Aromatase Inhibitors as Adjuvant Treatment for ER/PgR Positive Stage I Endometrial Carcinoma: A Retrospective Cohort Study
Abstract
1. Introduction
2. Results
2.1. Study Population
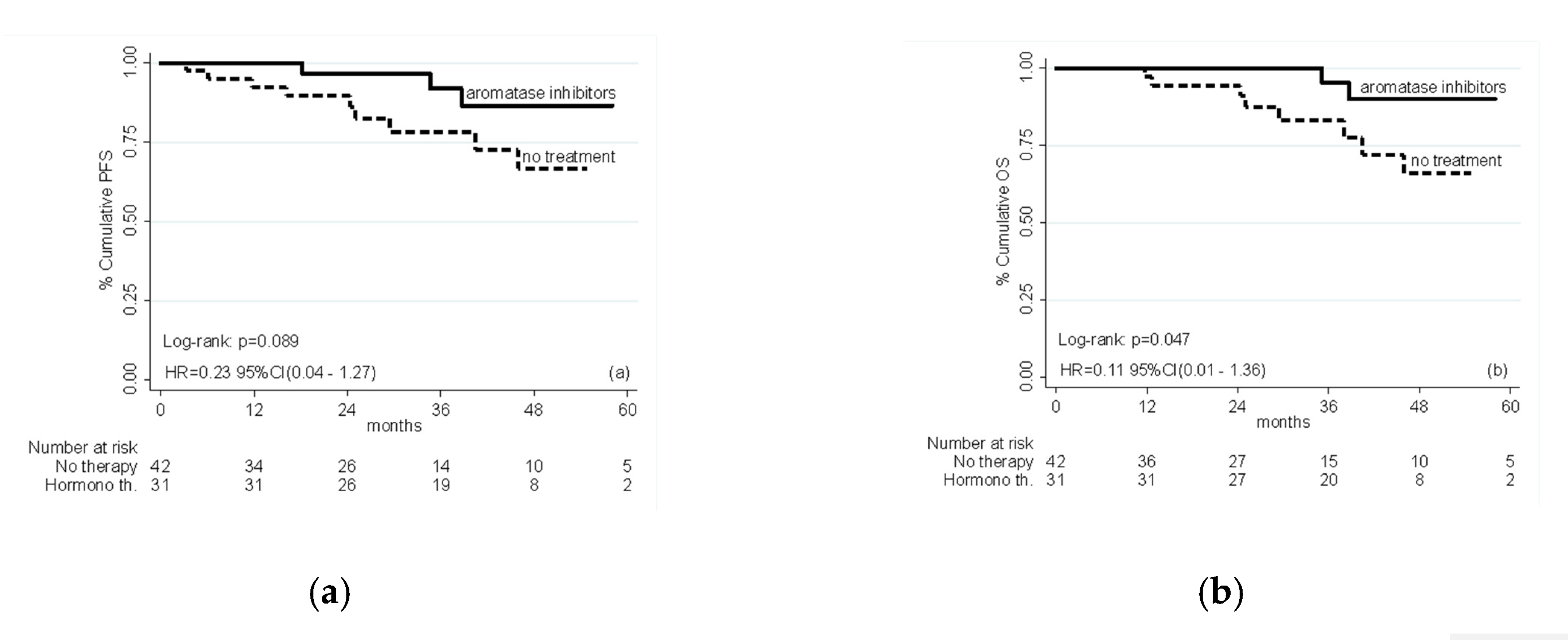
2.2. Efficacy
3. Discussion
4. Materials and Methods
4.1. Patients
4.2. Tissue Sampling, Histopathological Analysis and Immunohistochemistry (IHC)
4.3. Statistical Analysis
4.4. Assessment of Therapeutic Option and Patients Counselling
Author Contributions
Funding
Conflicts of Interest
References
- American Cancer Society, Cancer Statistics for USA. 2019. Available online: https://www.cancer.org/cancer/endometrial-cancer/about/key-statistics.html (accessed on 18 March 2020).
