Development of a Cartilage Oligomeric Matrix Protein Neo-Epitope Assay for the Detection of Intra-Thecal Tendon Disease
Abstract
1. Introduction
2. Results
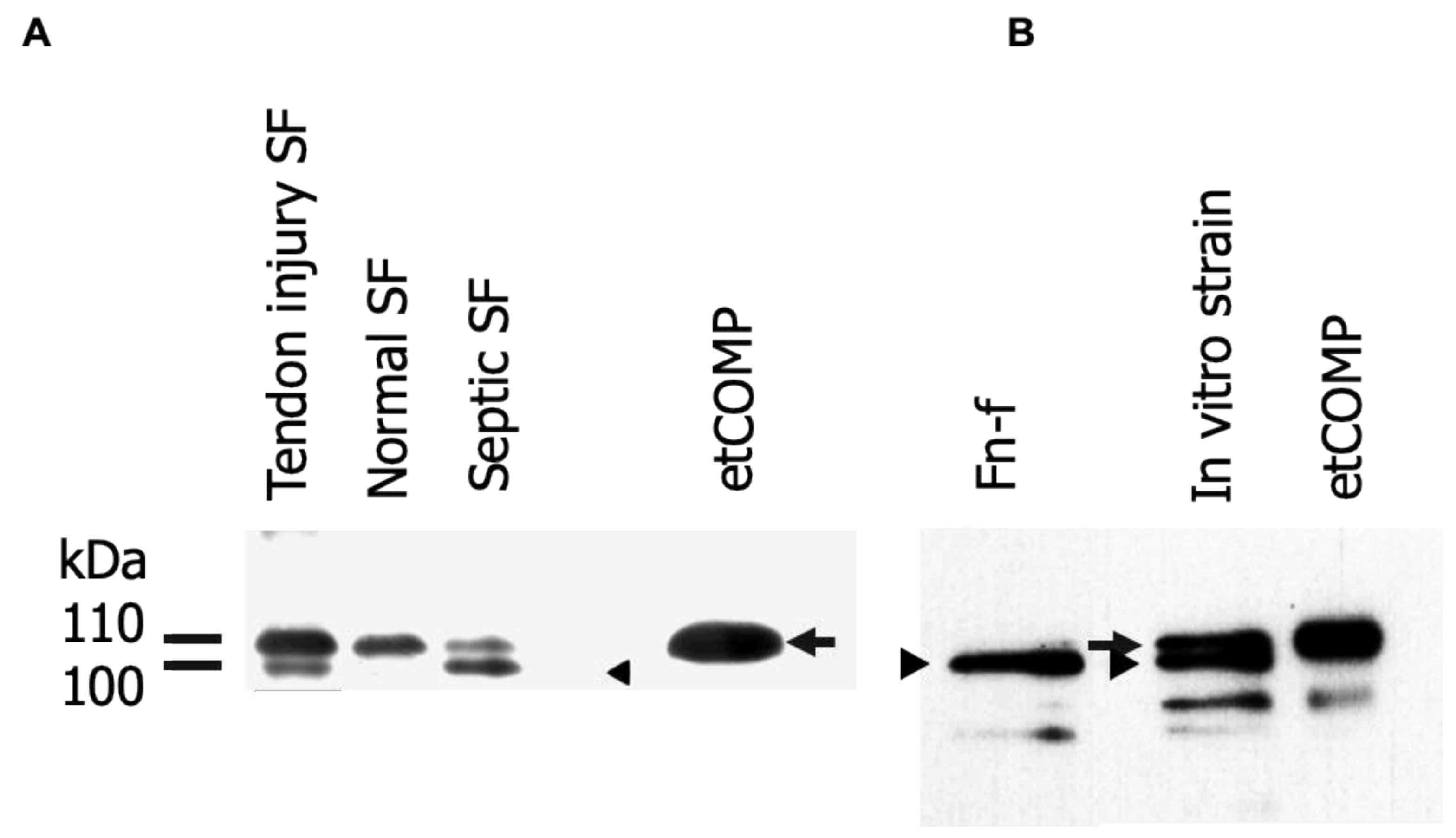
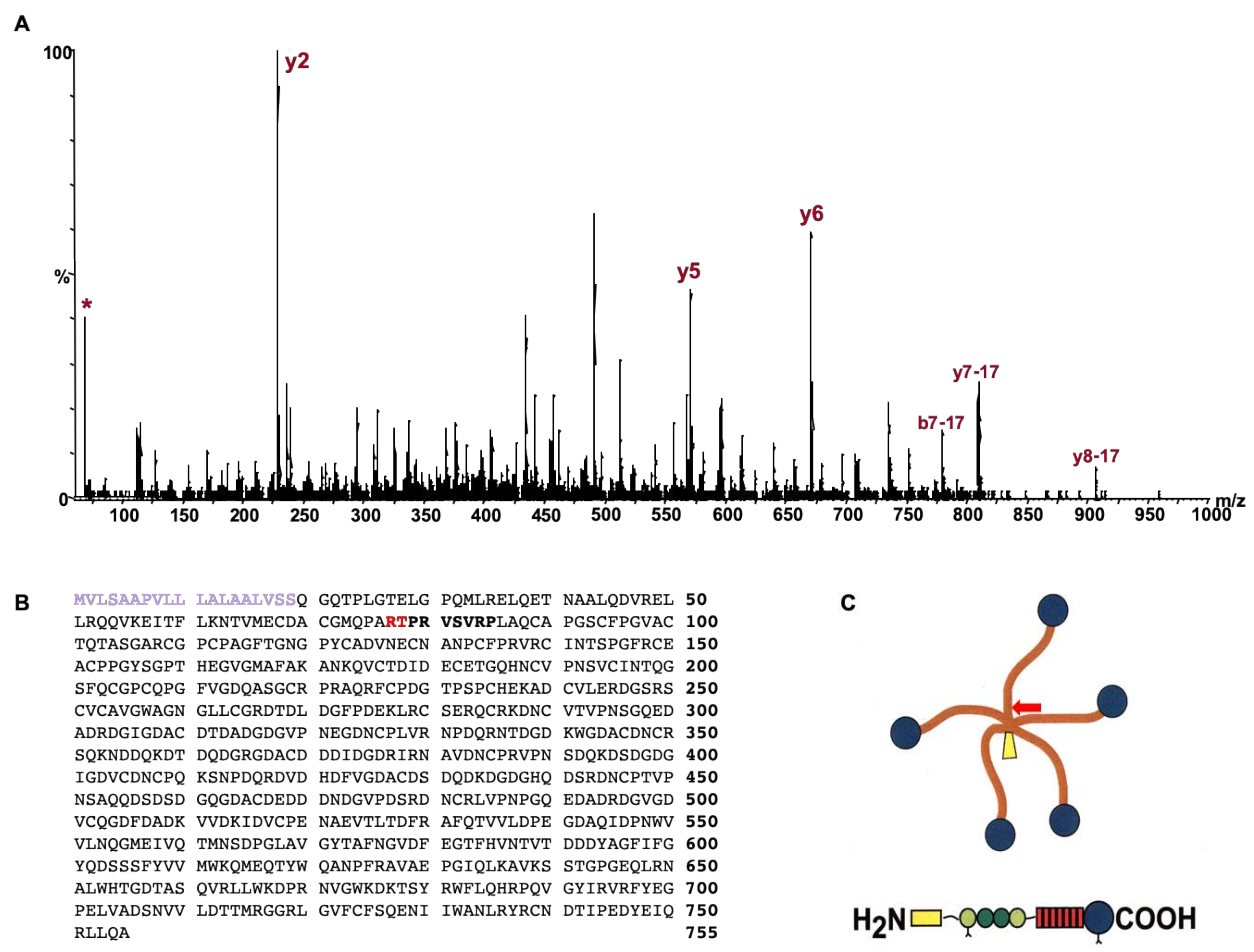
2.1. Identification and Analysis of Cartilage Oligomeric Matrix Protein (COMP) Fragments
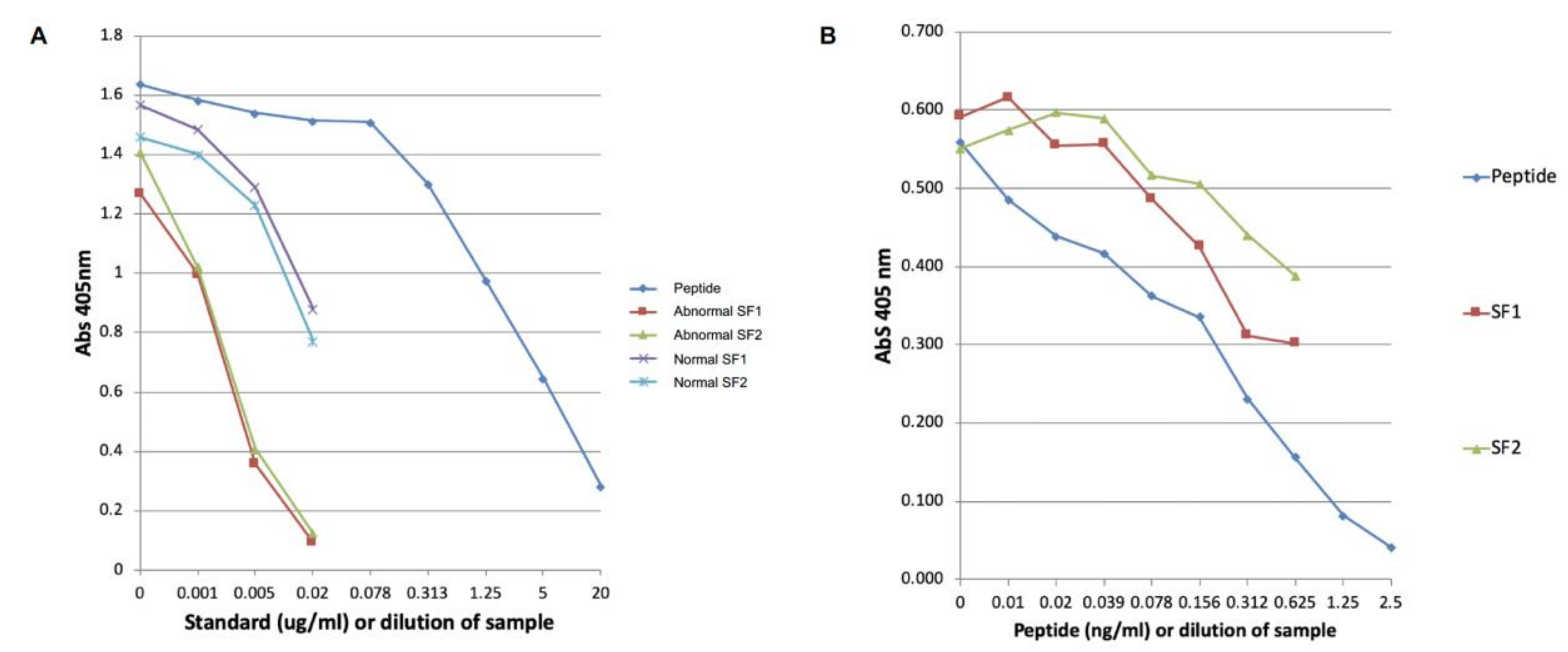
2.2. Development of the COMP Neoepitope Assay
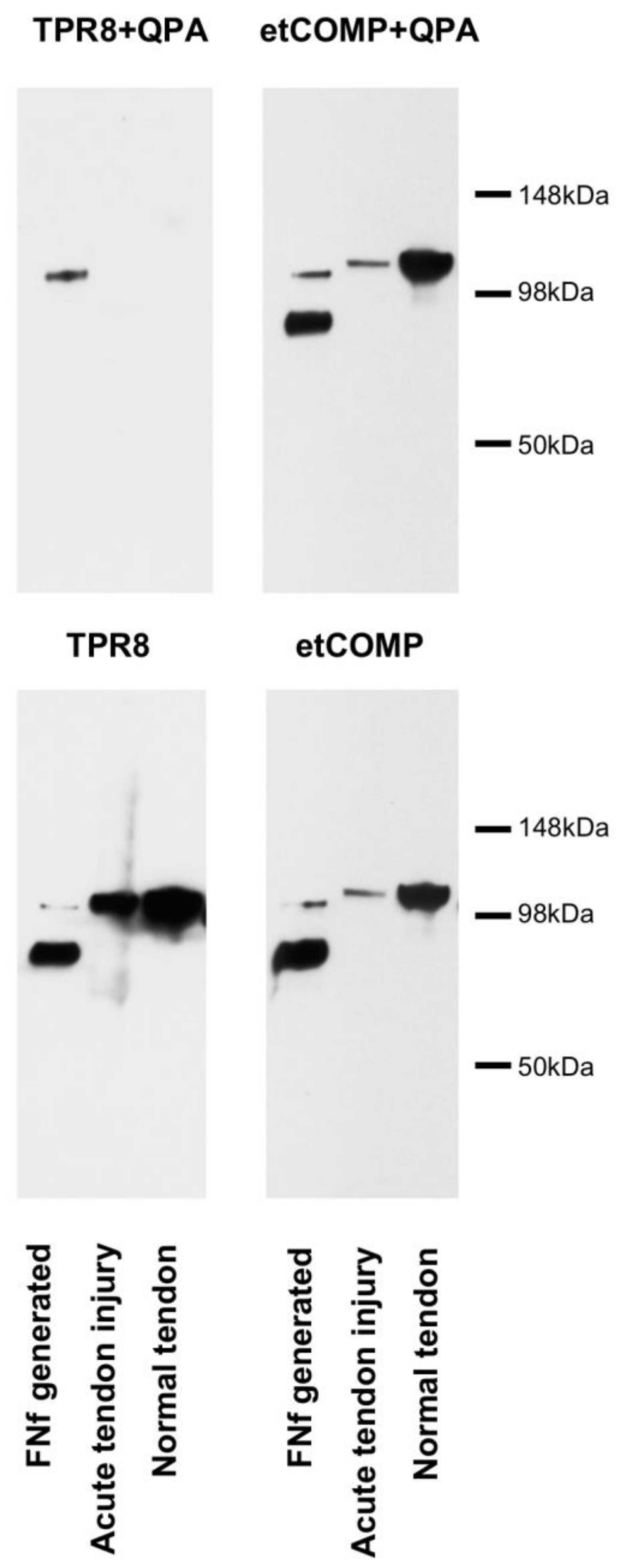
2.3. Cross-Reactivity of the Neo-epitope Antibody with Intact COMP
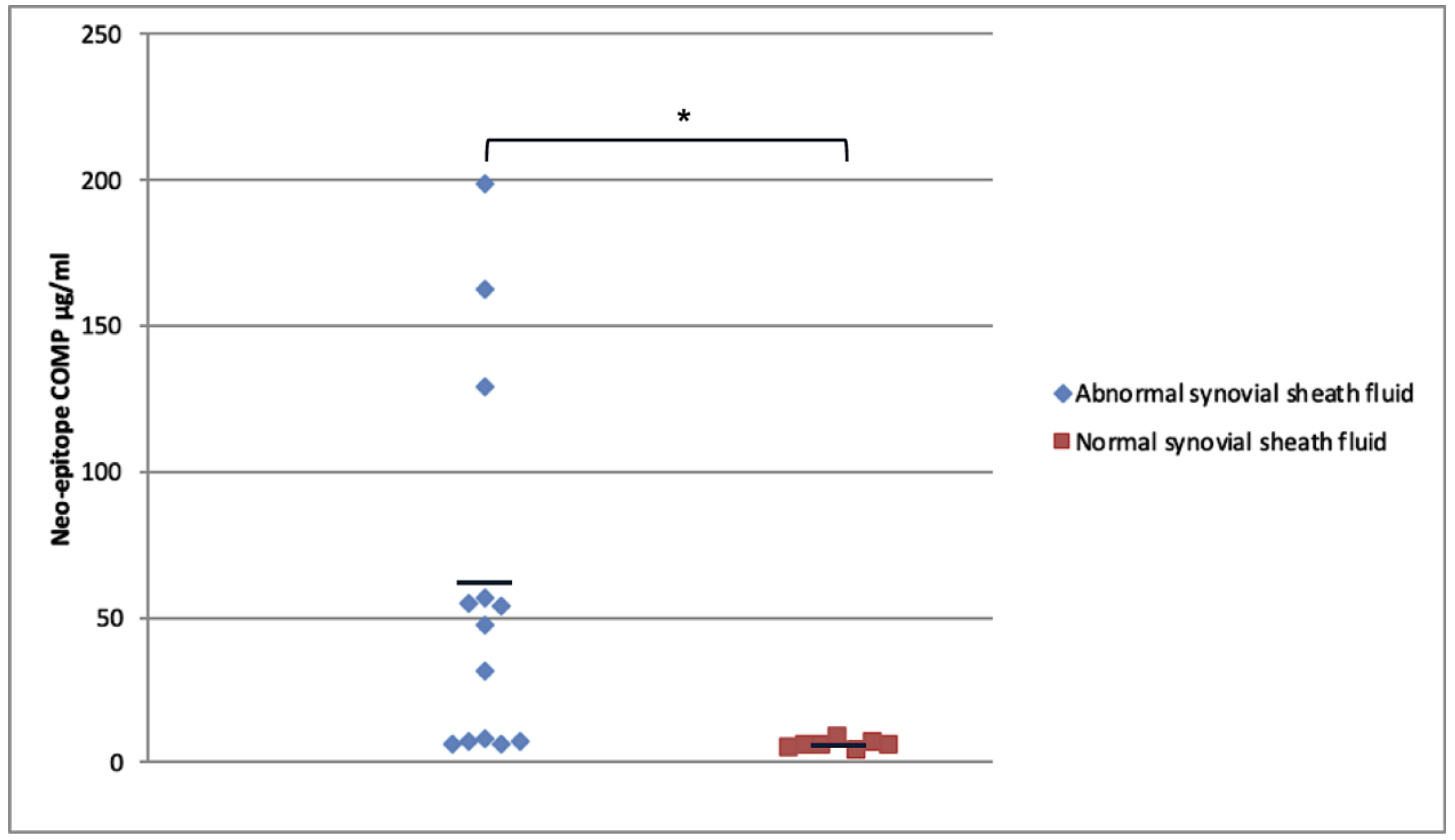
2.4. Modified COMP Neo-Epitope Assay
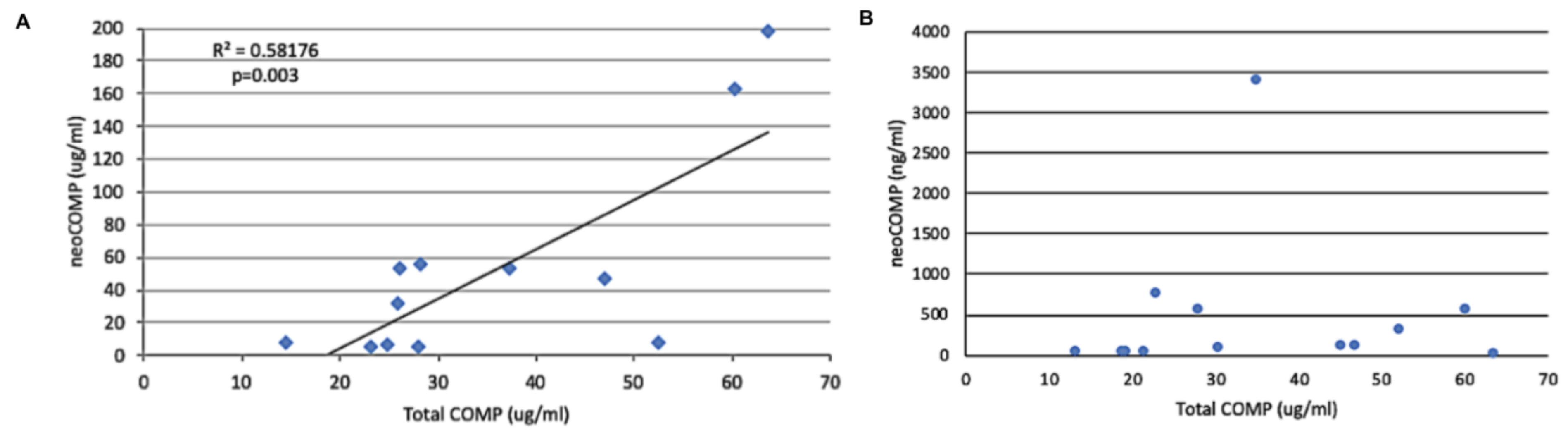
2.5. Relationship of Neo-Epitope Concentration to Total COMP
3. Discussion
4. Materials and Methods
4.1. Determination of COMP Cleavage Site
4.2. Identification of Cleavage Site Using Mass Spectrometry
4.3. Generation of Neo-Epitope Antiserum
4.4. Quantification of NeoCOMP in Digital Sheath Synovial Fluid
4.5. Quantification of Total COMP in the Same Tendon Sheath Synovial Fluids
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Avella, C.S.; Ely, E.R.; Verheyen, K.L.; Price, J.S.; Wood, J.L.; Smith, R.K. Ultrasonographic assessment of the superficial digital flexor tendons of National Hunt racehorses in training over two racing seasons. Equine Vet. J. 2009, 41, 449–454. [Google Scholar] [CrossRef]
- Suchak, A.A.; Bostick, G.; Reid, D.; Blitz, S.; Jomha, N. The incidence of Achilles tendon ruptures in Edmonton, Canada. Foot Ankle Int. 2005, 26, 932–936. [Google Scholar] [CrossRef]
