Internalization of miPEP165a into Arabidopsis Roots Depends on both Passive Diffusion and Endocytosis-Associated Processes
Abstract
1. Introduction
2. Results
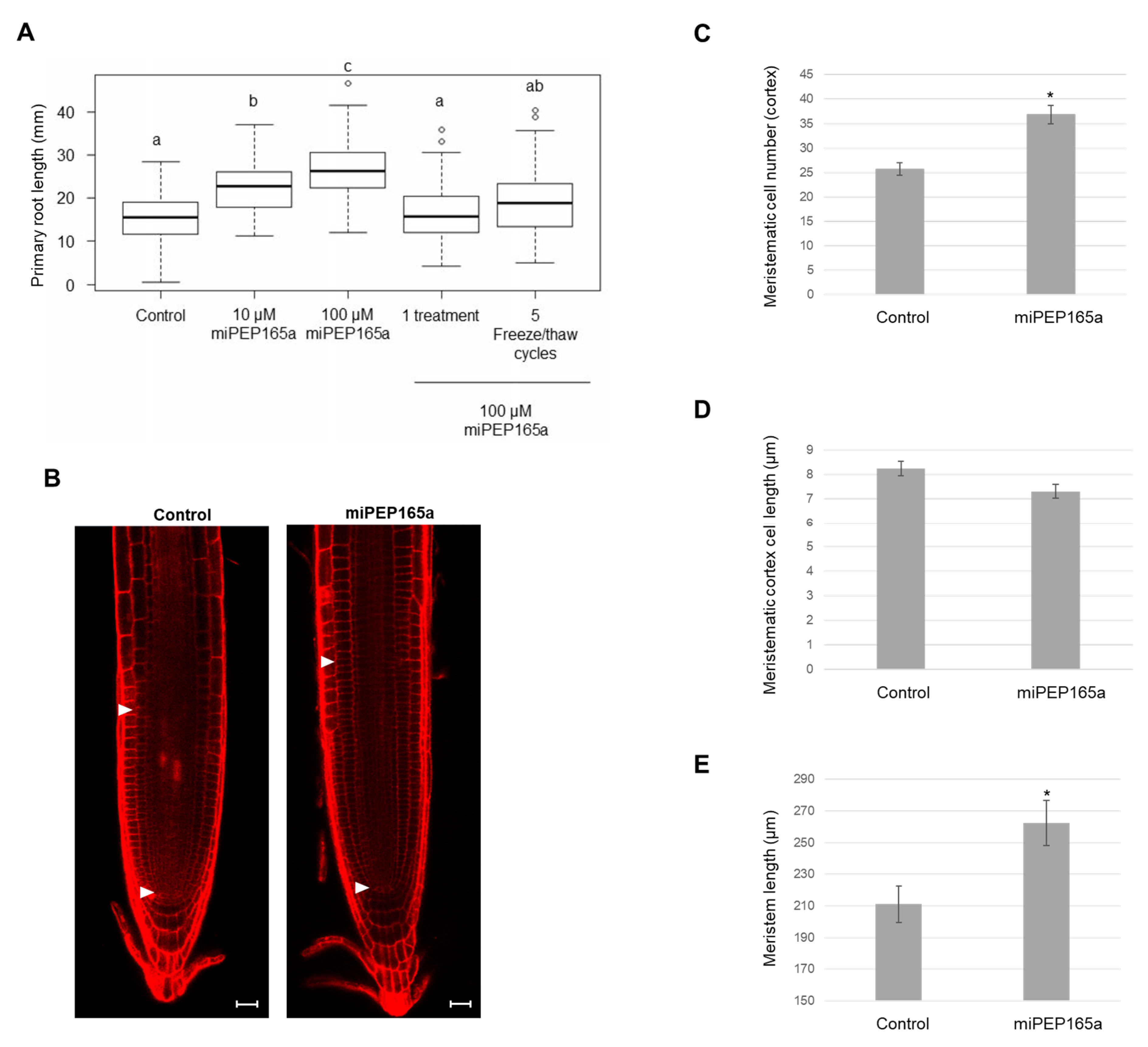
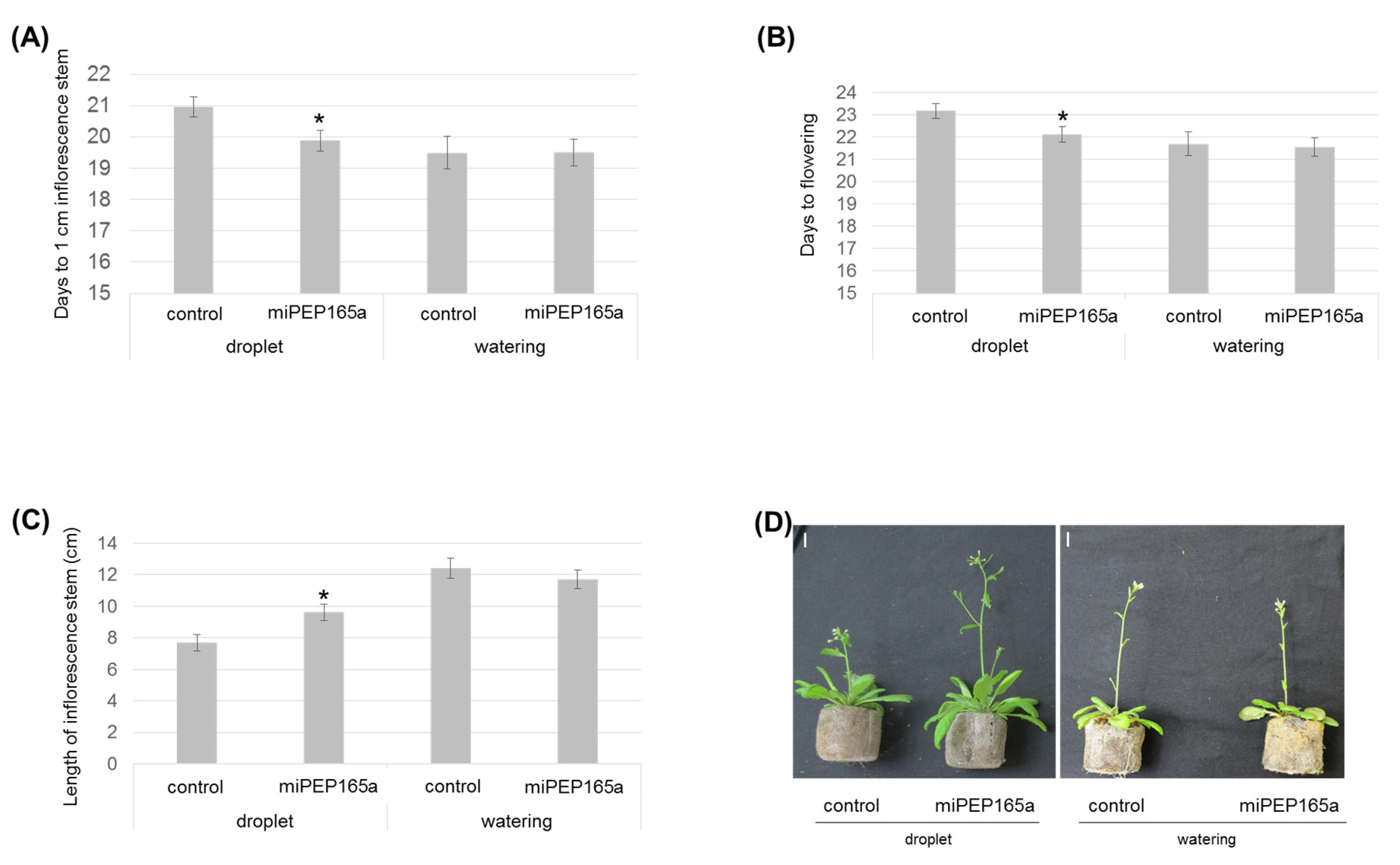
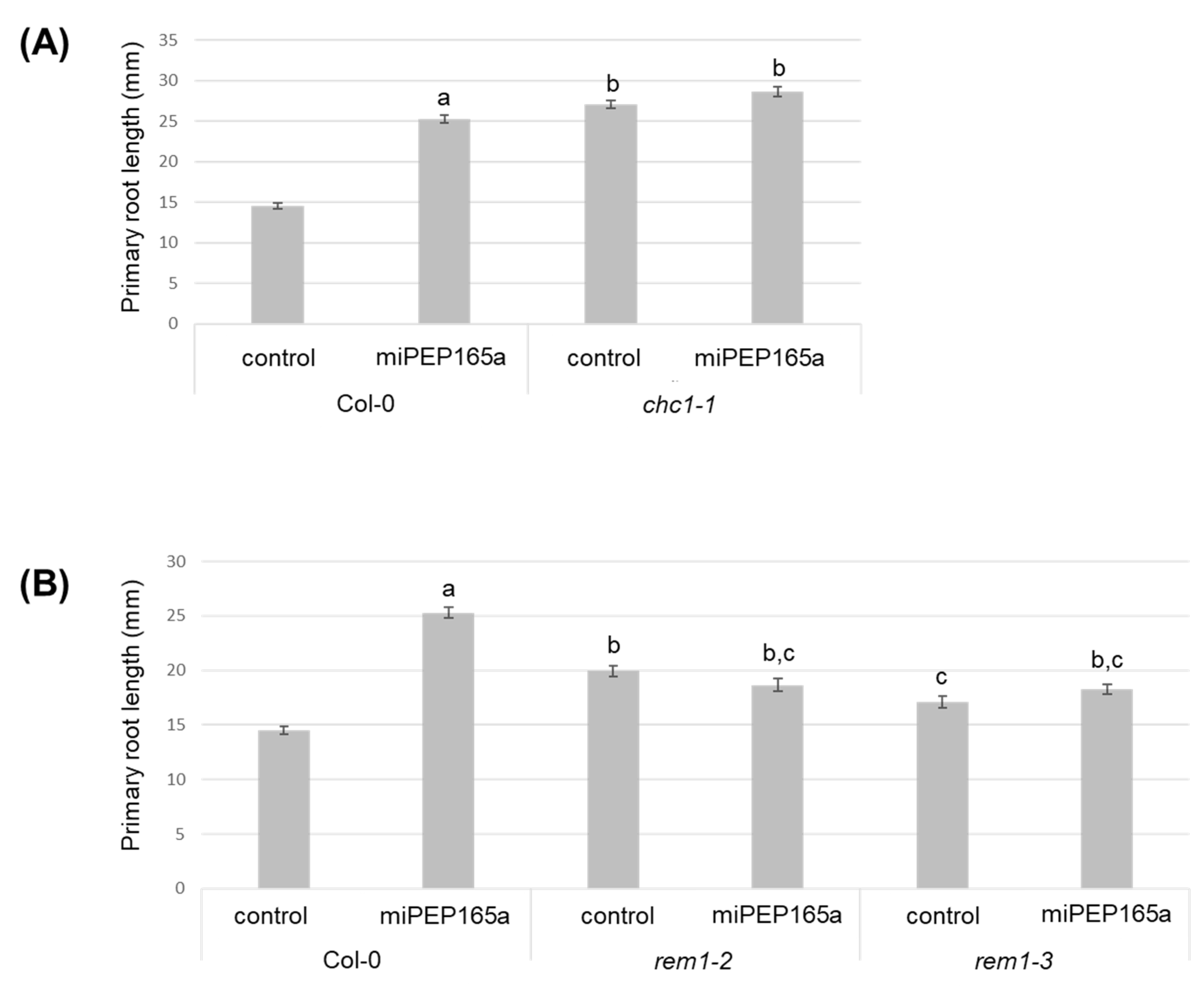
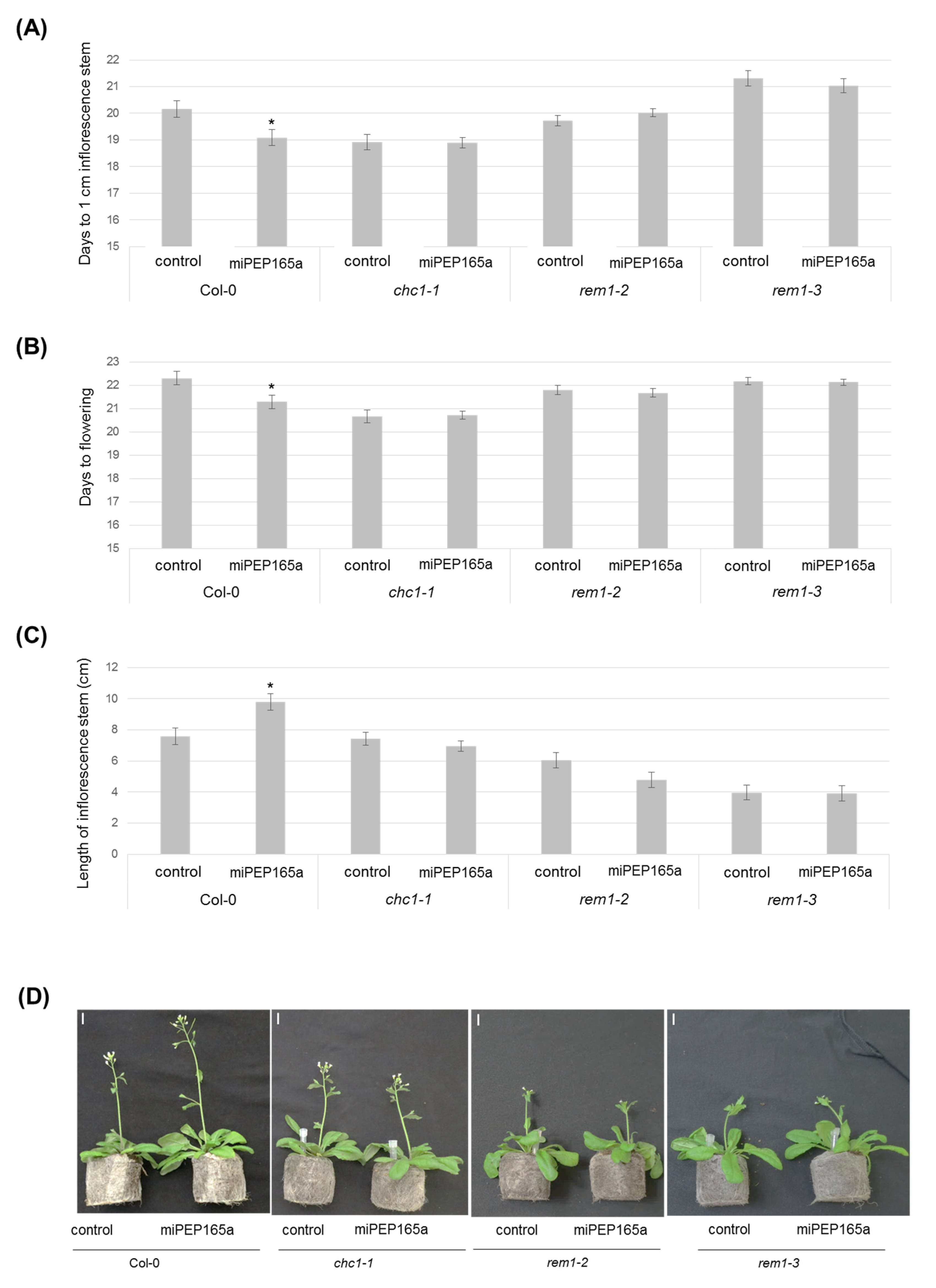
2.1. MiPEP165a Promotes Cell Division in the Meristematic Zone to Increase Primary Root Length and Acts on Flowering Time in Arabidopsis
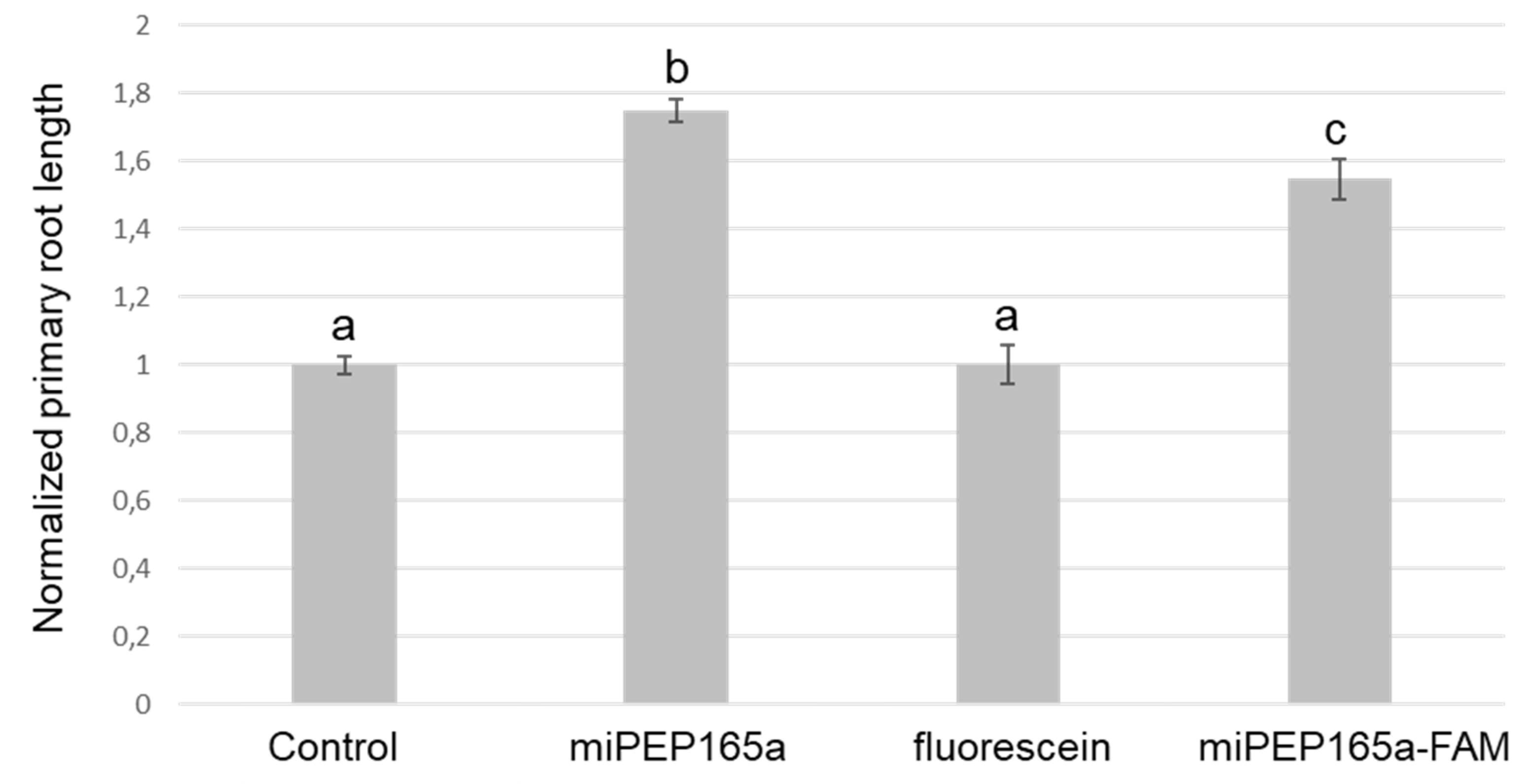
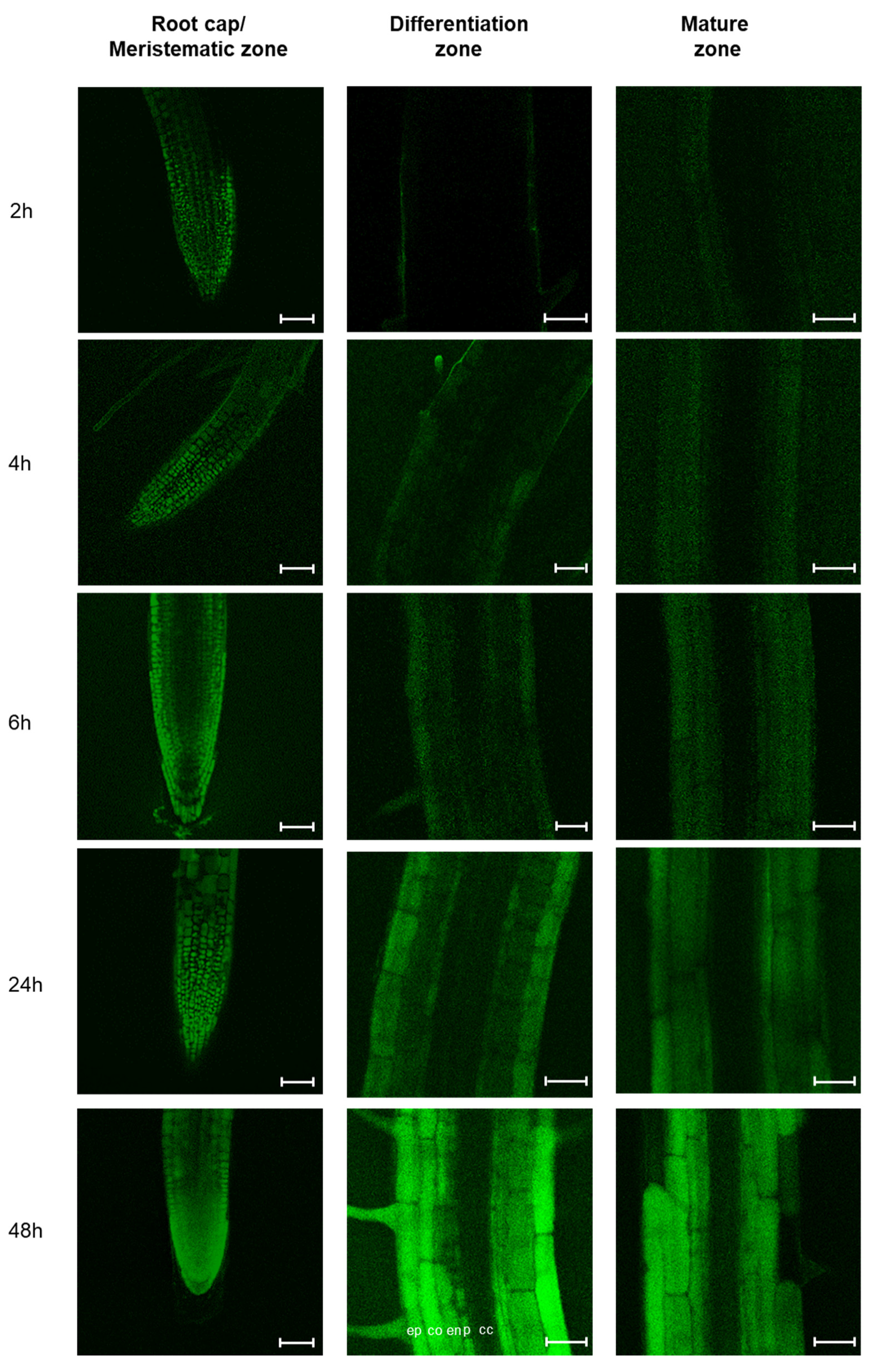
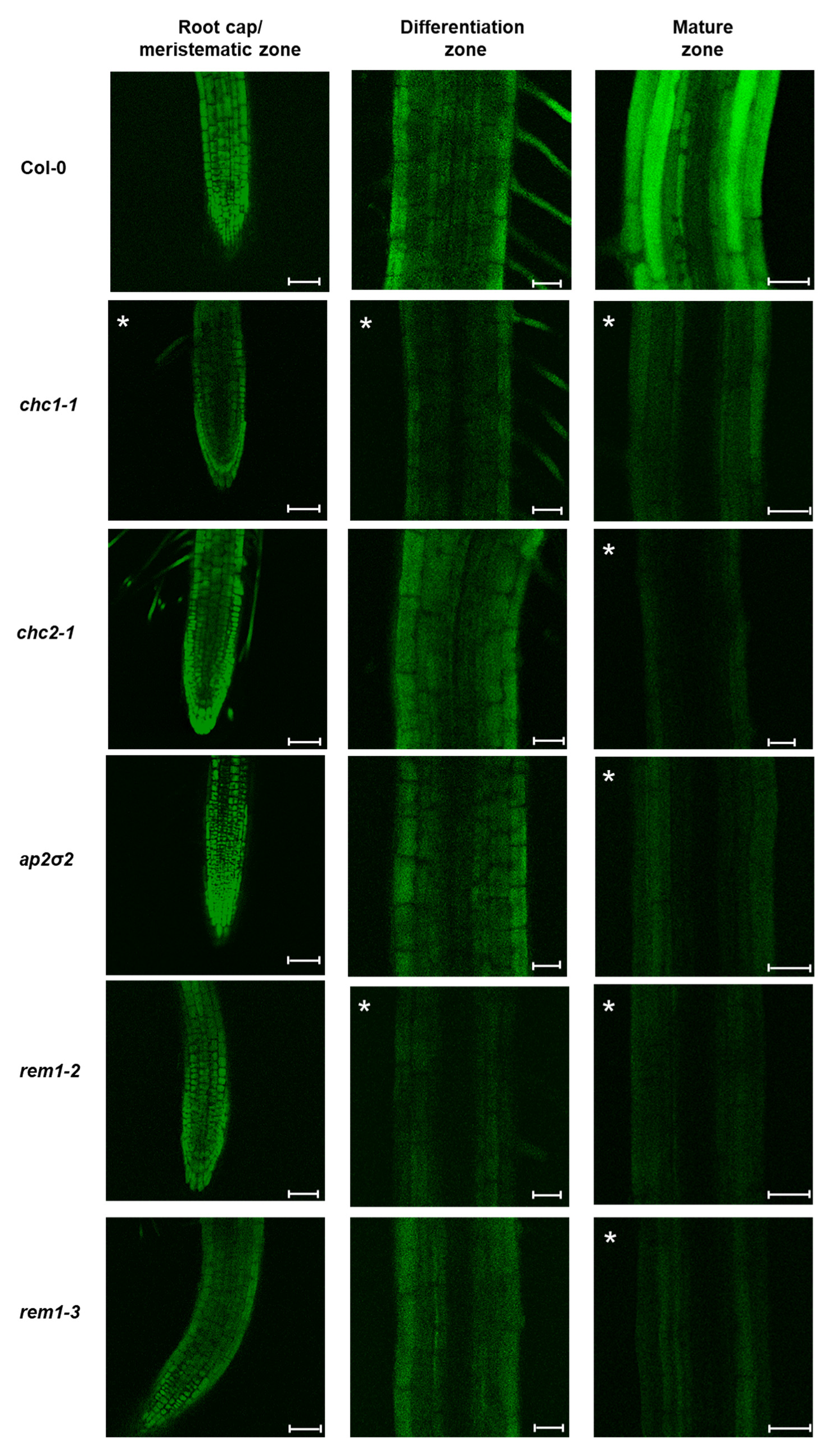
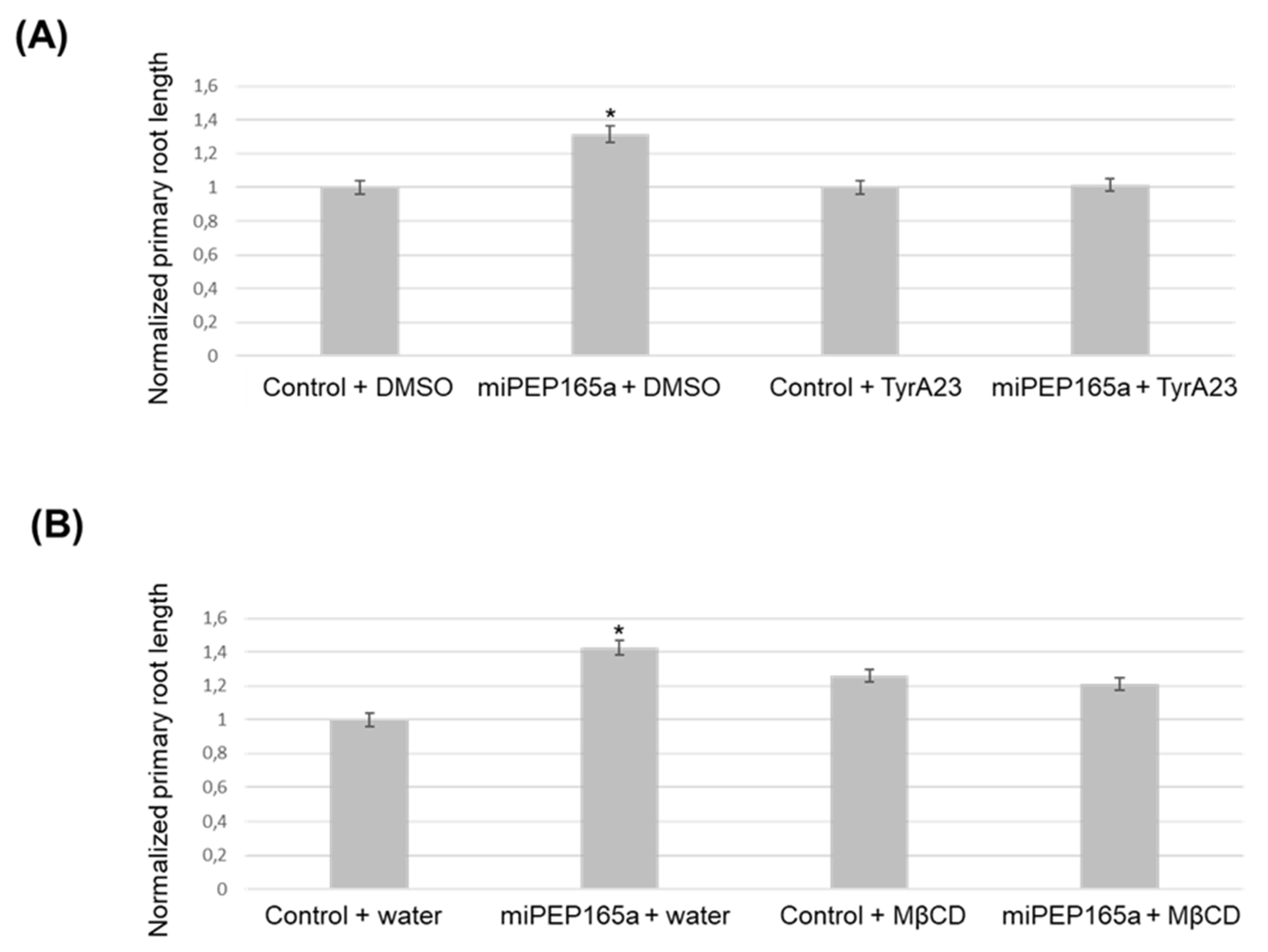
2.2. MiPEP165a Entry Involves both Passive Diffusion at the Root Apex and Endocytic Pathways in the Differentiation and Mature Zones
3. Discussion
4. Materials and methods
4.1. Peptide Synthesis
4.2. Plant Materials
4.3. Peptide Treatment of Arabidopsis Roots
4.4. Peptide Uptake in Arabidopsis Roots
4.5. Inhibitor Treatment
4.6. Flowering Phenotype
4.7. Propidium Iodide Staining
4.8. Immunoblots and RT-qPCR
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AGO1 | Argonaute 1 |
| AP2 | adaptor protein 2 |
| CAN/AtHB15 | CORONA |
| CHC | clathrin heavy chain |
| CLC | clathrin light chain |
| CME | clathrin-mediated endocytosis |
| DCL1 | dicer-like1 |
| FAM | 5-carboxyfluorescein |
| HB8 | homeobox gene 8 |
| HD-ZIP III | class III homeodomain-leucine zipper |
| MβCD | methyl-β-cyclodextrin |
| miPEP | miRNA-encoded peptide |
| miRNA | micro-RNA |
| MS | Murashige and Skoog medium |
| PHB | PHABULOSA |
| PHV | PHAVOLUTA |
| PIP2;1 | plasma membrane intrinsic protein 2 |
| pri-miRNA | primary-microRNA |
| pre-miRNA | precursor-microRNA |
| RbohD | respiratory burst oxidase protein D |
