Integrated Transcriptional and Proteomic Profiling Reveals Potential Amino Acid Transporters Targeted by Nitrogen Limitation Adaptation
Abstract
1. Introduction
2. Results
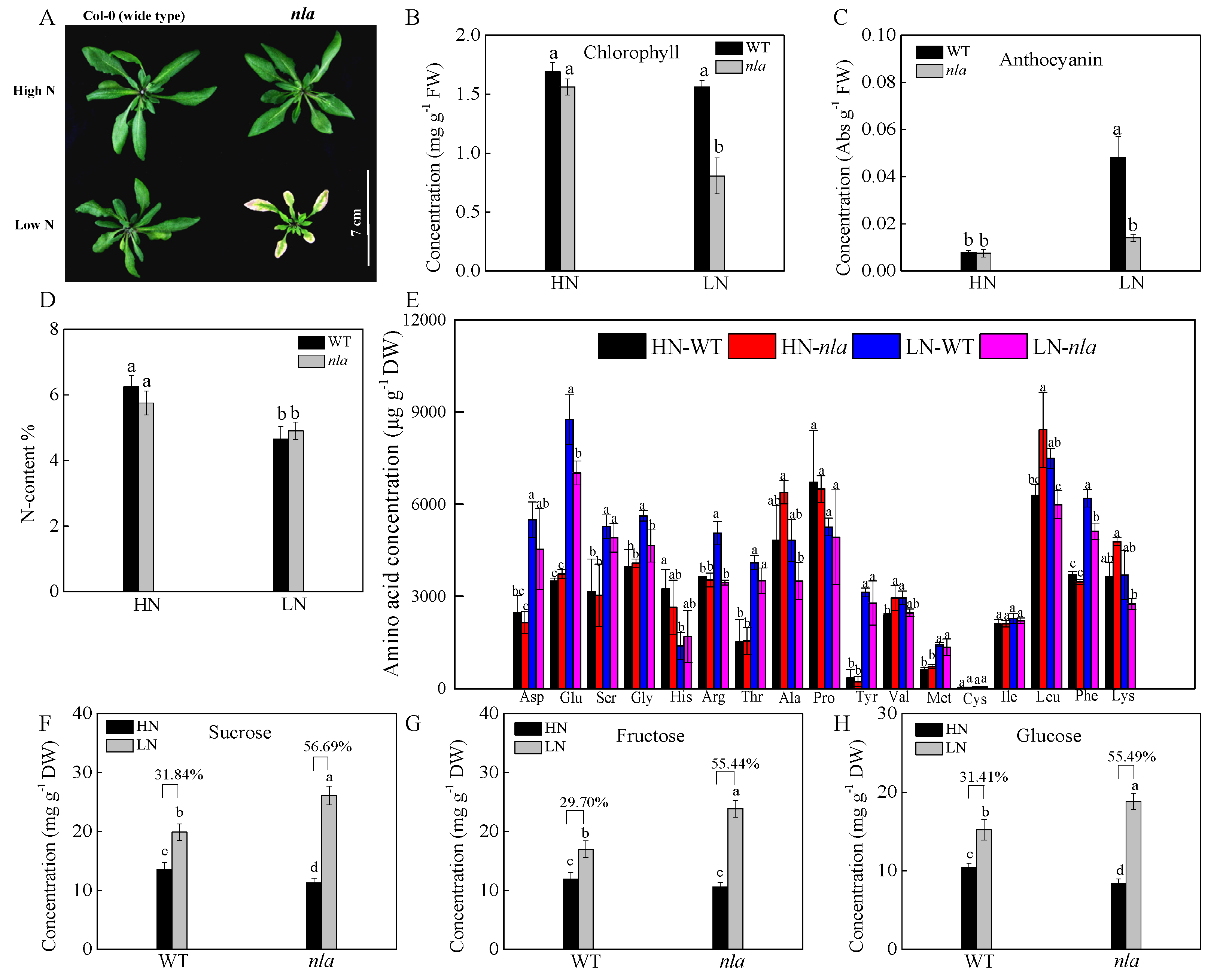
2.1. Differential Physiological Responses of the WT and nla Mutant Plants to N Limitation
2.2. Transcriptional Profiling Reveals Different Responses to N Limitation between the WT and nla Mutant Plants
2.3. iTRAQ Data Analysis and Protein Identification in the nla Mutant and WT under N-Sufficient and N-Limited Conditions
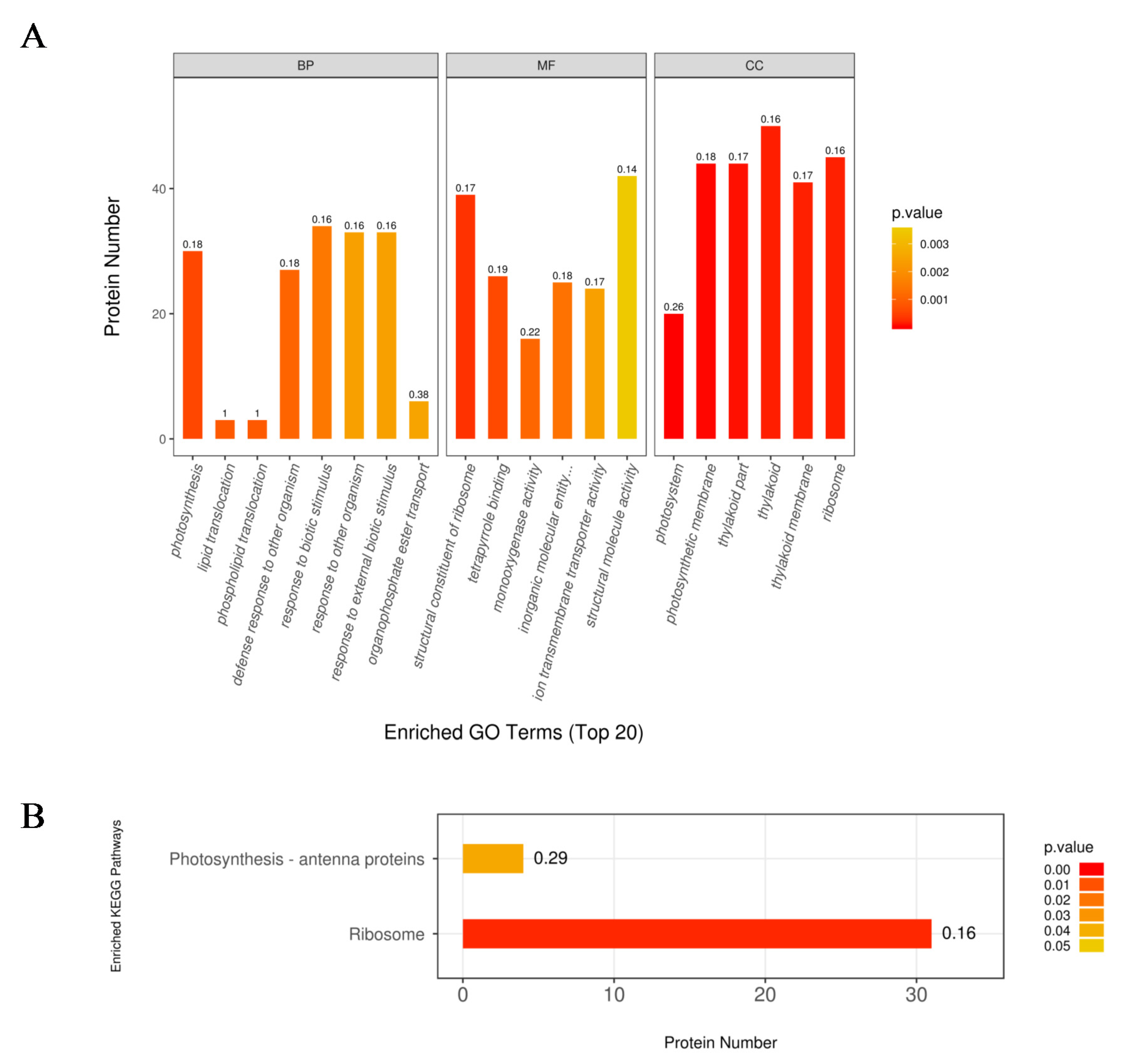
2.4. Functional Analysis of the DEPs in Response to N Limitation
2.5. N Limitation Represses Proteins Responsible for Photosynthesis and Protein Synthesis and Induced Proteins Related to Proteolysis and N Transport
2.6. LHT1, Responsible for Amino Acid Transport, Was the Sole Gene Identified Both in Transcriptional and Proteomic Profiling
2.7. Transcriptional Expression Patterns of Genes Regulated by NLA under N Limitation
3. Discussion
3.1. Differential Physiological and Molecular Responses of WT and nla Mutant Plants under N Limitation
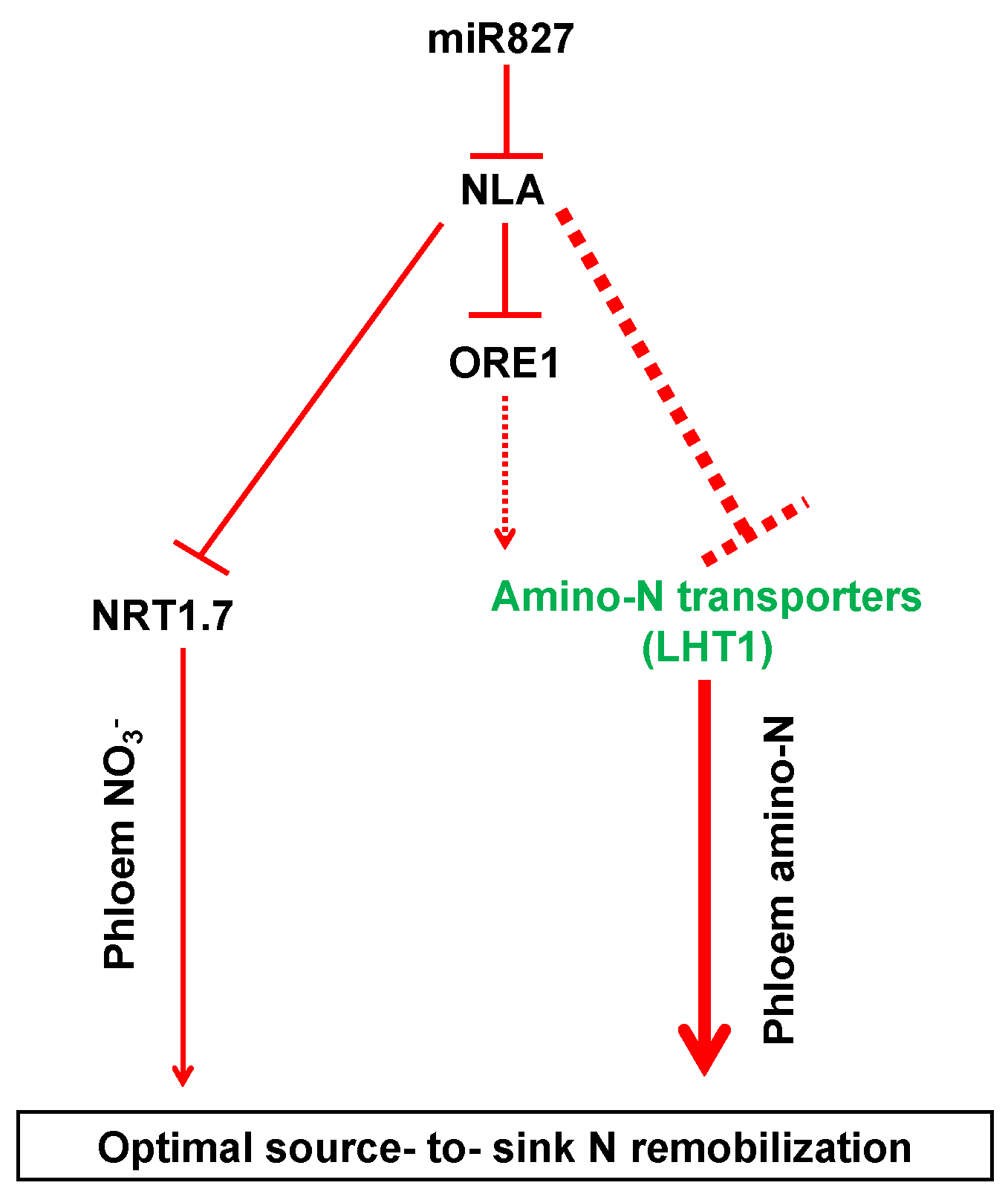
3.2. Amino Acid Transporters May Be Involved in Efficient N Remobilization Mediated by NLA
4. Materials and Methods
4.1. Plant Materials and Growth Conditions
4.2. Determination of Chlorophyll, Anthocyanin, and N Content
4.3. Free Amino Acid Determination
4.4. Determination of Sucrose, Fructose, and Glucose Content
4.5. Transcriptional Responses of nla to N Limitation
4.6. Protein Extraction, Digestion, and Isobaric Tags for Relative and Absolute Quantitation (iTRAQ) Labeling
4.7. Peptide Fractionation with Strong Cation Exchange (SCX) Chromatography
4.8. Liquid Chromatography-Tandem Mass Spectrometry (LC-MS/MS) Data Analysis, Protein Identification, and Quantification
4.9. Gene Ontology (GO) Annotation
4.10. Kyoto Encyclopedia of Genes and Genomes (KEGG) Pathway Annotation
4.11. Functional Enrichment Analysis
4.12. Quantitative Real-Time PCR (q-PCR) Assays
4.13. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| CLC | Chloride channel |
| DEP | Differentially expressed protein |
| GO | Gene Ontology |
| GOGAT | Glutamine synthetase/glutamate synthase |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| KO | KEGG orthology |
| NLA | N limitation adaption |
| NO3− | Nitrate |
| NT | Nitrate reductase |
| NRT | NO3− transporter |
| NUE | N use efficiency |
| Pi | Inorganic phosphate |
| q-PCR | Quantitative real-time PCR |
| WT | Wild-type |
References
- Xu, G.H. The basic and applied researches for improving crop nutrient use efficiency. Plant Physiol. J. 2016, 52, 1761–1763. [Google Scholar]
- Jin, J.Y. Changes in the efficiency of fertilizer use in China. J. Sci. Food Agr. 2012, 92, 1006–1009. [Google Scholar] [CrossRef]
- Masclaux-Daubresse, C.; Daniel-Vedele, F.; Dechorgnat, J.; Chardon, F.; Gaufichon, L.; Suzuki, A. Nitrogen uptake, assimilation and remobilization in plants: Challenges for sustainable and productive agriculture. Ann. Bot. 2010, 105, 1141–1157. [Google Scholar] [CrossRef]
- Schroeder, J.I.; Delhaize, E.; Frommer, W.B.; Guerinot, M.L.; Harrison, M.J.; Herrera-Estrella, L.; Horie, T.; Kochian, L.V.; Munns, R.; Nishizawa, N.K.; et al. Using membrane transporters to improve crops for sustainable food production. Nature 2013, 497, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Ding, G.; Li, L.; Cai, H.; Ye, X.; Zou, J.; Xu, F. Identification and characterization of improved nitrogen efficiency in interspecific hybridized new-type Brassica napus. Ann. Bot. 2014, 114, 549–559. [Google Scholar] [CrossRef] [PubMed]
- Kant, S. Understanding nitrate uptake, signaling and remobilization for improving plant nitrogen use efficiency. Semin. Cell Dev. Biol. 2018, 74, 89–96. [Google Scholar] [CrossRef]
- Tegeder, M.; Masclaux-Daubresse, C. Source and sink mechanisms of nitrogen transport and use. New Phytol. 2018, 217, 35–53. [Google Scholar] [CrossRef]
- Han, Y.L.; Song, H.X.; Liao, Q.; Yu, Y.; Jian, S.F.; Lepo, J.E.; Liu, Q.; Rong, X.M.; Tian, C.; Zeng, J.; et al. Nitrogen use efficiency is mediated by vacuolar nitrate sequestration capacity in roots of Brassica napus. Plant Physiol. 2016, 170, 1377. [Google Scholar] [CrossRef]
