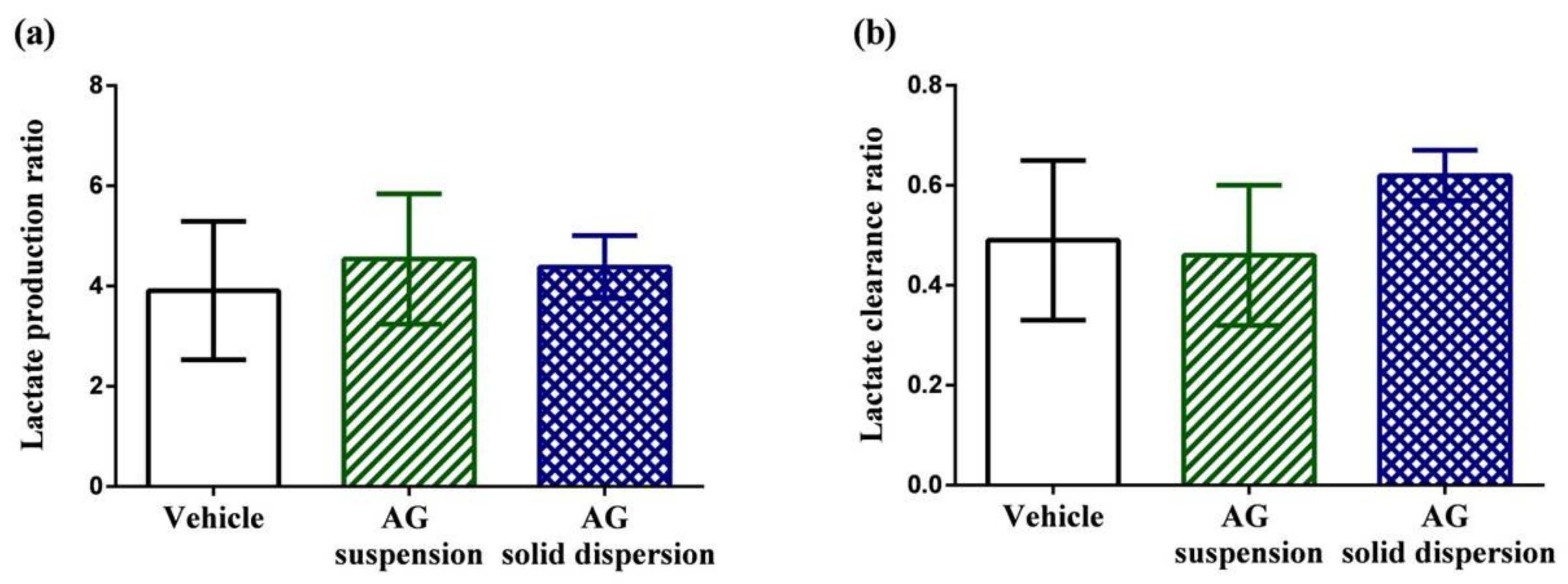
2.7.2. Lactate Production and Clearance Ratio
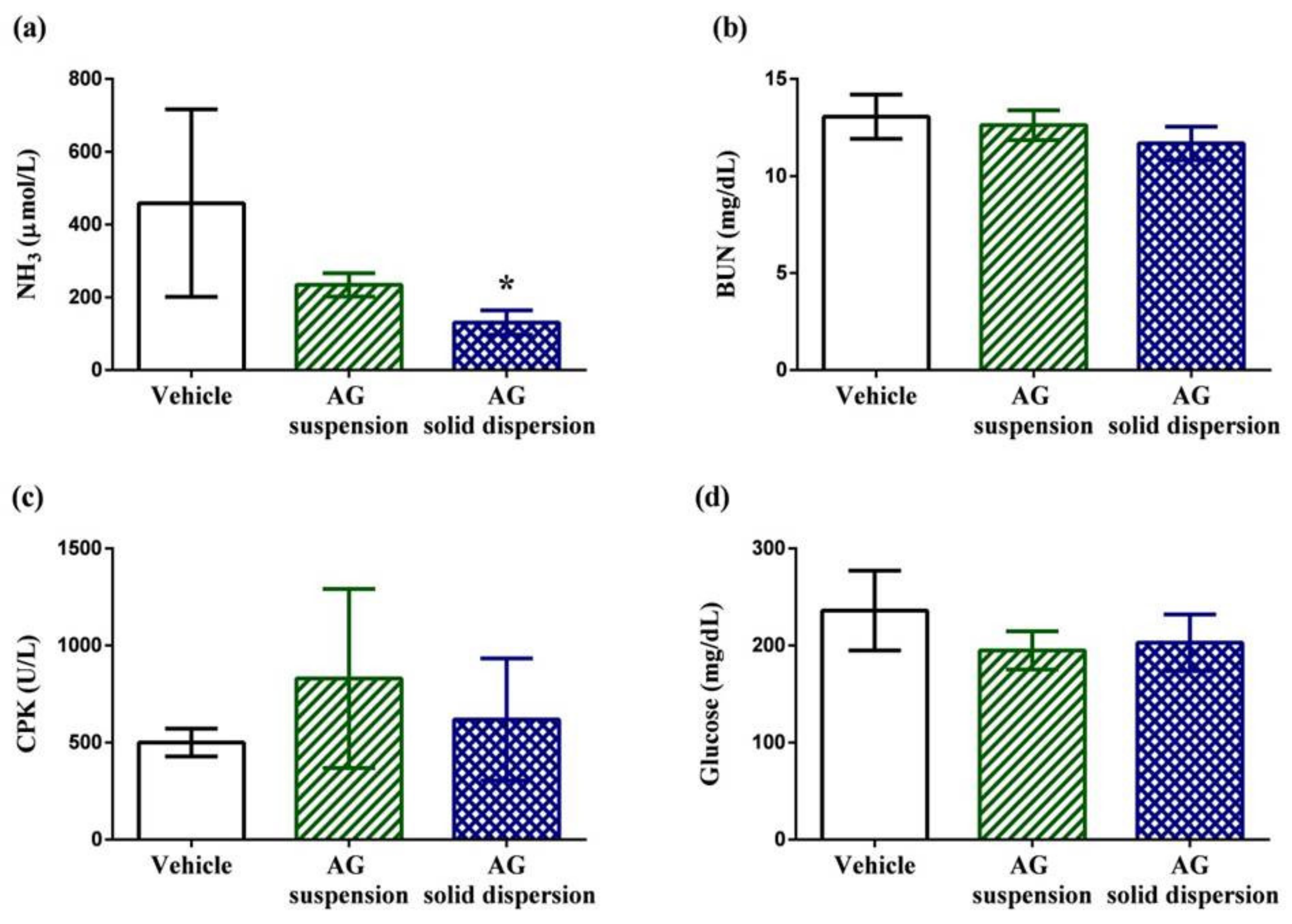
2.7.3. Fatigue-Related Indicator Determination
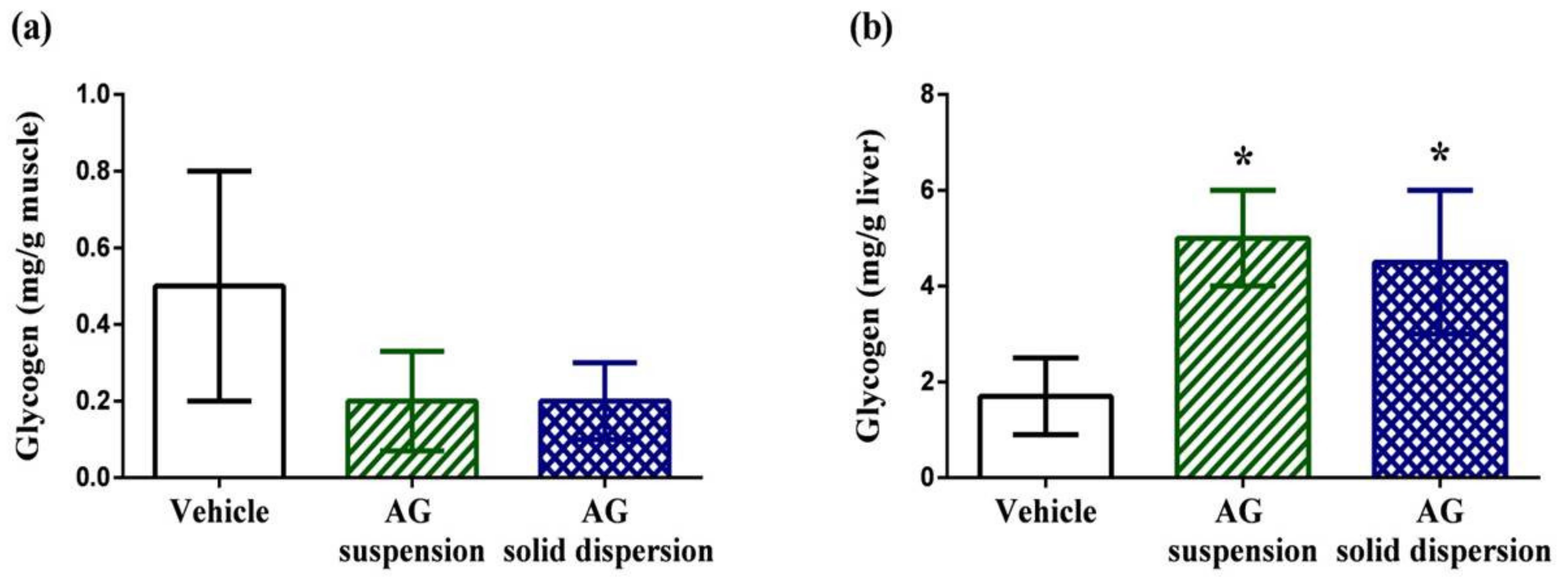
2.7.4. Tissue Glycogen Determination
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. HPLC Analysis of Andrographolide
3.3. Preparation of Andrographolide-Loaded Solid Dispersion and Physical Mixture
3.4. Formulation Characterization
3.4.1. In vitro Dissolution
3.4.2. Differential Scanning Calorimetric Analysis
3.4.3. Powder X-Ray Diffraction Analysis
3.4.4. Fourier Transform Infrared Spectroscopy
3.5. Animals
3.6. Ex vivo Permeability
3.7. Oral Bioavailability Evaluation
3.8. Anti-Fatigue Evaluation
3.9. Data Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chao, W.W.; Lin, B.F. Isolation and identification of bioactive compounds in Andrographis paniculata (chuanxinlian). Chin. Med. 2010, 5, 17. [Google Scholar] [CrossRef] [PubMed]
- Tan, W.D.; Liao, W.; Zhou, S.; Wong, W.F. Is there a future for andrographolide to be an anti-inflammatory drug? Deciphering its major mechanisms of action. Biochem. Pharmacol. 2017, 139, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Mussard, E.; Cesaro, A.; Lespessailles, E.; Legrain, B.; Berteina-Raboin, S.; Toumi, H. Andrographolide, A Natural Antioxidant: An Update. Antioxidants 2019, 8, 571. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Chen, S.R.; Chai, L.; Zhao, J.; Wang, Y.; Wang, Y. Overview of pharmacological activities of Andrographis paniculata and its major compound andrographolide. Crit. Rev. Food Sci. Nutr. 2019, 59 (Suppl. S1), S17–S29. [Google Scholar] [CrossRef]
- Fan, L.; Feng, Y.; Chen, G.C.; Qin, L.Q.; Fu, C.L.; Chen, L.H. Effects of coenzyme Q10 supplementation on inflammatory markers: A systematic review and meta-analysis of randomized controlled trials. Pharmacol. Res. 2017, 119, 128–136. [Google Scholar] [CrossRef] [PubMed]
- Castro-Marrero, J.; Sáez-Francàs, N.; Segundo, M.J.; Calvo, N.; Faro, M.; Aliste, L.; De Fernández Sevilla, T.; Alegre, J. Effect of coenzyme Q10 plus nicotinamide adenine dinucleotide supplementation on maximum heart rate after exercise testing in chronic fatigue syndrome—A randomized, controlled, double-blind trial. Clin. Nutr. 2016, 35, 826–834. [Google Scholar] [CrossRef]
