Chitosan Modified Zeolite Molecular Sieve Particles as a Filter for Ammonium Nitrogen Removal from Water
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Sorbent Media
2.3. Batch Adsorption Experiment
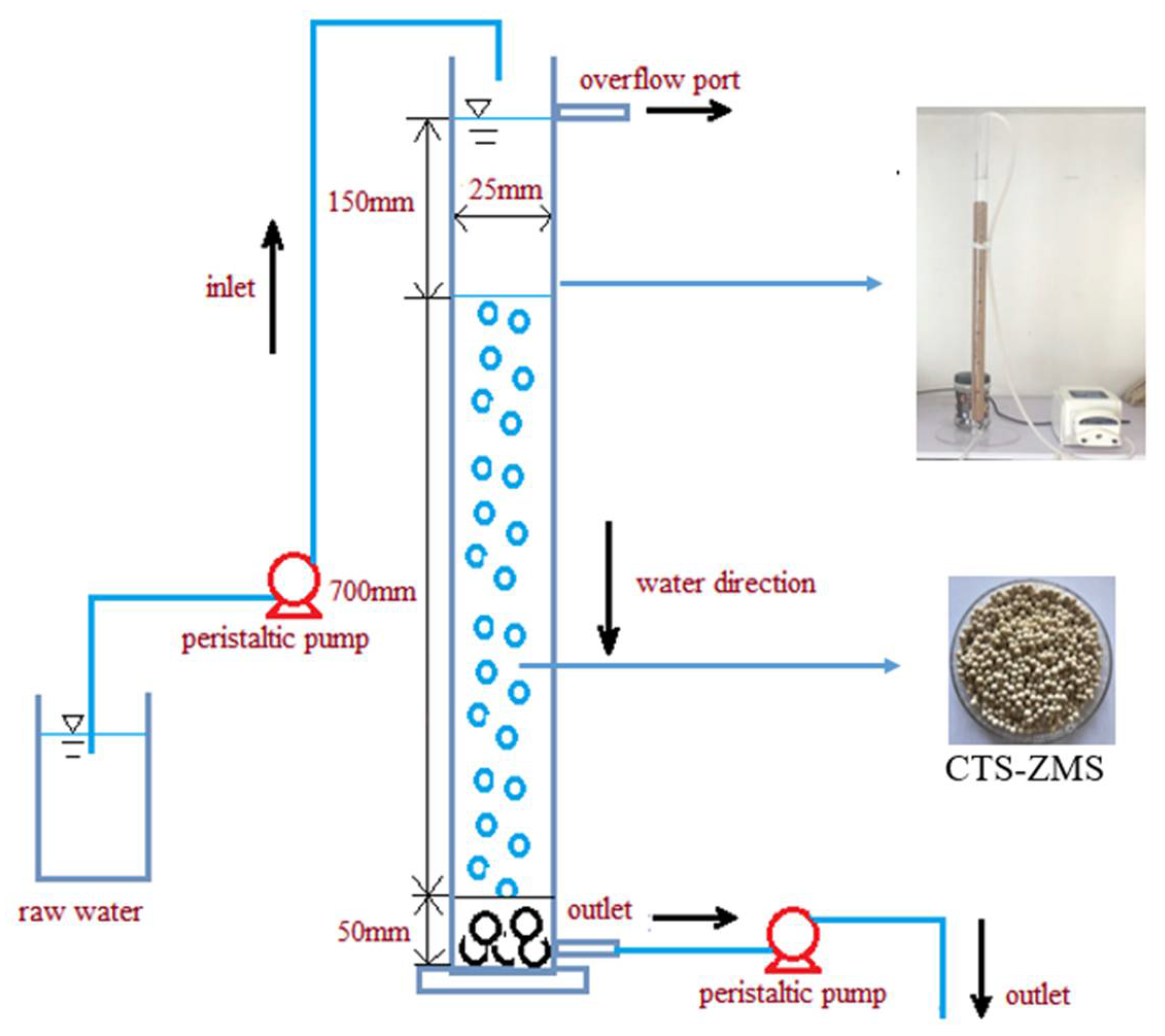
2.4. Dynamic Adsorption Experiment
2.5. Thomas Model
2.6. Characterizations
2.7. Regeneration
3. Results
3.1. Batch Experiment
3.2. Dynamic Experiment
3.2.1. Effect of Bed Depth
3.2.2. Effect of Flow Rate
3.2.3. Effect of Initial pH Value
3.2.4. Effect of Initial Ammonium Nitrogen Concentration
3.3. Dynamic Adsorption Model
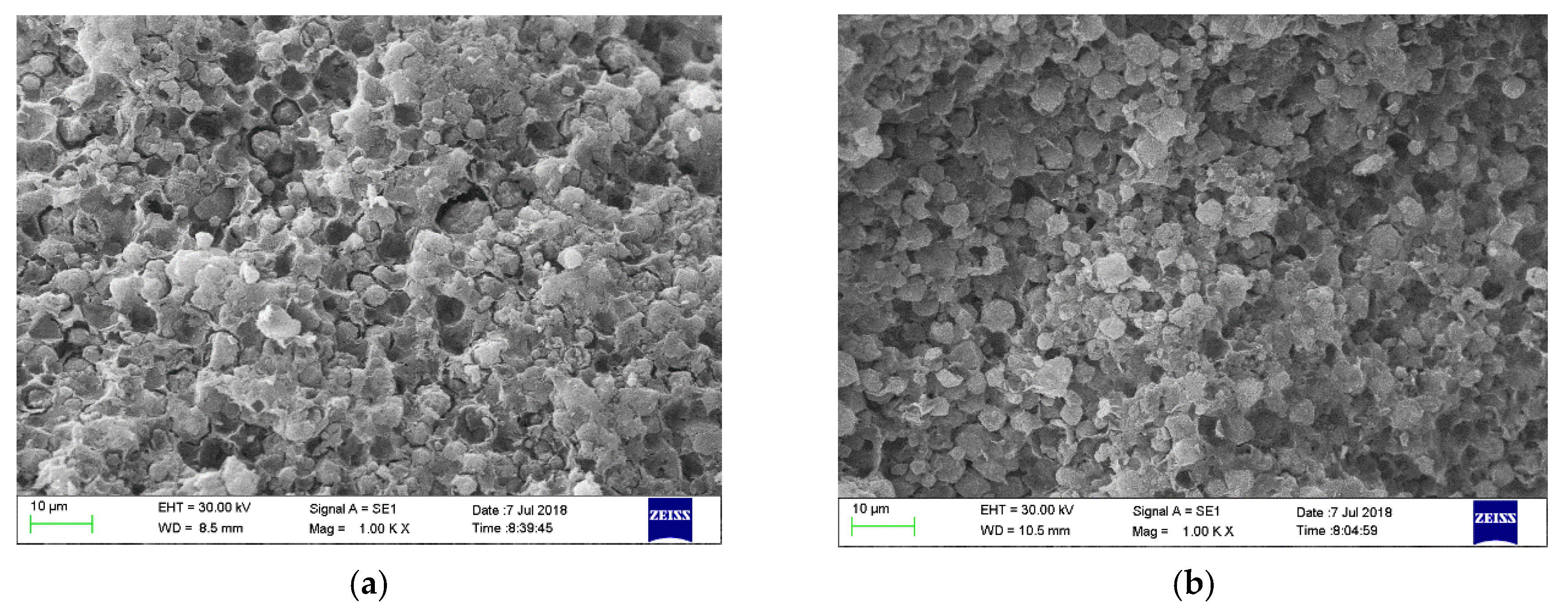
3.4. SEM
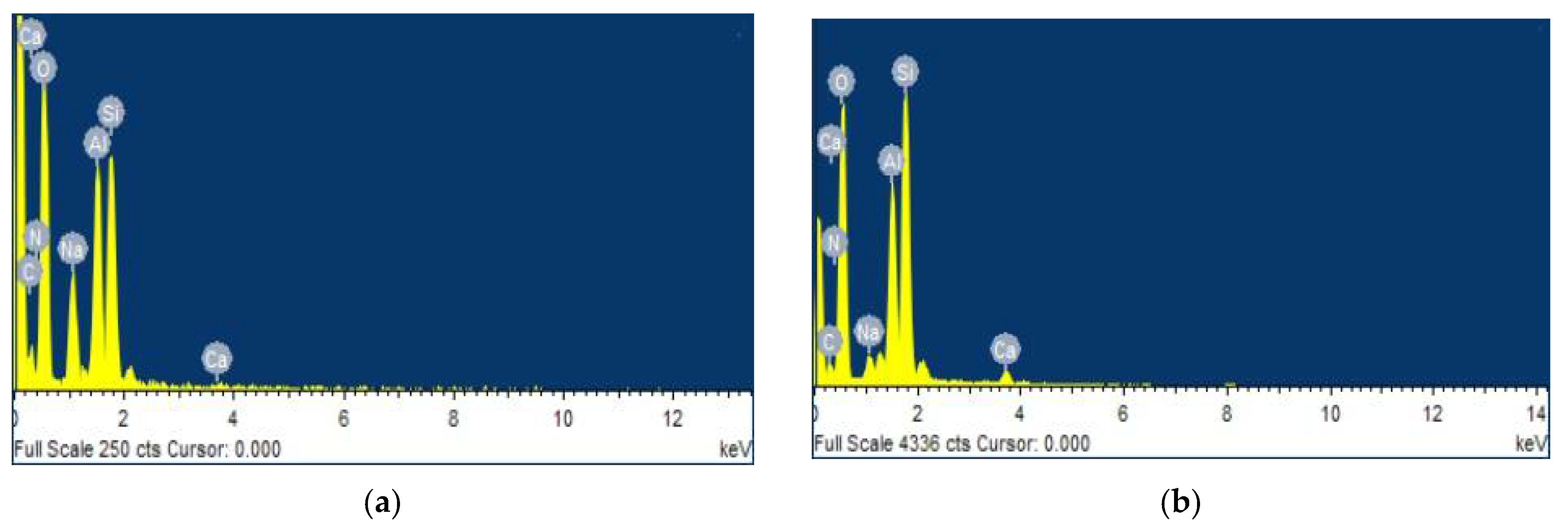
3.5. EDS
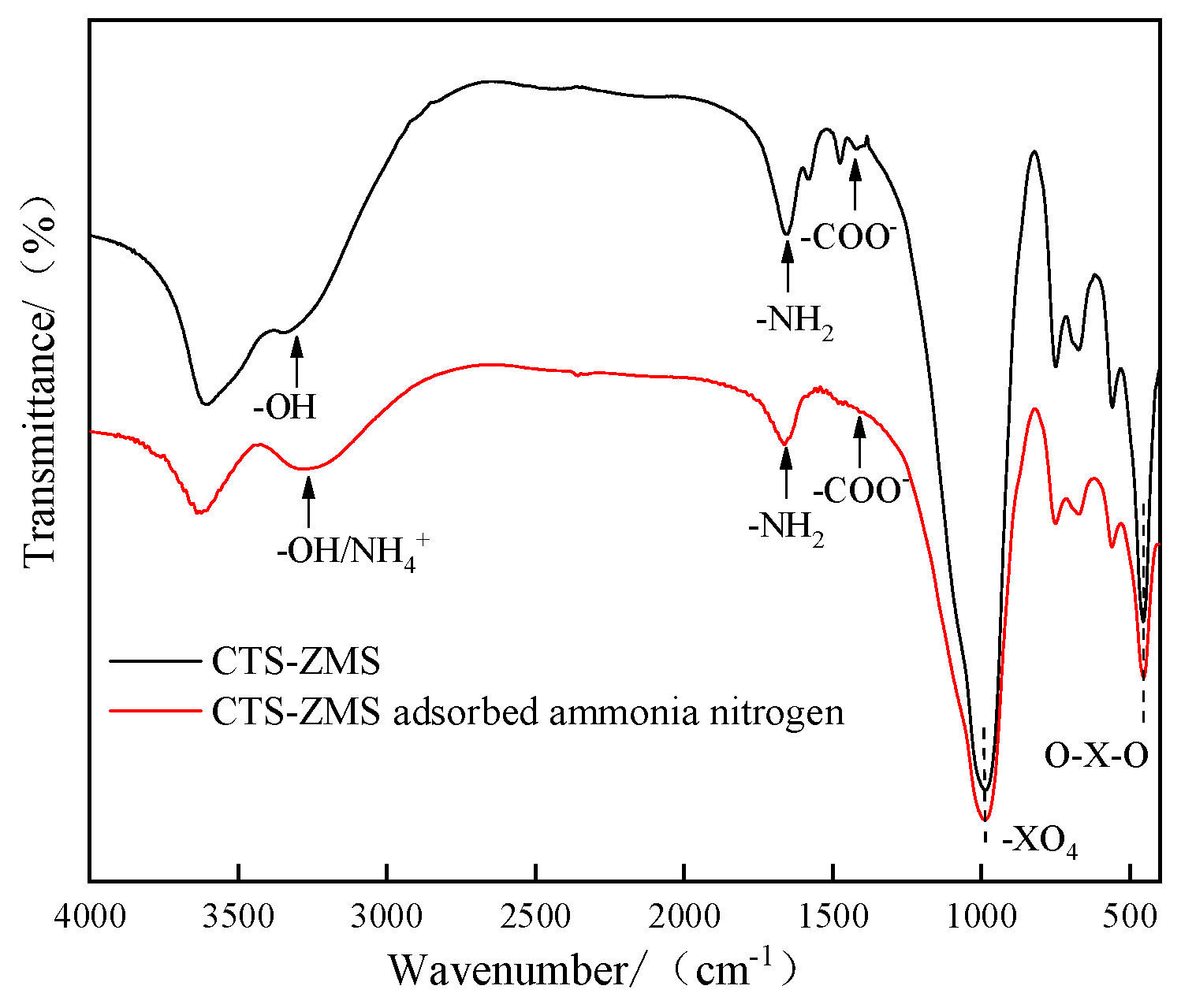
3.6. FTIR
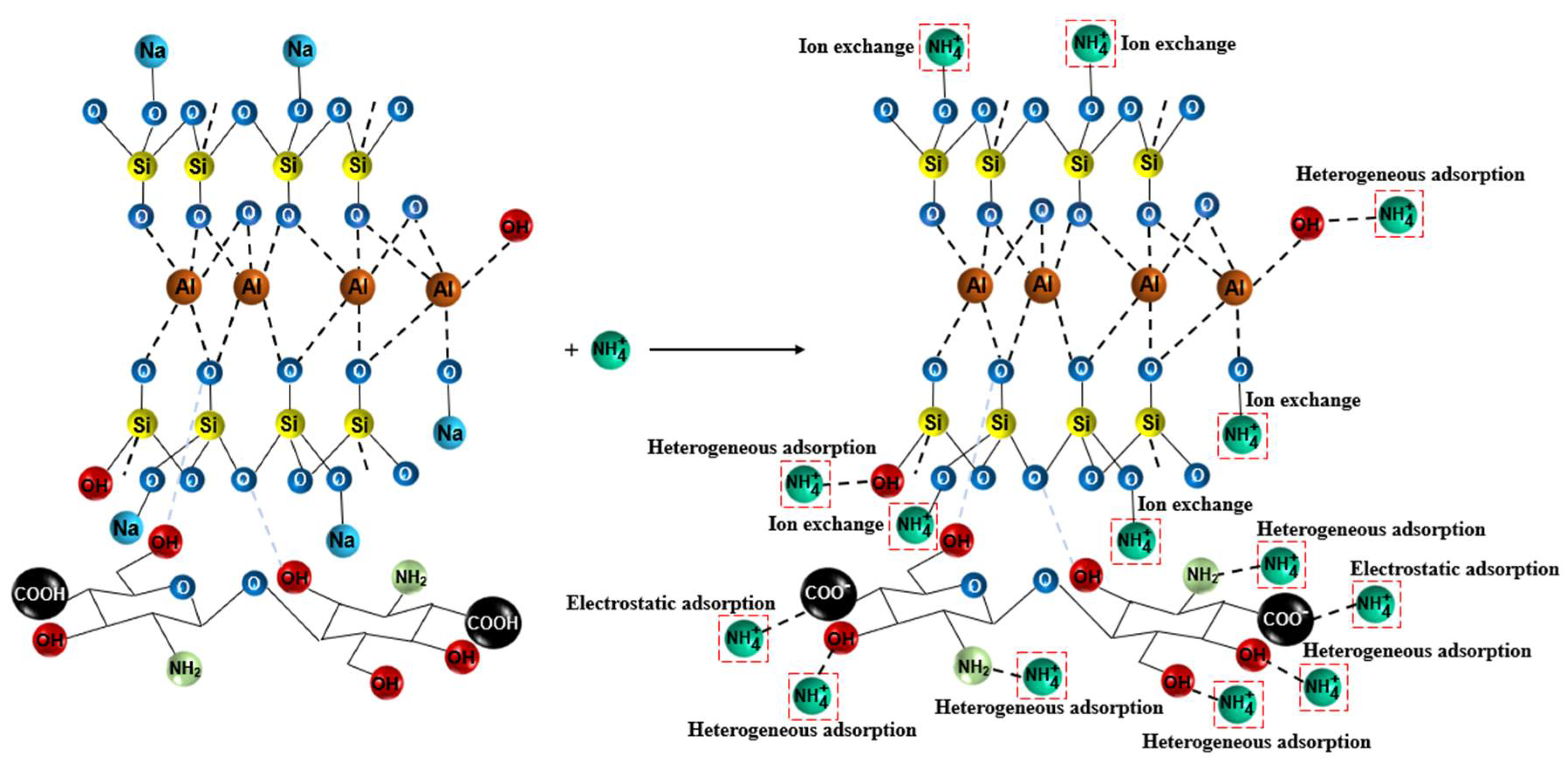
3.7. Adsorption Mechanism
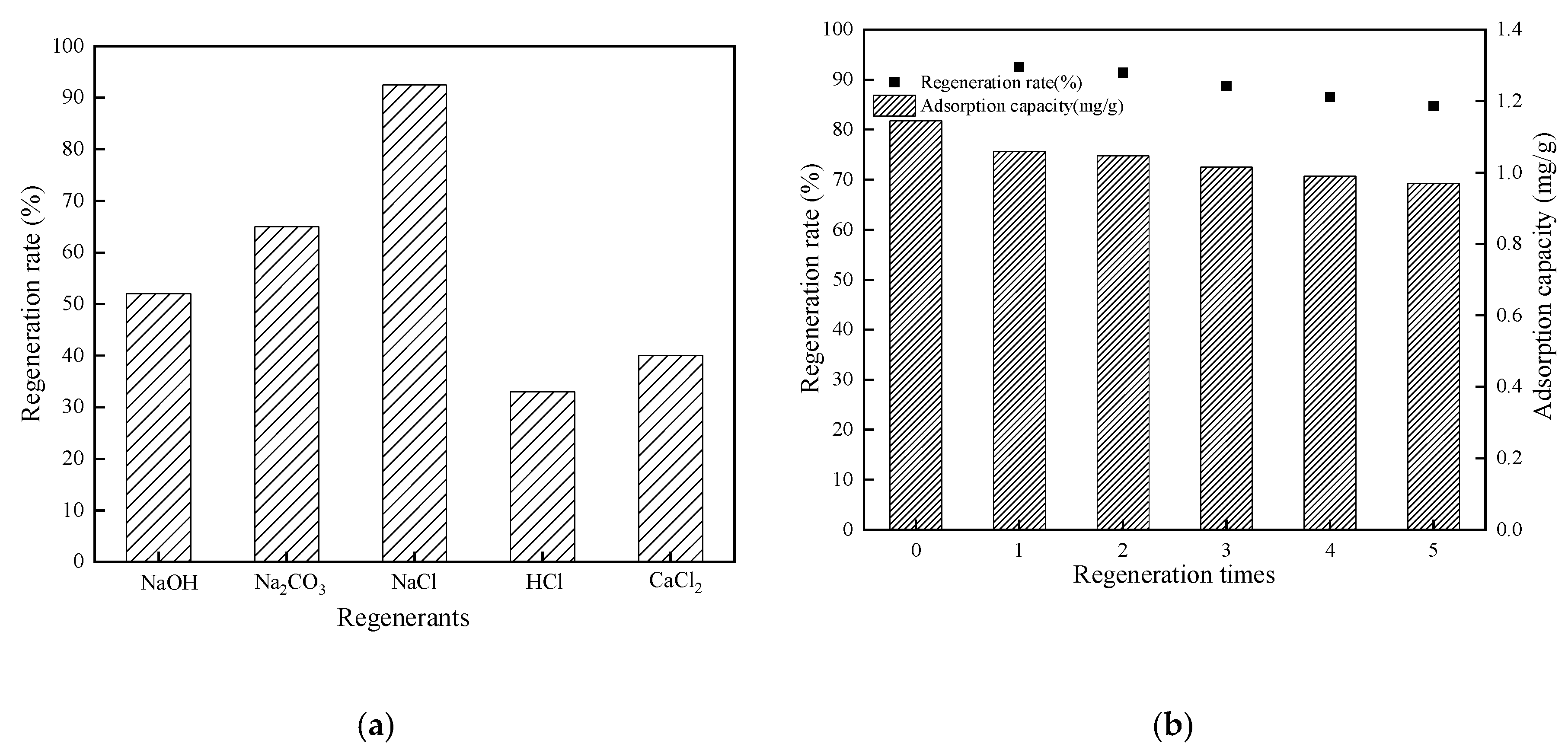
3.8. Regeneration
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fu, Q.; Zheng, B.; Zhao, X.; Wang, L.; Liu, C. Ammonia pollution characteristics of centralized drinking water sources in China. J. Environ. Sci. 2012, 24, 1739–1743. [Google Scholar] [CrossRef]
- Cheng, Q.; Huang, Y.; Nengzi, L.; Liu, J.; Zhang, J. Performance and microbial community profiles in pilot-scale biofilter for ammonia, iron and manganese removal at different dissolved oxygen concentrations. World J. Micro. Biotechnol. 2019, 35, 43. [Google Scholar]
- Körner, S.; Das, S.K.; Veenstra, S.; Vermaat, J.E. The effect of pH variation at the ammonium/ammonia equilibrium in wastewater and its toxicity to Lemna gibba. Aquat. Bot. 2001, 71, 71–78. [Google Scholar] [CrossRef]
- Adam, M.R.; Othman, M.H.D.; Samah, R.A.; Puteh, M.H.; Ismail, A.F.; Mustafa, A.; Rahman, M.A.; Jaafar, J. Current trends and future prospects of ammonia removal in wastewater: A comprehensive review on adsorptive membrane development. Sep. Purif. Technol. 2019, 213, 114–132. [Google Scholar]
