Vitamin D Suppresses Ovarian Cancer Growth and Invasion by Targeting Long Non-Coding RNA CCAT2
Abstract
1. Introduction
2. Results
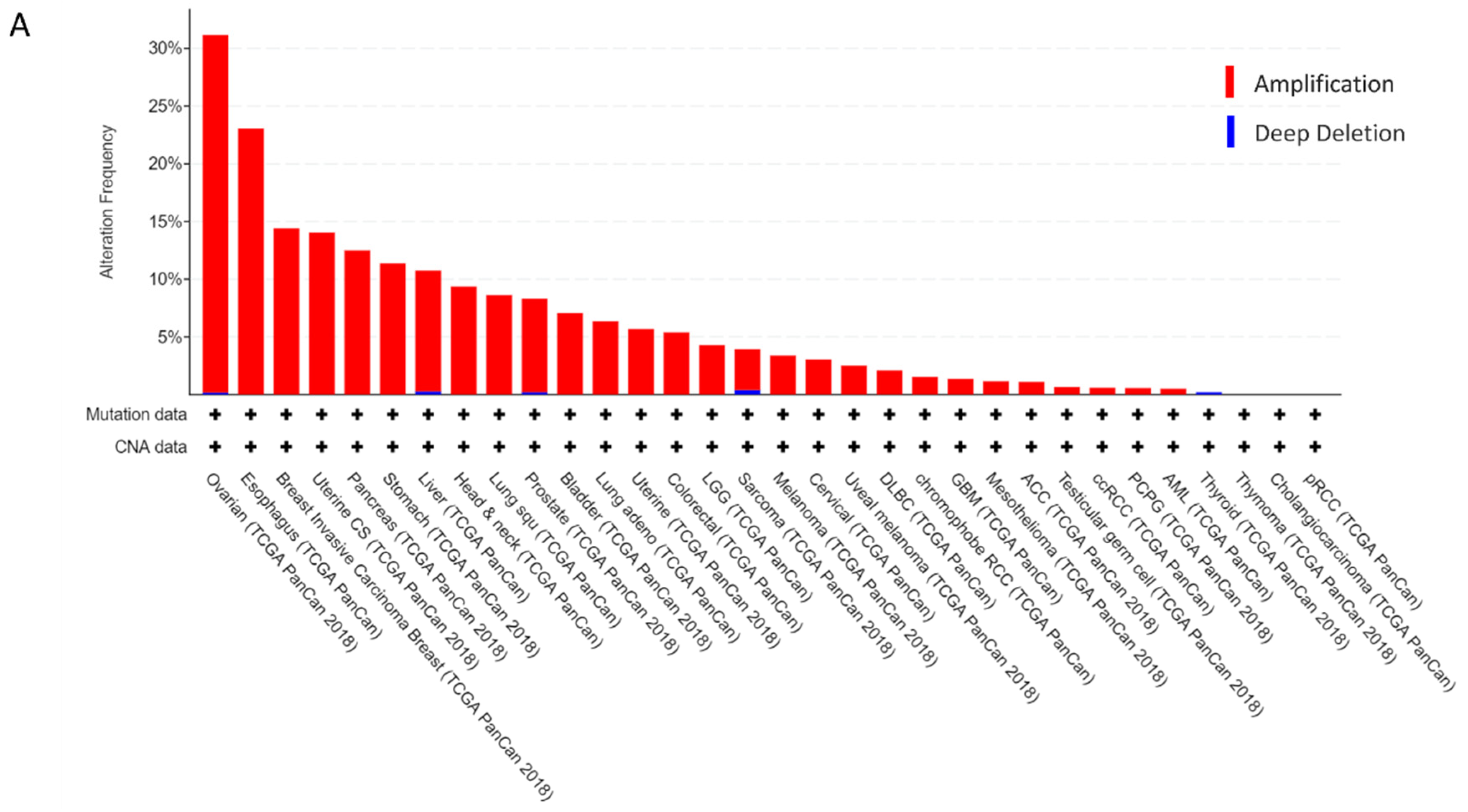
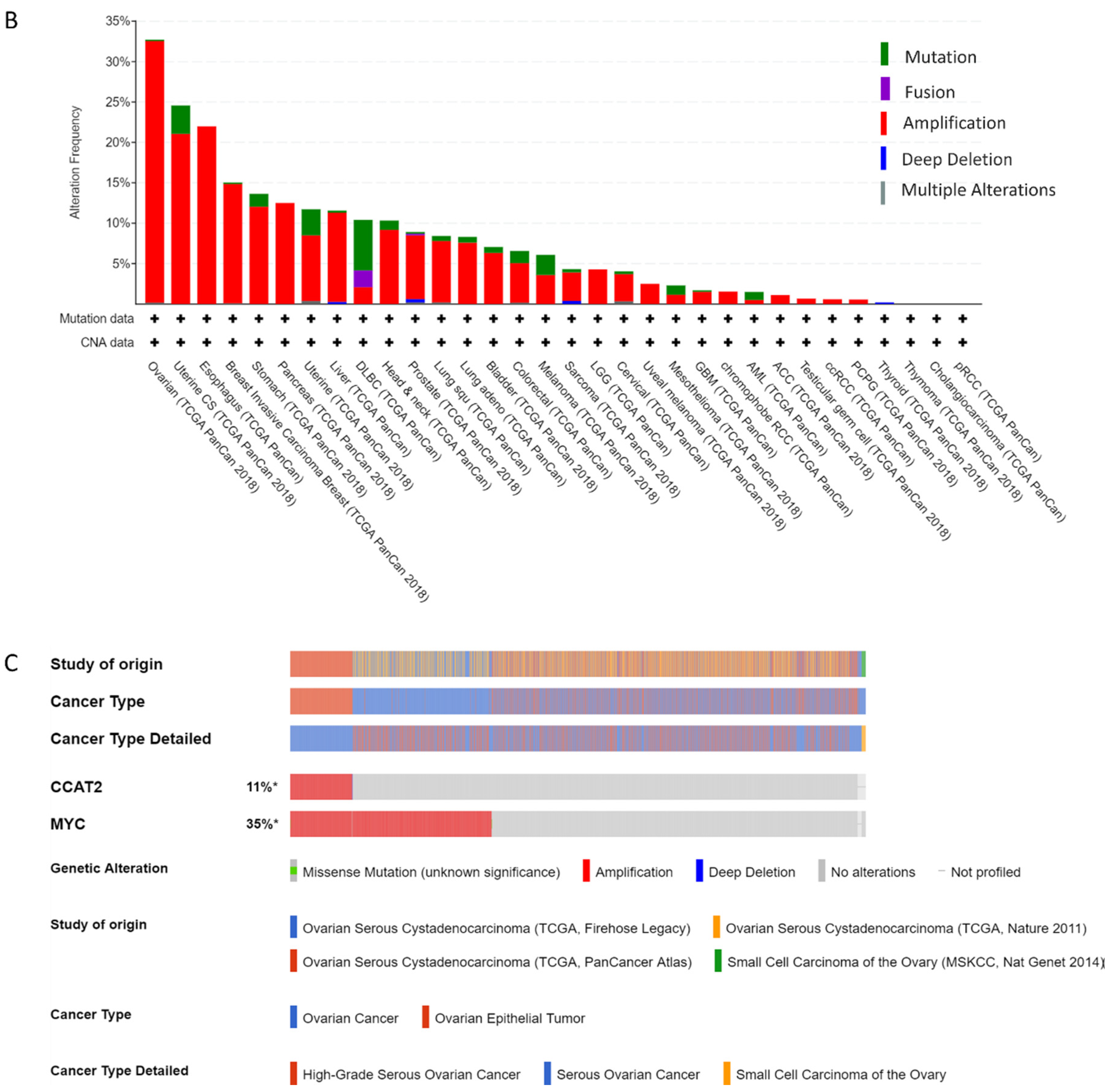
2.1. CCAT2 and MYC Genes are Overexpressed in Ovarian Cancer
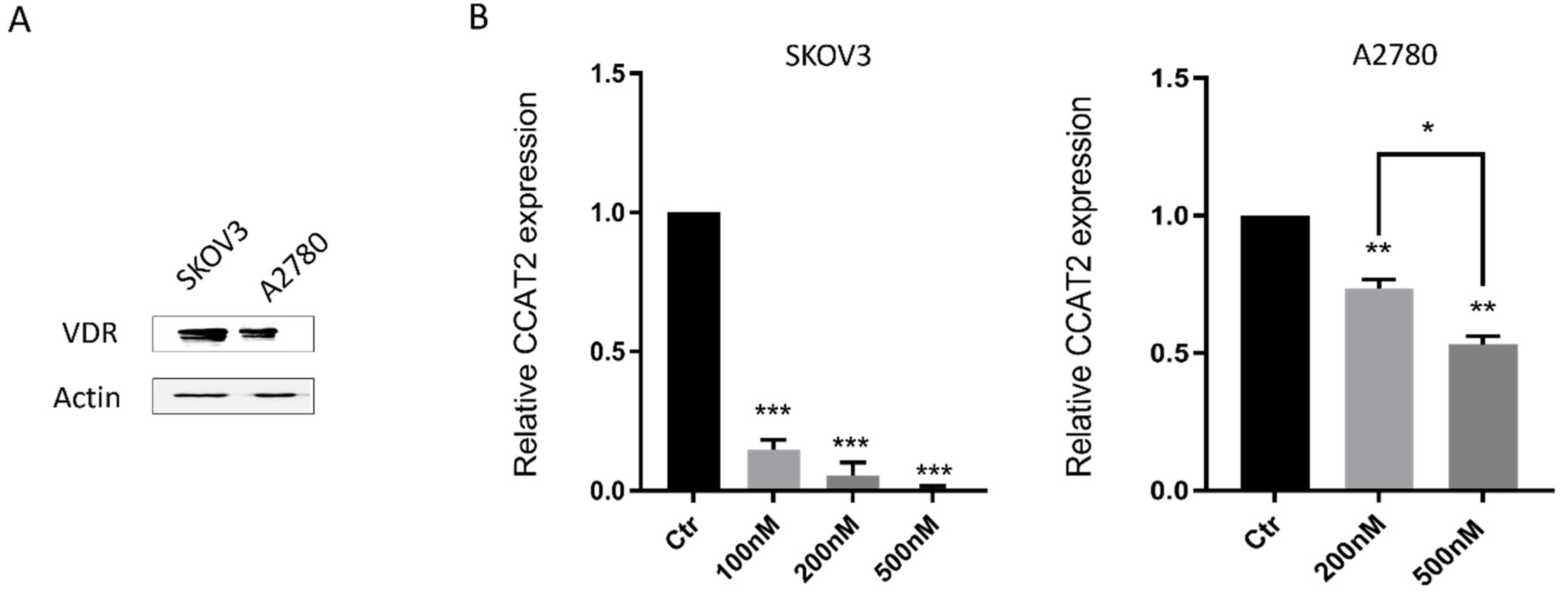
2.2. Calcitriol Down-Regulated CCAT2 in Ovarian Cancer Cell Lines
2.3. Calcitriol Inhibited the Proliferation and Changed Cell Cycle Distribution of Ovarian Cancer Cells
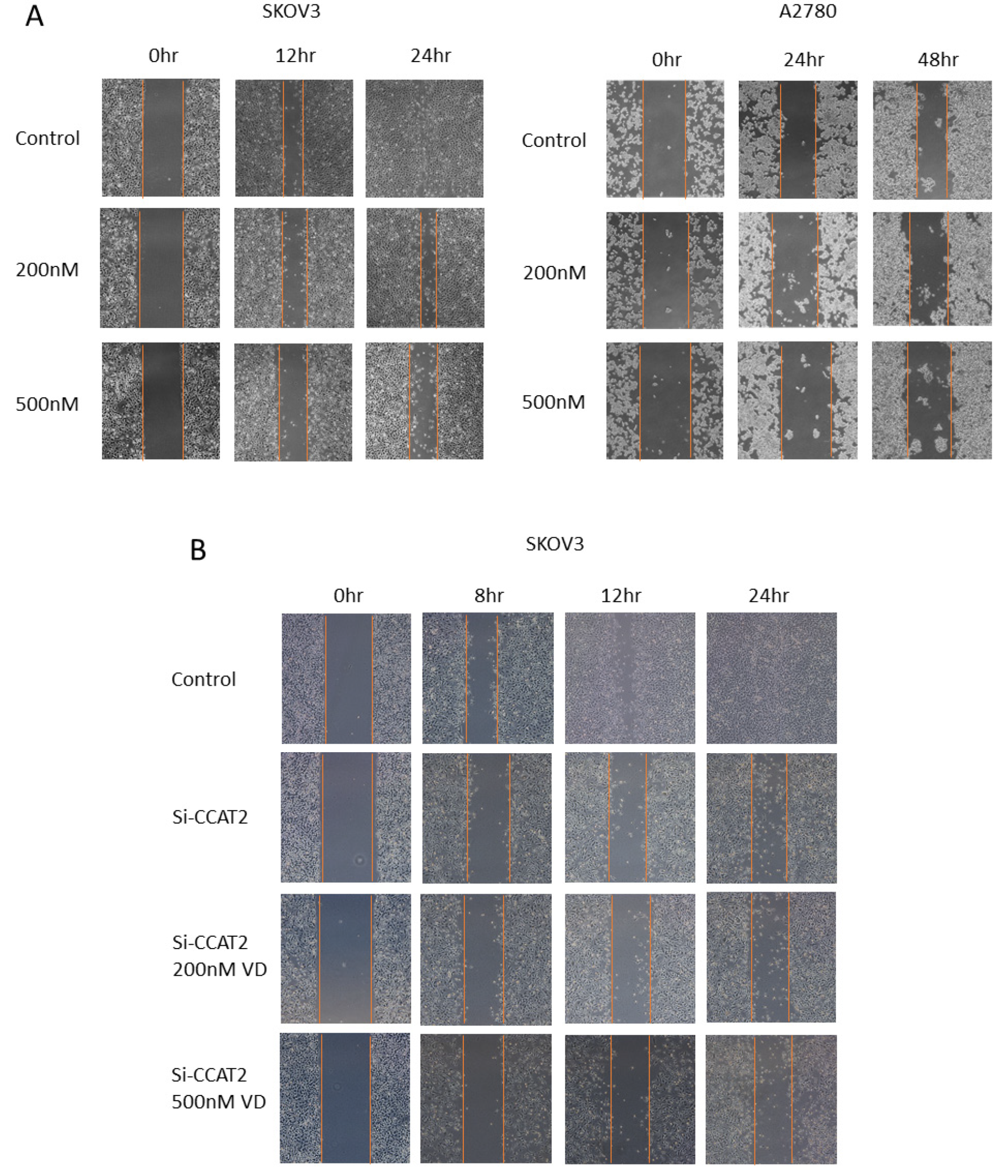
2.4. Calcitriol Suppressed the Migration and Invasion of Ovarian Cancer Cells
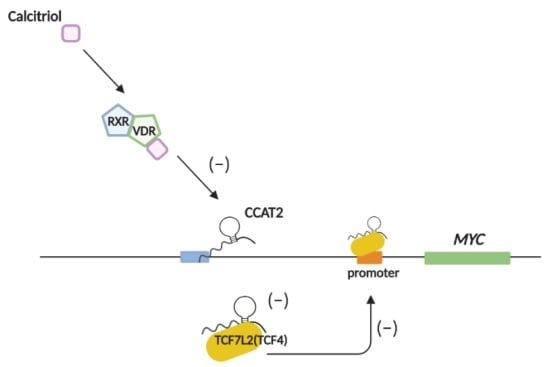
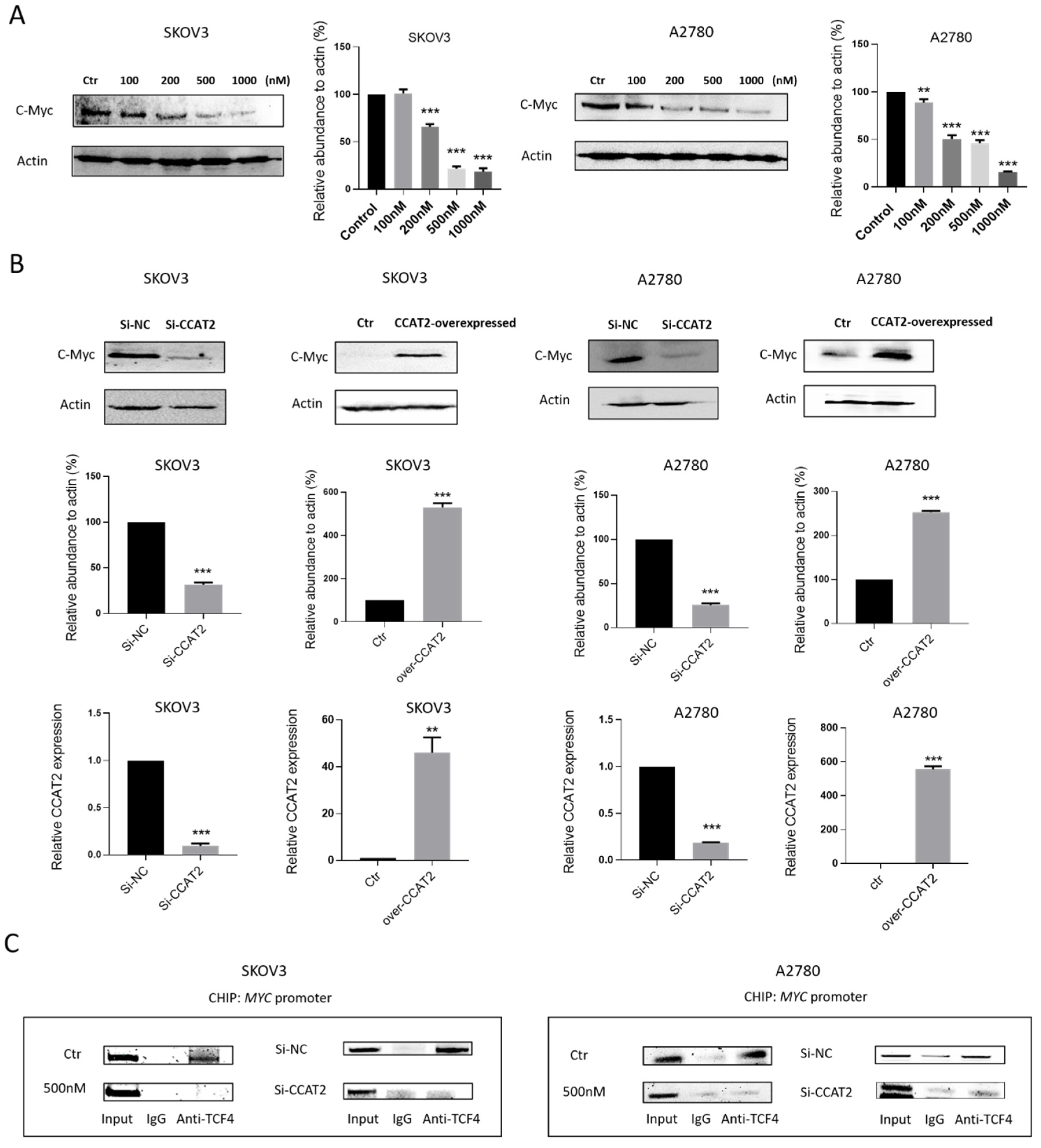
2.5. Calcitriol Inhibits c-Myc Expression by Targeting CCAT2 in Ovarian Cancer Cells
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Cell Proliferation Assay
4.3. Cell Cycle Assay
4.4. Small Interfering RNAs (siRNAs) Transfection
4.5. Monolayer Wound Healing Assay
4.6. Transwell Invasion and Migration Assay
4.7. Total RNA Isolation, cDNA Synthesis, and Quantification
4.8. Western Blotting
4.9. The Chromatin Immunoprecipitation (ChIP) Assay
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Huang, S.; Qing, C.; Huang, Z.; Zhu, Y. The long non-coding RNA CCAT2 is up-regulated in ovarian cancer and associated with poor prognosis. Diagn. Pathol. 2016, 11, 49. [Google Scholar] [CrossRef]
- Gao, Y.; Meng, H.; Liu, S.; Hu, J.; Zhang, Y.; Jiao, T.; Liu, Y.; Ou, J.; Wang, D.; Yao, L.; et al. LncRNA-HOST2 regulates cell biological behaviors in epithelial ovarian cancer through a mechanism involving microRNA let-7b. Hum. Mol. Genet. 2015, 24, 841–852. [Google Scholar] [CrossRef]