- Simioni, C.; Zauli, G.; Martelli, A.M.; Vitale, M.; Sacchetti, G.; Gonelli, A.; Neri, L.M. Oxidative stress: Role of physical exercise and antioxidant nutraceuticals in adulthood and aging. Oncotarget 2018, 9, 17181–17198. [Google Scholar] [CrossRef]
- Huang, W.C.; Chiu, W.C.; Chuang, H.L.; Tang, D.W.; Lee, Z.M.; Wei, L.; Huang, C.C. Effect of curcumin supplementation on physiological fatigue and physical performance in mice. Nutrients 2015, 7, 905–921. [Google Scholar] [CrossRef]
- Bertoglio, J.C.; Baumgartner, M.; Palma, R.; Ciampi, E.; Carcamo, C.; Cáceres, D.D.; Acosta-Jamett, G.; Hancke, J.L.; Burgos, R.A. Andrographis paniculata decreases fatigue in patients with relapsing-remitting multiple sclerosis: A 12-month double-blind placebo-controlled pilot study. BMC Neurol. 2016, 16, 77–84. [Google Scholar] [CrossRef]
- Chen, H.W.; Huang, C.S.; Li, C.C.; Lin, A.H.; Huang, Y.J.; Wang, T.S.; Yao, H.T.; Lii, C.K. Bioavailability of andrographolide and protection against carbon tetrachloride-induced oxidative damage in rats. Toxicol. Appl. Pharmacol. 2014, 280, 1–9. [Google Scholar] [CrossRef]
- Yen, C.C.; Chen, Y.C.; Wu, M.T.; Wang, C.C.; Wu, Y.T. Nanoemulsion as a strategy for improving the oral bioavailability and anti-inflammatory activity of andrographolide. Int. J. Nanomed. 2018, 13, 669–680. [Google Scholar] [CrossRef] [PubMed]
- Panossian, A.; Hovhannisyan, A.; Mamikonyan, G.; Abrahamian, H.; Hambardzumyan, E.; Gabrielian, E.; Goukasova, G.; Wikman, G.; Wagner, H. Pharmacokinetic and oral bioavailability of andrographolide from Andrographis paniculata fixed combination kan jang in rats and human. Phytomedicine 2000, 7, 351–364. [Google Scholar] [CrossRef]
- Lee, S.Y.; Abdullah, L.C.; Rahman, R.A.; Abas, F.; Chong, G.H. Role of polymers as crystal growth inhibitors in coprecipitation via solution-enhanced dispersion by supercritical fluids (seds) to improve andrographolide dissolution from standardized Andrographis paniculata extract. J. Drug Deliv. Sci. Technol. 2019, 50, 145–154. [Google Scholar] [CrossRef]
- Ren, K.; Zhang, Z.; Li, Y.; Liu, J.; Zhao, D.; Zhao, Y.; Gong, T. Physicochemical characteristics and oral bioavailability of andrographolide complexed with hydroxypropyl-beta-cyclodextrin. Pharmazie 2009, 64, 515–520. [Google Scholar] [PubMed]