- Du, Q.; Liu, S.; Cao, Z.; Wang, Y. Ammonium removal from aqueous solution using natural Chinese clinoptilolite. Sep. Purif. Technol. 2005, 44, 223–234. [Google Scholar]
- Seruga, P.; Krzywonos, M.; Pyźanowska, J.; Urbanowska, A.; Pawlak-Kruczek, H.; Niedźwiecki, Ł. Removal of ammonia from the municipal waste treatment effluents using natural minerals. Molecular 2019, 24, 3633. [Google Scholar] [CrossRef]
- Yadu, A.; Sahariah, B.P.; Anandkumar, J. Influence of COD/ammonia ratio on simultaneous of NH4+-N and COD in surface water using moving bed batch reactor. J. Water Process Eng. 2018, 22, 66–72. [Google Scholar] [CrossRef]
- Blute, N.; Ghosh, A.; Lytle, D. Assessing ammonia treatment options. Opflow Online 2012, 38, 14–17. [Google Scholar] [CrossRef]
- Xue, R.; Donovan, A.; Zhang, H.; Ma, Y.; Adam, C.; Yang, J.; Hua, B.; Inniss, E.; Eichholz, T.; Shi, H. Simultaneous removal of ammonia and N-nitrosamine precursors from high ammonia water by zeolite and powdered activated carbon. J. Environ. Sci. 2018, 64, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Soltani, R.D.C.; Rezaee, A.; Khataee, A.R.; Godini, H. Optimization of the operational parameters during a biological nitrification process using response surface methodology. Can. J. Chem. Eng. 2014, 92, 13–22. [Google Scholar] [CrossRef]
- Zhu, G.; Peng, Y.; Wang, S.; Wu, S.; Ma, B. Effect of influent flow rate distribution on the performance of step-feed biological nitrogen removal process. Chem. Eng. J. 2007, 131, 319–328. [Google Scholar] [CrossRef]
- Khabzina, Y.; Farrusseng, D. Unravelling ammonia adsorption mechanisms of adsorbents in humid conditions. Microporous Mesoporous Mater. 2018, 265, 142–148. [Google Scholar]
- Lapo, B.; Demey, H.; Carchi, T.; Sastre, M.A. Antimony removal from water by a chitosan-iron(III)[ChiFer(III)] biocomposit. Polymers 2019, 11, 351. [Google Scholar] [CrossRef] [PubMed]
- Demey, H.; Barron-Zambrano, J.; Mhadhbi, T.; Miloudi, H.; Yang, Z.; Ruiz, M.; Sastre, M.A. Boron removal from aqueous solutions by using a novel alginate-based sorbent: Comparison with Al2O3 particles. Polymers 2019, 11, 1509. [Google Scholar] [CrossRef]