- Shapira, I.; Oswald, M.; Lovecchio, J.; Khalili, H.; Menzin, A.; Whyte, J.; Dos Santos, L.; Liang, S.; Bhuiya, T.; Keogh, M.; et al. Circulating biomarkers for detection of ovarian cancer and predicting cancer outcomes. Br. J. Cancer 2014, 110, 976–983. [Google Scholar] [CrossRef]
- Feeley, K.M.; Wells, M. Precursor lesions of ovarian epithelial malignancy. Histopathology 2001, 38, 87–95. [Google Scholar] [CrossRef]
- Tripathi, M.K.; Doxtater, K.; Keramatnia, F.; Zacheaus, C.; Yallapu, M.M.; Jaggi, M.; Chauhan, S.C. Role of lncRNAs in ovarian cancer: Defining new biomarkers for therapeutic purposes. Drug Discov. Today 2018, 23, 1635–1643. [Google Scholar] [CrossRef]
- Wang, L.; Cho, K.B.; Li, Y.; Tao, G.; Xie, Z.; Guo, B. Long Noncoding RNA (lncRNA)-Mediated Competing Endogenous RNA Networks Provide Novel Potential Biomarkers and Therapeutic Targets for Colorectal Cancer. Int. J. Mol. Sci. 2019, 20, 5758. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, A.M.; Chang, H.Y. Long Noncoding RNAs in Cancer Pathways. Cancer Cell 2016, 29, 452–463. [Google Scholar] [CrossRef] [PubMed]
- Ling, H.; Spizzo, R.; Atlasi, Y.; Nicoloso, M.; Shimizu, M.; Redis, R.S.; Nishida, N.; Gafa, R.; Song, J.; Guo, Z.; et al. CCAT2, a novel noncoding RNA mapping to 8q24, underlies metastatic progression and chromosomal instability in colon cancer. Genome Res. 2013, 23, 1446–1461. [Google Scholar] [CrossRef] [PubMed]
- Qiu, M.; Xu, Y.; Yang, X.; Wang, J.; Hu, J.; Xu, L.; Yin, R. CCAT2 is a lung adenocarcinoma-specific long non-coding RNA and promotes invasion of non-small cell lung cancer. Tumour Biol.: J. Int. Soc. Oncodevelopmental Biol. Med. 2014, 35, 5375–5380. [Google Scholar] [CrossRef] [PubMed]