- Ye, L.; Wang, T.; Tang, L.; Liu, W.; Yang, Z.; Zhou, J.; Zheng, Z.; Cai, Z.; Hu, M.; Liu, Z. Poor oral bioavailability of a promising anticancer agent andrographolide is due to extensive metabolism and efflux by P-glycoprotein. J. Pharm. Sci. 2011, 100, 5007–5017. [Google Scholar] [CrossRef] [PubMed]
- Bothiraja, C.; Shinde, M.B.; Rajalakshmi, S.; Pawar, A.P. Evaluation of molecular pharmaceutical and in-vivo properties of spray-dried isolated andrographolide-PVP. J. Pharm. Pharmacol. 2009, 61, 1465–1472. [Google Scholar] [CrossRef]
- Zhao, G.; Zeng, Q.; Zhang, S.; Zhong, Y.; Wang, C.; Chen, Y.; Qu, L.; Liao, Z. Effect of Carrier Lipophilicity and Preparation Method on the Properties of Andrographolide–Solid Dispersion. Pharmaceutics 2019, 11, 74. [Google Scholar] [CrossRef]
- Zhang, D.; Lin, J.; Zhang, F.; Han, X.; Han, L.; Yang, M.; Zou, W. Preparation and evaluation of andrographolide solid dispersion vectored by silicon dioxide. Pharmacogn. Mag. 2016, 12, S245–S252. [Google Scholar]
- Ma, Y.; Yang, Y.; Xie, J.; Xu, J.; Yue, P.; Yang, M. Novel nanocrystal-based solid dispersion with high drug loading, enhanced dissolution, and bioavailability of andrographolide. Int. J. Nanomed. 2018, 13, 3763–3779. [Google Scholar] [CrossRef]
- Xu, J.; Ma, Y.; Xie, Y.; Chen, Y.; Liu, Y.; Yue, P.; Yang, M. Design and evaluation of novel solid self-nanodispersion delivery system for andrographolide. AAPS PharmSciTech 2017, 18, 1572–1584. [Google Scholar] [CrossRef]
- Jain, P.K.; Khurana, N.; Pounikar, Y.; Gajbhiye, A.; Kharya, M.D. Enhancement of absorption and hepatoprotective potential through soya-phosphatidylcholine-andrographolide vesicular system. J. Liposome Res. 2013, 23, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Tu, Y.S.; Sun, D.M.; Zhang, J.J.; Jiang, Z.Q.; Chen, Y.X.; Zeng, X.H.; Huang, D.E.; Yao, N. Preparation and characterisation of andrographolide niosomes and its anti-hepatocellular carcinoma activity. J. Microencapsul. 2014, 31, 307–316. [Google Scholar] [CrossRef] [PubMed]
- Parveen, R.; Ahmad, F.J.; Iqbal, Z.; Ahmad, S. Effect of andrographolide solid lipid nanoparticles (SLNs) on breast cancer. Planta Med. 2013, 79, P105. [Google Scholar] [CrossRef]
- Chellampillai, B.; Pawar, A.P. Improved bioavailability of orally administered andrographolide from pH-sensitive nanoparticles. Eur. Drug Metab. Pharmacokinet. 2011, 35, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Sheng, H.H.; Feng, N.P.; Wei, H.; Wang, Z.T.; Wang, C.H. Preparation of and rographolide-loaded solid lipid nanoparticles and their in vitro and in vivo evaluations: Characteristics, release, absorption, transports, pharmacokinetics, and antihyperlipidemic activity. J. Pharm. Sci. 2013, 102, 4414–4425. [Google Scholar] [CrossRef] [PubMed]
