Mapping the Gene Expression Spectrum of Mediator Subunits in Response to Viroid Infection in Plants
Abstract
1. Introduction
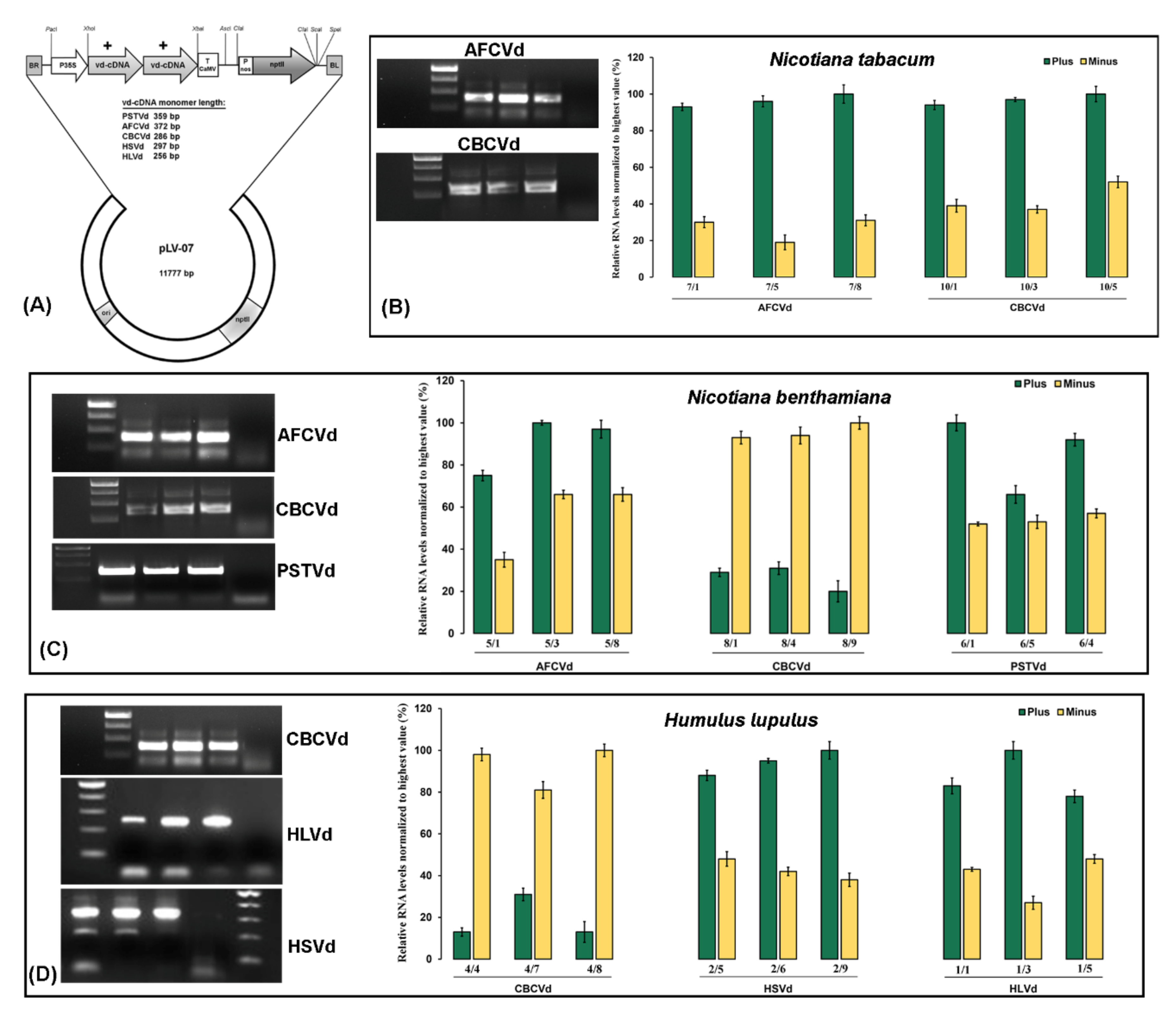
2. Results and Discussion
3. Materials and Methods
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dotson, M.R.; Yuan, C.X.; Roeder, R.G.; Myers, L.C.; Gustafsson, C.M.; Jiang, Y.W.; Li, Y.; Kornberg, R.D.; Asturias, F.J. Structural organization of yeast and mammalian mediator complexes. Proc. Natl. Acad. Sci. USA 2000, 97, 14307–14310. [Google Scholar] [CrossRef] [PubMed]
- Malik, S.; Roeder, R.G. The metazoan Mediator co-activator complex as an integrative hub for transcriptional regulation. Nat. Rev. Genet. 2010, 11, 761–772. [Google Scholar] [CrossRef] [PubMed]
- Conaway, R.C.; Conaway, J.W. Function and regulation of the Mediator complex. Curr. Opin. Genet. Dev. 2011, 21, 225–230. [Google Scholar] [CrossRef] [PubMed]
- Petrenko, N.; Jin, Y.; Wong, K.H.; Struhl, K. Mediator undergoes a compositional change during transcriptional activation. Mol. Cell 2016, 64, 443–454. [Google Scholar] [CrossRef]
- Soutourina, J. Transcription regulation by the Mediator complex. Nat. Rev. Mol. Cell Biol. 2018, 19, 262–274. [Google Scholar] [CrossRef]
- Knoll, E.R.; Zhu, Z.I.; Sarkar, D.; Landsman, D.; Morse, R.H. Role of the pre-initiation complex in Mediator recruitment and dynamics. eLife 2018, 7, e39633. [Google Scholar] [CrossRef]
- Tsai, K.-L.; Sato, S.; Tomomori-Sato, C.; Conaway, R.C.; Conaway, J.W.; Asturias, F.J. A conserved Mediator-CDK8 kinase module association regulates Mediator-RNA polymerase II interaction. Nat. Struct. Mol. Biol. 2013, 20, 611–619. [Google Scholar] [CrossRef]
- Bäckström, S.; Elfving, N.; Nilsson, R.; Wingsle, G.; Björklund, S. Purification of a Plant Mediator from Arabidopsis thaliana Identifies PFT1 as the Med25 Subunit. Mol. Cell 2007, 26, 717–729. [Google Scholar] [CrossRef]
- Malik, N.; Agarwal, P.; Tyagi, A. Emerging functions of multi-protein complex Mediator with special emphasis on plants. Crit. Rev. Biochem. Mol. Biol. 2017, 52, 475–502. [Google Scholar] [CrossRef]
- Chen, R.; Jiang, H.; Li, L.; Zhai, Q.; Qi, L.; Zhou, W.; Liu, X.; Li, H.; Zheng, W.; Sun, J.; et al. The Arabidopsis mediator subunit MED25 differentially regulates jasmonate and abscisic acid signaling through interacting with the MYC2 and ABI5 transcription factors. Plant Cell 2012, 24, 2898–2916. [Google Scholar] [CrossRef]
- Elfving, N.; Davoine, C.; Benlloch, R.; Blomberg, J.; Brannstrom, K.; Muller, D.; Nilsson, A.; Ulfstedt, M.; Ronne, H.; Wingsle, G.; et al. The Arabidopsis thaliana Med25 mediator subunit integrates environmental cues to control plant development. Proc. Natl. Acad. Sci. USA 2011, 108, 8245–8250. [Google Scholar] [CrossRef] [PubMed]