| REM | remorin |
| REV | REVOLUTA |
| TyrA23 | Tyrphostin A23. |
References
- Lagos-Quintana, M.; Rauhut, R.; Lendeckel, W.; Tuschl, T. Identification of novel genes coding for small expressed RNAs. Science 2001, 294, 853–858. [Google Scholar] [CrossRef]
- Lau, N.C.; Lim, L.P.; Weinstein, E.G.; Bartel, D.P. An abundant class of tiny RNAs with probable regulatory roles in Caenorhabditis elegans. Science 2001, 294, 858–862. [Google Scholar] [CrossRef] [PubMed]
- Lee, R.C.; Ambros, V. An extensive class of small RNAs in Caenorhabditis elegans. Science 2001, 294, 862–864. [Google Scholar] [CrossRef] [PubMed]
- Reinhart, B.J.; Weinstein, E.G.; Rhoades, M.W.; Bartel, B.; Bartel, D.P. MicroRNAs in plants. Genes Dev. 2002, 16, 1616–1626. [Google Scholar] [CrossRef]
- Lauressergues, D.; Couzigou, J.M.; San Clemente, H.; Martinez, Y.; Dunand, C.; Bécard, G.; Combier, J.P. Primary transcripts of microRNAs encode regulatory peptides. Nature 2015, 520, 90–93. [Google Scholar] [CrossRef] [PubMed]
- Couzigou, J.M.; André, O.; Guillotin, B.; Alexandre, M.; Combier, J.P. Use of microRNA-encoded peptide miPEP172c to stimulate nodulation in soybean. New Phytol. 2016, 211, 379–381. [Google Scholar] [CrossRef] [PubMed]
- Couzigou, J.M.; Lauressergues, D.; André, O.; Gutjahr, C.; Guillotin, B.; Bécard, G.; Combier, J.P. Positive Gene Regulation by a Natural Protective miRNA Enables Arbuscular Mycorrhizal Symbiosis. Cell Host Microbe 2017, 21, 106–112. [Google Scholar] [CrossRef]
- Fan, L.; Li, R.; Pan, J.; Ding, Z.; Lin, J. Endocytosis and its regulation in plants. Trends Plant Sci. 2015, 20, 388–397. [Google Scholar] [CrossRef]