- Redis, R.S.; Sieuwerts, A.M.; Look, M.P.; Tudoran, O.; Ivan, C.; Spizzo, R.; Zhang, X.; de Weerd, V.; Shimizu, M.; Ling, H.; et al. CCAT2, a novel long non-coding RNA in breast cancer: Expression study and clinical correlations. Oncotarget 2013, 4, 1748–1762. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.J.; Li, Y.; Wu, Y.Z.; Wang, Y.; Nian, W.Q.; Wang, L.L.; Li, L.C.; Luo, H.L.; Wang, D.L. Long non-coding RNA CCAT2 promotes the breast cancer growth and metastasis by regulating TGF-beta signaling pathway. Eur. Rev. Med Pharmacol. Sci. 2017, 21, 706–714. [Google Scholar]
- Hua, F.; Li, C.H.; Chen, X.G.; Liu, X.P. Long Noncoding RNA CCAT2 Knockdown Suppresses Tumorous Progression by Sponging miR-424 in Epithelial Ovarian Cancer. Oncol. Res. 2018, 26, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Liu, M.; Zhuang, R.; Jiang, J.; Gao, J.; Wang, H.; Chen, H.; Zhang, Z.; Kuang, Y.; Li, P. Long non-coding RNA CCAT2 promotes epithelial-mesenchymal transition involving Wnt/beta-catenin pathway in epithelial ovarian carcinoma cells. Oncol. Lett. 2018, 15, 3369–3375. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Liu, L.; Zhu, W. Up-regulation of long non-coding RNA CCAT2 correlates with tumor metastasis and poor prognosis in cervical squamous cell cancer patients. Int. J. Clin. Exp. Pathol. 2015, 8, 13261–13266. [Google Scholar] [PubMed]
- Wu, L.; Jin, L.; Zhang, W.; Zhang, L. Roles of Long Non-Coding RNA CCAT2 in Cervical Cancer Cell Growth and Apoptosis. Med Sci. Monit. 2016, 22, 875–879. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.Y.; Hua, L.; Yao, K.H.; Chen, J.T.; Zhang, J.J.; Hu, J.H. Long non-coding RNA CCAT2 is up-regulated in gastric cancer and associated with poor prognosis. Int. J. Clin. Exp. Pathol. 2015, 8, 779–785. [Google Scholar] [PubMed]
- Wang, Y.J.; Liu, J.Z.; Lv, P.; Dang, Y.; Gao, J.Y.; Wang, Y. Long non-coding RNA CCAT2 promotes gastric cancer proliferation and invasion by regulating the E-cadherin and LATS2. Am. J. Cancer Res. 2016, 6, 2651–2660. [Google Scholar]
