Proteomic Profiles and Biological Processes of Relapsed vs. Non-Relapsed Pediatric Hodgkin Lymphoma
Abstract
1. Introduction
2. Results
2.1. Plasma Protein Profiling
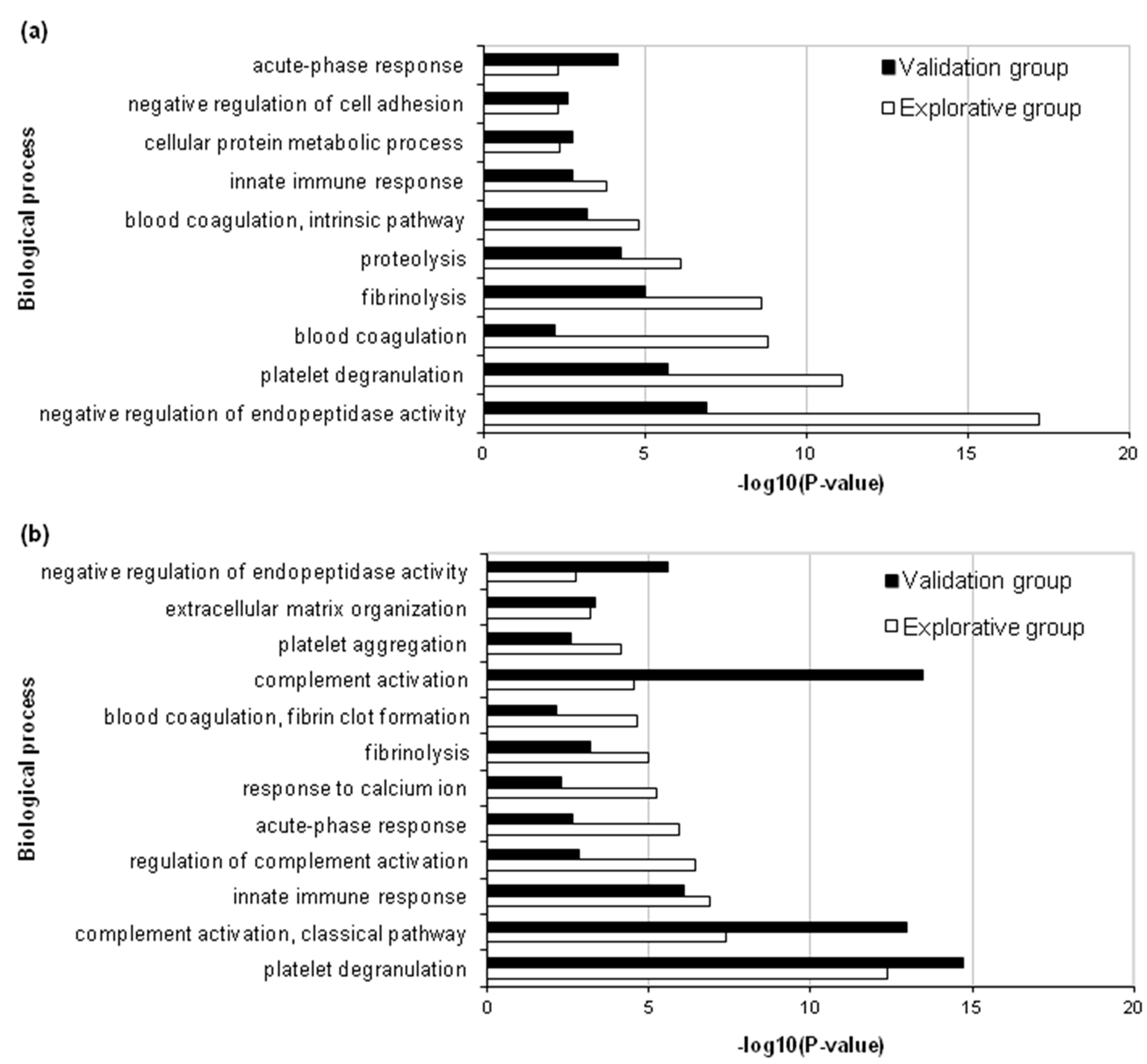
2.2. Functional Annotation of Differentially Abundant Proteins
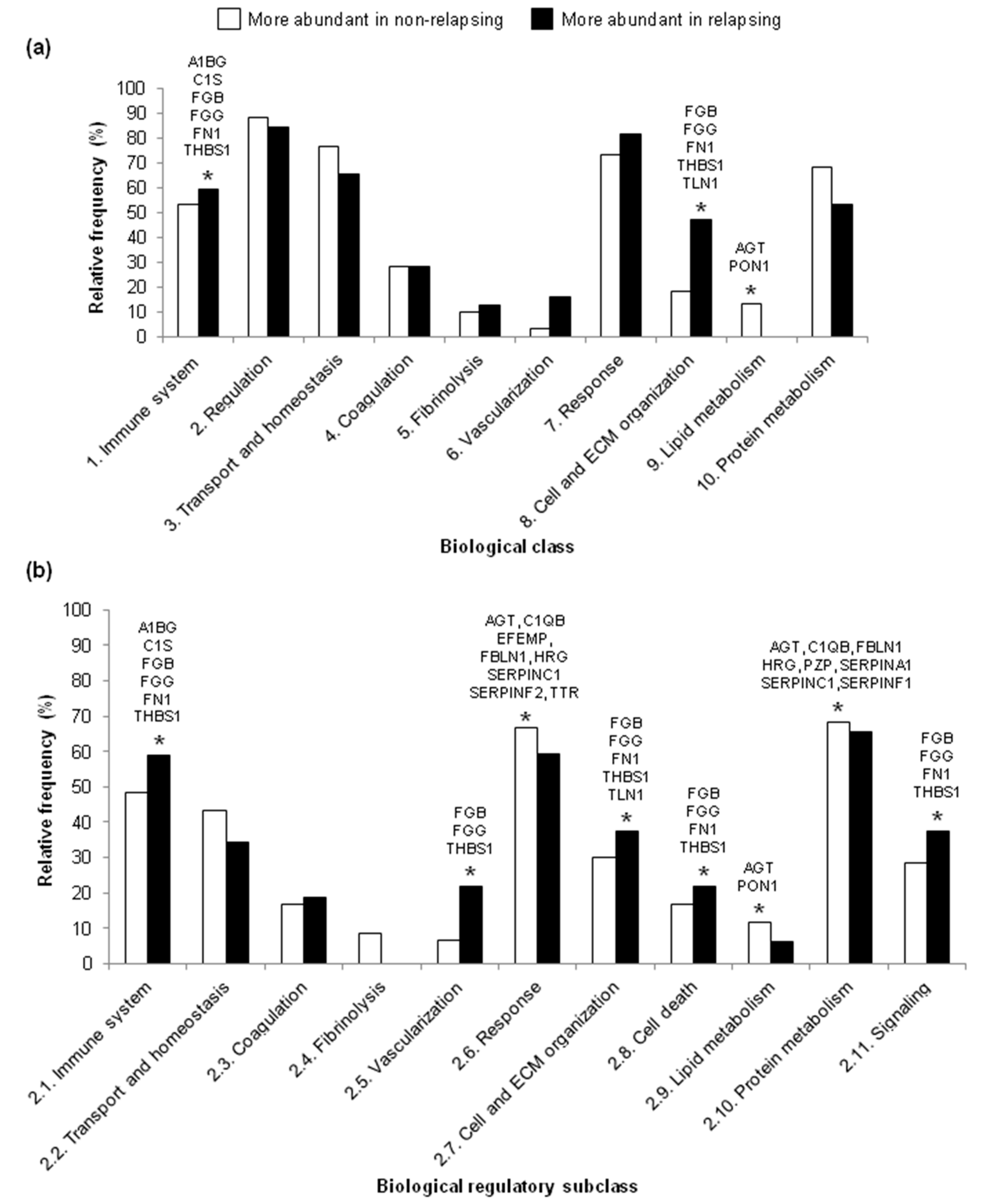
2.3. Analysis of Biological Processes
3. Discussion
4. Materials and Methods
4.1. Research Ethics Statement
4.2. Patients and Plasma Samples
4.3. Protein Extraction and Digestion
4.4. LC-MS/MS and Label-Free Proteomic Profiling
4.5. Immunoblotting
4.6. Protein Functional Annotation
4.7. Biological Process Classification
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| 2DE | two-dimensional electrophoresis |
| DIGE | difference gel electrophoresis |
| ECM | extracellular matrix |
| HL | Hodgkin lymphoma |
| LC-MS/MS | liquid chromatography mass spectrometry |
References
- Smith, M.A.; Altekruse, S.F.; Adamson, P.C.; Reaman, G.H.; Seibel, N.L. Declining childhood and adolescent cancer mortality. Cancer 2014, 120, 2497–2506. [Google Scholar] [CrossRef] [PubMed]
- Donaldson, S.S.; Link, M.P.; Weienstein, H.J.; Rai, S.N.; Brain, S.; Billett, A.L.; Hurwitz, C.A.; Krasin, M.; Kun, L.E.; Marcus, K.C.; et al. Final results of a prospective clinical trial with VAMP and low-dose involved-field radiation for children with low-risk Hodgkin’s disease. J. Clin. Oncol. 2007, 25, 332–337. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, C.L.; Constine, L.S.; Villaluna, D.; London, W.B.; Hutchison, R.E.; Sposto, R.; Lipshultz, S.E.; Turner, C.S.; de Alarcon, P.A.; Chauvenet, A. A risk-adapted, response-based approach using ABVE-PC for children and adolescents with intermediate and high-risk Hodgkin lymphoma: The results of P9425. Blood 2009, 114, 2051–2059. [Google Scholar] [CrossRef] [PubMed]
- Nagpal, P.; Akl, M.R.; Ayoub, N.M.; Tomiyama, T.; Cousins, T.; Tai, B.; Carroll, N.; Nyrenda, T.; Bhattacharyya, P.; Harris, M.B.; et al. Pediatric Hodgkin lymphoma: Biomarkers, drugs, and clinical trials for translational science and medicine. Oncotarget 2016, 7, 67551–67573. [Google Scholar] [CrossRef] [PubMed]
- Pui, C.H.; Hudson, M.; Luo, X.; Wilimas, J.; Evans, W.; Crist, W.M. Serum interleukin-2 receptor levels in Hodgkin disease and other solid tumors of childhood. Leukemia 1993, 7, 1242–1244. [Google Scholar] [PubMed]
- Shafat, I.; Barak, A.B.; Postovsky, S.; Elhasid, R.; Ilan, N.; Vlodavsky, I.; Arush, M.W. Heparanase levels are elevated in the plasma of pediatric cancer patients and correlate with response to anticancer treatment. Neoplasia 2007, 9, 909–916. [Google Scholar] [CrossRef]
- Ben Arush, M.W.; Shafat, I.; Ben Barak, A.; Shalom, R.B.; Vlodavsky, I.; Vlodavsky, E.; lan, N. Plasma heparanase as a significant marker of treatment response in children with Hodgkin lymphoma: Pilot study. Pediatr Hematol Oncol. 2009, 26, 157–164. [Google Scholar] [CrossRef]
- Qi, L.; Cazares, L.; Johnson, C.; de Alarcon, P.; Kupfer, G.M.; Semmes, O.J. Serum protein expression profiling in pediatric Hodgkin lymphoma: A report from the Children’s Oncology Group. Pediatr. Blood Cancer 2008, 51, 216–221. [Google Scholar] [CrossRef]
- Kamper, P.; Ludvigsen, M.; Bendix, K.; Hamilton-Dutoit, S.; Rabinovich, G.A.; Møller, M.B.; Nyengaard, J.R.; Honoré, B.; d’Amore, F. Proteomic analysis identifies galectin-1 as a predictive biomarker for relapsed/refractory disease in classical Hodgkin lymphoma. Blood 2011, 117, 6638–6649. [Google Scholar] [CrossRef]
