AMPK Regulates Developmental Plasticity through an Endogenous Small RNA Pathway in Caenorhabditis elegans
Abstract
1. Introduction
1.1. AMPK and Developmental Plasticity
1.2. C. elegans as a Model Organism to Study AMPK Signalling
2. Loss of AMPK Results in Germline Defects
2.1. Defects in the Dauer Germ Line Result in Post-Dauer Sterility in AMPK Mutants
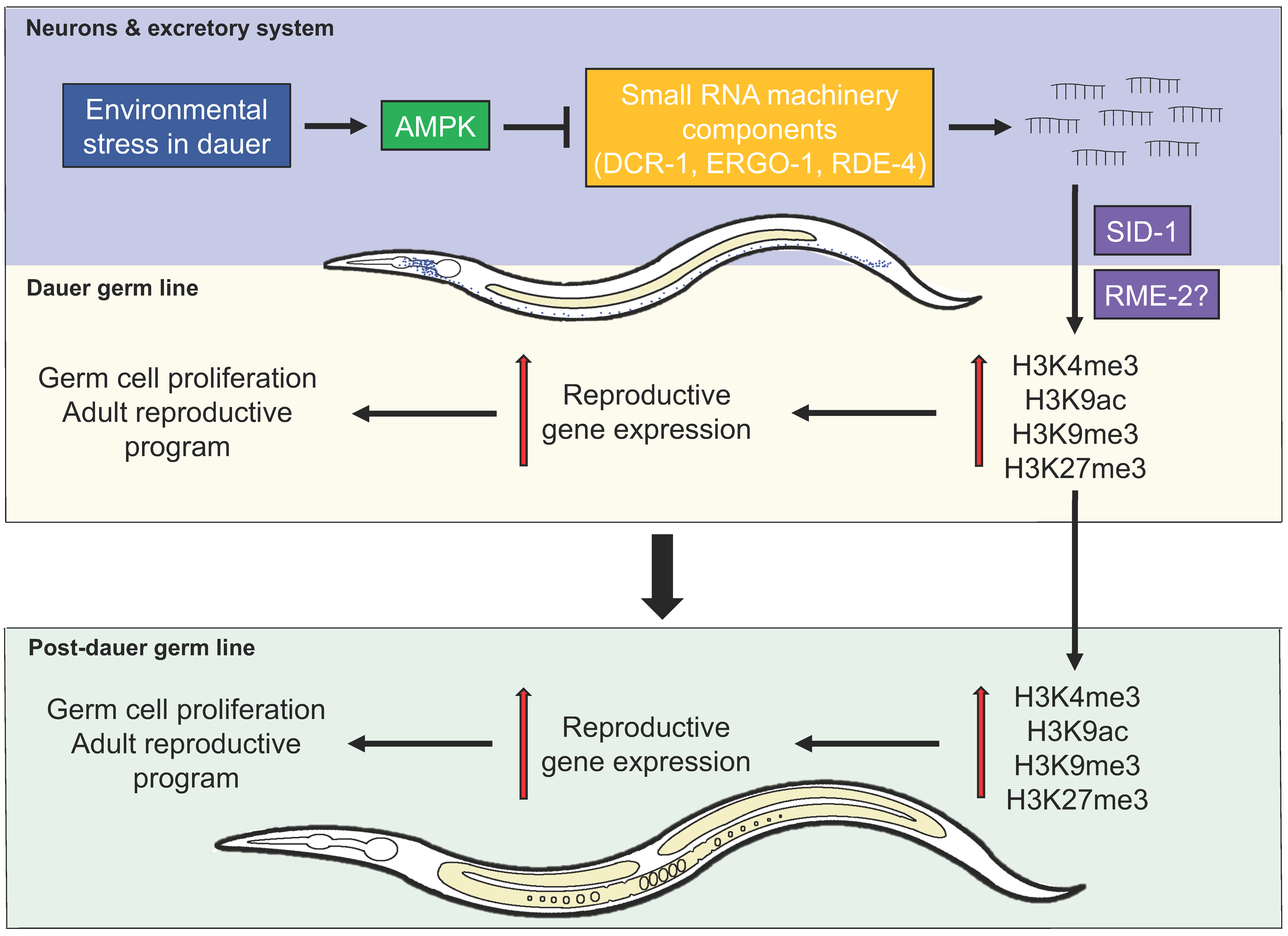
2.2. Misregulation of Chromatin Marks and Gene Expression in AMPK Mutants
3. AMPK Regulates Germline Stem Cell Quiescence and Integrity through an Endogenous Small RNA Pathway
4. Somatic Expression of AMPK Rescues the AMPK Mutant Defects
4.1. Neurons, Small RNAs, and the Weismann Barrier
4.2. Somatic Expression of AMPK is Sufficient to Restore Dauer Germline Quiescence and Post-Dauer Fertility in AMPK Mutants
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Narbonne, P.; Roy, R. Caenorhabditis elegans dauers need LKB1/AMPK to ration lipid reserves and ensure long-term survival. Nature 2009, 457, 210–214. [Google Scholar] [CrossRef] [PubMed]
- Narbonne, P.; Roy, R. Inhibition of germline proliferation during C. elegans dauer development requires PTEN, LKB1 and AMPK signalling. Development 2006, 133, 611–619. [Google Scholar] [CrossRef] [PubMed]
- Kadekar, P.; Roy, R. AMPK regulates germline stem cell quiescence and integrity through an endogenous small RNA pathway. PLoS Biol. 2019, 17, e3000309. [Google Scholar] [CrossRef] [PubMed]
- Narbonne, P.; Roy, R. Regulation of germline stem cell proliferation downstream of nutrient sensing. Cell Div. 2006, 1, 29. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, M.; Roy, R. 5′-AMP-Activated Protein Kinase Signaling in Caenorhabditis elegans. Exp. Suppl. 2016, 107, 375–388. [Google Scholar] [CrossRef]
- Beale, E.G. 5′-AMP-Activated Protein Kinase Signaling in Caenorhabditis elegans. Exp. Biol. Med. 2008, 233, 12–20. [Google Scholar] [CrossRef]
- Hubbard, E.J.; Korta, D.Z.; Dalfo, D. Physiological control of germline development. Adv. Exp. Med. Biol. 2013, 757, 101–131. [Google Scholar] [CrossRef]
- Riddle, D.L.; Swanson, M.M.; Albert, P.S. Interacting genes in nematode dauer larva formation. Nature 1981, 290, 668–671. [Google Scholar] [CrossRef]
- Dalley, B.K.; Golomb, M. Gene expression in the Caenorhabditis elegans dauer larva: Developmental regulation of Hsp90 and other genes. Dev. Biol. 1992, 151, 80–90. [Google Scholar] [CrossRef]
- Riddle, D.L.; Albert, P.S. Genetic and Environmental Regulation of Dauer Larva Development. In C. elegans, 2nd ed.; Riddle, D.L., Blumenthal, T., Meyer, B.J., Priess, J.R., Eds.; Cold Spring Harbor (NY): New York, NY, USA, 1997. [Google Scholar]