- De Angeli, A.; Monachello, D.; Ephritikhine, G.; Frachisse, J.M.; Thomine, S.; Gambale, F.; Barbier-Brygoo, H. The nitrate/proton antiporter AtCLCa mediates nitrate accumulation in plant vacuoles. Nature 2006, 442, 939–942. [Google Scholar] [CrossRef]
- Liao, Q.; Zhou, T.; Yao, J.Y.; Han, Q.F.; Song, H.X.; Guan, C.Y.; Hua, Y.P.; Zhang, Z.H. Genome-scale characterization of the vacuole nitrate transporter Chloride Channel (CLC) genes and their transcriptional responses to diverse nutrient stresses in allotetraploid rapeseed. PLoS ONE 2018, 13, 1–26. [Google Scholar] [CrossRef]
- Lin, S.H.; Kuo, H.-F.; Canivenc, G.; Lin, C.S.; Lepetit, M.; Hsu, P.-K.; Tillard, P.; Lin, H.-L.; Wang, Y.-Y.; Tsai, C.-B.; et al. Mutation of the Arabidopsis NRT1.5 Nitrate Transporter Causes Defective Root-to-Shoot Nitrate Transport. Plant Cell Online 2008, 20, 2514–2528. [Google Scholar] [CrossRef] [PubMed]
- Li, J.Y.; Fu, Y.L.; Pike, S.M.; Bao, J.; Tian, W.; Zhang, Y.; Chen, C.Z.; Zhang, Y.; Li, H.-M.; Huang, J.; et al. The Arabidopsis Nitrate Transporter NRT1.8 Functions in Nitrate Removal from the Xylem Sap and Mediates Cadmium Tolerance. Plant Cell Online 2010, 22, 1633–1646. [Google Scholar] [CrossRef] [PubMed]
- Fan, K.; Zhang, Q.; Liu, M.; Ma, L.; Shi, Y.; Ruan, J. Metabolomic and transcriptional analyses reveal the mechanism of C, N allocation from source leaf to flower in tea plant (Camellia sinensis. L). J. Plant Physiol. 2019, 232, 200–208. [Google Scholar] [CrossRef] [PubMed]
- Shin, L.J.; Lo, J.C.; Chen, G.H.; Callis, J.; Fu, H.; Yeh, K.C. IRT1 DEGRADATION FACTOR1, a RING E3 Ubiquitin Ligase, Regulates the Degradation of IRON-REGULATED TRANSPORTER1 in Arabidopsis. Plant Cell 2013, 25, 3039–3051. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Celma, J.; Chou, H.; Kobayashi, T.; Long, T.A.; Balk, J. Hemerythrin E3 Ubiquitin Ligases as Negative Regulators of Iron Homeostasis in Plants. Front. Plant Sci. 2019, 10, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Peng, M.; Hannam, C.; Gu, H.; Bi, Y.M.; Rothstein, S.J. A mutation in NLA, which encodes a RING-type ubiquitin ligase, disrupts the adaptability of Arabidopsis to nitrogen limitation. Plant J. 2007, 50, 320–337. [Google Scholar] [CrossRef] [PubMed]
- Yaeno, T.; Iba, K. BAH1/NLA, a RING-Type Ubiquitin E3 Ligase, Regulates the Accumulation of Salicylic Acid and Immune Responses to Pseudomonas syringae DC3000. Plant Physiol. 2008, 148, 1032–1041. [Google Scholar] [CrossRef]
- Kant, S.; Bi, Y.M.; Rothstein, S.J. Understanding plant response to nitrogen limitation for the improvement of crop nitrogen use efficiency. J. Exp. Bot. 2011, 62, 1499–1509. [Google Scholar] [CrossRef]
- Liu, W.; Sun, Q.; Wang, K.; Du, Q.; Li, W.X. Nitrogen Limitation Adaptation (NLA) is involved in source-to-sink remobilization of nitrate by mediating the degradation of NRT1.7 in Arabidopsis. New Phytol. 2017, 214, 734–744. [Google Scholar] [CrossRef]
- Peng, M.; Bi, Y.M.; Zhu, T.; Rothstein, S.J. Genome-wide analysis of Arabidopsis responsive transcriptome to nitrogen limitation and its regulation by the ubiquitin ligase gene NLA. Plant Mol. Biol. 2007, 65, 775–797. [Google Scholar] [CrossRef]
- Havé, M.; Marmagne, A.; Chardon, F.; Masclaux-Daubresse, C. Nitrogen remobilization during leaf senescence: Lessons from Arabidopsis to crops. J. Exp. Bot. 2017, 68, 2513–2529. [Google Scholar] [PubMed]
- Evans, J.R.; Clarke, V.C. The nitrogen cost of photosynthesis. J. Exp. Bot. 2018, 70, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Park, B.S.; Yao, T.; Seo, J.S.; Wong, E.C.C.; Mitsuda, N.; Huang, C.H.; Chua, N.H. Arabidopsis NITROGEN LIMITATION ADAPTATION regulates ORE1 homeostasis during senescence induced by nitrogen deficiency. Nat. Plants 2018, 4, 898–903. [Google Scholar] [CrossRef] [PubMed]
- Kant, S.; Peng, M.; Rothstein, S.J. Genetic regulation by NLA and microRNA827 for maintaining nitrate-dependent phosphate homeostasis in Arabidopsis. PLoS Genet. 2011, 7. [Google Scholar] [CrossRef]
