Alpha-Smooth Muscle Actin-Positive Perivascular Cells in Diabetic Retina and Choroid
Abstract
1. Introduction
2. Results
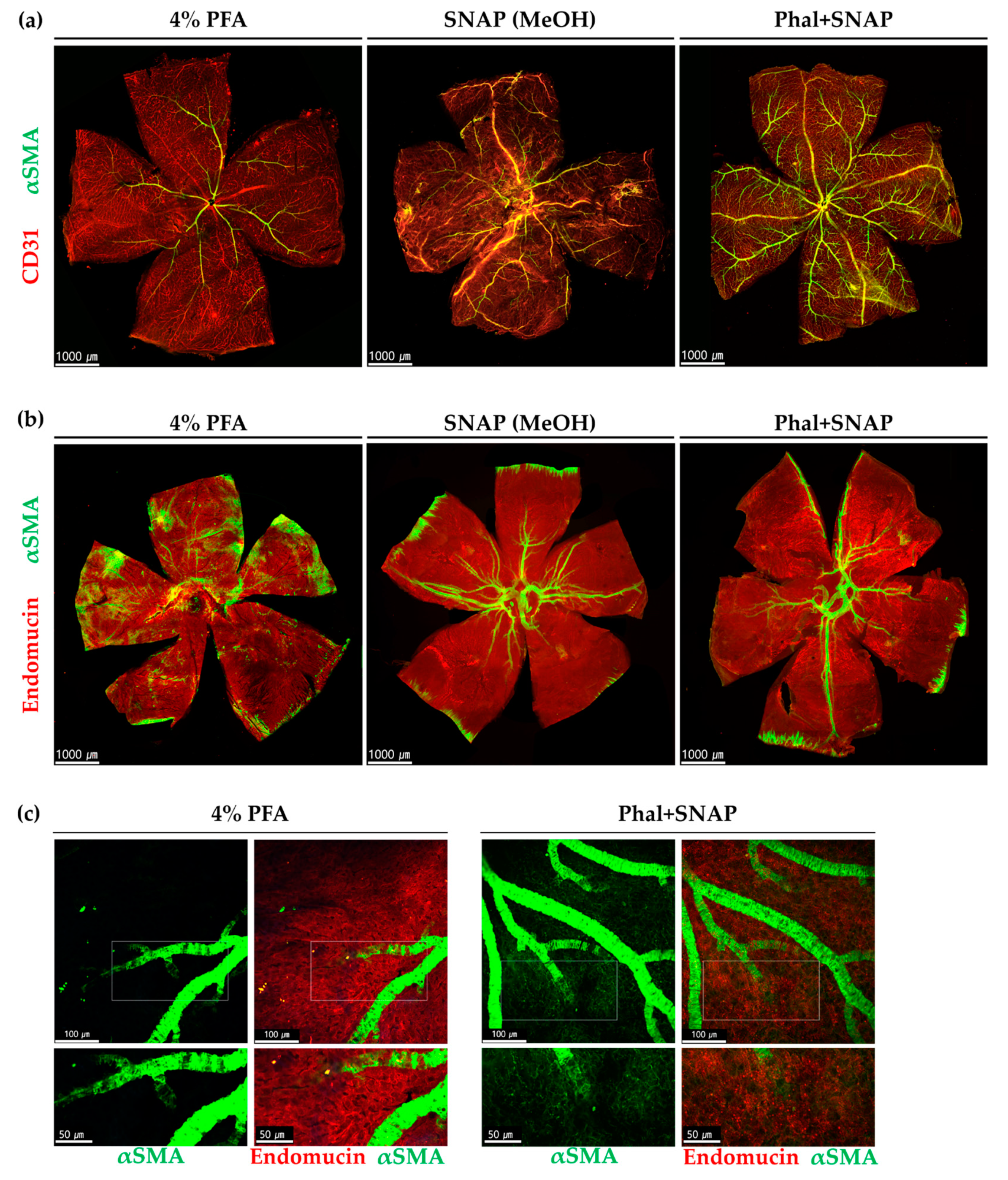
2.1. Snap Fixation for the Detection of αSMA Expression in Wild Type Mice
2.1.1. αSMA Positive Perivascular Cells in Large-Diameter Vessels in the Retina and Choroid
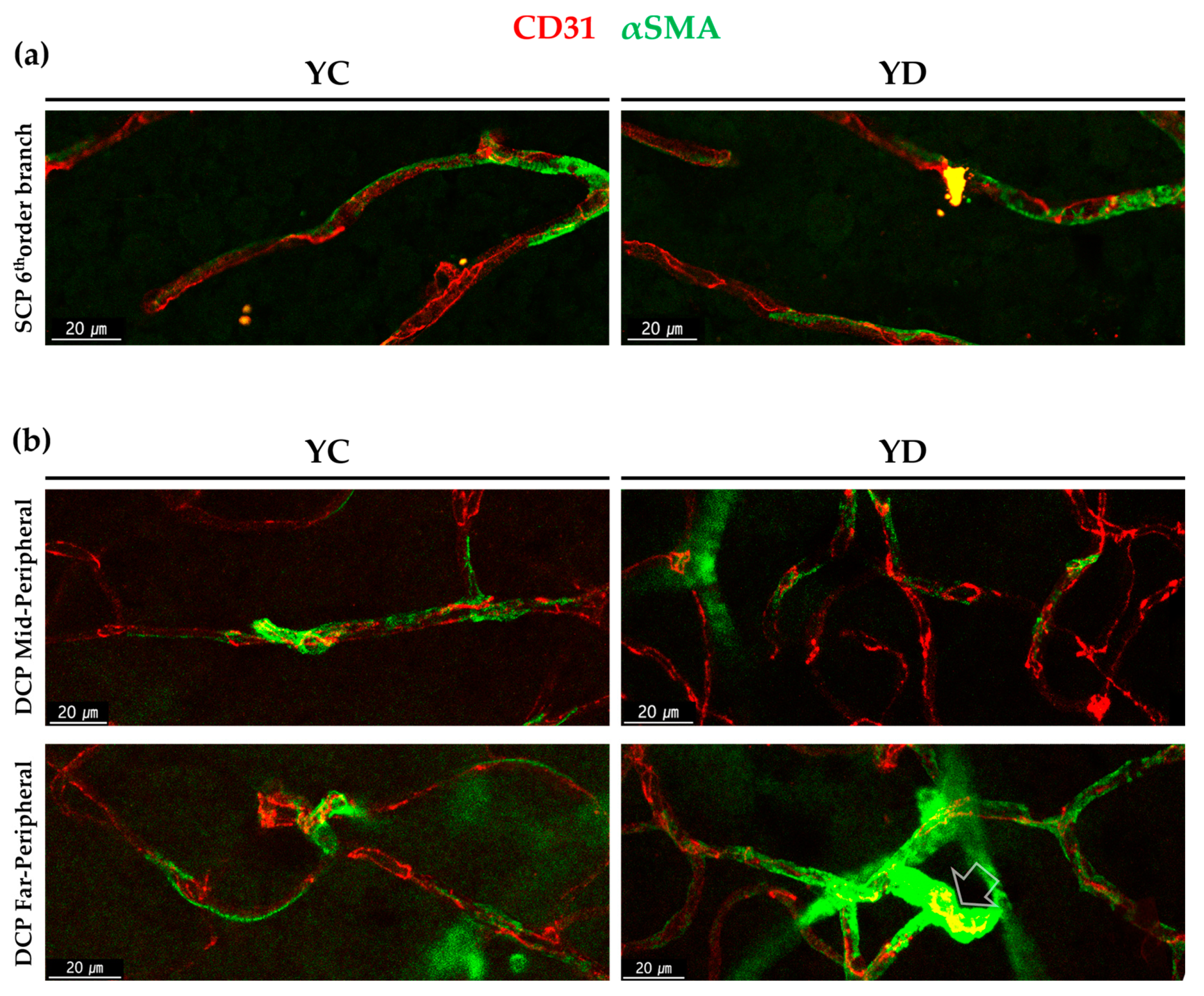
2.1.2. αSMA-Positive Perivascular Cells in Capillary Networks of the Retina and Choroid
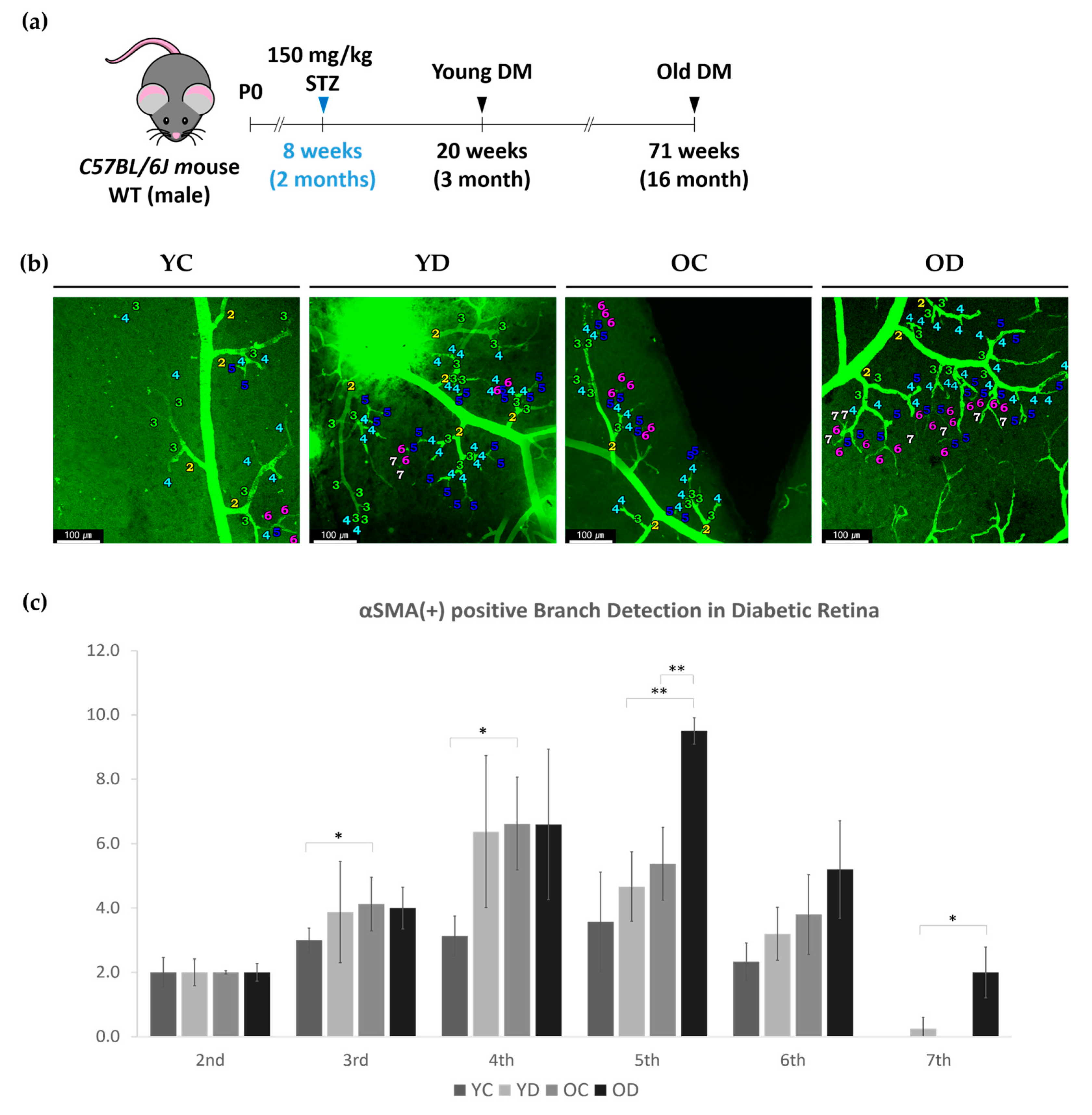
2.2. Retinal and Choroidal αSMA in STZ-Induced Diabetic Mouse Model
2.2.1. Regional Changes of Retinal αSMA in the Diabetic Model
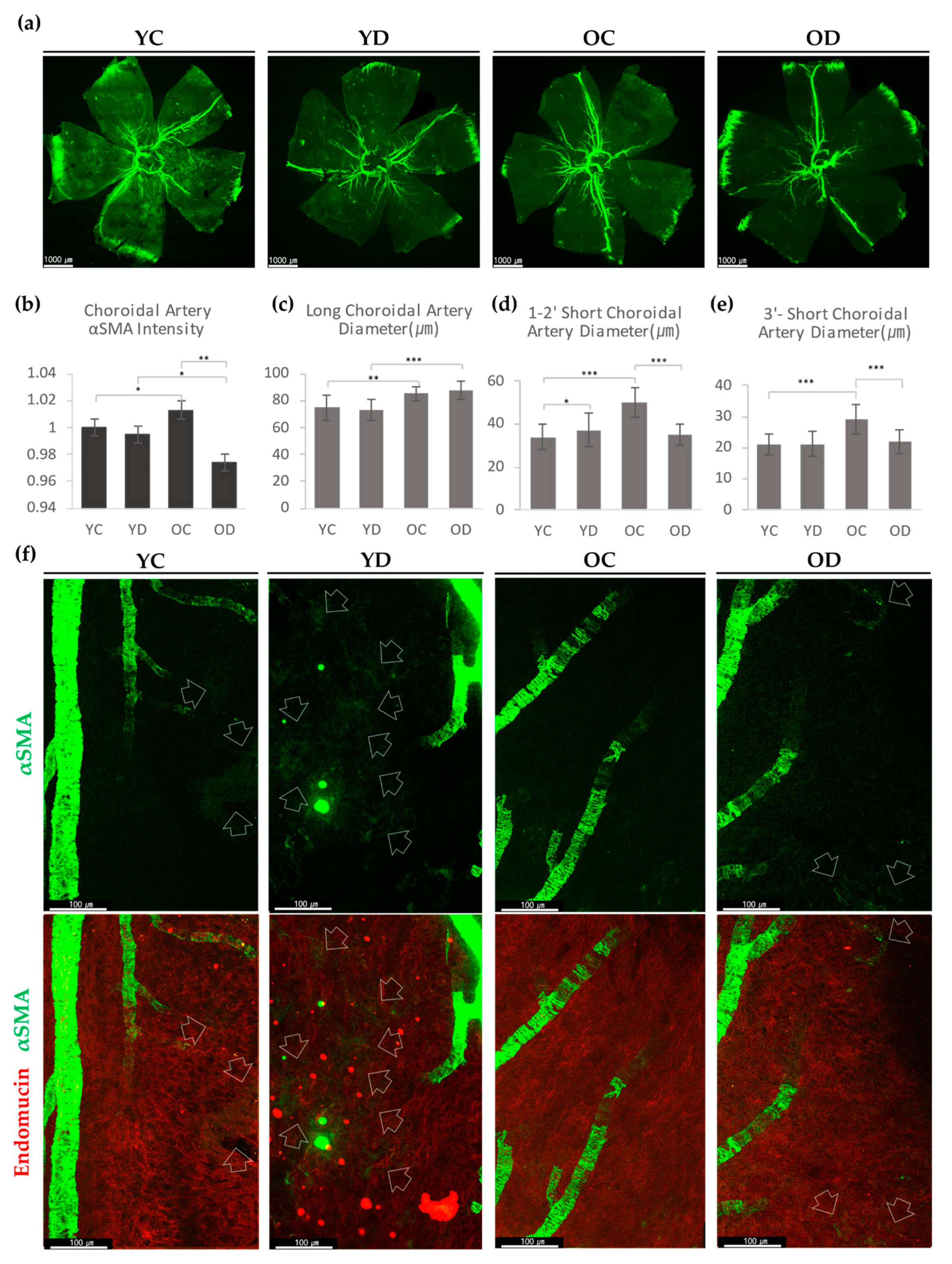
2.2.2. Choroidal αSMA in the Diabetic Model
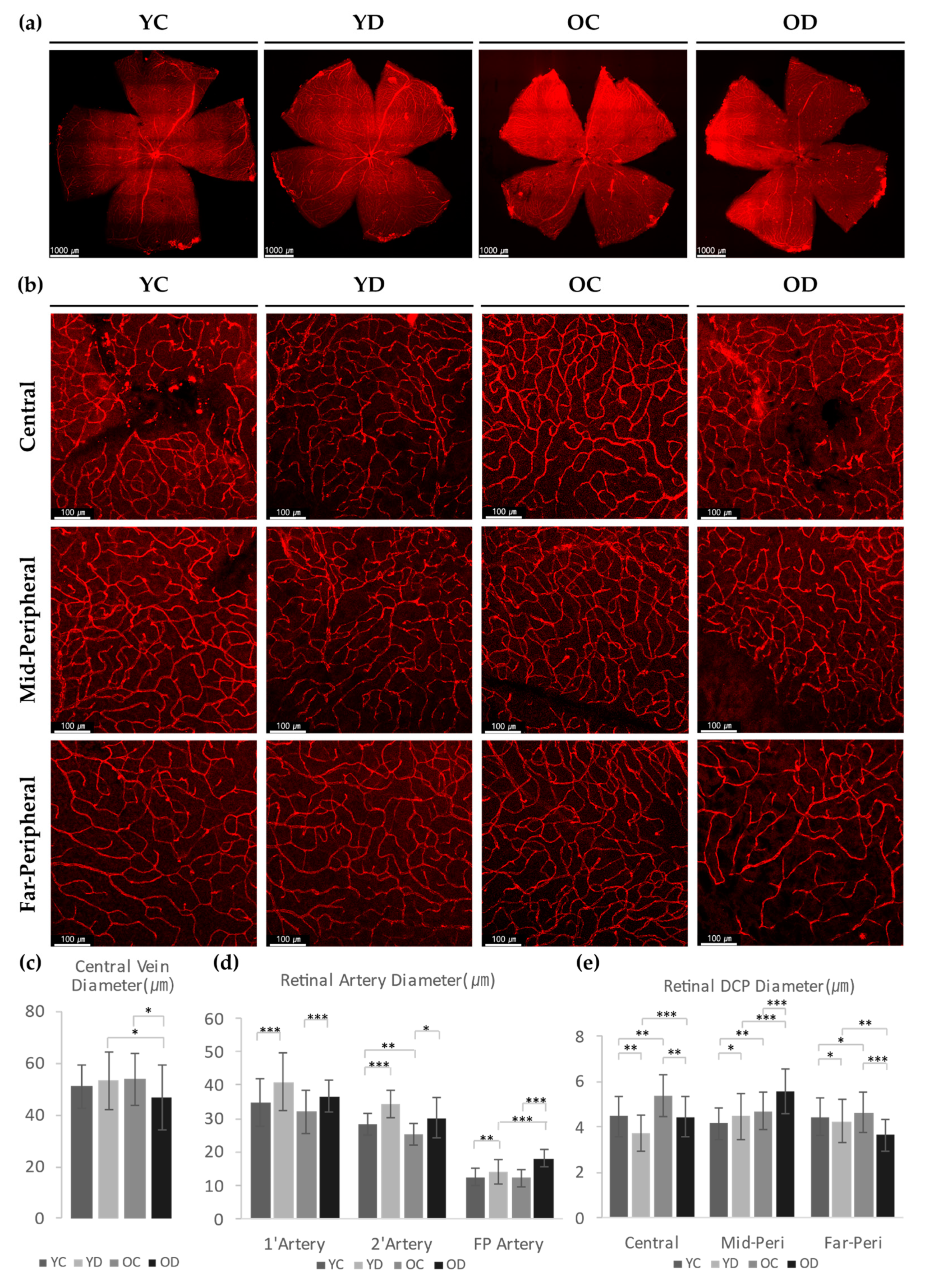
2.2.3. Evaluation of Retinal and Choroidal Vessels in Diabetic Model
3. Discussion
4. Materials and Methods
4.1. Normal Mice and STZ-Induced Diabetic Mice Model
4.2. Intravitreal Injection of Phalloidin
4.3. Wholemount Immunofluorescence and Confocal Microscopy of the Retina
4.4. Image Analysis
4.5. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| αSMA | Alpha-Smooth Muscle Actin |
| DCP | Deep Capillary Plexus |
| DM | Diabetes Mellitus |
| DR | Diabetic Retinopathy |
| DVP | Deep Vasculature Plexus, includes ICP and DCP |