- Repetto, O.; Mussolin, L.; Elia, C.; Martina, L.; Bianchi, M.; Buffardi, S.; Sala, A.; Burnelli, R.; Mascarin, M.; De Re, V. Proteomic identification of plasma biomarkers in children and adolescents with recurrent Hodgkin Lymphoma. J. Cancer 2018, 9, 4650–4658. [Google Scholar] [CrossRef]
- Unlü, M.; Morgan, M.E.; Minden, J.S. Difference gel electrophoresis: A single gel method for detecting changes in protein extracts. Electrophoresis 1997, 18, 2071–2077. [Google Scholar] [CrossRef] [PubMed]
- Abdallah, C.; Dumas-Gaudot, E.; Renaut, J.; Sergeant, K. Gel-based and gel-free quantitative proteomics approaches at a glance. Int. J. Plant Genom. 2012, 2012, 494572. [Google Scholar] [CrossRef] [PubMed]
- Sandin, M.; Chawade, A.; Levander, F. Is label-free LC-MS/MS ready for biomarker discovery? Proteom. Clin. Appl. 2015, 9, 289–294. [Google Scholar] [CrossRef] [PubMed]
- Sap, A.K.; Demmers, J.A. Labeling Methods in Mass Spectrometry Based Quantitative Proteomics. In Integrative Proteomics; Leung, H.C.E., Man, T.K., Flores, R.J., Eds.; InTech: Rijeka, Croatia, 2012; pp. 111–133. [Google Scholar]
- Beer, L.A.; Liu, P.; Ky, B.; Barnhart, K.T.; Speicher, D.W. Efficient Quantitative Comparisons of Plasma Proteomes Using Label-Free Analysis with MaxQuant. Methods Mol. Biol. 2017, 1619, 339–352. [Google Scholar] [PubMed]
- Bantscheff, M.; Schirle, M.; Sweetman, G.; Rick, J.; Kuster, B. Quantitative mass spectrometry in proteomics: a critical review. Anal. Bioanal. Chem. 2007, 389, 1017–1031. [Google Scholar] [CrossRef]
- Sandow, J.J.; Rainczuk, A.; Infusini, G.; Makanji, M.; Bilandzic, M.; Wilson, A.L.; Fairweather, N.; Stanton, P.G.; Garama, D.; Gough, D.; et al. Discovery and Validation of Novel Protein Biomarkers in Ovarian Cancer Patient Urine. Proteom. Clin. Appl. 2018, 12, e1700135. [Google Scholar] [CrossRef] [PubMed]
- Gámez-Pozo, A.; Trilla-Fuertes, L.; Prado-Vázquez, G.; Chiva, C.; López-Vacas, R.; Nanni, P.; Berges-Soria, J.; Grossmann, J.; Díaz-Almirón, M.; Ciruelos, E.; et al. Prediction of adjuvant chemotherapy response in triple negative breast cancer with discovery and targeted proteomics. PLoS ONE 2017, 12, e0178296. [Google Scholar] [CrossRef]
- Yoo, M.W.; Park, J.; Han, H.S.; Yun, Y.M.; Kang, J.W.; Choi, D.Y.; Lee, J.W.; Jung, J.H.; Lee, K.Y.; Kim, K.P. Discovery of gastric cancer specific biomarkers by the application of serum proteomics. Proteomics 2017, 17. [Google Scholar] [CrossRef]
- Tu, C.; Mojica, W.; Straubinger, R.M.; Li, J.; Shen, S.; Qu, M.; Nie, L.; Roberts, R.; An, B.; Qu, J. Quantitative proteomic profiling of paired cancerous and normal colon epithelial cells isolated freshly from colorectal cancer patients. Proteom. Clin. Appl. 2017, 11. [Google Scholar] [CrossRef]
- Carbone, P.P.; Kaplan, H.S.; Musshoff, K.; Smithers, D.W.; Tubiana, M. Report of the Committee on Hodgkin’s disease staging classification. Cancer Res. 1971, 31, 1860–1861. [Google Scholar]
- Lilley, K.S.; Friedman, D.B. All about DIGE: Quantification technology for differential-display 2D-gel proteomics. Expert Rev. Proteom. 2004, 1, 401–409. [Google Scholar] [CrossRef] [PubMed]
- Gundry, R.L.; White, M.Y.; Murray, C.I.; Kane, L.A.; Fu, Q.; Stanley, B.A.; Van Eyk, J.E. Preparation of proteins and peptides for mass spectrometry analysis in a bottom-up proteomics workflow. Curr. Protoc. Mol. Biol. 2009, 90. [Google Scholar] [CrossRef]