- Shen, P.; Pichler, M.; Chen, M.; Calin, G.A.; Ling, H. To Wnt or Lose: The Missing Non-Coding Linc in Colorectal Cancer. Int. J. Mol. Sci. 2017, 18, 2003. [Google Scholar] [CrossRef]
- Garland, C.F.; Mohr, S.B.; Gorham, E.D.; Grant, W.B.; Garland, F.C. Role of ultraviolet B irradiance and vitamin D in prevention of ovarian cancer. Am. J. Prev. Med. 2006, 31, 512–514. [Google Scholar] [CrossRef]
- Lefkowitz, E.S.; Garland, C.F. Sunlight, vitamin D, and ovarian cancer mortality rates in US women. Int. J. Epidemiol. 1994, 23, 1133–1136. [Google Scholar] [CrossRef]
- Armas, L.A.; Dowell, S.; Akhter, M.; Duthuluru, S.; Huerter, C.; Hollis, B.W.; Lund, R.; Heaney, R.P. Ultraviolet-B radiation increases serum 25-hydroxyvitamin D levels: The effect of UVB dose and skin color. J. Am. Acad. Dermatol. 2007, 57, 588–593. [Google Scholar] [CrossRef]
- Grant, W.B. Ecological studies of the UVB-vitamin D-cancer hypothesis. Anticancer Res. 2012, 32, 223–236. [Google Scholar] [PubMed]
- Padi, S.K.; Zhang, Q.; Rustum, Y.M.; Morrison, C.; Guo, B. MicroRNA-627 mediates the epigenetic mechanisms of vitamin D to suppress proliferation of human colorectal cancer cells and growth of xenograft tumors in mice. Gastroenterology 2013, 145, 437–446. [Google Scholar] [CrossRef] [PubMed]
- Gross, C.; Stamey, T.; Hancock, S.; Feldman, D. Treatment of early recurrent prostate cancer with 1,25-dihydroxyvitamin D3 (calcitriol). J. Urol. 1998, 159, 2035–2039; discussion 2039–2040. [Google Scholar] [CrossRef]