- Diaz, C. Characterization of Markers to Determine the Extent and Variability of Leaf Senescence in Arabidopsis. A Metabolic Profiling Approach. Plant Physiol. 2005, 138, 898–908. [Google Scholar] [CrossRef]
- Wingler, A.; Purdy, S.; MacLean, J.A.; Pourtau, N. The role of sugars in integrating environmental signals during the regulation of leaf senescence. J. Exp. Bot. 2006, 57, 391–399. [Google Scholar] [CrossRef]
- Maier, A.; Schrader, A.; Kokkelink, L.; Falke, C.; Welter, B.; Iniesto, E.; Rubio, V.; Uhrig, J.F.; Hülskamp, M.; Hoecker, U. Light and the E3 ubiquitin ligase COP1/SPA control the protein stability of the MYB transcription factors PAP1 and PAP2 involved in anthocyanin accumulation in Arabidopsis. Plant J. 2013, 74, 638–651. [Google Scholar] [CrossRef]
- Girondé, A.; Etienne, P.; Trouverie, J.; Bouchereau, A.; Le Cahérec, F.; Leport, L.; Orsel, M.; Niogret, M.F.; Nesi, N.; Carole, D.; et al. The contrasting N management of two oilseed rape genotypes reveals the mechanisms of proteolysis associated with leaf N remobilization and the respective contributions of leaves and stems to N storage and remobilization during seed filling. BMC Plant Biol. 2015, 15. [Google Scholar] [CrossRef]
- Wang, X.-F.; An, J.-P.; Liu, X.; Su, L.; You, C.-X.; Hao, Y.-J. The Nitrate-Responsive Protein MdBT2 Regulates Anthocyanin Biosynthesis by Interacting with the MdMYB1 Transcription Factor. Plant Physiol. 2018, 178, 890–906. [Google Scholar] [CrossRef]
- Shimizu, T.; Inoue, T.; Shiraishi, H. A senescence-associated S-like RNase in the multicellular green alga Volvox carteri. Gene 2001, 274, 227–235. [Google Scholar] [CrossRef]
- Seo, P.J.; Park, J.M.; Kang, S.K.; Kim, S.G.; Park, C.M. An Arabidopsis senescence-associated protein SAG29 regulates cell viability under high salinity. Planta 2011, 233, 189–200. [Google Scholar] [CrossRef] [PubMed]
- Deng, S.; Mai, Y.; Niu, J. Fruit characteristics, soluble sugar compositions and transcriptome analysis during the development of Citrus maxima “seedless” and identification of SUS and INV genes involved in sucrose degradation. Gene 2019, 689, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Kiba, T.; Krapp, A. Plant nitrogen acquisition under low availability: Regulation of uptake and root architecture. Plant Cell Physiol. 2016, 57, 707–714. [Google Scholar] [CrossRef] [PubMed]
- Adam, Z.; Adamska, I.; Nakabayashi, K.; Ostersetzer, O.; Haussuhl, K.; Manuell, A.; Zheng, B.; Vallon, O.; Rodermel, S.R.; Shinozaki, K.; et al. Chloroplast and Mitochondrial Proteases in Arabidopsis. A Proposed Nomenclature. Plant Physiol. 2001, 125, 1912–1918. [Google Scholar] [CrossRef] [PubMed]
- Bongue-Bartelsman, M.; Phillips, D.A. Nitrogen stress regulates gene expression of enzymes in the flflavonoid biosynthetic pathway of tomato. Plant Physiol. Bioch. 1995, 33, 539–546. [Google Scholar]
- Chalker-Scott, L. Environmental significance of anthocyanins in plant stress responses. Photochem. Photobiol. 1999, 70, 1–9. [Google Scholar] [CrossRef]
- Jun, J.H.; Liu, C.; Xiao, X.; Dixon, R.A. The Transcriptional Repressor MYB2 Regulates Both Spatial and Temporal Patterns of Proanthocyandin and Anthocyanin Pigmentation in Medicago truncatula. Plant Cell 2015, 27, 2860–2879. [Google Scholar]
- Avice, J.C.; Etienne, P. Leaf senescence and nitrogen remobilization efficiency in oilseed rape (Brassica napus L.). J. Exp. Bot. 2014, 65, 3813–3824. [Google Scholar] [CrossRef]
- Schulte, auf’m, Erley, G.; Begum, N.; Worku, M.; Bänzige, M.; Walter, J. Leaf senescence induced by nitrogen defciency as indicator of genotypic diferences in nitrogen efciency in tropical maize. J. Plant Nutr. Soil Sci. 2007, 170, 106–114. [Google Scholar] [CrossRef]
- Agüera, E.; Cabello, P.; de la Haba, P. Induction of leaf senescence by low nitrogen nutrition in sunflower (Helianthus annuus) plants. Physiol. Plant. 2010, 138, 256–267. [Google Scholar] [CrossRef]
- Meng, S.; Peng, J.S.; He, Y.N.; Zhang, G.B.; Yi, H.Y.; Fu, Y.L.; Gong, J.M. Arabidopsis NRT1.5 Mediates the Suppression of Nitrate Starvation-Induced Leaf Senescence by Modulating Foliar Potassium Level. Mol. Plant 2016, 9, 461–470. [Google Scholar] [CrossRef] [PubMed]