| F-actin | Filamentous Actin, Actin Filament |
| ICP | Intermediate Capillary Plexus |
| OC | Old Control |
| OD | Old Diabetes |
| PFA | Paraformaldehyde |
| Phal | Phalloidin |
| RPE | Retinal Pigment Epithelium |
| SCP | Superficial Capillary Plexus |
| VSMC | Vascular Smooth Muscle Cell |
| YC | Young Control |
| YD | Young Diabetes |
References
- Yamazaki, T.; Mukouyama, Y.S. Tissue Specific Origin, Development, and Pathological Perspectives of Pericytes. Front. Cardiovasc. Med. 2018, 5, 78. [Google Scholar] [CrossRef]
- Bergers, G.; Song, S. The role of pericytes in blood-vessel formation and maintenance. Neuro Oncol. 2005, 7, 452–464. [Google Scholar] [CrossRef] [PubMed]
- Murfee, W.L.; Skalak, T.C.; Peirce, S.M. Differential arterial/venous expression of NG2 proteoglycan in perivascular cells along microvessels: Identifying a venule-specific phenotype. Microcirculation 2005, 12, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Grant, R.I.; Hartmann, D.A.; Underly, R.G.; Berthiaume, A.A.; Bhat, N.R.; Shih, A.Y. Organizational hierarchy and structural diversity of microvascular pericytes in adult mouse cortex. J. Cereb. Blood Flow Metab. 2019, 39, 411–425. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, N.B.; Attwell, D.; Hall, C.N. Pericyte-mediated regulation of capillary diameter: A component of neurovascular coupling in health and disease. Front. Neuroenergetics 2010, 2, 5. [Google Scholar] [CrossRef]