- Sreedhar, A.; Zhao, Y. Dysregulated metabolic enzymes and metabolic reprogramming in cancer cells. Biomed. Rep. 2018, 8, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Rinaldi, G.; Rossi, M.; Fendt, S.M. Metabolic interactions in cancer: Cellular metabolism at the interface between the microenvironment, the cancer cell phenotype and the epigenetic landscape. Wiley Interdiscip. Rev. Syst. Biol. Med. 2018, 10. [Google Scholar] [CrossRef]
- Chang, I.W.; Lin, V.C.; Wu, W.J.; Liang, P.I.; Li, W.M.; Yeh, B.W.; He, H.L.; Liao, A.C.; Chan, T.C.; Li, C.F. Complement Component 1, s Subcomponent Overexpression is an Independent Poor Prognostic Indicator in Patients with Urothelial Carcinomas of the Upper Urinary Tract and Urinary Bladder. J. Cancer 2016, 7, 1396–1405. [Google Scholar] [CrossRef]
- Spiegel, R.J.; Schaefer, E.J.; Magrath, I.T.; Edwards, B.K. Plasma lipid alterations in leukemia and lymphoma. Am. J. Med. 1982, 72, 775–782. [Google Scholar] [CrossRef]
- Blackman, J.D.; Cabana, V.G.; Mazzone, T. The acute-phase response and associated lipoprotein abnormalities accompanying lymphoma. J. Intern. Med. 1993, 233, 201–204. [Google Scholar] [CrossRef]
- Hajjar, D.P.; Hajjar, K.A. Alterations of Cholesterol Metabolism in Inflammation-Induced Atherogenesis. J. Enzymol Metab. 2016, 1, 104. [Google Scholar]
- Naik, P.P.; Ghadge, M.S.; Raste, A.S. Lipid profile in leukemia and Hodgkin’s disease. Indian J. Clin. Biochem. 2006, 21, 100–102. [Google Scholar] [CrossRef]
- Annevon, Z.; Torsten, K. Dissecting the proteome of lipoproteins: New biomarkers for cardiovascular diseases? Transl. Proteom. 2015, 7, 30–39. [Google Scholar]
- Strazzullo, P.; Galletti, F. Impact of the renin-angiotensin system on lipid and carbohydrate metabolism. Curr. Opin. Nephrol. Hypertens. 2004, 13, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Meneses, M.J.; Silvestre, R.; Sousa-Lima, I.; Macedo, M.P. Paraoxonase-1 as a Regulator of Glucose and Lipid Homeostasis: Impact on the Onset and Progression of Metabolic Disorders. Int. J. Mol. Sci. 2019, 20, 4049. [Google Scholar] [CrossRef] [PubMed]
- Poltavets, V.; Kochetkova, M.; Pitson, S.M.; Samuel, M.S. The Role of the Extracellular Matrix and Its Molecular and Cellular Regulators in Cancer Cell Plasticity. Front. Oncol. 2018, 8, 431. [Google Scholar] [CrossRef]
- Werb, Z.; Lu, P. The Role of Stroma in Tumor Development. Cancer J. 2015, 21, 250–253. [Google Scholar] [CrossRef] [PubMed]
- Roger, P.; Pujol, P.; Lucas, A.; Baldet, P.; Rochefort, H. Increased immunostaining of fibulin-1, an estrogen-regulated protein in the stroma of human ovarian epithelial tumors. Am. J. Pathol. 1998, 153, 1579–1588. [Google Scholar] [CrossRef]
- Kischel, P.; Waltregny, D.; Greffe, Y.; Mazzucchelli, G.; De Pauw, E.; de Leval, L.; Castronovo, V. Identification of stromal proteins overexpressed in nodular sclerosis Hodgkin lymphoma. Proteome Sci. 2011, 9, 63. [Google Scholar] [CrossRef]
- Aldinucci, D.; Gloghini, A.; Pinto, A.; De Filippi, R.; Carbone, A. The classical Hodgkin’s lymphoma microenvironment and its role in promoting tumour growth and immune escape. J. Pathol. 2010, 221, 248–263. [Google Scholar] [CrossRef]
- De Re, V.; Caggiari, L.; Repetto, O.; Mussolin, L.; Mascarin, M. Classical Hodgkin’s Lymphoma in the Era of Immune Checkpoint Inhibition. J. Clin. Med. 2019, 8, 1596. [Google Scholar] [CrossRef]
- Liu, W.R.; Shipp, M.A. Signaling pathways and immune evasion mechanisms in classical Hodgkin lymphoma. Blood 2017, 130, 2265–2270. [Google Scholar] [CrossRef]
- Kennedy-Nasser, A.A.; Hanley, P.; Bollard, C.M. Hodgkin disease and the role of the immune system. Pediatr. Hematol. Oncol. 2011, 28, 176–186. [Google Scholar] [CrossRef]