- Rentsch, D.; Schmidt, S.; Tegeder, M. Transporters for uptake and allocation of organic nitrogen compounds in plants. FEBS Lett. 2007, 581, 2281–2289. [Google Scholar] [CrossRef] [PubMed]
- Perchlik, M.; Foster, J.; Tegeder, M. Different and overlapping functions of Arabidopsis LHT6 and AAP1 transporters in root amino acid uptake. J. Exp. Bot. 2014, 65, 5193–5204. [Google Scholar] [CrossRef] [PubMed]
- Jacquot, A.; Li, Z.; Gojon, A.; Schulze, W.; Lejay, L. Post-translational regulation of nitrogen transporters in plants and microorganisms. J. Exp. Bot. 2017, 68, 2567–2580. [Google Scholar] [CrossRef] [PubMed]
- Megha, S.; Basu, U.; Kav, N.N.V. Regulation of low temperature stress in plants by microRNAs. Plant Cell Environ. 2018, 41, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Nimeth, B.A.; Riegler, S.; Kalyna, M. Alternative Splicing and DNA Damage Response in Plants. Front. Plant Sci. 2020, 11, 91. [Google Scholar] [CrossRef] [PubMed]
- Casas-Vila, N.; Bluhm, A.; Sayols, S.; Dinges, N.; Dejung, M.; Altenhein, T.; Kappei, D.; Altenhein, B.; Roignant, J.Y.; Butter, F. The developmental proteome of Drosophila melanogaster. Genome Res. 2017, 27, 1273–1285. [Google Scholar] [CrossRef]
- Hirner, A.; Ladwig, F.; Stransky, H.; Okumoto, S.; Keinath, M.; Harms, A. Arabidopsis LHT1 Is a High-Affinity Transporter for Cellular Amino Acid Uptake in Both Root Epidermis and Leaf Mesophyll. Plant Cell. 2016, 18, 1931–1946. [Google Scholar] [CrossRef]
- Chen, L.S.; Bush, D.R. LHT1, a lysine- and histidine-specific amino acid transporter in Arabidopsis. Plant Physiol. 1997, 115, 1127–1134. [Google Scholar] [CrossRef]
- Svennerstam, H.; Ganeteg, U.; Bellini, C.; Näsholm, T. Comprehensive screening of Arabidopsis mutants suggests the lysine histidine transporter 1 to be involved in plant uptake of amino acids. Plant Physiol. 2007, 143, 1853–1860. [Google Scholar] [CrossRef]
- Ganeteg, U.; Ahmad, I.; Jämtgård, S.; Aguetoni-Cambui, C.; Inselsbacher, E.; Svennerstam, H. Amino acid transporter mutants of Arabidopsis provides evidence that a non-mycorrhizal plant acquires organic nitrogen from agricultural soil. Plant Cell Environ. 2017, 40, 413–423. [Google Scholar] [CrossRef] [PubMed]
- Mancinelli, A.A.L.; Yang, C.H.; Lindquist, P.; Anderson, O.R. Photocontrol of Anthocyanin Synthesis: III. the Action of Streptomycin on the Synthesis of Chlorophyll and Anthocyanin. Plant Physiol. 1975, 55, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.M.; Xu, W.F.; Li, S.M.; Zhao, X.Q.; Dong, G.Q. Responses of two rice cultivars differing in seedling-stage nitrogen use efficiency to growth under low-nitrogen conditions. Plant Soil 2010, 326, 291–302. [Google Scholar] [CrossRef]
- Lu, K.; Wu, B.; Wang, J.; Zhu, W.; Nie, H.; Qian, J.; Huang, W.; Fang, Z. Blocking amino acid transporter OsAAP3 improves grain yield by promoting outgrowth buds and increasing tiller number in rice. Plant Biotechnol. J. 2018, 16, 1710–1722. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhang, S.; Qin, G.; Wang, L.; Wu, T.; Qi, K.; Zhang, S. Molecular cloning and expression analysis of a gene for sucrose transporter from pear (Pyrus bretschneideri Rehd.) fruit. Plant Physiol. Biochem. 2013, 73, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Zhu, T.; Budworth, P.; Han, B.; Brown, D.; Chang, H.S.; Zou, G.Z. Toward elucidating the global gene expression patterns of developing Arabidopsis: Parallel analysis of 8 300 genes by a high-density oligonucleotide probe array. Plant Physiol. Biochem. 2001, 39, 221–242. [Google Scholar] [CrossRef]
- Hu, X.; Li, N.; Wu, L.; Li, C.; Li, C.; Zhang, L.; Liu, T.; Wang, W. Quantitative iTRAQ-based proteomic analysis of phosphoproteins and ABA-regulated phosphoproteins in maize leaves under osmotic stress. Sci. Rep. 2015, 5, 1–26. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, Y.; Yang, H.; Wang, W.; Wu, J.; Hu, X. Quantitative Proteomic Analyses Identify ABA-Related Proteins and Signal Pathways in Maize Leaves under Drought Conditions. Front. Plant Sci. 2016, 7. [Google Scholar] [CrossRef]
- Wiśniewski, J.R.; Zougman, A.; Nagaraj, N.; Mann, M. Universal sample preparation method for proteome analysis. Nat. Methods 2009, 6, 359–362. [Google Scholar] [CrossRef]