- Trost, A.; Lange, S.; Schroedl, F.; Bruckner, D.; Motloch, K.A.; Bogner, B.; Kaser-Eichberger, A.; Strohmaier, C.; Runge, C.; Aigner, L.; et al. Brain and Retinal Pericytes: Origin, Function and Role. Front. Cell Neurosci. 2016, 10, 20. [Google Scholar] [CrossRef]
- Kur, J.; Newman, E.A.; Chan-Ling, T. Cellular and physiological mechanisms underlying blood flow regulation in the retina and choroid in health and disease. Prog. Retin. Eye Res. 2012, 31, 377–406. [Google Scholar] [CrossRef]
- Alarcon-Martinez, L.; Yilmaz-Ozcan, S.; Yemisci, M.; Schallek, J.; Kilic, K.; Can, A.; Di Polo, A.; Dalkara, T. Capillary pericytes express alpha-smooth muscle actin, which requires prevention of filamentous-actin depolymerization for detection. Elife 2018, 7, e34861. [Google Scholar] [CrossRef]
- Forrester, J.V.; Dick, A.D.; McMenamin, P.G.; Roberts, F.; Pearlma, E. The Eye: Basic Sciences in Practice, 2nd ed.; Saunders-Elsevier Limited: Edinburgh, UK, 2002; pp. 48–52. [Google Scholar]
- Moshfeghi, A.A.; Kim, E.L. Wide-field Imaging of Retinal Diseases. US Ophthalmic Rev. 2015, 8, 125–131. [Google Scholar] [CrossRef]