- Jiang, X.; Wang, J.; Deng, X.; Xiong, F.; Ge, J.; Xiang, B.; Wu, X.; Ma, J.; Zhou, M.; Li, X.; et al. Role of the tumor microenvironment in PD-L1/PD-1-mediated tumor immune escape. Mol. Cancer 2019, 18, 10. [Google Scholar] [CrossRef] [PubMed]
- Farruggia, P.; Puccio, G.; Sala, A.; Todesco, A.; Buffardi, S.; Garaventa, A.; Bottigliero, G.; Bianchi, M.; Zecca, M.; Locatelli, F.; et al. The prognostic value of biological markers in paediatric Hodgkin lymphoma. Eur. J. Cancer Oxf. Engl. 2016, 52, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Campo, E.; Swerdlow, S.H.; Harris, N.L.; Pileri, S.; Stein, H.; Jaffe, E.S. The 2008 WHO classification of lymphoid neoplasms and beyond: Evolving concepts and practical applications. Blood 2011, 117, 5019–5032. [Google Scholar] [CrossRef] [PubMed]
- Old, W.M.; Meyer-Arendt, K.; Aveline-Wolf, L.; Pierce, K.G.; Mendoza, A.; Sevinsky, J.R.; Resing, K.A.; Ahn, N.G. Comparison of label-free methods for quantifying human proteins by shotgun proteomics. Mol. Cell Proteom. 2005, 4, 1487–1502. [Google Scholar] [CrossRef]
- Huang da, W.; Sherman, B.T.; Lempicki, R.A. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protoc. 2009, 4, 44–57. [Google Scholar] [CrossRef]
- Szklarczyk, D.; Morris, J.H.; Cook, H.; Kuhn, M.; Wyder, S.; Simonovic, M.; Santos, A.; Doncheva, N.T.; Roth, A.; Bork, P.; et al. The STRING database in 2017: Quality-controlled protein-protein association networks, made broadly accessible. Nucleic Acids Res. 2017, 45, D362–D368. [Google Scholar] [CrossRef]
- Srivastava, A.; Creek, D.J. Discovery and Validation of Clinical Biomarkers of Cancer: A Review Combining Metabolomics and Proteomics. Proteomics 2019, 19, e1700448. [Google Scholar] [CrossRef]

| Group | Disease Status | Patient no. | Sex a | Age at Diagnosis, Years | Stage b | Systemic Symptoms | LH2004 Therapeutic Group |
|---|---|---|---|---|---|---|---|
| Explorative | NR | 1 | M | 16 | 4 | Yes | 3 |
| 2 | F | 14 | 4 | No | 3 | ||
| 3 | F | 15 | 2 | No | 1 | ||
| R | 1 | M | 13 | 2 | Yes | 3 | |
| 2 | F | 15 | 2 | No | 3 | ||
| 3 | M | 12 | 2 | No | 3 | ||
| Validation | NR | 1 | M | 16 | 4 | Yes | 3 |
| 2 | F | 13 | 2 | No | 2 | ||
| 3 | F | 15 | 2 | No | 1 | ||
| R | 1 | M | 13 | 2 | Yes | 3 | |
| 2 | F | 15 | 2 | No | 3 | ||
| 3 | M | 12 | 2 | No | 3 |
| UniProtKB ID | Gene | Protein | Subcellular Localization | FC |
|---|---|---|---|---|
| More abundant in non-relapsing HL | ||||
| A0A0J9YXX1 | IGHV5-10-1 | Immunoglobulin heavy variable 5-10-1 | secreted, cell membrane | 0.80 |
| P01861 | IGHG4 | Immunoglobulin heavy constant γ 4 | secreted, cell membrane | 0.78 |
| P08603 | CFH | Complement factor H | secreted | 0.79 |
| P02765 | AHSG | α-2-HS-glycoprotein | secreted | 0.79 |
| P01871 | IGHM | Immunoglobulin heavy constant mu | secreted, cell membrane | 0.78 |
| P01619 | IGKV3-20 | Immunoglobulin kappa variable 3-20 | secreted, cell membrane | 0.78 |
| P02649 | APOE | Apolipoprotein E | secreted | 0.78 |
| P02654 | APOC1 | Apolipoprotein C-I | secreted | 0.77 |
| P01031 | C5 | Complement C5 | secreted | 0.77 |
| P27918 | CFP | Properdin | secreted | 0.76 |
| P02766 | TTR | Transthyretin° | secreted, lysosome | 0.75 |
| P00751 | CFB | Complement factor B | secreted | 0.73 |
| P04196 | HRG | Histidine-rich glycoprotein° | secreted | 0.71 |
| P02790 | HPX | Hemopexin | secreted | 0.68 |
| P49959 | MRE11 | Double-strand break repair protein MRE11 | nucleus | 0.68 |
| P19827 | ITIH1 | Inter-α-trypsin inhibitor heavy chain H1 | secreted | 0.68 |