- Attar, K.; Demey, H.; Bouazza, D.; Sastre, M.A. Sorption and desorption studies of Pb(II) and Ni(II) from aqueous solutions by a new composites based on alginate and magadiite materials. Polymers 2019, 11, 340. [Google Scholar] [CrossRef]
- Xie, J.; Li, C.; Chi, L.; Wu, D. Chitosan modified zeolite as a versatile adsorbent for the removal of different pollutants from water. Fuel 2013, 103, 480–485. [Google Scholar] [CrossRef]
- Wang, J.; Chen, C. Chitosan-based biosorbents: Modification and application for biosorption of heavy metals and radionuclides. Bioresour. Technol. 2014, 160, 129–141. [Google Scholar] [CrossRef]
- Bernardi, F.; Zadinelo, I.V.; Alves, H.J.; Meurer, F.; dos Santos, L.D. Chitins and chitosans for the removal of total ammonia of aquaculture effluents. Aquaculture 2018, 483, 203–212. [Google Scholar] [CrossRef]
- Zadinelo, I.V.; Alves, H.J.; Moesch, A.; Colpini, L.M.S.; da Silva, L.C.R.; dos Santos, L.D. Influence of the chemical composition of smectites on the removal of ammonium ions from aquaculture effluents. J. Mater. Sci. 2015, 50, 1865–1875. [Google Scholar] [CrossRef]
- Chung, Y.C.; Li, Y.H.; Chen, C.C. Pollutant removal from aquaculture wastewater using the biopolymer chitosan at different molecular weights. J. Environ. Sci. Health A 2005, 40, 1775–1790. [Google Scholar] [CrossRef]
- Davis, S.P. Chitosan: Manufacture, Properties, and Usage; Series: Biotechnology in Agriculture, Industry and Medicine; Nova Science Publishers: Hauppauge, NY, USA, 2011; pp. 134–140. [Google Scholar]
- Chassary, P.; Vincent, T.; Guibal, E. Metal anion sorption on chitosan and derivative materials: A strategy for polymer modification and optimum use. React. Funct. Polym. 2004, 60, 137–149. [Google Scholar] [CrossRef]