- Ross, P.L.; Huang, Y.N.; Marchese, J.N.; Williamson, B.; Parker, K.; Hattan, S.; Khainovski, N.; Pillai, S.; Dey, S.; Daniels, S.; et al. Multiplexed protein quantitation in Saccharomyces cerevisiae using amine-reactive isobaric tagging reagents. Mol. Cell. Proteom. 2004, 3, 1154–1169. [Google Scholar] [CrossRef]
- Ji, W.; Cong, R.; Li, S.; Li, R.; Qin, Z.; Li, Y.; Zhou, X.; Chen, S.; Li, J. Comparative Proteomic Analysis of Soybean Leaves and Roots by iTRAQ Provides Insights into Response Mechanisms to Short-Term Salt Stress. Front. Plant Sci. 2016, 7, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Chu, P.; Yan, G.X.; Yang, Q.; Zhai, L.N.; Zhang, C.; Zhang, F.Q.; Guan, R.Z. ITRAQ-based quantitative proteomics analysis of Brassica napus leaves reveals pathways associated with chlorophyll deficiency. J. Proteom. 2015, 113, 244–259. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Fu, X.; Mei, X.; Zhou, Y.; Cheng, S.; Zeng, L.; Dong, F.; Yang, Z. Proteolysis of chloroplast proteins is responsible for accumulation of free amino acids in dark-treated tea (Camellia sinensis) leaves. J. Proteom. 2017, 157, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Götz, S.; García-Gómez, J.M.; Terol, J.; Williams, T.D.; Nagaraj, S.H.; Nueda, M.J.; Robles, M.; Talón, M.; Dopazo, J.; Conesa, A. High-throughput functional annotation and data mining with the Blast2GO suite. Nucleic Acids Res. 2008, 36, 3420–3435. [Google Scholar] [CrossRef]
- Quevillon, E.; Silventoinen, V.; Pillai, S.; Harte, N.; Mulder, N.; Apweiler, R.; Lopez, R. InterProScan: Protein domains identifier. Nucleic Acids Res. 2005, 33, 116–120. [Google Scholar] [CrossRef]
- Moriya, Y.; Itoh, M.; Okuda, S.; Yoshizawa, A.C.; Kanehisa, M. KAAS: An automatic genome annotation and pathway reconstruction server. Nucleic Acids Res. 2007, 35, W182–W185. [Google Scholar] [CrossRef]
- Luo, J.S.; Yang, Y.; Gu, T.; Wu, Z.; Zhang, Z. The Arabidopsis defensin gene AtPDF2.5 mediates cadmium tolerance and accumulation. Plant Cell Environ. 2019, 42, 2681–2695. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]


| Gene ID | Description | WT | nla | ||
|---|---|---|---|---|---|
| Fold Change | p-Value | Fold Change | p-Value | ||
| Photosynthesis | |||||
| At1g55670 | subunit G of photosystem I | −1.90 | 0.002 | −4.32 | 0.003 |
| At1g49380 | cytochrome c biogenesis protein family | −2.10 | 0.002 | −8.01 | 0.005 |
| At5g11450 | 23 kDa polypeptide of water-oxidizing complex of photosystem II | −2.18 | 0.003 | −3.64 | 0.005 |
| At3g48730 | glutamate-1-semialdehyde 2,1-aminomutase 2 (GSA 2) | −2.25 | 0.003 | −3.63 | 0.003 |
| At1g03130 | photosystem I reaction center subunit II | −2.41 | 0.002 | −4.53 | 0.005 |
| At4g28660 | photosystem II reaction center W (PsbW) family protein | −2.53 | 0.004 | −5.08 | 0.002 |
| At3g26060 | peroxiredoxin Q | −2.71 | 0.003 | −7.15 | 0.002 |
| At3g14930 | uroporphyrinogen decarboxylase | −3.42 | 0.001 | −2.75 | 0.005 |
| At2g40490 | uroporphyrinogen decarboxylase | −3.51 | 0.002 | −3.11 | 0.002 |
| At4g18480 | magnesium-chelatase subunit chlI | −3.74 | 0.002 | −4.90 | 0.003 |
| Protein synthesis | |||||
| At1g74970 | ribosomal protein S9 | −1.99 | 0.002 | −4.85 | 0.003 |
| At2g38140 | chloroplast 30S ribosomal protein S31 | −2.03 | 0.003 | −4.16 | 0.004 |
| At1g32990 | ribosomal protein L11 family protein | −2.11 | 0.004 | −6.02 | 0.002 |
| At5g13510 | ribosomal protein L10 family protein | −2.18 | 0.003 | −4.72 | 0.002 |
| At5g27820 | ribosomal protein L18 family protein | −2.25 | 0.002 | −3.16 | 0.001 |
| At5g47190 | ribosomal protein L19 family protein | −2.26 | 0.003 | −5.64 | 0.004 |
| At1g79850 | chloroplast 30S ribosomal protein S17 | −2.36 | 0.002 | −5.52 | 0.001 |
| At4g29060 | elongation factor Ts family protein | −2.52 | 0.003 | −4.68 | 0.004 |
| At3g15190 | chloroplast 30S ribosomal protein S20 | −2.53 | 0.002 | −7.40 | 0.003 |
| At2g24090 | ribosomal protein L35 family protein | −2.54 | 0.001 | −6.40 | 0.003 |
| At4g24770 | RNA-binding protein cp31 | −2.59 | 0.001 | −5.50 | 0.004 |
| At3g52380 | RNA-binding protein cp33 | −2.78 | 0.003 | −4.46 | 0.003 |