- Kujala, U.M.; Sarna, S.; Kaprio, J. Cumulative incidence of achilles tendon rupture and tendinopathy in male former elite athletes. Clin. J. Sport Med. 2005, 15, 133–135. [Google Scholar] [CrossRef] [PubMed]
- Jarvinen, T.A.; Kannus, P.; Maffulli, N.; Khan, K.M. Achilles tendon disorders: Etiology and epidemiology. Foot Ankle Clin. 2005, 10, 255–266. [Google Scholar] [CrossRef] [PubMed]
- Patterson-Kane, J.C.; Rich, T. Achilles tendon injuries in elite athletes: Lessons in pathophysiology from their equine counterparts. ILAR J. 2014, 55, 86–99. [Google Scholar] [CrossRef] [PubMed]
- Lui, P.P.; Maffulli, N.; Rolf, C.; Smith, R.K. What are the validated animal models for tendinopathy? Scand J. Med. Sci. Sports 2011, 21, 3–17. [Google Scholar] [CrossRef]
- Happonen, K.E.; Heinegard, D.; Saxne, T.; Blom, A.M. Interactions of the complement system with molecules of extracellular matrix: Relevance for joint diseases. Immunobiology 2012, 217, 1088–1096. [Google Scholar] [CrossRef] [PubMed]
- Garvican, E.R.; Salavati, M.; Smith, R.K.W.; Dudhia, J. Exposure of a tendon extracellular matrix to synovial fluid triggers endogenous and engrafted cell death: A mechanism for failed healing of intrathecal tendon injuries. Connect. Tissue Res. 2017, 58, 438–446. [Google Scholar] [CrossRef]
- Smith, M.R.; Wright, I.M.; Smith, R.K. Endoscopic assessment and treatment of lesions of the deep digital flexor tendon in the navicular bursae of 20 lame horses. Equine Vet. J. 2007, 39, 18–24. [Google Scholar] [CrossRef]