- Teimouri, A.; Nasab, S.G.; Vahdatpoor, N.; Habibollahi, S.; Salavati, H.; Chermahini, A.N. Chitosan/Zeolite Y/Nano ZrO2 nanocomposite as an adsorbent for the removal of nitrate from the aqueous solution. Int. J. Biol. macromol. 2016, 93, 254–266. [Google Scholar] [CrossRef]
- Dehghani, M.H.; Dehghan, A.; Alidadi, H.; Dolatabadi, M.; Mehrabpour, M.; Converti, A. Removal of methylene blue dye from aqueous solutions by a new chitosan/zeolite composite from shrimp waste: Kinetic and equilibrium study. J. Chem. Eng. 2017, 34, 1699–1707. [Google Scholar] [CrossRef]
- Kumpanenkoa, I.V.; Ivanova, N.A.; Dyubanov, M.V.; Skryl’nikov, A.M.; Kovaleva, N.Y.; Roshchin, A.V. Removal of mercury (II) from aqueous solutions via dynamic column adsorption. Chem. Phys. Ecol. Process. 2019, 38, 59–70. [Google Scholar] [CrossRef]
- Vakili, M.; Mojiri, A.; Kindaichi, T.; Cagnetta, G.; Yuan, J.; Wang, B.; Giwa, A.S. Cross-linked chitosan/zeolite as a fixed-bed column for organic micropollutants removal from aqueous solution, optimization with RSM and artificial neural network. J. Environ. Manag. 2019, 250, 109434. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Ru, Y.; Zhou, L. Preparation and characterization of chitosan-zeolite molecular sieve composite for ammonia and nitrate removal. Adv. Compos. Lett. 2018, 27, 185–192. [Google Scholar] [CrossRef]
- Langmuir, I. The adsorption of gases on plane surfaces of glass. mica and platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef]
- Freundlich, H.M.F. Über die Adsorption in Lösungen. Zeitschrift für physikalische Chemie 1906, 57, 1996–2001. [Google Scholar] [CrossRef]
- Paudyal, H.; Pangeni, B.; Inoue, K.; Kawakita, H.; Alam, K.O.S. Adsorptive removal of fluoride from aqueous medium using a fixed bed column packed with Zr(IV) loaded dried orange juice residue. Bioresour. Technol. 2013, 146, 713–720. [Google Scholar] [CrossRef]
- Thomas, H.C. Heterogeneous ion exchange in a flowing system. J. Am. Chem. Soc. 1944, 66, 1664–1671. [Google Scholar] [CrossRef]