- Dhonukshe, P.; Aniento, F.; Hwang, I.; Robinson, D.G.; Mravec, J.; Stierhof, Y.D.; Friml, J. Clathrin-mediated constitutive endocytosis of PIN auxin efflux carriers in Arabidopsis. Curr. Biol. 2007, 17, 520–527. [Google Scholar] [CrossRef]
- Yamaoka, S.; Shimono, Y.; Shirakawa, M.; Fukao, Y.; Kawase, T.; Hatsugai, N.; Tamura, K.; Shimada, T.; Hara-Nishimura, I. Identification and dynamics of Arabidopsis adaptor protein-2 complex and its involvement in floral organ development. Plant Cell 2013, 25, 2958–2969. [Google Scholar] [CrossRef]
- Barberon, M.; Zelazny, E.; Robert, S.; Conéjéro, G.; Curie, C.; Friml, J.; Vert, G. Monoubiquitin-dependent endocytosis of the iron-regulated transporter 1 (IRT1) transporter controls iron uptake in plants. Proc. Natl. Acad. Sci. USA 2011, 108, 450–458. [Google Scholar] [CrossRef] [PubMed]
- Kitakura, S.; Vanneste, S.; Robert, S.; Löfke, C.; Teichmann, T.; Tanaka, H.; Friml, J. Clathrin mediates endocytosis and polar distribution of PIN auxin transporters in Arabidopsis. Plant Cell 2011, 23, 1920–1931. [Google Scholar] [CrossRef] [PubMed]
- Gadeyne, A.; Sánchez-Rodríguez, C.; Vanneste, S.; Di Rubbo, S.; Zauber, H.; Vanneste, K.; Van Leene, J.; De Winne, N.; Eeckhout, D.; Persiau, G.; et al. The TPLATE adaptor complex drives clathrin-mediated endocytosis in plants. Cell 2014, 156, 691–704. [Google Scholar] [CrossRef] [PubMed]
- Zwiewka, M.; Nodzyński, T.; Robert, S.; Vanneste, S.; Friml, J. Osmotic Stress Modulates the Balance between Exocytosis and Clathrin-Mediated Endocytosis in Arabidopsis thaliana. Mol. Plant 2015, 8, 117–1187. [Google Scholar] [CrossRef]
- Mbengue, M.; Bourdais, G.; Gervasi, F.; Beck, M.; Zhou, J.; Spallek, T.; Bartels, S.; Boller, T.; Ueda, T.; Kuhn, H.; et al. Clathrin-dependent endocytosis is required for immunity mediated by pattern recognition receptor kinases. Proc. Natl. Acad. Sci. USA 2016, 113, 11034–11039. [Google Scholar] [CrossRef]