- Smith, M.R.; Wright, I.M. Noninfected tenosynovitis of the digital flexor tendon sheath: A retrospective analysis of 76 cases. Equine Vet. J. 2006, 38, 134–141. [Google Scholar] [CrossRef]
- Smith, M.R.W.; Wright, I.M.; Smith, R.K.W.; Bladon, B.; Schramme, M.C. Treatment options for DDFT lesions: Evidence based results. In Proceedings of the 2009 ECVS Annual Conference, Nantes, France, 2–4 July 2009. [Google Scholar]
- Young-Min, S.; Cawston, T.; Marshall, N.; Coady, D.; Christgau, S.; Saxne, T.; Robins, S.; Griffiths, I. Biomarkers predict radiographic progression in early rheumatoid arthritis and perform well compared with traditional markers. Arthritis Rheum. 2007, 56, 3236–3247. [Google Scholar] [CrossRef] [PubMed]
- Lindqvist, E.; Eberhardt, K.; Bendtzen, K.; Heinegard, D.; Saxne, T. Prognostic laboratory markers of joint damage in rheumatoid arthritis. Ann. Rheum. Dis. 2005, 64, 196–201. [Google Scholar] [CrossRef] [PubMed]
- Mansson, B.; Geborek, P.; Saxne, T. Cartilage and bone macromolecules in knee joint synovial fluid in rheumatoid arthritis: Relation to development of knee or hip joint destruction. Ann. Rheum. Dis. 1997, 56, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Mansson, B.; Carey, D.; Alini, M.; Ionescu, M.; Rosenberg, L.C.; Poole, A.R.; Heinegard, D.; Saxne, T. Cartilage and bone metabolism in rheumatoid arthritis. Differences between rapid and slow progression of disease identified by serum markers of cartilage metabolism. J. Clin. Investig. 1995, 95, 1071–1077. [Google Scholar] [CrossRef]