- Sermkaew, N.; Ketjinda, W.; Boonme, P.; Phadoongsombut, N.; Wiwattanapatapee, R. Liquid and solid self-microemulsifying drug delivery systems for improving the oral bioavailability of andrographolide from a crude extract of Andrographis paniculata. Eur. J. Pharm. Sci. 2013, 50, 459–466. [Google Scholar] [CrossRef]
- Leuner, C.; Dressman, J. Improving drug solubility for oral delivery using solid dispersions. Eur. J. Pharm. Biopharm. 2000, 50, 47–60. [Google Scholar] [CrossRef]
- Fitriani, L.; Rismawati, E.; Umar, S.; Zaini, E. Solid Dispersion of Usnic Acid-PVP K30 and Evaluation of Antioxidant Activity. Rasayan J. Chem. 2018, 11, 1643–1648. [Google Scholar] [CrossRef]
- Nadal, J.M.; Gomes, M.L.S.; Borsato, D.M.; Almeida, M.A.; Barboza, F.M.; Zawadzki, S.F.; Farago, P.V.; Zanin, S.M.W. Spray-dried solid dispersions containing ferulic acid: Comparative analysis of three carriers, in vitro dissolution, antioxidant potential and in vivo anti-platelet effect. Drug Dev. Ind. Pharm. 2016, 42, 1813–1824. [Google Scholar] [CrossRef]
- Yen, C.C.; Chang, C.W.; Hsu, M.C.; Wu, Y.T. Self-nanoemulsifying drug delivery system for resveratrol: Enhanced oral bioavailability and reduced physical fatigue in rats. Int. J. Mol. Sci. 2017, 18, 1853. [Google Scholar] [CrossRef]
- Ghaderi, R.; Artursson, P.; Carlfors, J. Preparation of biodegradable microparticles using solution-enhanced dispersion by supercritical fluids (seds). Pharm. Res. 1999, 16, 676–681. [Google Scholar] [CrossRef] [PubMed]
- Franco, M.; Trapani, G.; Latrofa, A.; Tullio, C.; Provenzano, M.R.; Serra, M.; Muggironi, M.; Biggio, G.; Liso, G. Dissolution properties and anticonvulsant activity of phenytoin-polyethylene glycol 6000 and -polyvinylpyrrolidone K-30 solid dispersions. Int. J. Pharm. 2001, 225, 63–73. [Google Scholar] [CrossRef]
- Hilton, J.E.; Summers, M.P. The effect of wetting agents on the dissolution of indomethacin solid dispersion systems. Int. J. Pharm. 1986, 31, 157–164. [Google Scholar] [CrossRef]