- Iñigo, S.; Alvarez, M.J.; Strasser, B.; Califano, A.; Cerdán, P.D. PFT1, the MED25 subunit of the plant Mediator complex, promotes flowering through CONSTANS dependent and independent mechanisms in Arabidopsis. Plant J. 2012, 69, 601–612. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Chen, X. The plant Mediator and its role in noncoding RNA production. Front. Biol. 2011, 6, 125. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pérez-Martín, F.; Yuste-Lisbona, F.J.; Pineda, B.; García-Sogo, B.; Del Olmo, I.; de Dios Alché, J.; Egea, I.; Flores, F.B.; Piñeiro, M.; Jarillo, J.A.; et al. Developmental role of the tomato Mediator complex subunit MED18 in pollen ontogeny. Plant J. 2018, 96, 300–315. [Google Scholar] [CrossRef]
- Bonawitz, N.D.; Soltau, W.L.; Blatchley, M.R.; Powers, B.L.; Hurlock, A.K.; Seals, L.A.; Weng, J.-K.; Stout, J.; Chapple, C. REF4 and RFR1, subunits of the transcriptional coregulatory complex mediator, are required for phenylpropanoid homeostasis in Arabidopsis. J. Biol. Chem. 2012, 287, 5434–5445. [Google Scholar] [CrossRef]
- Dhawan, R.; Luo, H.; Foerster, A.M.; Abuqamar, S.; Du, H.-N.; Briggs, S.D.; Mittelsten Scheid, O.; Mengiste, T. HISTONE MONOUBIQUITINATION1 interacts with a subunit of the mediator complex and regulates defense against necrotrophic fungal pathogens in Arabidopsis. Plant Cell 2009, 21, 1000–1019. [Google Scholar] [CrossRef]
- Kidd, B.N.; Edgar, C.I.; Kumar, K.K.; Aitken, E.A.; Schenk, P.M.; Manners, J.M.; Kazan, K. The mediator complex subunit PFT1 is a key regulator of jasmonate-dependent defense in Arabidopsis. Plant Cell 2009, 21, 2237–2252. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, C.; Zhang, Y.; Sun, Y.; Mou, Z. The Arabidopsis mediator complex subunit16 positively regulates salicylate-mediated systemic acquired resistance and jasmonate/ethylene-induced defense pathways. Plant Cell 2012, 24, 4294–4309. [Google Scholar] [CrossRef]
- Zhu, Y.; Schluttenhoffer, C.M.; Wang, P.; Fu, F.; Thimmapuram, J.; Zhu, J.-K.; Lee, S.Y.; Yun, D.-J.; Mengiste, T. CYCLIN-DEPENDENT KINASE8 differentially regulates plant immunity to fungal pathogens through kinase-dependent and -independent functions in Arabidopsis. Plant Cell 2014, 26, 4149–4170. [Google Scholar] [CrossRef]
- Çevik, V.; Kidd, B.N.; Zhang, P.; Hill, C.; Kiddle, S.; Denby, K.J.; Holub, E.B.; Cahill, D.M.; Manners, J.M.; Schenk, P.M.; et al. MEDIATOR25 Acts as an Integrative Hub for the Regulation of Jasmonate-Responsive Gene Expression in Arabidopsis. Plant Physiol. 2012, 160, 541–555. [Google Scholar] [CrossRef]
- Flores, R.; Hernández, C.; de Alba, A.E.M.; Daròs, J.-A.; Di Serio, F. Viroids and Viroid-Host Interactions. Annu. Rev. Phytopathol. 2005, 43, 117–139. [Google Scholar] [CrossRef] [PubMed]
- Di Serio, F.; Flores, R.; Verhoeven, J.T.J.; Li, S.-F.; Pallás, V.; Randles, J.W.; Sano, T.; Vidalakis, G.; Owens, R.A. Current status of viroid taxonomy. Arch. Virol. 2014, 159, 3467–3478. [Google Scholar] [CrossRef] [PubMed]