- Ehlers, J.P.; Jiang, A.C.; Boss, J.D.; Hu, M.; Figueiredo, N.; Babiuch, A.; Talcott, K.; Sharma, S.; Hach, J.; Le, T.; et al. Quantitative Ultra-Widefield Angiography and Diabetic Retinopathy Severity: An Assessment of Panretinal Leakage Index, Ischemic Index and Microaneurysm Count. Ophthalmology 2019, 126, 1527–1532. [Google Scholar] [CrossRef]
- Abcouwer, S.F. Angiogenic Factors and Cytokines in Diabetic Retinopathy. J. Clin. Cell Immunol. 2013. [Google Scholar] [CrossRef]
- Beltramo, E.; Porta, M. Pericyte loss in diabetic retinopathy: Mechanisms and consequences. Curr. Med. Chem. 2013, 20, 3218–3225. [Google Scholar] [CrossRef] [PubMed]
- Hall, A.P. Review of the pericyte during angiogenesis and its role in cancer and diabetic retinopathy. Toxicol. Pathol. 2006, 34, 763–775. [Google Scholar] [CrossRef] [PubMed]
- Kusuhara, S.; Fukushima, Y.; Ogura, S.; Inoue, N.; Uemura, A. Pathophysiology of Diabetic Retinopathy: The Old and the New. Diabetes Metab. J. 2018, 42, 364–376. [Google Scholar] [CrossRef] [PubMed]
- Park, D.Y.; Lee, J.; Kim, J.; Kim, K.; Hong, S.; Han, S.; Kubota, Y.; Augustin, H.G.; Ding, L.; Kim, J.W.; et al. Plastic roles of pericytes in the blood-retinal barrier. Nat. Commun. 2017, 8, 15296. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, E.; Kovacs-Oller, T.; Sagdullaev, B.T. Vascular Pericyte Impairment and Connexin43 Gap Junction Deficit Contribute to Vasomotor Decline in Diabetic Retinopathy. J. Neurosci. 2017, 37, 7580–7594. [Google Scholar] [CrossRef]