| P08697 | SERPINF2 | α-2-antiplasmin° | secreted | 0.67 |
| P00747 | PLG | Plasminogen | secreted | 0.67 |
| Q03591 | CFHR1 | Complement factor H-related protein 1 | secreted | 0.66 |
| P0C0L5 | C4B | Complement C4-B | secreted | 0.64 |
| O75636 | FCN3 | Ficolin-3 | secreted | 0.63 |
| P15169 | CPN1 | Carboxypeptidase N catalytic chain | extracellular space | 0.61 |
| P04004 | VTN | Vitronectin* | extracellular space | 0.61 |
| P06396 | GSN | Gelsolin | cytoskeleton, secreted | 0.56 |
| Q12805 | EFEMP1 | EGF-containing fibulin-like extracellular matrix protein 1° | extracellular space, extracellular matrix (ECM) | 0.58 |
| P0C0L4 | C4A | Complement C4-A | secreted | 0.59 |
| O14791 | APOL1 | Apolipoprotein L1 | secreted | 0.58 |
| P00734 | F2 | Prothrombin | extracellular space | 0.58 |
| P07358 | C8B | Complement component C8 β chain | secreted | 0.57 |
| P10909 | CLU | Clusterin | nucleus, microsome, endoplasmic reticulum, cytosol, mitochondrion, nucleus | 0.56 |
| Q08380 | LGALS3BP | Galectin-3-binding protein | secreted, ECM | 0.55 |
| P23142 | FBLN1 | Fibulin-1° | ECM | 0.54 |
| Q06033 | ITIH3 | Inter-α-trypsin inhibitor heavy chain H3 | secreted | 0.52 |
| P00736 | C1R | Complement C1r subcomponent | secreted | 0.51 |
| Q15485 | FCN2 | Ficolin-2 | secreted, ECM | 0.50 |
| P05546 | SERPIND1 | Heparin cofactor 2 | endoplasmic reticulum, extracellular exosome | 0.50 |
| P02746 | C1QB | Complement C1q subcomponent subunit B° | secreted | 0.50 |
| P02747 | C1QC | Complement C1q subcomponent subunit C | secreted | 0.48 |
| P01591 | JCHAIN | Immunoglobulin J chain | secreted | 0.47 |
| P02760 | AMBP | Protein AMBP | secreted | 0.46 |
| Q9BXR6 | CFHR5 | Complement factor H-related protein 5 | secreted | 0.45 |
| P07225 | PROS1 | Vitamin K-dependent protein S | secreted | 0.44 |
| P02652 | APOA2 | Apolipoprotein A-II | secreted | 0.42 |
| P01008 | SERPINC1 | Antithrombin III*° | extracellular space | 0.39 |
| P00748 | F12 | Coagulation factor XII | secreted | 0.36 |
| P20742 | PZP | Pregnancy zone protein° | secreted | 0.36 |
| P02745 | C1QA | Complement C1q subcomponent subunit A | secreted | 0.31 |
| P01019 | AGT | Angiotensinogen° | secreted | 0.28 |
| A0A0C4DH68 | IGKV2-24 | Immunoglobulin kappa variable 2-24 | secreted, cell membrane | 0.26 |
| P04180 | LCAT | Phosphatidylcholine-sterol acyltransferase | secreted | 0.26 |
| P24593 | IGFBP5 | Insulin-like growth factor-binding protein 5 | secreted | 0.26 |
| P22792 | CPN2 | Carboxypeptidase N subunit 2 | secreted | 0.26 |
| P68871 | HBB | Hemoglobin subunit β | cytosol, extracellular region, secreted | 0.26 |
| P0DP03 | IGHV3-30-5 | Immunoglobulin heavy variable 3-30-5 | secreted, cell membrane | 0.26 |
| P08709 | F7 | Coagulation factor VII | secreted | 0.23 |
| P01009 | SERPINA1 | α-1-antitrypsin*° | secreted, endoplasmic reticulum | 0.23 |
| P19823 | ITIH2 | Inter-α-trypsin inhibitor heavy chain H2 | secreted | 0.10 |
| Q92496 | CFHR4 | Complement factor H-related protein 4 | secreted | 0.09 |
| P48740 | MASP1 | Mannan-binding lectin serine protease 1 | secreted | 0.08 |
| P27169 | PON1 | Serum paraoxonase/arylesterase 1° | extracellular space | 0.08 |
| More abundant in relapsing HL | ||||
| P02751 | FN1 | Fibronectin° | ECM | 19.61 |
| P06702 | S100A9 | Protein S100-A9 | cytoskeleton, extracellular region, cytoskeleton, secreted, cell membrane | 15.33 |
| P35908 | KRT2 | Keratin, type II cytoskeletal 2 epidermal | cytoskeleton, cytosol, endoplasmic reticulum, nucleus, cell membrane | 9.45 |