- Ortiz-Morea, F.A.; Savatin, D.V.; Dejonghe, W.; Kumar, R.; Luo, Y.; Adamowski, M.; Van den Begin, J.; Dressano, K.; Pereira de Oliveira, G.; Zhao, X.; et al. Danger-associated peptide signaling in Arabidopsis requires clathrin. Proc. Natl. Acad. Sci. USA 2016, 113, 11028–11033. [Google Scholar] [CrossRef]
- Li, X.; Wang, X.; Yang, Y.; Li, R.; He, Q.; Fang, X.; Luu, D.-T.; Maurel, C.; Lin, J. Single-molecule analysis of PIP2;1 dynamics and partitioning reveals multiple modes of Arabidopsis plasma membrane aquaporin regulation. Plant Cell 2011, 23, 3780–3797. [Google Scholar] [CrossRef]
- Baral, A.; Irani, N.G.; Fujimoto, M.; Nakano, A.; Mayor, S.; Mathew, M.K. Salt-induced remodeling of spatially restricted clathrin-independent endocytic pathways in Arabidopsis root. Plant Cell 2015, 27, 1297–1315. [Google Scholar] [CrossRef]
- Li, R.; Liu, P.; Wan, Y.; Chen, T.; Wang, Q.; Mettbach, U.; Baluska, F.; Samaj, J.; Fang, X.; Lucas, W.J.; et al. A membrane microdomain-associated protein, Arabidopsis Flot1, is involved in a clathrin-independent endocytic pathway and is required for seedling development. Plant Cell 2012, 24, 2105–2122. [Google Scholar] [CrossRef]
- Yu, M.; Liu, H.; Dong, Z.; Xiao, J.; Su, B.; Fan, L.; Komis, G.; Samaj, J.; Lin, J.; Li, R. The dynamics and endocytosis of Flot1 protein in response to flg22 in Arabidopsis. J. Plant Physiol. 2017, 215, 73–84. [Google Scholar] [CrossRef]
- Lefebvre, B.; Timmers, T.; Mbengue, M.; Moreau, S.; Hervé, C.; Tóth, K.; Bittencourt-Silvestre, J.; Klaus, D.; Deslandes, L.; Godiard, L.; et al. A remorin protein interacts with symbiotic receptors and regulates bacterial infection. Proc. Natl. Acad. Sci. USA 2010, 107, 2343–2348. [Google Scholar] [CrossRef] [PubMed]