- Flores, R.; Delgado, S.; Gas, M.-E.; Carbonell, A.; Molina, D.; Gago, S.; De la Pena, M. Viroids: The minimal non-coding RNAs with autonomous replication. FEBS Lett. 2004, 567, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Tsagris, E.M.; Martínez de Alba, Á.E.; Gozmanova, M.; Kalantidis, K. Viroids. Cell. Microbiol. 2008, 10, 2168–2179. [Google Scholar] [CrossRef]
- Maniataki, E.; Tabler, M.; Tsagris, M. Viroid RNA systemic spread may depend on the interaction of a 71-nucleotide bulged hairpin with the host protein VirP1. RNA 2003, 9, 346–354. [Google Scholar] [CrossRef]
- Dalakouras, A.; Dadami, E.; Wassenegger, M. Viroid-induced DNA methylation in plants. Biomol. Concepts 2013, 4, 557–565. [Google Scholar] [CrossRef]
- Zheng, Y.; Wang, Y.; Ding, B.; Fei, Z. Comprehensive transcriptome analyses reveal that Potato spindle tuber viroid triggers genome-wide changes in alternative splicing, inducible trans-acting activity of phased secondary small interfering RNAs, and immune responses. J. Virol. 2017, 91, e00247-17. [Google Scholar] [CrossRef]
- Navarro, B.; Gisel, A.; Rodio, M.E.; Delgado, S.; Flores, R.; Di Serio, F. Small RNAs containing the pathogenic determinant of a chloroplast-replicating viroid guide the degradation of a host mRNA as predicted by RNA silencing. Plant J. 2012, 70, 991–1003. [Google Scholar] [CrossRef]
- Kappagantu, M.; Nelson, M.E.; Bullock, J.M.; Kenny, S.T.; Eastwell, K.C. Hop stunt viroid: Effects on vegetative growth and yield of hop cultivars, and its distribution in Central Washington State. Plant Dis. 2017, 101, 607–612. [Google Scholar] [CrossRef]
- Mishra, K.A.; Kumar, A.; Mishra, D.; Nath, S.V.; Jakše, J.; Kocábek, T.; Killi, K.U.; Morina, F.; Matoušek, J. Genome-Wide Transcriptomic Analysis Reveals Insights into the Response to Citrus bark cracking viroid (CBCVd) in Hop (Humulus lupulus L.). Viruses 2018, 10, 570. [Google Scholar] [CrossRef]
- Stajner, N.; Radisek, S.; Mishra, A.K.; Nath, V.S.; Matousek, J.; Jakse, J. Evaluation of disease severity and global transcriptome response induced by Citrus bark cracking viroid, Hop latent viroid, and their co-infection in hop (Humulus lupulus L.). Int. J. Mol. Sci. 2019, 20, 3154. [Google Scholar] [CrossRef] [PubMed]
- Samanta, S.; Thakur, J.K. Importance of Mediator complex in the regulation and integration of diverse signaling pathways in plants. Front. Plant Sci. 2015, 6, 757. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Li, L.; Qu, L.-J. Plant Mediator complex and its critical functions in transcription regulation. J. Integr. Plant Biol. 2016, 58, 106–118. [Google Scholar] [CrossRef] [PubMed]