| At2g33450 | chloroplast 50S ribosomal protein L28 | −3.27 | 0.001 | −5.32 | 0.001 |
| At3g13120 | chloroplast 30S ribosomal protein S10 | −3.75 | 0.002 | −6.71 | 0.003 |
| At2g33450 | chloroplast 50S ribosomal protein L28 | −4.20 | 0.001 | −7.45 | 0.001 |
| At3g08740 | elongation factor P (EF-P) family protein, | −4.87 | 0.001 | −5.12 | 0.001 |
| Proteolytic degradation | |||||
| At3g57680 | peptidase S41 family protein | 7.48 | 0.002 | 5.82 | 0.005 |
| At5g37540 | aspartyl protease | 4.23 | 0.002 | 13.36 | 0.005 |
| At5g13800 | hydrolase, alpha/beta fold family | 4.15 | 0.001 | 5.86 | 0.003 |
| At2g05630 | autophagy protein APG8d (AtAPG8d) | 3.87 | 0.001 | 3.91 | 0.002 |
| At4g21980 | autophagy protein APG8a (AtAPG8a) | 3.13 | 0.001 | 5.27 | 0.001 |
| At4g04620 | autophagy protein APG8b (AtAPG8b) | 2.90 | 0.003 | 4.52 | 0.005 |
| At4g01610 | cathepsin B-like cysteine protease | 2.79 | 0.001 | 5.11 | 0.002 |
| At5g51070 | ATP-dependent Clp protease ATP-binding subunit (ClpD) | 2.19 | 0.004 | 4.27 | 0.003 |
| At1g11910 | aspartyl protease | 2.13 | 0.003 | 2.20 | 0.005 |
| At3g15580 | autophagy protein APG8i (AtAPG8i) | 2.04 | 0.003 | 2.26 | 0.002 |
| Gene ID | Description | WT | nla | ||
|---|---|---|---|---|---|
| Fold Change | p-Value | Fold Change | p-Value | ||
| Nitrogen transport | |||||
| At5g40780 | lysine and histidine specific transporter (AtLHT1) | NC | 18.65 | 0.001 | |
| At4g35180 | amino acid transporter family protein (AtLHT7) | NC | 15.02 | 0.001 | |
| At4g21120 | cationic amino acid transporter (AtCAT1) | NC | 6.21 | 0.002 | |
| At2g38290 | high-affinity ammonium transporter 2 (AMT2) | NC | 3.75 | 0.004 | |
| At1g31830 | amino acid permease family protein (AtPUT2/AtPQR2) | NC | 2.93 | 0.002 | |
| At5g63850 | amino acid transporter 4 (AAP4) | NC | 2.55 | 0.002 | |
| At3g56200 | amino acid transporter | NC | 2.36 | 0.001 | |
| At4g13510 | ammonium transporter 1 (AMT1.1) | NC | 2.22 | 0.004 | |
| At1g58360 | neutral amino acid transporter (AtAAP1) | NC | 2.07 | 0.002 | |
| Genes involved in anthocyanin synthesis | |||||
| At2g37040 | phenylalanine ammonia lyase (PAL1) | 9.00 | 0.002 | 4.03 | 0.003 |
| At2g30490 | cinnamic acid 4-hydroxylase | 5.41 | 0.003 | 4.23 | 0.001 |
| At5g13930 | chalcone synthase (CHS) | 7.61 | 0.004 | 2.28 | 0.002 |
| At3g51240 | flavanone 3-hydroxylase (F3H) | 22.64 | 0.003 | 4.98 | 0.005 |
| At5g42800 | dihydroflavonol 4-reductase (DFR) | 31.11 | 0.005 | 8.47 | 0.004 |
| At4g22880 | anthocyanidin synthase (ANS) | 49.24 | 0.005 | 8.85 | 0.005 |
| At4g22870 | anthocyanidin synthase (ANS) | 33.89 | 0.003 | 7.43 | 0.004 |
| At3g21560 | UDP-glycosyltransferase | 8.48 | 0.001 | 3.82 | 0.005 |
| At5g17050 | glycosyltransferase family | 10.65 | 0.003 | NS | |
| At3g53260 | phenylalanine ammonia-lyase (PAL2) | 3.47 | 0.003 | NC | |
| At1g65060 | 4-coumaroyl-CoA synthase 3 (4CL3) | 9.50 | 0.001 | NS | |
| At5g05270 | chalcone-flavanone isomerase family | 17.38 | 0.004 | NS | |
| At1g66390 | MYB domain containing transcription factor (MYB90) | 29.11 | 0.005 | 35.98 | 0.005 |
| At2g47190 | myb family transcription factor (MYB2) | NC | 24.55 | 0.001 | |
| At1g56650 | MYB domain containing transcription factor (MYB75) | 12.70 | 0.004 | NSc | |
| At4g34990 | myb family transcription factor (MYB32) | 3.60 | 0.002 | NC | |
| Senescence-associated genes | |||||
| At2g19190 | senescence-responsive receptor-like serine/threonine kinase (SIRK) | NC | 9.56 | 0.005 | |
| At5g45890 | senescence-specific SAG12 protein (SAG12) | NC | 62.49 | 0.004 | |
| At5g14930 | leaf senescence-associated protein (SAG101) | NC | 6.54 | 0.002 | |
| At5g66170 | senescence-associated family protein | NC | 6.26 | 0.001 | |
| At3g10980 | senescence-associated protein. | NC | 2.37 | 0.004 | |
| Protein ID | Description | Fold Change | p-Value |
|---|---|---|---|
| Photosynthesis | |||
| P56780 | Photosystem II reaction center protein H | −2.46 | 0 |
| Q8LC58 | Photosystem I reaction center subunit IV B, chloroplast (PSI-E B) | −2.16 | 0 |
| Q9SA56 | Photosystem I reaction center subunit II−2, chloroplastic | −1.93 | 0.01 |
| A0A178W7I8 | PSAG | −1.88 | 0.003 |
| P10796 | Ribulose bisphosphate carboxylase small chain 1B, chloroplastic | −1.84 | 0.004 |