- Lortet-Tiulent, J.; Ferlay, J.; Bray, F.; Jemal, A. International pattern and trends in endometrial cancer incidence, 1978–2013. J. Natl. Cancer Inst. 2018, 110, 354–361. [Google Scholar] [CrossRef] [PubMed]
- Onstand, M.A.; Schmandt, R.E.; Lu, K.H. Addressing the role of obesity in endometrial cancer risk, prevention, and treatment. J. Clin. Oncol. 2016, 34, 4225–4230. [Google Scholar] [CrossRef] [PubMed]
- Kandoth, N.C.; Schultz, N.; Cherniack, A.D.; Akbani, R.; Liu, Y.; Shen, H.; Robertson, A.G.; Pashtan, I.; Shen, R.; Cancer Genome Atlas Research; et al. Integrated genomic characterization of endometrial carcinoma. Nature 2013, 497, 67–73. [Google Scholar]
- Ito, K.; Utsunomiya, H.; Yaegashi, N.; Sasano, H. Biological roles of estrogen and progesterone in human endometrial carcinoma—New development in potential endocrine therapy for endometrial cancer. Endocr. J. 2007, 54, 667–679. [Google Scholar] [CrossRef] [PubMed]
- Grady, D.; Gebretsadik, T.; Kerlikowske, K.; Ernster, V.; Petitti, D. Hormone replacement therapy and endometrial cancer risk: A meta-analysis. Obstet. Gynecol. 1995, 85, 304–313. [Google Scholar] [CrossRef]
- Brinton, L.A.; Trabert, B.; Anderson, G.L.; Falk, R.T.; Felix, A.S.; Fuhrman, B.J.; Gass, M.L.; Kuller, L.H.; Pfeiffer, R.M.; Rohan, T.E.; et al. Serum estrogens and estrogen metabolites and endometrial cancer risk among postmenopausal women. Cancer Epidemiol. Biomark. Prev. 2016, 25, 1081–1099. [Google Scholar] [CrossRef]
- Allen, N.E.; Key, T.J.; Dossus, L.; Rinaldi, S.; Cust, A.; Lukanova, A.; Peeters, P.H.; Onland-Moret, N.C.; Lahmann, P.H.; Berrino, F.; et al. Endogenous sex hormones and endometrial cancer risk in women in the European Prospective Investigation into Cancer and Nutrition (EPIC). Endocr. Relat. Cancer 2008, 15, 485–497. [Google Scholar] [CrossRef]
- Gao, C.; Wang, Y.; Tian, W.; Zhu, Y.; Xue, F. The therapeutic significance of aromatase inhibitors in endometrial carcinoma. Gynecol. Oncol. 2014, 134, 190–195. [Google Scholar] [CrossRef]
- Watanabe, K.; Sasano, H.; Harada, N.; Ozaki, M.; Niikura, H.; Sato, S.; Yajima, A. Aromatase in human endometrial carcinoma and hyperplasia: Immunohisto chemical, in situ hybridization, and biochemical studies. Am. J. Pathol. 1995, 146, 491–500. [Google Scholar]
- Zhao, H.; Zhou, L.; Shangguan, A.J.; Bulun, S.E. Most of the estrogen in premenopausal women is synthesized by the ovaries, while extraovarian subcutaneous adipose tissue is the predominant tissue source of estrogen after menopause. J. Mol. Endocrinol. 2016, 57, 19–33. [Google Scholar] [CrossRef]
- Nieves-Neira, W.; Kim, J.J.; Matei, D. Hormonal strategies in gynecologic cancer: Bridging biology and therapy. Gynecol. Oncol. 2018, 150, 207–210. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.nccn.org/professionals/physician_gls/pdf/uterine.pdf (accessed on 19 March 2020).
- Yerushalmi, R.; Woods, R.; Ravdin, P.M.; Hayes, M.M.; Gelmon, K.A. Ki67 in breast cancer: Prognostic and predictive potential. Lancet Oncol. 2010, 11, 174–183. [Google Scholar] [CrossRef]
- Colombo, N.; Creutzberg, C.; Amant, F.; Bosse, T.; González-Martín, A.; Ledermann, J.; Marth, C.; Nout, R.; Querleu, D.; Mirza, M.R.; et al. ESMO-ESGO-ESTRO Consensus conference on endometrial cancer: Diagnosis, treatment and follow-up. Ann. Oncol. 2016, 27, 16–41. [Google Scholar] [CrossRef] [PubMed]