- Moreno, J.; Krishnan, A.V.; Swami, S.; Nonn, L.; Peehl, D.M.; Feldman, D. Regulation of prostaglandin metabolism by calcitriol attenuates growth stimulation in prostate cancer cells. Cancer Res. 2005, 65, 7917–7925. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, G.C.; Turbov, J.; Rosales, R.; Yoo, J.; Hunn, J.; Zappia, K.J.; Lund, K.; Barry, C.P.; Rodriguez, I.V.; Pike, J.W.; et al. Progestins inhibit calcitriol-induced CYP24A1 and synergistically inhibit ovarian cancer cell viability: An opportunity for chemoprevention. Gynecol. Oncol. 2016, 143, 159–167. [Google Scholar] [CrossRef]
- Saunders, D.E.; Christensen, C.; Williams, J.R.; Wappler, N.L.; Lawrence, W.D.; Malone, J.M.; Malviya, V.K.; Deppe, G. Inhibition of breast and ovarian carcinoma cell growth by 1,25-dihydroxyvitamin D3 combined with retinoic acid or dexamethasone. Anti-Cancer Drugs 1995, 6, 562–569. [Google Scholar] [CrossRef]
- Swami, S.; Krishnan, A.V.; Wang, J.Y.; Jensen, K.; Horst, R.; Albertelli, M.A.; Feldman, D. Dietary vitamin D(3) and 1,25-dihydroxyvitamin D(3) (calcitriol) exhibit equivalent anticancer activity in mouse xenograft models of breast and prostate cancer. Endocrinology 2012, 153, 2576–2587. [Google Scholar] [CrossRef]
- Srinivas, S.; Feldman, D. A phase II trial of calcitriol and naproxen in recurrent prostate cancer. Anticancer Res. 2009, 29, 3605–3610. [Google Scholar]
- Trump, D.L.; Potter, D.M.; Muindi, J.; Brufsky, A.; Johnson, C.S. Phase II trial of high-dose, intermittent calcitriol (1,25 dihydroxyvitamin D3) and dexamethasone in androgen-independent prostate cancer. Cancer 2006, 106, 2136–2142. [Google Scholar] [CrossRef]
- Muindi, J.R.; Peng, Y.; Potter, D.M.; Hershberger, P.A.; Tauch, J.S.; Capozzoli, M.J.; Egorin, M.J.; Johnson, C.S.; Trump, D.L. Pharmacokinetics of high-dose oral calcitriol: Results from a phase 1 trial of calcitriol and paclitaxel. Clin. Pharmacol. Ther. 2002, 72, 648–659. [Google Scholar] [CrossRef] [PubMed]