- vom Hagen, F.; Feng, Y.; Hillenbrand, A.; Hoffmann, S.; Shani, M.; Deutsch, U.; Hammes, H.P. Early loss of arteriolar smooth muscle cells: More than just a pericyte loss in diabetic retinopathy. Exp. Clin. Endocrinol. Diabetes 2005, 113, 573–576. [Google Scholar] [CrossRef]
- Lutty, G.A. Diabetic choroidopathy. Vision Res. 2017, 139, 161–167. [Google Scholar] [CrossRef]
- Melancia, D.; Vicente, A.; Cunha, J.P.; Abegao Pinto, L.; Ferreira, J. Diabetic choroidopathy: A review of the current literature. Graefes Arch. Clin. Exp. Ophthalmol. 2016, 254, 1453–1461. [Google Scholar] [CrossRef]
- Holm, A.; Heumann, T.; Augustin, H.G. Microvascular Mural Cell Organotypic Heterogeneity and Functional Plasticity. Trends Cell Biol. 2018, 28, 302–316. [Google Scholar] [CrossRef]
- Hartmann, D.A.; Underly, R.G.; Grant, R.I.; Watson, A.N.; Lindner, V.; Shih, A.Y. Pericyte structure and distribution in the cerebral cortex revealed by high-resolution imaging of transgenic mice. Neurophotonics 2015, 2, 041402. [Google Scholar] [CrossRef] [PubMed]