| P0DJI8 | SAA1 | Serum amyloid A-1 protein | secreted | 5.37 |
| Q15848 | ADIPO | Adiponectin | secreted | 4.73 |
| P36955 | SERPINF1 | Pigment epithelium-derived factor | secreted | 3.51 |
| Q9H5I5 | PIEZO2 | Piezo-type mechanosensitive ion channel component 2 | membrane | 3.10 |
| Q9Y490 | TLN1 | Talin-1° | cytoskeleton, cell membrane, cell surface | 3.10 |
| P0DJI9 | SAA2 | Serum amyloid A-2 protein | secreted | 3.06 |
| P09871 | C1S | Complement C1s subcomponent° | extracellular space | 3.05 |
| P04264 | KRT1 | Keratin, type II cytoskeletal 1 | cell membrane | 3.02 |
| P02753 | RBP4 | Retinol-binding protein 4 | secreted | 2.77 |
| Q86YZ3 | HRNR | Hornerin | cytoplasmic granules | 2.62 |
| P02671 | FGA | Fibrinogen α chain* | secreted | 2.27 |
| P02741 | CRP | C-reactive protein | secreted | 2.19 |
| P02656 | APOC3 | Apolipoprotein C-III | secreted | 2.12 |
| P02675 | FGB | Fibrinogen β chain*° | secreted | 2.06 |
| P01700 | IGLV1-47 | Immunoglobulin lambda variable 1-47 | secreted, membrane | 2.03 |
| P05160 | F13B | Coagulation factor XIII B chain | secreted | 2.03 |
| P35527 | KRT9 | Keratin, type I cytoskeletal 9 | cytosol, extracellular exosome, nucleus, membrane | 2.02 |
| P00450 | CP | Ceruloplasmin* | secreted | 2.01 |
| P05156 | CFI | Complement factor I | secreted | 1.95 |
| P10643 | C7 | Complement component C7 | secreted | 1.95 |
| P02679 | FGG | Fibrinogen γ chain*° | secreted | 1.94 |
| P07360 | C8G | Complement component C8 γ chain | secreted | 1.86 |
| P02748 | C9 | Complement component C9 | secreted | 1.80 |
| P07996 | THBS1 | Thrombospondin-1° | endoplasmic reticulum secreted, ECM, cell surface | 1.73 |
| P63261 | ACTG1 | Actin, cytoplasmic 2 | cytoskeleton | 1.57 |
| IGLC2_HUMAN | IGLC2 | Immunoglobulin lambda constant 2 | secreted, cell membrane | 1.37 |
| P18428 | LBP | Lipopolysaccharide-binding protein | secreted, cytoplasmic granule membrane | 1.36 |
| P04217 | A1BG | α-1B-glycoprotein° | secreted | 1.35 |
| LV39_HUMAN | IGLV3-9 | Immunoglobulin lambda variable 3-9 | secreted, cell membrane | 1.34 |
| UniProtKB ID | Protein Name (Gene Symbol) (a) | Biological Processes (DAVID) (p < 0.01) (b) | Biological Classes | Regulatory Subclasses |
|---|---|---|---|---|
| More abundant in non-relapsing HL (n = 11) | ||||
| P01019 | Angiotensinogen (AGT) | negative regulation of endopeptidase activity, regulation of blood vessel size by renin-angiotensin | transport and homeostasis, regulation, vascularization, response, cell and ECM organization, lipid metabolism, protein metabolism | immune system, transport and homeostasis, vascularization, response, cell and ECM organization, cell death, lipid metabolism, protein metabolism, signaling |
| P02746 | Complement C1q subcomponent subunit B (C1QB) | complement activation, proteolysis, complement activation (classical pathway), innate immune response | immune system, regulation, transport and homeostasis, response, protein metabolism | immune system, response, protein metabolism |
| Q12805 | EGF-containing fibulin-like extracellular matrix protein 1 (EFEMP1) | NA | regulation, transport and homeostasis, response, protein metabolism | response, signaling |
| P23142 | Fibulin-1 (FBLN1) | negative regulation of cell adhesion | regulation, transport and homeostasis, coagulation, response, cell and ECM organization, protein metabolism | immune system, transport and homeostasis, response, cell and ECM organization, protein metabolism, signaling |