| Q8LCA1 | Protein CURVATURE THYLAKOID 1B, chloroplastic | −1.78 | 0.003 |
| Q8VY52 | PsbP domain-containing protein 2, chloroplastic | −1.77 | 0.001 |
| Q39195 | Photosystem II 5 kDa protein, chloroplastic | −1.72 | 0.001 |
| A0A178WK60 | Chlorophyll a-b binding protein, chloroplastic | −1.69 | 0.001 |
| Q9XF88 | Chlorophyll a-b binding protein CP29.2, chloroplastic | −1.68 | 0 |
| A0A178UXI3 | Photosystem II reaction center Psb28 protein | −1.66 | 0.008 |
| A8MS75 | Chlorophyll a-b binding protein, chloroplastic | −1.64 | 0.001 |
| P49107 | Photosystem I reaction center subunit N, chloroplastic | −1.63 | 0 |
| Q8H112 | PGR5-like protein 1A, chloroplastic | −1.62 | 0 |
| Q9SY97 | Photosystem I chlorophyll a/b-binding protein 3-1, chloroplastic | −1.55 | 0 |
| Q9SYX1 | Light-harvesting complex-like protein 3 isotype 1, chloroplastic | −1.54 | 0 |
| Q9FPI3 | Chlorophyll a-b binding protein, chloroplastic | −1.54 | 0.032 |
| P82538 | PsbP-like protein 1, chloroplastic | −1.53 | 0.001 |
| Q9S7W1 | Chlorophyll a-b binding protein CP29.3, chloroplastic | −1.51 | 0.003 |
| Chlorophyll biosynthesis | |||
| and organization | |||
| Q9SKT0 | Protein THYLAKOID FORMATION 1, chloroplastic | −1.96 | 0 |
| Q9M591 | Magnesium-protoporphyrin IX monomethyl ester [oxidative] cyclase, chloroplastic | −1.5 | 0.002 |
| Protein ID | Description | Fold Change | p-Value |
|---|---|---|---|
| Protein synthesis and folding | |||
| Q9LJE4 | Chaperonin 60 subunit beta 2, chloroplastic | −1.90 | 0.001 |
| A0A178VU06 | Peptidyl-prolyl cis-trans isomerase | −1.65 | 0.001 |
| Q9SMQ9 | DnaJ-like protein | −1.62 | 0.001 |
| A0A1P8ART2 | Molecular chaperone Hsp40/DnaJ family protein | −1.59 | 0.004 |
| P21240 | Chaperonin 60 subunit beta 1, chloroplastic | −1.51 | 0.002 |
| A0A178VZ96 | Peptidyl-prolyl cis-trans isomerase | −1.51 | 0.000 |
| Proteolysis | |||
| F4HX35 | Autophagy-related protein | 1.61 | 0.005 |
| Q9T075 | Protein RMD5 homolog | 2.09 | 0.000 |
| Ubiquitination pathway | |||
| Q42202 | Ubiquitin-60S ribosomal protein L40-2 | −2.14 | 0.023 |
| Q9CA23 | Ubiquitin-fold modifier 1 | −1.68 | 0.044 |
| P59232 | Ubiquitin-40S ribosomal protein S27a-2 | −1.61 | 0.005 |
| P25865 | Ubiquitin-conjugating enzyme E2 1 | −1.52 | 0.004 |
| Nitrogen metabolism and transport | |||
| Q9M390 | Protein NRT1/PTR FAMILY 8.1 | 1.67 | 0.003 |
| P11832 | Nitrate reductase [NADH] 1 | 1.81 | 0.008 |
| O04907 | Nitrilase 2 | 1.88 | 0.030 |
| A0A178UFA7 | LHT1 | 2.09 | 0.003 |
| Q9FGS5 | High-affinity nitrate transporter 3.1 | 2.15 | 0.027 |
| Gene ID | Protein ID | Description | Fold Changea | p-Valuea | Fold Changeb | p-Valueb |
|---|---|---|---|---|---|---|
| AT3G12500 | P19171 | Basic endochitinase B | 3.15 | 0.01 | 3.74 | 0.00 |
| At4g37430 | Q8H137 | Putative cytochrome P450 monooxygenase (CYP91A2) | 2.67 | 0.00 | 5.20 | 0.00 |
| At1g59710 | A0A1P8AQI0 | Actin cross-linking protein (DUF569) | 2.54 | 0.01 | 3.48 | 0.00 |
| AT2G43510 | Q42328 | Defensin-like protein 195 | 2.33 | 0.04 | 44.29 | 0.00 |
| At4g16260 | A0A1P8B3U2 | Glycosyl hydrolase superfamily protein | 2.28 | 0.01 | 16.58 | 0.00 |
| AT1G21250 | Q39191 | Wall-associated receptor kinase 1 | 2.20 | 0.00 | 4.43 | 0.00 |
| AT5G40780 | A0A178UFA7 | LHT1 | 2.09 | 0.00 | 18.65 | 0.00 |
| AT1G02920 | Q9SRY5 | Glutathione S-transferase F7 | 2.02 | 0.01 | 13.55 | 0.00 |
| At2g16710 | A8MR92 | Iron-sulfur cluster biosynthesis family protein | 2.00 | 0.00 | 3.51 | 0.00 |
| At5g27760 | A0A1P8BCY5 | Hypoxia-responsive family protein | 1.87 | 0.03 | 4.57 | 0.00 |
| At2g37130 | F4IQ05 | Peroxidase | 1.86 | 0.04 | 4.35 | 0.00 |
| AT1G59820 | Q9XIE6 | Phospholipid-transporting ATPase 3 | 1.85 | 0.00 | 2.67 | 0.00 |
| AT1G70690 | Q8GUJ2 | Plasmodesmata-located protein 5 | 1.82 | 0.01 | 8.92 | 0.00 |
| At5g15870 | Q9LFT3 | Glycosyl hydrolase family 81 protein | 1.77 | 0.02 | 2.65 | 0.00 |
| AT1G11310 | A0A1P8AMJ7 | MLO-like protein | 1.76 | 0.00 | 3.55 | 0.00 |
| AT3G48090 | B2BDD6 | Enhanced disease susceptibility 1 | 1.73 | 0.02 | 4.86 | 0.00 |
| AT4G32690 | Q67XG0 | Two-on-two hemoglobin-3 | 1.72 | 0.00 | 2.29 | 0.00 |
| AT5G04930 | P98204 | Phospholipid-transporting ATPase 1 | 1.71 | 0.00 | 3.29 | 0.00 |