- Mura, P.; Moyano, J.R.; Gonzalez-Rodriguez, M.L.; Rabasco-Alvarez, A.M.; Cirri, M.; Maestrelli, F. Characterization and dissolution properties of ketoprofen in binary and ternary solid dispersions with polyethylene glycol and surfactants. Drug Dev. Ind. Pharm. 2005, 31, 425–434. [Google Scholar] [CrossRef] [PubMed]
- Okonogi, S.; Puttipipatkhachorn, S. Dissolution improvement of high drug-loaded solid dispersion. AAPS PharmSciTech 2006, 7, E148–E153. [Google Scholar] [CrossRef] [PubMed]
- Vasconcelos, T.; Sarmento, B.; Costa, P. Solid dispersions as strategy to improve oral bioavailability of poor water soluble drugs. Drug Discov. Today 2007, 12, 1068–1075. [Google Scholar] [CrossRef]
- Maiti, K.; Mukherjee, K.; Murugan, V.; Saha, B.P.; Mukherjee, P.K. Enhancing bioavailability and hepatoprotective activity of andrographolide from Andrographis paniculata, a well-known medicinal food, through its herbosome. J. Sci. Food Agric. 2010, 90, 43–51. [Google Scholar] [CrossRef]
- Frizon, F.; De Oliveira Eloy, J.; Donaduzzi, C.M.; Mitsui, M.L.; Marchetti, J.M. Dissolution rate enhancement of loratadine in polyvinylpyrrolidone K-30 solid dispersions by solvent methods. Powder Technol. 2013, 235, 532–539. [Google Scholar] [CrossRef]
- Mendes, C.; Buttchevitz, A.; Kruger, J.H.; Caon, T.; De Oliveira Benedet, P.; Lemos-Senna, E.; Silva, M.A.S. Self-nanoemulsified drug delivery system of hydrochlorothiazide for increasing dissolution rate and diuretic activity. AAPS PharmSciTech 2017, 18, 2494–2504. [Google Scholar] [CrossRef]
- Mistry, P.; Mohapatra, S.; Gopinath, T.; Vogt, F.G.; Suryanarayanan, R. Role of the strength of drug-polymer interactions on the molecular mobility and crystallization inhibition in ketoconazole solid dispersions. Mol. Pharm. 2015, 12, 3339–3350. [Google Scholar] [CrossRef]
- Shono, Y.; Nishihara, H.; Matsuda, Y.; Furukawa, S.; Okada, N.; Fujita, T.; Yamamoto, A. Modulation of intestinal P-glycoprotein function by cremophor el and other surfactants by an in vitro diffusion chamber method using the isolated rat intestinal membranes. J. Pharm. Sci. 2004, 93, 877–885. [Google Scholar] [CrossRef] [PubMed]
- Evans, W.J.; Lambert, C.P. Physiological basis of fatigue. Am. J. Phys. Med. Rehabil. 2007, 86, S29–S46. [Google Scholar] [CrossRef] [PubMed]
- Coombes, J.S.; Rowell, B.; Dodd, S.L.; Demirel, H.A.; Naito, H.; Shanely, A.R.; Powers, S.K. Effects of vitamin e deficiency on fatigue and muscle contractile properties. Eur. J. Appl. Physiol. 2002, 87, 272–277. [Google Scholar] [CrossRef]