- Hao, H.; Fan, L.; Chen, T.; Li, R.; Li, X.; He, Q.; Botella, M.; Lin, J. Clathrin and membrane microdomains cooperatively regulate RbohD dynamics and activity in Arabidopsis. Plant Cell 2014, 26, 1729–1745. [Google Scholar] [CrossRef] [PubMed]
- Carlsbecker, A.; Lee, J.Y.; Roberts, C.J.; Dettmer, J.; Lehesranta, S.; Zhou, J.; Lindgren, O.; Moreno-Risueno, M.A.; Vatén, A.; Thitamadee, S.; et al. Cell signalling by microRNA165/6 directs gene dose-dependent root cell fate. Nature 2010, 465, 316–321. [Google Scholar] [CrossRef] [PubMed]
- Perilli, S.; Di Mambro, R.; Sabatini, S. Growth and development of the root apical meristem. Curr. Opin. Plant Biol. 2012, 15, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Dello Ioio, R.; Linhares, F.S.; Scacchi, E.; Casamitjana-Martinez, E.; Heidstra, R.; Costantino, P.; Sabatini, S. Cytokinins determine Arabidopsis root-meristem size by controlling cell differentiation. Curr. Biol. 2007, 17, 678–682. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Lai, N.; Gao, K.; Chen, F.; Yuan, L.; Mi, G. Ammonium inhibits primary root growth by reducing the length of meristem and elongation zone and decreasing elemental expansion rate in the root apex in Arabidopsis thaliana. PLoS ONE 2013, 8, e61031. [Google Scholar] [CrossRef]
- French, A.P.; Wilson, M.H.; Kenobi, K.; Dietrich, D.; Voβ, U.; Ubeda-Tomás, S.; Pridmore, T.P.; Wells, D.M. Identifying biological landmarks using a novel cell measuring image analysis tool: Cell-o-Tape. Plant Methods 2012, 8, 7. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, B.