| At3g15810 | Q9LVZ8 | Protein LURP-one-related 12 | 1.69 | 0.01 | 2.52 | 0.00 |
| AT1G76150 | Q8VYI3 | Enoyl-CoA hydratase 2, peroxisomal | 1.64 | 0.01 | 4.48 | 0.00 |
| AT1G20630 | Q96528 | Catalase-1 | 1.63 | 0.00 | 7.12 | 0.00 |
| At5g16450 | Q9FFE0 | Putative 4-hydroxy-4-methyl-2-oxoglutarate aldolase 2 | 1.63 | 0.01 | 3.12 | 0.00 |
| At3g05230 | Q9MA96 | Signal peptidase complex subunit 3A | 1.61 | 0.00 | 3.76 | 0.00 |
| AT4G08850 | Q8VZG8 | MDIS1-interacting receptor like kinase 2 | 1.56 | 0.00 | 6.29 | 0.00 |
| AT4G28390 | O49447 | ADP,ATP carrier protein 3, mitochondrial | 1.55 | 0.00 | 5.61 | 0.00 |
| AT3G17790 | Q9SCX8 | Purple acid phosphatase 17 | 1.52 | 0.01 | 7.23 | 0.00 |
| AT1G17840 | Q8RXN0 | ABC transporter G family member 11 | 1.51 | 0.03 | 0.43 | 0.00 |
| AT4G16760 | O65202 | Peroxisomal acyl-coenzyme A oxidase 1 | 1.50 | 0.03 | 4.60 | 0.00 |
| At1g63010 | Q2V4F9 | SPX domain-containing membrane protein At1g63010 | 1.50 | 0.01 | 2.47 | 0.00 |
| AT1G23740 | Q9ZUC1 | NADPH-dependent alkenal/one oxidoreductase, chloroplastic | 0.66 | 0.00 | 0.43 | 0.00 |
| At5g27560 | A0A1R7T377 | DUF1995 domain protein, putative (DUF1995) | 0.66 | 0.00 | 0.32 | 0.00 |
| At3g47070 | Q94CB6 | Uncharacterized protein At3g47070 | 0.65 | 0.00 | 0.25 | 0.00 |
| AT5G02120 | O81208 | Light-harvesting complex-like protein OHP1, chloroplastic | 0.64 | 0.00 | 0.30 | 0.00 |
| AT5G43750 | Q9FG89 | Photosynthetic NDH subunit of subcomplex B 5, chloroplastic | 0.63 | 0.01 | 0.09 | 0.00 |
| At4g21210 | B9DHI2 | AT4G21210 protein | 0.63 | 0.00 | 0.32 | 0.00 |
| AT1G10370 | Q9FUS7 | Glutathione S-transferase | 0.62 | 0.04 | 0.34 | 0.00 |
| AT4G22890 | Q8H112 | PGR5-like protein 1A, chloroplastic | 0.62 | 0.00 | 0.39 | 0.00 |
| AT1G70410 | Q94CE4 | Beta carbonic anhydrase 4 | 0.61 | 0.00 | 0.19 | 0.00 |
| AT1G64750 | Q9XIR8 | Protein DELETION OF SUV3 SUPPRESSOR 1(I) | 0.61 | 0.00 | 2.31 | 0.00 |
| At1g74730 | Q94F10 | Transmembrane protein, putative (DUF1118) | 0.61 | 0.00 | 0.43 | 0.00 |
| At3g61870 | F4IX01 | Plant/protein | 0.60 | 0.00 | 0.20 | 0.00 |
| AT5G07440 | O82179 | Glycine cleavage system H protein 2, mitochondrial | 0.60 | 0.00 | 11.46 | 0.00 |
| AT3G07390 | Q94BT2 | Auxin-induced in root cultures protein 12 | 0.59 | 0.00 | 5.75 | 0.00 |
| At4g33500 | Q93V88 | Probable protein phosphatase 2C 62 | 0.59 | 0.01 | 0.39 | 0.00 |
| AT2G23670 | O64835 | At2g23670/F26B6.32 | 0.57 | 0.02 | 0.31 | 0.00 |
| AT2G26540 | A0A1P8AZL4 | Uroporphyrinogen-III synthase family protein | 0.57 | 0.00 | 0.34 | 0.00 |
| AT5G66570 | P23321 | Oxygen-evolving enhancer protein 1-1, chloroplastic | 0.56 | 0.00 | 0.35 | 0.00 |
| At5g52780 | Q9LTD9 | Uncharacterized protein PAM68-like | 0.53 | 0.01 | 0.38 | 0.00 |
| AT2G20890 | Q9SKT0 | Protein THYLAKOID FORMATION 1, chloroplastic | 0.51 | 0.00 | 0.23 | 0.00 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liao, Q.; Tang, T.-j.; Zhou, T.; Song, H.-x.; Hua, Y.-p.; Zhang, Z.-h. Integrated Transcriptional and Proteomic Profiling Reveals Potential Amino Acid Transporters Targeted by Nitrogen Limitation Adaptation. Int. J. Mol. Sci. 2020, 21, 2171. https://doi.org/10.3390/ijms21062171
Liao Q, Tang T-j, Zhou T, Song H-x, Hua Y-p, Zhang Z-h. Integrated Transcriptional and Proteomic Profiling Reveals Potential Amino Acid Transporters Targeted by Nitrogen Limitation Adaptation. International Journal of Molecular Sciences. 2020; 21(6):2171. https://doi.org/10.3390/ijms21062171
Chicago/Turabian StyleLiao, Qiong, Tian-jiao Tang, Ting Zhou, Hai-xing Song, Ying-peng Hua, and Zhen-hua Zhang. 2020. "Integrated Transcriptional and Proteomic Profiling Reveals Potential Amino Acid Transporters Targeted by Nitrogen Limitation Adaptation" International Journal of Molecular Sciences 21, no. 6: 2171. https://doi.org/10.3390/ijms21062171
APA StyleLiao, Q., Tang, T.-j., Zhou, T., Song, H.-x., Hua, Y.-p., & Zhang, Z.-h. (2020). Integrated Transcriptional and Proteomic Profiling Reveals Potential Amino Acid Transporters Targeted by Nitrogen Limitation Adaptation. International Journal of Molecular Sciences, 21(6), 2171. https://doi.org/10.3390/ijms21062171