- Smith, I.; Yardley, D.; Burris, H.; De Boer, R.; Amadori, D.; McIntyre, K.; Ejlertsen, B.; Gnant, M.; Jonat, W.; Pritchard, K.I.; et al. Comparative efficacy and safety of adjuvant letrozole versus anastrozole in postmenopausal patients with hormone receptor-positive, node-positive early breast cancer: Final results of the randomized phase III Femara Versus Anastrozole Clinical Evaluation (FACE) trial. J. Clin. Oncol. 2017, 35, 1041–1048. [Google Scholar] [PubMed]
- Goss, P.E.; Ingle, J.N.; Pritchard, K.I.; Ellis, M.J.; Sledge, G.W.; Budd, G.T.; Rabaglio, M.; Ansari, R.H.; Johnson, D.B.; Tozer, R.; et al. Exemestane versus anastrozole in postmenopausal women with early breast cancer: NCIC CTG MA.27—A randomized controlled phase III trial. J. Clin. Oncol. 2013, 31, 1398–1404. [Google Scholar] [CrossRef]
- Guerrieri-Gonzaga, A.; Sestak, I.; Lazzeroni, M.; Serrano, D.; Rotmensz, N.; Cazzaniga, M.; Varricchio, C.; Pruneri, G.; Leonardi, M.C.; Orecchia, R.; et al. Benefit of low-dose tamoxifen in a large observational cohort of high risk ER positive breast DCIS. Int. J. Cancer 2016, 139, 2127–2134. [Google Scholar] [CrossRef]
- DeCensi, A.; Puntoni, M.; Guerrieri-Gonzaga, A.; Caviglia, S.; Avino, F.; Cortesi, L.; Taverniti, C.; Pacquola, M.G.; Falcini, F.; Gulisano, M.; et al. Randomized Placebo Controlled Trial of Low-Dose Tamoxifen to Prevent Local and Contralateral Recurrence in Breast Intraepithelial Neoplasia. J. Clin. Oncol. 2019, 37, 1629–1637. [Google Scholar] [CrossRef]
- Available online: https://clinicaltrials.gov/ct2/results?cond=Endometrial+Cancer+Stage+I&term=hormone+therapy&cntry=&state=&city=&dist= (accessed on 21 December 2018).
- De Palo, G.; Mangioni, C.; Periti, P.; del Vecchio, M.; Marubini, E. Treatment of FIGO (1971) stage I endometrial carcinoma with intensive surgery, radiotherapy and hormonotherapy according to pathological prognostic groups. Long-term results of a randomized multicentre study. Eur. J. Cancer 1993, 29, 1133–1140. [Google Scholar] [CrossRef]
- Dowsett, M.; Nielsen, T.O.; A’Hern, R.; Bartlett, J.; Coombes, R.C.; Cuzick, J.; Ellis, M.; Henry, N.L.; Hugh, J.C.; Lively, T.; et al. Assessment of Ki67 in breast cancer: Recommendations from the international Ki67 in Breast Cancer working group. J. Natl. Cancer Inst. 2011, 103, 1656–1664. [Google Scholar] [CrossRef]
- de Azambuja, E.; Cardoso, F.; de Castro, G., Jr.; Colozza, M.; Mano, M.S.; Durbecq, V.; Sotiriou, C.; Larsimont, D.; Piccart-Gebhart, M.J.; Paesmans, M. Ki-67 as prognostic marker in early breast cancer: A meta-analysis of published studies involving 12,155 patients. Br. J. Cancer 2007, 96, 1504–1513. [Google Scholar] [CrossRef]
- Kitson, S.; Sivalingam, V.N.; JBolton, J.; McVey, R.; Nickkho-Amiry, M.; Powell, M.E.; Leary, A.; Nijman, H.W.; Nout, R.A.; Bosse, T. Ki-67 in endometrial cancer: Scoring optimization and prognostic relevance for window studies. Mod. Pathol. 2017, 30, 459–468. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.esmo.org/Guidelines/Gynaecological-Cancers/ESMO-ESGO-ESTRO-Consensus-Conference-on-Endometrial-Cancer/eUpdate-Algorithms (accessed on 30 September 2019).