| P04196 | Histidine-rich glycoprotein (HRG) | negative regulation of endopeptidase activity, platelet degranulation, negative regulation of fibrinolysis, negative regulation of cell adhesion, fibrinolysis | immune system, regulation, transport and homeostasis, coagulation, fibrinolysis, response, cell and ECM organization | immune system, transport and homeostasis, coagulation, fibrinolysis, response, cell and ECM organization, cell death, protein metabolism, signaling |
| P27169 | Serum paraoxonase/ arylesterase 1 (PON1) | negative regulation of plasma lipoprotein particle oxidation, cholesterol metabolic process, phosphatidylcholine metabolic process, | regulation, response, lipid metabolism | transport and homeostasis |
| P20742 | Pregnancy zone protein (PZP) | negative regulation of endopeptidase activity | regulation | protein metabolism |
| P01009 | α-1-antitrypsin* (SERPINA1) | acute-phase response, ER to Golgi vesicle-mediated transport, platelet degranulation, blood coagulation, negative regulation of endopeptidase activity | immune system, regulation, transport and homeostasis, coagulation, response, protein metabolism | transport and homeostasis, protein metabolism |
| P01008 | Antithrombin III* (SERPINC1) | negative regulation of endopeptidase activity, blood coagulation | immune system, regulation, transport and homeostasis, coagulation, response, protein metabolism | coagulation, response, protein metabolism |
| P08697 | α-2-antiplasmin (SERPINF2) | acute-phase response, negative regulation of endopeptidase activity, platelet degranulation, fibrinolysis, regulation of blood vessel size by renin-angiotensin | immune system, regulation, transport and homeostasis, coagulation, fibrinolysis, response, cell and ECM organization, protein metabolism | immune system, transport and homeostasis, coagulation, fibrinolysis, vascularization, response, cell and ECM organization, protein metabolism, signaling |
| P02766 | Transthyretin (TTR) | retinoid metabolic process, cellular protein metabolic process, | immune system, regulation, transport and homeostasis, response, cell and ECM organization, protein metabolism | transport and homeostasis, response, signaling |
| More abundant in relapsing HL (n = 7) | ||||
| P04217 | α-1B-glycoprotein (A1BG) | platelet degranulation | immune system, transport and homeostasis, coagulation, response | none |
| P09871 | Complement C1s subcomponent (C1S) | proteolysis, complement activation, complement activation (classical pathway), innate immune response | immune system, regulation, response, protein metabolism | immune system, response, protein metabolism |
| P02675 | Fibrinogen β chain* (FGB) | platelet degranulation, innate immune response, response to calcium ion, fibrinolysis, blood coagulation, fibrin clot formation, platelet aggregation, positive regulation of peptide hormone secretion, plasminogen activation, positive regulation of heterotypic cell-cell adhesion, protein polymerization, cellular protein complex assembly, ECM organization, positive regulation of exocytosis, negative regulation of endothelial cell apoptotic process, platelet activation, positive regulation of vasoconstriction, positive regulation of substrate adhesion-dependent cell spreading, negative regulation of extrinsic apoptotic signaling pathway via death domain receptors, induction of bacterial agglutination | immune system, regulation, transport and homeostasis, coagulation, fibrinolysis, response, cell and ECM organization, protein metabolism | immune system, transport and homeostasis, coagulation, vascularization, response, cell and ECM organization, cell death, protein metabolism, signaling |