- Gladden, L.B. Lactate metabolism: A new paradigm for the third millennium. J. Physiol. 2004, 558, 5–30. [Google Scholar] [CrossRef] [PubMed]
- Ni, W.; Gao, T.; Wang, H.; Du, Y.; Li, J.; Li, C.; Wei, L.; Bi, H. Anti-fatigue activity of polysaccharides from the fruits of four tibetan plateau indigenous medicinal plants. J. Ethnopharmacol. 2013, 150, 529–535. [Google Scholar] [CrossRef]
- Sanchez-Medina, L.; González-Badillo, J.J. Velocity loss as an indicator of neuromuscular fatigue during resistance training. Med. Sci. Sports Exerc. 2011, 43, 1725–1734. [Google Scholar] [CrossRef]
- Banister, E.W.; Cameron, B.J. Exercise-induced hyperammonemia: Peripheral and central effects. Int. J. Sports Med. 1990, 11, 129–142. [Google Scholar] [CrossRef]
- Jing, L.; Cui, G.; Feng, Q.; Xiao, Y. Orthogonal test design for optimization of the extraction of polysaccharides from lycium barbarum and evaluation of its anti-athletic fatigue activity. J. Med. Plants Res. 2009, 3, 433–437. [Google Scholar]
- Horng, C.T.; Huang, J.K.; Wang, H.Y.; Huang, C.C.; Chen, F.A. Antioxidant and antifatigue activities of Polygonatum Alte-lobatum Hayata rhizomes in rats. Nutrients 2014, 6, 5327–5337. [Google Scholar] [CrossRef]
- Warren, G.L.; Ingalls, C.P.; Lowe, D.A.; Armstrong, R.B. Excitation-contraction uncoupling: Major role in contraction-induced muscle injury. Exerc. Sport Sci. Rev. 2001, 29, 82–87. [Google Scholar]
- Suh, S.H.; Paik, I.Y.; Jacobs, K. Regulation of blood glucose homeostasis during prolonged exercise. Mol. Cells 2007, 23, 272–279. [Google Scholar] [PubMed]
- McClung, J.M.; DeRuisseau, K.C.; Whidden, M.A.; Van Remmen, H.; Richardson, A.; Song, W.; Vrabas, I.S.; Powers, S.K. Overexpression of antioxidant enzymes in diaphragm muscle does not alter contraction-induced fatigue or recovery. Exp. Physiol. 2010, 95, 222–231. [Google Scholar] [CrossRef] [PubMed]
- Wu, R.E.; Huang, W.C.; Liao, C.C.; Chang, Y.K.; Kan, N.W.; Huang, C.C. Resveratrol protects against physical fatigue and improves exercise performance in mice. Molecules 2013, 18, 4689–4702. [Google Scholar] [CrossRef] [PubMed]
- Norenberg, M.D. Oxidative and nitrosative stress in ammonia neurotoxicity. Hepatology 2003, 37, 245–248. [Google Scholar] [CrossRef] [PubMed]
- Sahlin, K.; Tonkonogi, M.; Söderlund, K. Energy supply and muscle fatigue in humans. Acta Physiol. Scand. 1998, 162, 261–266. [Google Scholar] [CrossRef]