- Armulik, A.; Genove, G.; Betsholtz, C. Pericytes: Developmental, physiological, and pathological perspectives, problems, and promises. Dev. Cell 2011, 21, 193–215. [Google Scholar] [CrossRef] [PubMed]
- Perrin, B.J.; Ervasti, J.M. The actin gene family: Function follows isoform. Cytoskeleton 2010, 67, 630–634. [Google Scholar] [CrossRef] [PubMed]
- Davis-Dusenbery, B.N.; Wu, C.; Hata, A. Micromanaging vascular smooth muscle cell differentiation and phenotypic modulation. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 2370–2377. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.A.; Kim, S.J.; Choi, Y.A.; Yoon, H.J.; Kim, A.; Lee, J. Retinal VEGFA maintains the ultrastructure and function of choriocapillaris by preserving the endothelial PLVAP. Biochem. Biophys. Res. Commun. 2020, 522, 240–246. [Google Scholar] [CrossRef] [PubMed]
- Condren, A.B.; Kumar, A.; Mettu, P.; Liang, K.J.; Zhao, L.; Tsai, J.Y.; Fariss, R.N.; Wong, W.T. Perivascular mural cells of the mouse choroid demonstrate morphological diversity that is correlated to vasoregulatory function. PLoS ONE 2013, 8, e53386. [Google Scholar] [CrossRef]
- Chang, S.; Song, S.; Lee, J.; Yoon, J.; Park, J.; Choi, S.; Park, J.K.; Choi, K.; Choi, C. Phenotypic modulation of primary vascular smooth muscle cells by short-term culture on micropatterned substrate. PLoS ONE 2014, 9, e88089. [Google Scholar] [CrossRef]