- Saxne, T.; Heinegard, D. Cartilage oligomeric matrix protein: A novel marker of cartilage turnover detectable in synovial fluid and blood. Br. J. Rheumatol. 1992, 31, 583–591. [Google Scholar] [CrossRef]
- Ahrman, E.; Lorenzo, P.; Holmgren, K.; Grodzinsky, A.J.; Dahlberg, L.E.; Saxne, T.; Heinegard, D.; Onnerfjord, P. Novel cartilage oligomeric matrix protein (COMP) neoepitopes identified in synovial fluids from patients with joint diseases using affinity chromatography and mass spectrometry. J. Biol. Chem. 2014, 289, 20908–20916. [Google Scholar] [CrossRef]
- Lorenzo, P.; Aspberg, A.; Saxne, T.; Onnerfjord, P. Quantification of cartilage oligomeric matrix protein (COMP) and a COMP neoepitope in synovial fluid of patients with different joint disorders by novel automated assays. Osteoarthr. Cartil. 2017, 25, 1436–1442. [Google Scholar] [CrossRef]
- Arai, K.; Misumi, K.; Carter, S.D.; Shinbara, S.; Fujiki, M.; Sakamoto, H. Analysis of cartilage oligomeric matrix protein (COMP) degradation and synthesis in equine joint disease. Equine Vet. J. 2005, 37, 31–36. [Google Scholar] [CrossRef]
- Arai, K.; Tagami, M.; Hatazoe, T.; Nishimatsu, E.; Shimizu, Y.; Fujiki, M.; Misumi, K. Analysis of cartilage oligomeric matrix protein (COMP) in synovial fluid, serum and urine from 51 racehorses with carpal bone fracture. J. Vet. Med. Sci. 2008, 70, 915–921. [Google Scholar] [CrossRef]