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Ji, L.; Liu, X.; Yan, J.; Wang, W.; Yumul, R.E.; Kim, Y.J.; Dinh, T.T.; Liu, J.; Cui, X.; Zheng, B.; et al. ARGONAUTE10 and ARGONAUTE1 regulate the termination of floral stem cells through two microRNAs in Arabidopsis. PLoS Genet. 2011, 7, e1001358. [Google Scholar] [CrossRef]
- Balcerowicz, D.; Schoenaers, S.; Vissenberg, K. Cell fate determination and the switch from diffuse growth to planar polarity in Arabidopsis root epidermal cells. Front. Plant Sci. 2015, 6, 1163. [Google Scholar] [CrossRef]
- Fan, L.; Hao, H.; Xue, Y.; Zhang, L.; Song, K.; Ding, Z.; Botella, M.A.; Wang, H.; Lin, J. Dynamic analysis of Arabidopsis AP2 σ subunit reveals a key role in clathrin-mediated endocytosis and plant development. Development 2013, 140, 3826–3837. [Google Scholar] [CrossRef] [PubMed]
- Mongrand, S.; Morel, J.; Laroche, J.; Claverol, S.; Carde, J.P.; Hartmann, M.A.; Bonneu, M.; Simon-Plas, F.; Lessire, R.; Bessoule, J.J. Lipid rafts in higher plant cells: Purification and characterization of Triton X-100-insoluble microdomains from tobacco plasma membrane. J. Biol. Chem. 2004, 279, 36277–36286. [Google Scholar] [CrossRef] [PubMed]
- Kierszniowska, S.; Seiwert, B.; Schulze, W.X. Definition of Arabidopsis sterol-rich membrane microdomains by differential treatment with methyl-beta-cyclodextrin and quantitative proteomics. Mol. Cell Proteom. 2009, 8, 612–623. [Google Scholar] [CrossRef]
- Jarsch, I.K.; Ott, T. Perspectives on remorin proteins, membrane rafts, and their role during plant–microbe interactions. Mol. Plant Microbe Interact. 2011, 24, 7–12. [Google Scholar] [CrossRef]