- Duh, E.J.; Sun, J.K.; Stitt, A.W. Diabetic retinopathy: Current understanding, mechanisms, and treatment strategies. JCI Insight 2017, 2, 93751. [Google Scholar] [CrossRef]
- Alarcon-Martinez, L.; Yilmaz-Ozcan, S.; Yemisci, M.; Schallek, J.; Kilic, K.; Villafranca-Baughman, D.; Can, A.; Di Polo, A.; Dalkara, T. Retinal ischemia induces alpha-SMA-mediated capillary pericyte contraction coincident with perivascular glycogen depletion. Acta Neuropathol. Commun. 2019, 7, 134. [Google Scholar] [CrossRef]
- Cho, J.G.; Lee, A.; Chang, W.; Lee, M.S.; Kim, J. Endothelial to Mesenchymal Transition Represents a Key Link in the Interaction between Inflammation and Endothelial Dysfunction. Front. Immunol. 2018, 9, 294. [Google Scholar] [CrossRef]
- Lee, J.; Kim, K.E.; Choi, D.K.; Jang, J.Y.; Jung, J.J.; Kiyonari, H.; Shioi, G.; Chang, W.; Suda, T.; Mochizuki, N.; et al. Angiopoietin-1 guides directional angiogenesis through integrin alphavbeta5 signaling for recovery of ischemic retinopathy. Sci. Transl. Med. 2013, 5, 203ra127. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Park, D.Y.; Park, D.Y.; Park, I.; Chang, W.; Nakaoka, Y.; Komuro, I.; Yoo, O.J.; Koh, G.Y. Angiopoietin-1 suppresses choroidal neovascularization and vascular leakage. Investig. Ophthalmol. Vis. Sci. 2014, 55, 