- Misumi, K.; Tagami, M.; Kamimura, T.; Miyakoshi, D.; Helal, I.E.; Arai, K.; Fujiki, M. Urine cartilage oligomeric matrix protein (COMP) measurement is useful in discriminating the osteoarthritic Thoroughbreds. Osteoarthr. Cartil. 2006, 14, 1174–1180. [Google Scholar] [CrossRef][Green Version]
- Taylor, S.E.; Weaver, M.P.; Pitsillides, A.A.; Wheeler, B.T.; Wheeler-Jones, C.P.; Shaw, D.J.; Smith, R.K. Cartilage oligomeric matrix protein and hyaluronan levels in synovial fluid from horses with osteoarthritis of the tarsometatarsal joint compared to a control population. Equine Vet. J. 2006, 38, 502–507. [Google Scholar] [CrossRef] [PubMed]
- Misumi, K.; Vilim, V.; Hatazoe, T.; Murata, T.; Fujiki, M.; Oka, T.; Sakamoto, H.; Carter, S.D. Serum level of cartilage oligomeric matrix protein (COMP) in equine osteoarthritis. Equine Vet. J. 2002, 34, 602–608. [Google Scholar] [CrossRef]
- Misumi, K.; Vilim, V.; Clegg, P.D.; Thompson, C.C.; Carter, S.D. Measurement of cartilage oligomeric matrix protein (COMP) in normal and diseased equine synovial fluids. Osteoarthr. Cartil. 2001, 9, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Yamanokuchi, K.; Tagami, M.; Nishimatsu, E.; Shimizu, Y.; Hirose, Y.; Komatsu, K.; Misumi, K. Sandwich ELISA system for cartilage oligomeric matrix protein in equine synovial fluid and serum. Equine Vet. J. 2009, 41, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Skioldebrand, E.; Lorenzo, P.; Zunino, L.; Rucklidge, G.J.; Sandgren, B.; Carlsten, J.; Ekman, S. Concentration of collagen, aggrecan and cartilage oligomeric matrix protein (COMP) in synovial fluid from equine middle carpal joints. Equine Vet. J. 2001, 33, 394–402. [Google Scholar] [CrossRef]