- Gouran-Orimi, R.; Mirzayi, B.; Nematollahzadeh, A.; Tardast, A. Competitive adsorption of nitrate in fixed-bed column packed with bio-inspired polydopamine coated zeolite. J. Environ. Chem. Eng. 2018, 6, 2232–2240. [Google Scholar] [CrossRef]
- Ghosh, A.; Chakrabarti, S.; Ghosh, U.C. Fixed-bed column performance of Mn-incorporated iron (III) oxide nanoparticle agglomerates on As (III) removal from the spiked groundwater in lab bench scale. Chem. Eng. J. 2014, 248, 18–26. [Google Scholar] [CrossRef]
- Moussavi, G.; Talebi, S.; Farrokhi, M.; Sabouti, R.M. The investigation of mechanism, kinetic and isotherm of ammonia and humic acid co-adsorption onto natural zeolite. Chem. Eng. J. 2011, 171, 1159–1169. [Google Scholar] [CrossRef]
- Yang, K.; Zhang, X.; Chao, C.; Zhang, B.; Liu, J. In-situ preparation of NaA zeolite/chitosan porous hybrid beads for removal of ammonium from aqueous solution. Carbohyd. Polym. 2014, 107, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Xing, X.; Gao, B.Y.; Zhong, Q.Q.; Yue, Q.Y.; Li, Q. Sorption of nitrate onto amine crosslinked wheat straw: Characteristics, column sorption and desorption properties. J. Hazard. Mater. 2011, 186, 206–211. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Wen, X.; Ke, Q.F.; Xie, X.T.; Guo, Y.P. pH-responsive mesoporous ZSM-5 zeolite/chitosan core-cell nanodisks loaded with doxorubicin against osteosarcoma. Mater. Sci. Eng. C 2018, 85, 142–153. [Google Scholar]
- Luna, M.D.G.; Futalan, C.M.; Jurado, C.A., Jr.; Colades, J.I.; Wan, M.W. Removal of ammonium-nitrogen from aqueous solution using chitosan-coated bentonite: Mechanism and effect of operating parameters. Appl. Polym. Sci. 2018, 135, 45924(1–11). [Google Scholar] [CrossRef]
- Baral, S.; Das, N.; Ramalu, T.S.; Sahoo, S.K.; Das, S.N.; Chaudhary, G.R. Removal of Cr(VI) by thermally activated weed Salvinia cucullata in a fixed-bed column. J. Hazard. Mater. 2009, 161, 1427–1435. [Google Scholar] [CrossRef]