2191–2199. [Google Scholar] [CrossRef] [PubMed]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Bolte, S.; Cordelères, F.P. A guided tour into subcellular colocalization analysis in light microscopy. J. Microsc. 2006, 224, 213–232. [Google Scholar] [CrossRef] [PubMed]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.J.; Kim, S.A.; Choi, Y.A.; Park, D.Y.; Lee, J. Alpha-Smooth Muscle Actin-Positive Perivascular Cells in Diabetic Retina and Choroid. Int. J. Mol. Sci. 2020, 21, 2158. https://doi.org/10.3390/ijms21062158
Kim SJ, Kim SA, Choi YA, Park DY, Lee J. Alpha-Smooth Muscle Actin-Positive Perivascular Cells in Diabetic Retina and Choroid. International Journal of Molecular Sciences. 2020; 21(6):2158. https://doi.org/10.3390/ijms21062158
Chicago/Turabian StyleKim, Soo Jin, Sang A. Kim, Yeong A. Choi, Do Young Park, and Junyeop Lee. 2020. "Alpha-Smooth Muscle Actin-Positive Perivascular Cells in Diabetic Retina and Choroid" International Journal of Molecular Sciences 21, no. 6: 2158. https://doi.org/10.3390/ijms21062158
APA StyleKim, S. J., Kim, S. A., Choi, Y. A., Park, D. Y., & Lee, J. (2020). Alpha-Smooth Muscle Actin-Positive Perivascular Cells in Diabetic Retina and Choroid. International Journal of Molecular Sciences, 21(6), 2158. https://doi.org/10.3390/ijms21062158