- Subramanian, R.; Asmawi, M.Z.; Sadikun, A. Effect of andrographolide and ethanol extract of Andrographis paniculata on liver glycolytic, gluconeogenic, and lipogenic enzymes in a type 2 diabetic rat model. Pharm. Biol. 2008, 46, 772–780. [Google Scholar] [CrossRef][Green Version]
- Chang, C.W.; Wang, C.Y.; Wu, Y.T.; Hsu, M.C. Enhanced solubility, dissolution, and absorption of lycopene by a solid dispersion technique: The dripping pill delivery system. Powder Technol. 2016, 301, 641–648. [Google Scholar] [CrossRef]
- Gurunath, S.; Nanjwade, B.K.; Patila, P.A. Enhanced solubility and intestinal absorption of candesartan cilexetil solid dispersions using everted rat intestinal sacs. Saudi Pharm. J. 2014, 22, 246–257. [Google Scholar] [CrossRef]
- Thrivikraman, K.V.; Huot, R.L.; Plotsky, P.M. Jugular vein catheterization for repeated blood sampling in the unrestrained conscious rat. Brain Res. Protoc. 2002, 10, 84–94. [Google Scholar] [CrossRef]
- Gibaldi, M.; Perrier, D. Noncompartmental analysis based on statistical moment theory. Pharmacokinetics 1982, 2, 409–417. [Google Scholar]
- Barron, M.G.; Stehly, G.R.; Hayton, W.L. Pharmacokinetic modeling in aquatic animals I. Models and concepts. Aquat. Toxicol. 1990, 17, 187–212. [Google Scholar] [CrossRef]
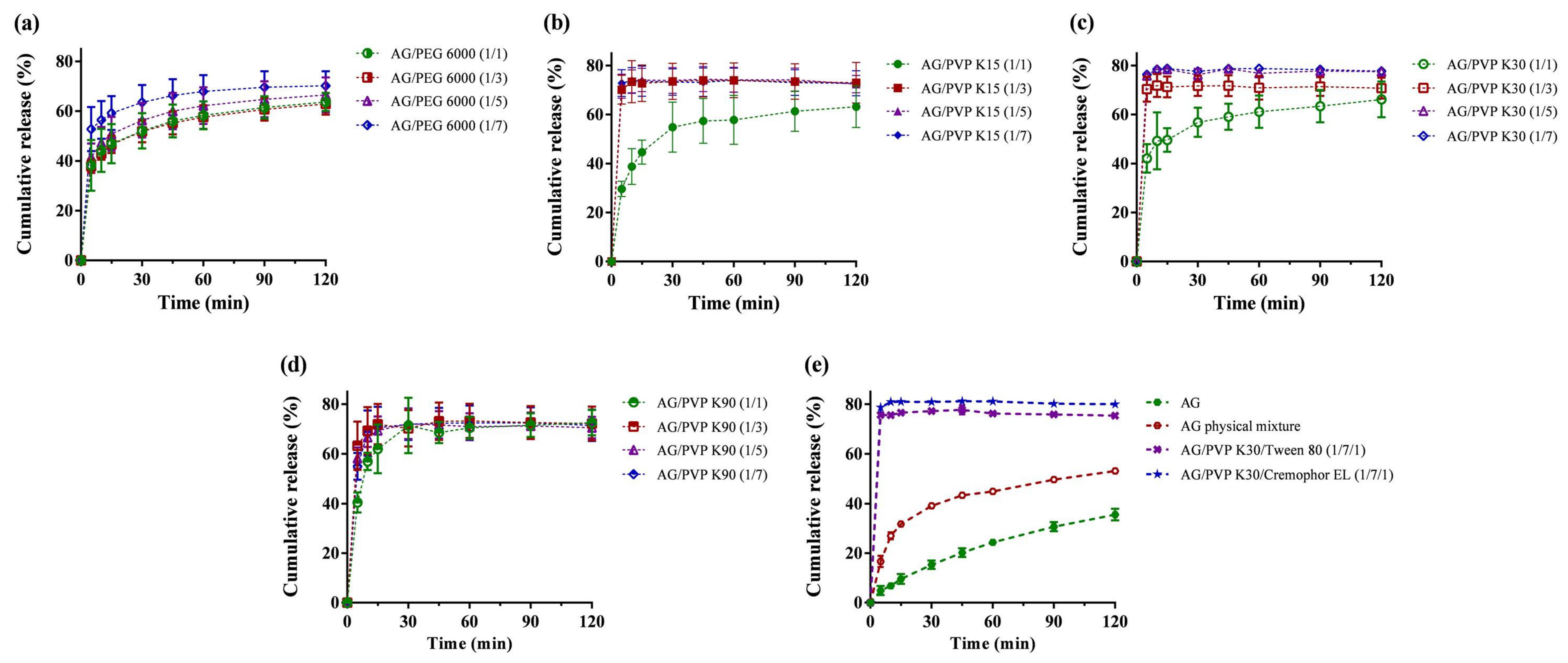
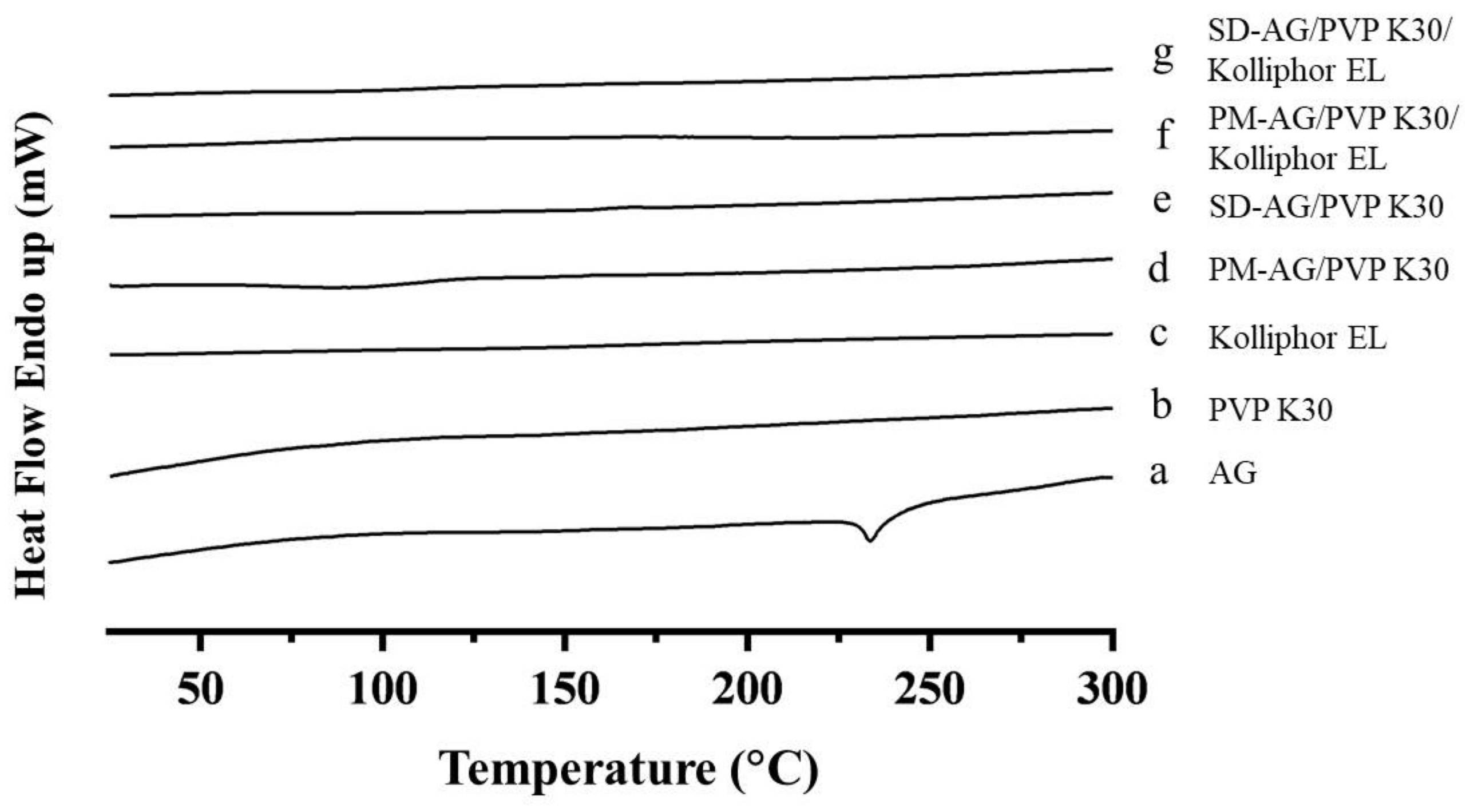
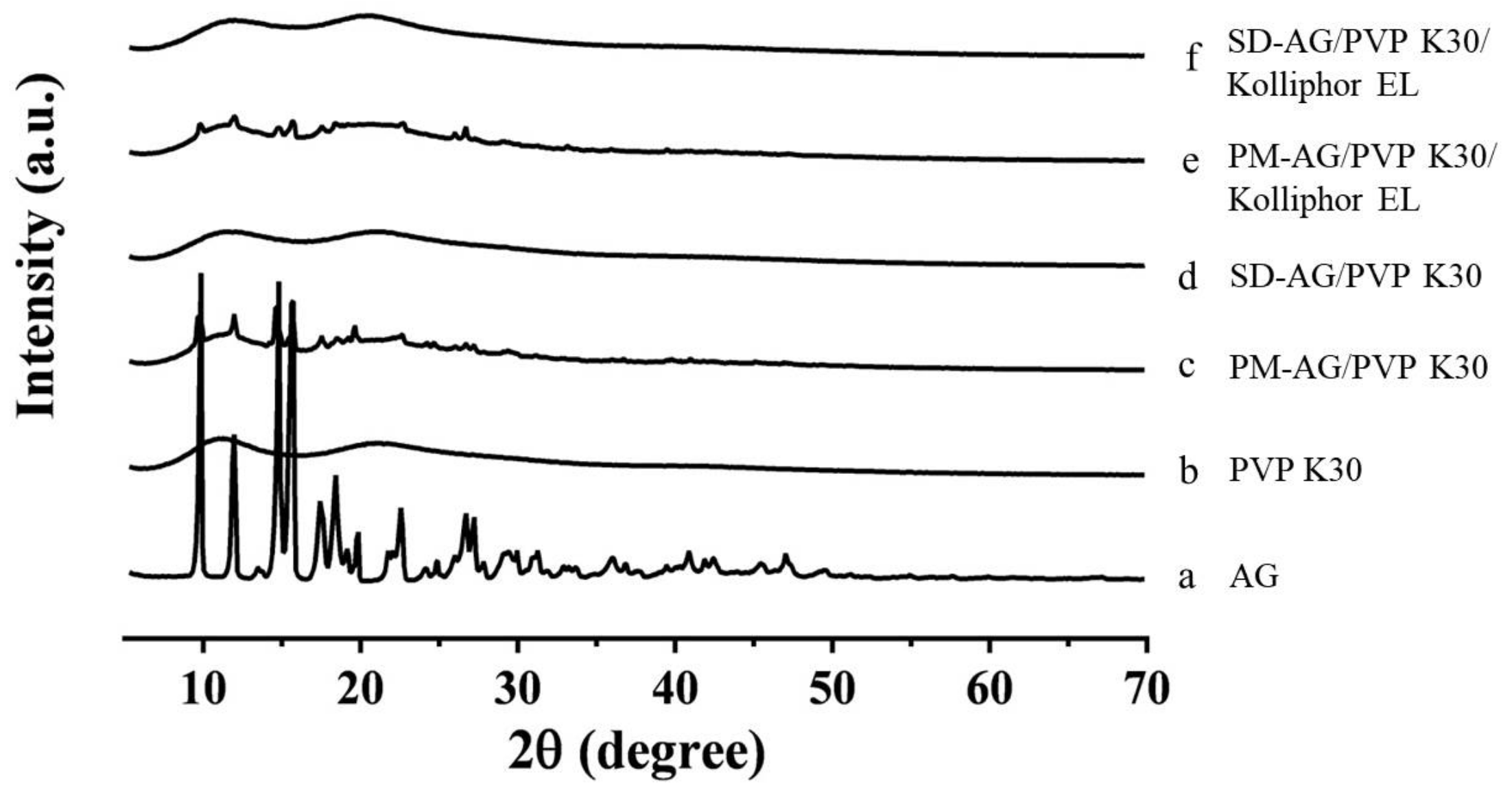
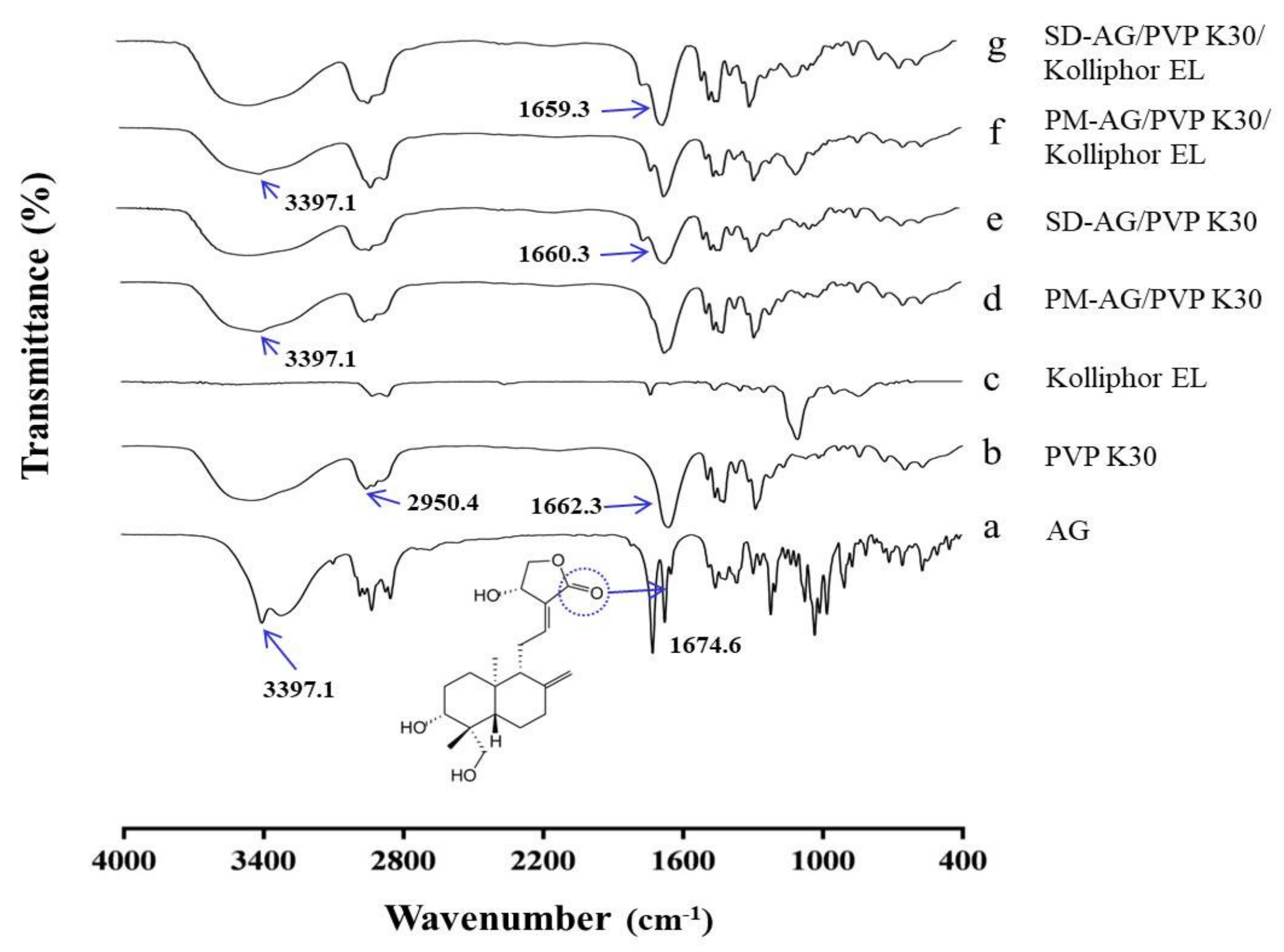

| Formulation | Ingredients | Weight Ratio (w/w) | Dissolution | |||
|---|---|---|---|---|---|---|
| Q5min | Q120min | D.E. | t70% (min) | |||
| 1 | AG | NA | 4.9 ± 1.8 | 35.6 ± 2.3 | 22.4 ± 1.7 | NA |
| 2 | AG, PEG 6000 | 1/1 | 38.2 ± 10.2 * | 63.7 ± 3.6 * | 55.1 ± 5.8 * | NA |
| 3 | AG, PEG 6000 | 1/3 | 37.9 ± 2.7 * | 62.9 ± 4.3 * | 54.5 ± 4.2 * | NA |
| 4 | AG, PEG 6000 | 1/5 | 41.5 ± 5.6 * | 66.6 ± 7.0 * | 58.7 ± 6.9 * | NA |
| 5 | AG, PEG 6000 | 1/7 | 52.8 ± 8.9 * | 70.3 ± 5.9 * | 64.7 ± 6.5 * | 120 |
| 6 | AG, PVP K15 | 1/1 | 29.6 ± 3.1 * | 63.2 ± 8.6 * | 54.7 ± 7.8 * | NA |
| 7 | AG, PVP K15 | 1/3 | 70.2 ± 5.9 * | 73.0 ± 8.3 * | 71.9 ± 8.2 * | 5 |
| 8 | AG, PVP K15 | 1/5 | 71.6 ± 4.9 * | 72.4 ± 3.6 * | 72.3 ± 4.6 * | 5 |
| 9 | AG, PVP K15 | 1/7 | 72.9 ± 5.5 * | 72.8 ± 5.1 * | 71.8 ± 5.2* | 5 |
| 10 | AG, PVP K30 | 1/1 | 42.1 ± 5.8 * | 66.2 ± 7.3 * | 58.0 ± 6.2 * | NA |
| 11 | AG, PVP K30 | 1/3 | 70.4 ± 5.1 * | 70.8 ± 4.3 * | 69.8 ± 4.2 * | 5 |