| Variable | Overall N = 73 (100%) | Hormone Therapy N = 31 (42.5%) | No Treatment N = 42 (57.5%) | p-Value | |
|---|---|---|---|---|---|
| Age, mean (SD) | 74.6 (10.4) | 73.4 (9.7) | 75.5 (10.9) | 0.396 2 | |
| Age, N (%) | <70 yrs | 25 (34.3) | 11 (35.5) | 14 (33.3) | 1.000 1 |
| >70 yrs | 48 (65.8) | 20 (64.5) | 28 (66.7) | ||
| BMI, N (%) | healthy weight | 22 (30.1%) | 9 (29.0%) | 13 (30.9%) | 0.912 1 |
| over weight | 31 (42.5%) | 14 (45.2%) | 17 (40.5%) | ||
| obesity | 20 (27.4%) | 8 (25.8%) | 12 (28.6%) | ||
| Adjuvant treatment: Exemestane Letrozole | 22 (71%) 9 (29%) | 22 (71%) 9 (29%) | 0 (0%) 0 (0%) | - | |
| Depth of myometrial invasion, N (%) | <50% | 40 (54.8) | 18 (58.1) | 22 (52.4) | 0.644 1 |
| >50% | 33 (45.2) | 13 (41.9) | 20 (47.6) | ||
| Stage, N (%) | I | 56 (82.4) | 26 (86.7) | 30 (79.0) | 0.720 1 |
| II | 7 (10.3) | 2 (6.7) | 5 (13.2) | ||
| III | 5 (7.4) | 2 (6.7) | 3 (7.9) | ||
| Grade, N (%) | <G2 | 36 (50.7) | 14 (46.7) | 22 (53.7) | 0.617 1 |
| >G3 | 35 (49.3) | 16 (53.3) | 19 (46.3) | ||
| Lymphovascular space invasion, N (%) | No | 69 (94.5) | 29 (93.5) | 40 (95.2) | 1.000 1 |
| Yes | 4 (5.5) | 2 (6.5) | 2 (4.8) | ||
| ER, median (IQR) | 80 (20–90) | 80 (40–90) | 80 (20–90) | 0.499 3 | |
| PgR, median (IQR) | 70 (30–90) | 80 (50–90) | 70 (10–90) | 0.358 3 | |
| Ki-67, median (IQR) | 40 (30–65) | 40 (20–60) | 50 (30–70) | 0.308 3 | |
| PFS | HR | 95% CI | p-Value |
|---|---|---|---|
| AI vs. no therapy | 0.23 | 0.04–1.27 | 0.092 |
| Age > 70 | 9.89 | 0.93–105.53 | 0.058 |
| BMI 25–29 vs. BMI < 25 | 0.28 | 0.05–1.55 | 0.144 |
| BMI > 30 vs. BMI < 25 | 0.44 | 0.06–3.12 | 0.409 |
| Myometrium invasion (yes vs. no) | 0.87 | 0.19–3.94 | 0.854 |
| Grade > G2 | 1.02 | 0.23–4.53 | 0.974 |
| Lymphovascular space invasion (yes vs. no) | 3.33 | 0.32–34.49 | 0.314 |
| ER | 0.97 | 0.93–1.01 | 0.178 |
| PgR | 1.03 | 0.98–1.09 | 0.209 |
| Ki67 | 1.04 | 1.01–1.09 | 0.026 |
| OS | HR | 95% CI | p-Value |
|---|---|---|---|
| AI vs. no therapy | 0.11 | 0.01–1.36 | 0.085 |
| Age > 70 | Na * | Na * | Na * |
| BMI 25–29 vs. BMI < 25 | 0.18 | 0.03–0.97 | 0.046 |
| BMI > 30 vs. BMI < 25 | 0.21 | 0.01–3.03 | 0.253 |
| Myometrium invasion (yes vs. no) | 1.04 | 0.22–4.92 | 0.962 |
| Grade > G2 | 0.80 | 0.14–4.71 | 0.803 |
| Lymphovascular space invasion (yes vs. no) | 2.66 | 0.21–33.14 | 0.446 |
| ER | 0.96 | 0.90–1.02 | 0.199 |
| PgR | 1.05 | 0.97–1.13 | 0.204 |
| Ki67 | 1.06 | 1.01–1.11 | 0.027 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paleari, L.; Rutigliani, M.; Siri, G.; Provinciali, N.; Colombo, N.; Decensi, A. Aromatase Inhibitors as Adjuvant Treatment for ER/PgR Positive Stage I Endometrial Carcinoma: A Retrospective Cohort Study. Int. J. Mol. Sci. 2020, 21, 2227. https://doi.org/10.3390/ijms21062227
Paleari L, Rutigliani M, Siri G, Provinciali N, Colombo N, Decensi A. Aromatase Inhibitors as Adjuvant Treatment for ER/PgR Positive Stage I Endometrial Carcinoma: A Retrospective Cohort Study. International Journal of Molecular Sciences. 2020; 21(6):2227. https://doi.org/10.3390/ijms21062227
Chicago/Turabian StylePaleari, Laura, Mariangela Rutigliani, Giacomo Siri, Nicoletta Provinciali, Nicoletta Colombo, and Andrea Decensi. 2020. "Aromatase Inhibitors as Adjuvant Treatment for ER/PgR Positive Stage I Endometrial Carcinoma: A Retrospective Cohort Study" International Journal of Molecular Sciences 21, no. 6: 2227. https://doi.org/10.3390/ijms21062227
APA StylePaleari, L., Rutigliani, M., Siri, G., Provinciali, N., Colombo, N., & Decensi, A. (2020). Aromatase Inhibitors as Adjuvant Treatment for ER/PgR Positive Stage I Endometrial Carcinoma: A Retrospective Cohort Study. International Journal of Molecular Sciences, 21(6), 2227. https://doi.org/10.3390/ijms21062227