- Skioldebrand, E.; Heinegard, D.; Eloranta, M.L.; Nilsson, G.; Dudhia, J.; Sandgren, B.; Ekman, S. Enhanced concentration of COMP (cartilage oligomeric matrix protein) in osteochondral fractures from racing Thoroughbreds. J. Orthop. Res. 2005, 23, 156–163. [Google Scholar] [CrossRef]
- Skioldebrand, E.; Ekman, S.; Mattsson Hulten, L.; Svala, E.; Bjorkman, K.; Lindahl, A.; Lundqvist, A.; Onnerfjord, P.; Sihlbom, C.; Ruetschi, U. Cartilage oligomeric matrix protein neoepitope in the synovial fluid of horses with acute lameness: A new biomarker for the early stages of osteoarthritis. Equine Vet. J. 2017, 49, 662–667. [Google Scholar] [CrossRef]
- Smith, R.K.; Zunino, L.; Webbon, P.M.; Heinegard, D. The distribution of cartilage oligomeric matrix protein (COMP) in tendon and its variation with tendon site, age and load. Matrix Biol. 1997, 16, 255–271. [Google Scholar] [CrossRef]
- Smith, R.K.; Birch, H.L.; Goodman, S.; Heinegard, D.; Goodship, A.E. The influence of ageing and exercise on tendon growth and degeneration—Hypotheses for the initiation and prevention of strain-induced tendinopathies. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2002, 133, 1039–1050. [Google Scholar] [CrossRef]