- Jain, M.; Garg, V.K.; Kadirvelu, K. Cadmium(II) sorption and desorption in a fixed bed column using sunflower waste carbon calcium–alginate beads. Bioresour. Technol. 2013, 129, 242–248. [Google Scholar] [CrossRef]
- Singh, A.; Kumar, D.; Gaur, J.P. Continuous metal removal from solution and industrial effluents using Spirogyra biomass-packed column reactor. Water Res. 2012, 46, 779–788. [Google Scholar] [CrossRef]
- Aksu, Z.; Gönen, F. Biosorption of phenol by immobilized activated sludge in a continuous packed bed: Prediction of breakthrough curves. Process Biochem. 2004, 39, 599–613. [Google Scholar] [CrossRef]
- Cooney, E.L.; Booker, N.A.; Shallcross, D.C.; Stevens, G.W. Ammonia removal from wastewaters using natural Australian zeolite. Pilot-Scale study using continuous packed column process. Sep. Sci. Technol. 1999, 34, 2741–2760. [Google Scholar] [CrossRef]
- Alshameri, A.; Yan, C.; Al-Ani, Y.; Salman, A.; Ibrahim, A.; Zhou, C.; Wang, H. An investigation into the adsorption removal of ammonium by salt activated Chinese (Hulaodu) natural zeolite: Kinetics, isotherms and thermodynamics. J. Taiwan Inst. Chem. E 2014, 45, 554–564. [Google Scholar] [CrossRef]
- Hu, Z.; Qin, Y.; Lu, S.; Huang, C.; Chen, Z. Preparation and properties of chitosan-tranexamic acid salts. Mater. Sci. Forum. 2019, 943, 129–134. [Google Scholar] [CrossRef]
- Drenchev, N.; Hadjiivanov, K. Interaction of H2(D2) with OH (OD) groups in a ZSM-5 Zeolite: FTIR study of the isotopic effects. J. Phys. Chem. 2014, 118, 25118–25123. [Google Scholar] [CrossRef]
- Wu, Z.; An, Y.; Wang, Z.; Yang, S.; Chen, H.; Zhou, Z.; Mai, S. Study on zeolite enhanced contact–adsorption regeneration–stabilization process for nitrogen removal. J. Hazard Mater. 2008, 156, 317–326. [Google Scholar] [CrossRef]
- Guo, H.; Tang, L.; Yan, B.; Wan, K.; Li, P. NaA zeolite derived from blast furnace slag: Its application for ammonium removal. Water Sci. Tech. 2017, 76, 1140–1149. [Google Scholar]