| P02679 | Fibrinogen γ chain* (FGG) | platelet degranulation, innate immune response, response to calcium ion, fibrinolysis, blood coagulation, fibrin clot formation, platelet aggregation, positive regulation of peptide hormone secretion, plasminogen activation, positive regulation of heterotypic cell-cell adhesion, protein polymerization, cellular protein complex assembly, ECM organization, positive regulation of exocytosis, negative regulation of endothelial cell apoptotic process, platelet activation, positive regulation of vasoconstriction, positive regulation of substrate adhesion-dependent cell spreading, negative regulation of extrinsic apoptotic signaling pathway via death domain receptors, induction of bacterial agglutination | immune system, regulation, transport and homeostasis, coagulation, fibrinolysis, response, cell and ECM organization, protein metabolism | immune system, transport and homeostasis, coagulation, vascularization, response, cell and ECM organization, cell death, signaling, protein metabolism |
| P02751 | Fibronectin (FN1) | platelet degranulation, acute-phase response, ECM organization | immune system, regulation, transport and homeostasis, coagulation, response, cell and ECM organization | immune system, transport and homeostasis, response, cell and ECM organization, cell death, lipid metabolism, signaling |
| P07996 | Thrombospondin-1 (THBS1) | ECM organization, response to calcium ion, immune response, platelet degranulation, response to calcium ion, inflammatory response | immune system, regulation, transport and homeostasis, coagulation, response, cell and ECM organization, protein metabolism | immune system, transport and homeostasis, coagulation, vascularization, response, cell and ECM organization, cell death, protein metabolism, signaling |
| Q9Y490 | Talin-1 (TLN1) | platelet degranulation, platelet aggregation | regulation, transport and homeostasis, coagulation, response, cell and ECM organization, protein metabolism | cell and ECM organization |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Repetto, O.; De Re, V.; Mussolin, L.; Tedeschi, M.; Elia, C.; Bianchi, M.; Buffardi, S.; Sala, A.; Burnelli, R.; Mascarin, M. Proteomic Profiles and Biological Processes of Relapsed vs. Non-Relapsed Pediatric Hodgkin Lymphoma. Int. J. Mol. Sci. 2020, 21, 2185. https://doi.org/10.3390/ijms21062185
Repetto O, De Re V, Mussolin L, Tedeschi M, Elia C, Bianchi M, Buffardi S, Sala A, Burnelli R, Mascarin M. Proteomic Profiles and Biological Processes of Relapsed vs. Non-Relapsed Pediatric Hodgkin Lymphoma. International Journal of Molecular Sciences. 2020; 21(6):2185. https://doi.org/10.3390/ijms21062185
Chicago/Turabian StyleRepetto, Ombretta, Valli De Re, Lara Mussolin, Massimo Tedeschi, Caterina Elia, Maurizio Bianchi, Salvatore Buffardi, Alessandra Sala, Roberta Burnelli, and Maurizio Mascarin. 2020. "Proteomic Profiles and Biological Processes of Relapsed vs. Non-Relapsed Pediatric Hodgkin Lymphoma" International Journal of Molecular Sciences 21, no. 6: 2185. https://doi.org/10.3390/ijms21062185
APA StyleRepetto, O., De Re, V., Mussolin, L., Tedeschi, M., Elia, C., Bianchi, M., Buffardi, S., Sala, A., Burnelli, R., & Mascarin, M. (2020). Proteomic Profiles and Biological Processes of Relapsed vs. Non-Relapsed Pediatric Hodgkin Lymphoma. International Journal of Molecular Sciences, 21(6), 2185. https://doi.org/10.3390/ijms21062185