- Smith, R.K.; Birch, H.; Patterson-Kane, J.; Firth, E.C.; Williams, L.; Cherdchutham, W.; van Weeren, P.; Goodship, A.E. Should equine athletes commence training during skeletal development? Changes in tendon matrix associated with development, ageing, function and exercise. Equine Vet. J. Suppl. 1999, 30, 201–209. [Google Scholar] [CrossRef]
- Smith, R.K.; Heinegard, D. Cartilage oligomeric matrix protein (COMP) levels in digital sheath synovial fluid and serum with tendon injury. Equine Vet. J. 2000, 32, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.R.; Wright, I.M.; Minshall, G.J.; Dudhia, J.; Verheyen, K.; Heinegard, D.; Smith, R.K. Increased cartilage oligomeric matrix protein concentrations in equine digital flexor tendon sheath synovial fluid predicts intrathecal tendon damage. Vet. Surg. 2011, 40, 54–58. [Google Scholar] [CrossRef] [PubMed]
- Johnson, A.; Smith, R.; Saxne, T.; Hickery, M.; Heinegard, D. Fibronectin fragments cause release and degradation of collagen-binding molecules from equine explant cultures. Osteoarthr. Cartil. 2004, 12, 149–159. [Google Scholar] [CrossRef] [PubMed]
- Dakin, S.G.; Smith, R.K.; Heinegard, D.; Onnerfjord, P.; Khabut, A.; Dudhia, J. Proteomic analysis of tendon extracellular matrix reveals disease stage-specific fragmentation and differential cleavage of COMP (cartilage oligomeric matrix protein). J. Biol. Chem. 2014, 289, 4919–4927. [Google Scholar] [CrossRef]
- Peffers, M.J.; Thornton, D.J.; Clegg, P.D. Characterization of neopeptides in equine articular cartilage degradation. J. Orthop. Res. 2016, 34, 106–120. [Google Scholar] [CrossRef]