- Tan, B.; Luo, Y.; Bi, X.; Liang, X.; Wang, S.; Gao, X.; Zhang, Z.; Fang, Y. Improved desorption performance of NaA zeolite by rare earth (Re=La, Nd) ion exchange. Heat Mass Transf. 2019, 55, 3179–3187. [Google Scholar] [CrossRef]
- Al-Ghouti, M.; Khraisheh, M.A.M.; Ahmad, M.N.M.; Allen, S. Thermodynamic behaviour and the effect of temperature on the removal of dyes from aqueous solution using modified diatomite: A kinetic study. J. Colloid Inter. Sci. 2005, 287, 6–13. [Google Scholar]
- Zheng, Y.; Wang, A. Enhanced adsorption of ammonium using hydrogel composites based on chitosan and halloysite. J. Macromol. Sci. 2010, 47, 33–38. [Google Scholar] [CrossRef]
- Zheng, Y.; Zhang, J.; Wang, A. Fast removal of ammonium nitrogen from aqueous solution using chitosan-g-poly(acrylic acid)/attapulgite composite. Chem. Eng. J. 2009, 155, 215–222. [Google Scholar] [CrossRef]


| Langmuir | Freundlich | |||||
|---|---|---|---|---|---|---|
| Type | qm/(mg/g) | b/(L/mg) | R2 | Kf/[(mg/g)/(mg/L)1/n] | 1/n | R2 |
| CTS-ZMS | 1.145 | 0.703 | 0.9504 | 0.224 | 0.137 | 0.6314 |
| Adsorbent | Experimental Conditions | NH4+-N Removal Efficiency | References |
|---|---|---|---|
| CTS | pH: 7.5 Concentration: 22.91 mg/L | 29.74% | [18] |
| Iranian natural zeolite | pH: 7.0 Concentration: 50 mg/L Temperature: 20 °C | 69% | [34] |
| CTS-ZMS | pH: 7.0 Concentration: 4–5 mg/L Temperature: 25 °C | 0.636 mg/g 81.60% | [27] |
| NaA zeolite/chitosan | Adsorbent dose: 0.7 g/L Concentrationrage: 100 mg/L Temperature: 25 °C | 5.84 mg/g 82.80% | [35] |
| Experimental Conditions | Thomas Parameters | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No. | Z (cm) | Q (mL/min) | pH | C0 (mg/L) | ta (min) | tb (min) | Δt (min) | KTh × 10−3 (L/min·mg) | R2 | q0/(mg/g) |
| 1 | 30 | 32 | 6.5 | 5 | 6 | 391 | 385 | 1.88 | 0.931 | 0.225 |
| 2 | 50 | 32 | 6.5 | 5 | 9 | 509 | 500 | 1.53 | 0.962 | 0.228 |
| 3 | 70 | 32 | 6.5 | 5 | 45 | 687 | 642 | 1.32 | 0.988 | 0.255 |
| 4 | 70 | 32 | 6.5 | 5 | 45 | 687 | 642 | 1.32 | 0.989 | 0.255 |