- Lee, H.; Cho, J.S.; Lambacher, N.; Lee, J.; Lee, S.J.; Lee, T.H.; Gartner, A.; Koo, H.S. The Caenorhabditis elegans AMP-activated protein kinase AAK-2 is phosphorylated by LKB1 and is required for resistance to oxidative stress and for normal motility and foraging behavior. J. Biol. Chem. 2008, 283, 14988–14993. [Google Scholar] [CrossRef]
- Fukuyama, M.; Rougvie, A.E.; Rothman, J.H. C. elegans DAF-18/PTEN mediates nutrient-dependent arrest of cell cycle and growth in the germline. Curr. Biol. 2006, 16, 773–779. [Google Scholar] [CrossRef] [PubMed]
- Rossi, D.J.; Jamieson, C.H.; Weissman, I.L. Stems cells and the pathways to aging and cancer. Cell 2008, 132, 681–696. [Google Scholar] [CrossRef] [PubMed]
- Seidel, H.S.; Kimble, J. Cell-cycle quiescence maintains Caenorhabditis elegans germline stem cells independent of GLP-1/Notch. Elife 2015, 4. [Google Scholar] [CrossRef] [PubMed]
- Kadekar, P.; Chaouni, R.; Clark, E.; Kazanets, A.; Roy, R. Genome-wide surveys reveal polarity and cytoskeletal regulators mediate LKB1-associated germline stem cell quiescence. BMC Genom. 2018, 19, 462. [Google Scholar] [CrossRef] [PubMed]
- Hall, S.E.; Beverly, M.; Russ, C.; Nusbaum, C.; Sengupta, P. A cellular memory of developmental history generates phenotypic diversity in C. elegans. Curr. Biol. 2010, 20, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Hall, S.E.; Chirn, G.W.; Lau, N.C.; Sengupta, P. RNAi pathways contribute to developmental history-dependent phenotypic plasticity in C. elegans. RNA 2013, 19, 306–319. [Google Scholar] [CrossRef] [PubMed]
- Bungard, D.; Fuerth, B.J.; Zeng, P.Y.; Faubert, B.; Maas, N.L.; Viollet, B.; Carling, D.; Thompson, C.B.; Jones, R.G.; Berger, S.L. Signaling kinase AMPK activates stress-promoted transcription via histone H2B phosphorylation. Science 2010, 329, 1201–1205. [Google Scholar] [CrossRef]
- Demoinet, E.; Li, S.; Roy, R. AMPK blocks starvation-inducible transgenerational defects in Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA 2017, 114, E2689–E2698. [Google Scholar] [CrossRef]
- Gonzalez-Aguilera, C.; Palladino, F.; Askjaer, P. C. elegans epigenetic regulation in development and aging. Brief. Funct. Genom. 2014, 13, 223–234. [Google Scholar] [CrossRef]
- Moazed, D. Small RNAs in transcriptional gene silencing and genome defence. Nature 2009, 457, 413–420. [Google Scholar] [CrossRef]
- van Wolfswinkel, J.C.; Ketting, R.F. The role of small non-coding RNAs in genome stability and chromatin organization. J. Cell Sci. 2010, 123, 1825–1839. [Google Scholar] [CrossRef]
- Faehnle, C.R.; Joshua-Tor, L. Argonautes confront new small RNAs. Curr. Opin. Chem. Biol. 2007, 11, 569–577. [Google Scholar] [CrossRef]