| 12 | AG, PVP K30 | 1/5 | 75.9 ± 0.2 * | 77.5 ± 0.1 * | 75.9 ± 0.7 * | 5 |
| 13 | AG, PVP K30 | 1/7 | 76.3 ± 0.9 * | 77.8 ± 1.4 * | 77.8 ± 1.7 * | 5 |
| 14 | AG, PVP K90 | 1/1 | 40.5 ± 4.1* | 72.5 ± 5.1 * | 66.9 ± 5.3 * | 30 |
| 15 | AG, PVP K90 | 1/3 | 63.3 ± 9.7 * | 72.2 ± 6.9 * | 70.3 ± 7.0* | 15 |
| 16 | AG, PVP K90 | 1/5 | 58.3 ± 4.3 * | 70.6 ± 4.4 * | 68.9 ± 5.1 * | 30 |
| 17 | AG, PVP K90 | 1/7 | 55.0 ± 5.4 * | 71.5 ± 6.3 * | 69.7 ± 6.4 * | 15 |
| 18 | AG, PVP K30, Tween 80 | 1/7/1 | 75.7 ± 1.2 * | 75.4 ± 0.6 * | 74.8 ± 0.1 * | 5 |
| 19 | AG, PVP K30, Kolliphor EL | 1/7/1 | 78.7 ± 0.1 * | 80.0 ± 0.7 * | 78.9 ± 0.5 * | 5 |
| 20 | Physical mixture (AG, PVP K30, Kolliphor EL) | 1/7/1 | 16.7 ± 2.3 * | 53.2 ± 0.2 * | 43.2 ± 0.5 * | NA |
| Parameters | Units | AG Suspension (300 mg/kg, p.o.) | AG solid Dispersion (100 mg/kg, p.o.) |
|---|---|---|---|
| Tmax | h | 2.1 ± 1.8 | 0.4 ± 0.3 |
| t1/2 | h | 7.1 ± 3.7 | 7.0 ± 3.1 |
| Cmax | ng/mL | 206.6 ± 57.6 | 254.0 ± 59.7 |
| AUC0-t | h ng/mL | 935.3 ± 130.3 | 928.2 ± 181.1 |
| Cmax/dose | (ng/mL)/(mg/kg) | 0.7 ± 0.2 | 2.5 ± 0.6 * |
| AUC0-t/dose | (h ng/mL)/(mg/kg) | 3.1 ± 0.4 | 9.3 ± 1.8 * |
| Relative bioavailability | % | - | 297.7 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yen, C.-C.; Liang, Y.-K.; Cheng, C.-P.; Hsu, M.-C.; Wu, Y.-T. Oral Bioavailability Enhancement and Anti-Fatigue Assessment of the Andrographolide Loaded Solid Dispersion. Int. J. Mol. Sci. 2020, 21, 2506. https://doi.org/10.3390/ijms21072506
Yen C-C, Liang Y-K, Cheng C-P, Hsu M-C, Wu Y-T. Oral Bioavailability Enhancement and Anti-Fatigue Assessment of the Andrographolide Loaded Solid Dispersion. International Journal of Molecular Sciences. 2020; 21(7):2506. https://doi.org/10.3390/ijms21072506
Chicago/Turabian StyleYen, Ching-Chi, Yu-Kai Liang, Chao-Pei Cheng, Mei-Chich Hsu, and Yu-Tse Wu. 2020. "Oral Bioavailability Enhancement and Anti-Fatigue Assessment of the Andrographolide Loaded Solid Dispersion" International Journal of Molecular Sciences 21, no. 7: 2506. https://doi.org/10.3390/ijms21072506
APA StyleYen, C.-C., Liang, Y.-K., Cheng, C.-P., Hsu, M.-C., & Wu, Y.-T. (2020). Oral Bioavailability Enhancement and Anti-Fatigue Assessment of the Andrographolide Loaded Solid Dispersion. International Journal of Molecular Sciences, 21(7), 2506. https://doi.org/10.3390/ijms21072506