| 5 | 70 | 49 | 6.5 | 5 | 12 | 389 | 377 | 1.94 | 0.913 | 0.182 |
| 6 | 70 | 65 | 6.5 | 5 | 7 | 215 | 208 | 3.59 | 0.958 | 0.123 |
| 7 | 70 | 32 | 4.5 | 5 | 19 | 539 | 520 | 1.57 | 0.979 | 0.215 |
| 8 | 70 | 32 | 6.5 | 5 | 45 | 687 | 642 | 1.32 | 0.989 | 0.255 |
| 9 | 70 | 32 | 8.5 | 5 | 9 | 471 | 462 | 1.62 | 0.958 | 0.190 |
| 10 | 70 | 32 | 6.5 | 3 | 8 | 450 | 442 | 3.58 | 0.991 | 0.095 |
| 11 | 70 | 32 | 6.5 | 5 | 45 | 687 | 642 | 1.32 | 0.989 | 0.255 |
| 12 | 70 | 32 | 6.5 | 7 | 215 | 746 | 531 | 1.30 | 0.964 | 0.489 |
| Element (wt.%) | C | N | O | Na | Al | Si | Ca | Total Amount | |
|---|---|---|---|---|---|---|---|---|---|
| CTS-ZMS | Weight percentage | 10.33 | 5.16 | 46.77 | 4.68 | 10.48 | 22.35 | 0.23 | 100.00 |
| Atomic percentage | 14.99 | 6.55 | 52.40 | 3.51 | 6.69 | 13.35 | 0.10 | ||
| NH4+-N adsorbed CTS-ZMS | Weight percentage | 3.26 | 10.25 | 50.22 | 1.20 | 13.34 | 21.43 | 0.30 | 100.00 |
| Atomic percentage | 5.15 | 13.67 | 57.45 | 0.94 | 8.89 | 13.77 | 0.13 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, Y.; Zhang, J. Chitosan Modified Zeolite Molecular Sieve Particles as a Filter for Ammonium Nitrogen Removal from Water. Int. J. Mol. Sci. 2020, 21, 2383. https://doi.org/10.3390/ijms21072383
Gao Y, Zhang J. Chitosan Modified Zeolite Molecular Sieve Particles as a Filter for Ammonium Nitrogen Removal from Water. International Journal of Molecular Sciences. 2020; 21(7):2383. https://doi.org/10.3390/ijms21072383
Chicago/Turabian StyleGao, Yunan, and Jiayu Zhang. 2020. "Chitosan Modified Zeolite Molecular Sieve Particles as a Filter for Ammonium Nitrogen Removal from Water" International Journal of Molecular Sciences 21, no. 7: 2383. https://doi.org/10.3390/ijms21072383
APA StyleGao, Y., & Zhang, J. (2020). Chitosan Modified Zeolite Molecular Sieve Particles as a Filter for Ammonium Nitrogen Removal from Water. International Journal of Molecular Sciences, 21(7), 2383. https://doi.org/10.3390/ijms21072383