- Shuaib, M.; Parsi, K.M.; Thimma, M.; Adroub, S.A.; Kawaji, H.; Seridi, L.; Ghosheh, Y.; Fort, A.; Fallatah, B.; Ravasi, T.; et al. Nuclear AGO1 Regulates Gene Expression by Affecting Chromatin Architecture in Human Cells. Cell Syst. 2019, 9, 446–458.e6. [Google Scholar] [CrossRef]
- Burkewitz, K.; Morantte, I.; Weir, H.J.M.; Yeo, R.; Zhang, Y.; Huynh, F.K.; Ilkayeva, O.R.; Hirschey, M.D.; Grant, A.R.; Mair, W.B. Neuronal CRTC-1 governs systemic mitochondrial metabolism and lifespan via a catecholamine signal. Cell 2015, 160, 842–855. [Google Scholar] [CrossRef]
- Posner, R.; Toker, I.A.; Antonova, O.; Star, E.; Anava, S.; Azmon, E.; Hendricks, M.; Bracha, S.; Gingold, H.; Rechavi, O. Neuronal Small RNAs Control Behavior Transgenerationally. Cell 2019, 177, 1814–1826.e15. [Google Scholar] [CrossRef]
- Marre, J.; Traver, E.C.; Jose, A.M. Extracellular RNA is transported from one generation to the next in Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA 2016, 113, 12496–12501. [Google Scholar] [CrossRef]
- Tonkin, L.A.; Bass, B.L. Mutations in RNAi rescue aberrant chemotaxis of ADAR mutants. Science 2003, 302, 1725. [Google Scholar] [CrossRef]
- Geison, G.L. Darwin and heredity: The evolution of his hypothesis of pangenesis. J. Hist. Med. Allied Sci. 1969, 24, 375–411. [Google Scholar] [CrossRef]
- Grant, B.; Hirsh, D. Receptor-mediated endocytosis in the Caenorhabditis elegans oocyte. Mol. Biol. Cell 1999, 10, 4311–4326. [Google Scholar] [CrossRef]
- Fazal, F.M.; Han, S.; Parker, K.R.; Kaewsapsak, P.; Xu, J.; Boettiger, A.N.; Chang, H.Y.; Ting, A.Y. Atlas of Subcellular RNA Localization Revealed by APEX-Seq. Cell 2019, 178, 473–490.e6. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wong, C.; Roy, R. AMPK Regulates Developmental Plasticity through an Endogenous Small RNA Pathway in Caenorhabditis elegans. Int. J. Mol. Sci. 2020, 21, 2238. https://doi.org/10.3390/ijms21062238
Wong C, Roy R. AMPK Regulates Developmental Plasticity through an Endogenous Small RNA Pathway in Caenorhabditis elegans. International Journal of Molecular Sciences. 2020; 21(6):2238. https://doi.org/10.3390/ijms21062238
Chicago/Turabian StyleWong, Christopher, and Richard Roy. 2020. "AMPK Regulates Developmental Plasticity through an Endogenous Small RNA Pathway in Caenorhabditis elegans" International Journal of Molecular Sciences 21, no. 6: 2238. https://doi.org/10.3390/ijms21062238
APA StyleWong, C., & Roy, R. (2020). AMPK Regulates Developmental Plasticity through an Endogenous Small RNA Pathway in Caenorhabditis elegans. International Journal of Molecular Sciences, 21(6), 2238. https://doi.org/10.3390/ijms21062238