- Cantin, G.T.; Stevens, J.L.; Berk, A.J. Activation domain-mediator interactions promote transcription preinitiation complex assembly on promoter DNA. Proc. Natl. Acad. Sci. USA 2003, 100, 12003–12008. [Google Scholar] [CrossRef] [PubMed]
- Baek, H.J.; Kang, Y.K.; Roeder, R.G. Human mediator enhances basal transcription by facilitating recruitment of transcription factor IIB during preinitiation complex assembly. J. Biol. Chem. 2006, 281, 15172–15181. [Google Scholar] [CrossRef]
- Kim, M.J.; Jang, I.-C.; Chua, N.-H. The Mediator complex MED15 subunit mediates activation of downstream lipid-related genes by the WRINKLED1 transcription factor. Plant Physiol. 2016, 171, 1951–1964. [Google Scholar] [CrossRef]
- Owens, R.A.; Tech, K.B.; Shao, J.Y.; Sano, T.; Baker, C.J. Global analysis of tomato gene expression during Potato spindle tuber viroid infection reveals a complex array of changes affecting hormone signaling. Mol. Plant Microbe Interact. 2012, 25, 582–598. [Google Scholar] [CrossRef]
- Katsarou, K.; Wu, Y.; Zhang, R.; Bonar, N.; Morris, J.; Hedley, P.E.; Bryan, G.J.; Kalantidis, K.; Hornyik, C. Insight on Genes Affecting Tuber Development in Potato upon Potato spindle tuber viroid (PSTVd) Infection. PLoS ONE 2016, 11, e0150711. [Google Scholar] [CrossRef]
- Sukumari Nath, V.; Kumar Mishra, A.; Kumar, A.; Matoušek, J.; Jakše, J. Revisiting the role of transcription factors in coordinating the defense response against Citrus bark cracking viroid infection in commercial hop (Humulus Lupulus L.). Viruses 2019, 11, 419. [Google Scholar] [CrossRef]
- An, C.; Mou, Z. The function of the Mediator complex in plant immunity. Plant Signal. Behav. 2013, 8, e23182. [Google Scholar] [CrossRef]
- Lai, Z.; Schluttenhofer, C.M.; Bhide, K.; Shreve, J.; Thimmapuram, J.; Lee, S.Y.; Yun, D.-J.; Mengiste, T. MED18 interaction with distinct transcription factors regulates multiple plant functions. Nat. Commun. 2014, 5, 3064. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yao, J.; Zhang, Y.; Sun, Y.; Mou, Z. The Arabidopsis Mediator complex subunits MED14/SWP and MED16/SFR6/IEN1 differentially regulate defense gene expression in plant immune responses. Plant J. 2013, 75, 484–497. [Google Scholar] [CrossRef]
- Wathugala, D.L.; Hemsley, P.A.; Moffat, C.S.; Cremelie, P.; Knight, M.R.; Knight, H. The Mediator subunit SFR6/MED16 controls defence gene expression mediated by salicylic acid and jasmonate responsive pathways. New Phytol. 2012, 195, 217–230. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Zheng, B.; Yu, Y.; Won, S.Y.; Mo, B.; Chen, X. The role of Mediator in small and long noncoding RNA production in Arabidopsis thaliana. EMBO J. 2011, 30, 814–822. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.K.; Duraisamy, G.S.; Matoušek, J.; Radisek, S.; Javornik, B.; Jakse, J. Identification and characterization of microRNAs in Humulus lupulus using high-throughput sequencing and their response to Citrus bark cracking viroid (CBCVd) infection. BMC Genom. 2016, 17. [Google Scholar] [CrossRef]