- Haussler, M.R.; Jurutka, P.W.; Mizwicki, M.; Norman, A.W. Vitamin D receptor (VDR)-mediated actions of 1alpha,25(OH)(2)vitamin D(3): Genomic and non-genomic mechanisms. Best Pract. Res. Clin. Endocrinol. Metab. 2011, 25, 543–559. [Google Scholar] [CrossRef] [PubMed]
- Thill, M.; Woeste, A.; Reichert, K.; Fischer, D.; Rody, A.; Friedrich, M.; Koster, F. Vitamin D inhibits ovarian cancer cell line proliferation in combination with celecoxib and suppresses cyclooxygenase-2 expression. Anticancer Res. 2015, 35, 1197–1203. [Google Scholar] [PubMed]
- Bikle, D.D.; Jiang, Y. The protective role of vitamin d signaling in non-melanoma skin cancer. Cancers 2013, 5, 1426–1438. [Google Scholar] [CrossRef]
- Ma, Y.; Trump, D.L.; Johnson, C.S. Vitamin D and miRNAs in cancer. Curr. Gene Ther. 2014, 14, 269–275. [Google Scholar] [CrossRef]
- Jiang, Y.J.; Bikle, D.D. LncRNA: A new player in 1alpha, 25(OH)(2) vitamin D(3)/VDR protection against skin cancer formation. Exp. Dermatol. 2014, 23, 147–150. [Google Scholar] [CrossRef]
- Pahlevan Kakhki, M.; Nikravesh, A.; Shirvani Farsani, Z.; Sahraian, M.A.; Behmanesh, M. HOTAIR but not ANRIL long non-coding RNA contributes to the pathogenesis of multiple sclerosis. Immunology 2018, 153, 479–487. [Google Scholar] [CrossRef]
- Cerami, E.; Gao, J.; Dogrusoz, U.; Gross, B.E.; Sumer, S.O.; Aksoy, B.A.; Jacobsen, A.; Byrne, C.J.; Heuer, M.L.; Larsson, E.; et al. The cBio cancer genomics portal: An open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012, 2, 401–404. [Google Scholar] [CrossRef]
- Gao, J.; Aksoy, B.A.; Dogrusoz, U.; Dresdner, G.; Gross, B.; Sumer, S.O.; Sun, Y.; Jacobsen, A.; Sinha, R.; Larsson, E.; et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci. Signal. 2013, 6, pl1. [Google Scholar] [CrossRef]