- Banbury, D.N.; Oakley, J.D.; Sessions, R.B.; Banting, G. Tyrphostin A23 inhibits internalization of the transferrin receptor by perturbing the interaction between tyrosine motifs and the medium chain subunit of the AP-2 adaptor complex. J. Biol. Chem. 2003, 278, 12022–12028. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.K.; Kubo, M.; Zhong, R.; Demura, T.; Ye, Z.H. Overexpression of miR165 affects apical meristem formation, organ polarity establishment and vascular development in Arabidopsis. Plant Cell Physiol. 2007, 48, 391–404. [Google Scholar] [CrossRef] [PubMed]
- Dello Ioio, R.; Galinha, C.; Fletcher, A.G.; Grigg, S.P.; Molnar, A.; Willemsen, V.; Scheres, B.; Sabatini, S.; Baulcombe, D.; Maini, P.K.; et al. A PHABULOSA/cytokinin feedback loop controls root growth in Arabidopsis. Curr. Biol. 2012, 22, 1699–1704. [Google Scholar] [CrossRef]
- Singh, A.; Singh, S.; Panigrahi, K.C.; Reski, R.; Sarkar, A.K. Balanced activity of microRNA166/165 and its target transcripts from the class III homeodomain-leucine zipper family regulates root growth in Arabidopsis thaliana. Plant Cell Rep. 2014, 33, 945–953. [Google Scholar] [CrossRef]
- Chen, A.; Komives, E.A.; Schroeder, J.I. An improved grafting technique for mature Arabidopsis plants demonstrates long-distance shoot-to-root transport of phytochelatins in Arabidopsis. Plant Physiol. 2006, 141, 108–120. [Google Scholar] [CrossRef]
- Delay, C.; Imin, N.; Djordjevic, M.A. Regulation of Arabidopsis root development by small signaling peptides. Front. Plant Sci. 2013, 4, 352. [Google Scholar] [CrossRef] [PubMed]
- Ko, D.; Helariutta, Y.A. Shoot—Root communication in flowering plants. Curr. Biol. 2017, 27, 973–978. [Google Scholar] [CrossRef] [PubMed]