- Uthe, H.; Vanselow, J.T.; Schlosser, A. Proteomic analysis of the Mediator complex interactome in Saccharomyces cerevisiae. Sci. Rep. 2017, 7, 43584. [Google Scholar] [CrossRef]
- Matoušek, J.; Siglová, K.; Jakše, J.; Radišek, S.; Brass, J.R.J.; Tsushima, T.; Guček, T.; Duraisamy, G.S.; Sano, T.; Steger, G. Propagation and some physiological effects of Citrus bark cracking viroid and Apple fruit crinkle viroid in multiple infected hop (Humulus lupulus L.). J. Plant Physiol. 2017, 213, 166–177. [Google Scholar] [CrossRef]
- Kalantidis, K.; Denti, M.A.; Tzortzakaki, S.; Marinou, E.; Tabler, M.; Tsagris, M. Virp1 is a host protein with a major role in Potato spindle tuber viroid infection in Nicotiana plants. J. Virol. 2007, 81, 12872–12880. [Google Scholar] [CrossRef]
- Štajner, N.; Cregeen, S.; Javornik, B. Evaluation of reference genes for RT-qPCR expression studies in hop (Humulus lupulus L.) during infection with vascular pathogen Verticillium albo-atrum. PLoS ONE 2013, 8, e68228. [Google Scholar] [CrossRef]
- Mathur, S.; Vyas, S.; Kapoor, S.; Tyagi, A.K. The Mediator complex in plants: Structure, phylogeny, and expression profiling of representative genes in a dicot (Arabidopsis) and a monocot (Rice) during reproduction and abiotic Stress. Plant Physiol. 2011, 157, 1609–1627. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nath, V.S.; Shrestha, A.; Awasthi, P.; Mishra, A.K.; Kocábek, T.; Matoušek, J.; Sečnik, A.; Jakše, J.; Radišek, S.; Hallan, V. Mapping the Gene Expression Spectrum of Mediator Subunits in Response to Viroid Infection in Plants. Int. J. Mol. Sci. 2020, 21, 2498. https://doi.org/10.3390/ijms21072498
Nath VS, Shrestha A, Awasthi P, Mishra AK, Kocábek T, Matoušek J, Sečnik A, Jakše J, Radišek S, Hallan V. Mapping the Gene Expression Spectrum of Mediator Subunits in Response to Viroid Infection in Plants. International Journal of Molecular Sciences. 2020; 21(7):2498. https://doi.org/10.3390/ijms21072498
Chicago/Turabian StyleNath, Vishnu Sukumari, Ankita Shrestha, Praveen Awasthi, Ajay Kumar Mishra, Tomáš Kocábek, Jaroslav Matoušek, Andrej Sečnik, Jernej Jakše, Sebastjan Radišek, and Vipin Hallan. 2020. "Mapping the Gene Expression Spectrum of Mediator Subunits in Response to Viroid Infection in Plants" International Journal of Molecular Sciences 21, no. 7: 2498. https://doi.org/10.3390/ijms21072498
APA StyleNath, V. S., Shrestha, A., Awasthi, P., Mishra, A. K., Kocábek, T., Matoušek, J., Sečnik, A., Jakše, J., Radišek, S., & Hallan, V. (2020). Mapping the Gene Expression Spectrum of Mediator Subunits in Response to Viroid Infection in Plants. International Journal of Molecular Sciences, 21(7), 2498. https://doi.org/10.3390/ijms21072498