- Silvagno, F.; Poma, C.B.; Realmuto, C.; Ravarino, N.; Ramella, A.; Santoro, N.; D’Amelio, P.; Fuso, L.; Pescarmona, G.; Zola, P. Analysis of vitamin D receptor expression and clinical correlations in patients with ovarian cancer. Gynecol. Oncol. 2010, 119, 121–124. [Google Scholar] [CrossRef]
- Muindi, J.R.; Modzelewski, R.A.; Peng, Y.; Trump, D.L.; Johnson, C.S. Pharmacokinetics of 1alpha,25-dihydroxyvitamin D3 in normal mice after systemic exposure to effective and safe antitumor doses. Oncology 2004, 66, 62–66. [Google Scholar] [CrossRef] [PubMed]
- Kissmeyer, A.M.; Binderup, L. Calcipotriol (MC 903): Pharmacokinetics in rats and biological activities of metabolites. A comparative study with 1,25(OH)2D3. Biochem. Pharmacol. 1991, 41, 1601–1606. [Google Scholar] [CrossRef]
- Shaw, T.J.; Senterman, M.K.; Dawson, K.; Crane, C.A.; Vanderhyden, B.C. Characterization of intraperitoneal, orthotopic, and metastatic xenograft models of human ovarian cancer. Mol. Ther.: J. Am. Soc. Gene Ther. 2004, 10, 1032–1042. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Cai, J.; Zhang, Y.; Zhu, Y.; Yang, P.; Wang, Z. Establishment of two ovarian cancer orthotopic xenograft mouse models for in vivo imaging: A comparative study. Int. J. Oncol. 2017, 51, 1199–1208. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Dong, Q.; Yan, Z.; Lu, W.; Feng, L.; Xie, C.; Xie, Z.; Su, B.; Liu, M. MPEG-DSPE polymeric micelle for translymphatic chemotherapy of lymph node metastasis. Int. J. Pharm. 2015, 487, 8–16. [Google Scholar] [CrossRef]
- Glowka, E.; Stasiak, J.; Lulek, J. Drug Delivery Systems for Vitamin D Supplementation and Therapy. Pharmaceutics 2019, 11, 347. [Google Scholar] [CrossRef]