- Raffaele, S.; Mongrand, S.; Gamas, P.; Niebel, A.; Ott, T. Genome-wide annotation of remorins, a plant-specific protein family: Evolutionary and functional perspectives. Plant Physiol. 2007, 145, 593–600. [Google Scholar] [CrossRef] [PubMed]
- Meijering, E.; Jacob, M.; Sarria, J.C.; Steiner, P.; Hirling, H.; Unser, M. Design and validation of a tool for neurite tracing and analysis in fluorescence microscopy images. Cytom. A 2004, 58, 167–176. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ormancey, M.; Le Ru, A.; Duboé, C.; Jin, H.; Thuleau, P.; Plaza, S.; Combier, J.-P. Internalization of miPEP165a into Arabidopsis Roots Depends on both Passive Diffusion and Endocytosis-Associated Processes. Int. J. Mol. Sci. 2020, 21, 2266. https://doi.org/10.3390/ijms21072266
Ormancey M, Le Ru A, Duboé C, Jin H, Thuleau P, Plaza S, Combier J-P. Internalization of miPEP165a into Arabidopsis Roots Depends on both Passive Diffusion and Endocytosis-Associated Processes. International Journal of Molecular Sciences. 2020; 21(7):2266. https://doi.org/10.3390/ijms21072266
Chicago/Turabian StyleOrmancey, Mélanie, Aurélie Le Ru, Carine Duboé, Hailing Jin, Patrice Thuleau, Serge Plaza, and Jean-Philippe Combier. 2020. "Internalization of miPEP165a into Arabidopsis Roots Depends on both Passive Diffusion and Endocytosis-Associated Processes" International Journal of Molecular Sciences 21, no. 7: 2266. https://doi.org/10.3390/ijms21072266
APA StyleOrmancey, M., Le Ru, A., Duboé, C., Jin, H., Thuleau, P., Plaza, S., & Combier, J.-P. (2020). Internalization of miPEP165a into Arabidopsis Roots Depends on both Passive Diffusion and Endocytosis-Associated Processes. International Journal of Molecular Sciences, 21(7), 2266. https://doi.org/10.3390/ijms21072266