- Reitsma, P.H.; Rothberg, P.G.; Astrin, S.M.; Trial, J.; Bar-Shavit, Z.; Hall, A.; Teitelbaum, S.L.; Kahn, A.J. Regulation of myc gene expression in HL-60 leukaemia cells by a vitamin D metabolite. Nature 1983, 306, 492–494. [Google Scholar] [CrossRef]
- Fu, D.; Zhang, Y.; Cui, H. Long noncoding RNA CCAT2 is activated by E2F1 and exerts oncogenic properties by interacting with PTTG1 in pituitary adenomas. Am. J. Cancer Res. 2018, 8, 245–255. [Google Scholar]
- Clinckspoor, I.; Verlinden, L.; Overbergh, L.; Korch, C.; Bouillon, R.; Mathieu, C.; Verstuyf, A.; Decallonne, B. 1,25-dihydroxyvitamin D3 and a superagonistic Analog. In combination with paclitaxel or suberoylanilide hydroxamic acid have potent antiproliferative effects on anaplastic thyroid cancer. J. Steroid Biochem. Mol. Biol. 2011, 124, 1–9. [Google Scholar] [CrossRef]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; Zhou, S.; Guo, B. Vitamin D Suppresses Ovarian Cancer Growth and Invasion by Targeting Long Non-Coding RNA CCAT2. Int. J. Mol. Sci. 2020, 21, 2334. https://doi.org/10.3390/ijms21072334
Wang L, Zhou S, Guo B. Vitamin D Suppresses Ovarian Cancer Growth and Invasion by Targeting Long Non-Coding RNA CCAT2. International Journal of Molecular Sciences. 2020; 21(7):2334. https://doi.org/10.3390/ijms21072334
Chicago/Turabian StyleWang, Liye, Shuang Zhou, and Bin Guo. 2020. "Vitamin D Suppresses Ovarian Cancer Growth and Invasion by Targeting Long Non-Coding RNA CCAT2" International Journal of Molecular Sciences 21, no. 7: 2334. https://doi.org/10.3390/ijms21072334
APA StyleWang, L., Zhou, S., & Guo, B. (2020). Vitamin D Suppresses Ovarian Cancer Growth and Invasion by Targeting Long Non-Coding RNA CCAT2. International Journal of Molecular Sciences, 21(7), 2334. https://doi.org/10.3390/ijms21072334