- Thur, J.; Rosenberg, K.; Nitsche, D.P.; Pihlajamaa, T.; Ala-Kokko, L.; Heinegard, D.; Paulsson, M.; Maurer, P. Mutations in cartilage oligomeric matrix protein causing pseudoachondroplasia and multiple epiphyseal dysplasia affect binding of calcium and collagen I, II, and IX. J. Biol. Chem. 2001, 276, 6083–6092. [Google Scholar] [CrossRef]
- Danfelter, M.; Onnerfjord, P.; Heinegard, D. Fragmentation of proteins in cartilage treated with interleukin-1: Specific cleavage of type IX collagen by matrix metalloproteinase 13 releases the NC4 domain. J. Biol. Chem. 2007, 282, 36933–36941. [Google Scholar] [CrossRef]
- Gough, M.R.; Munroe, G.A.; Mayhew, G. Urea as a measure of dilution of equine synovial fluid. Equine Vet. J. 2002, 34, 76–79. [Google Scholar] [CrossRef]
- Brink, P.; Smith, R.K.; Tverdal, A.; Dolvik, N.I. Changes in synovial fluid biomarker concentrations following arthroscopic surgery in horses with osteochondritis dissecans of the distal intermediate ridge of the tibia. Am. J. Vet. Res. 2015, 76, 599–607. [Google Scholar] [CrossRef]
- Wright, I.M.; McMahon, P.J. Tenosynovitis associated with longitudinal tears of the digital flexor tendons in horses: A report of 20 cases. Equine Vet. J. 1999, 31, 12–18. [Google Scholar] [CrossRef]
- Jackson, B.E.; Smith, R.K.; Price, J.S. A molecular marker of type I collagen metabolism reflects changes in connective tissue remodelling associated with injury to the equine superficial digital flexor tendon. Equine Vet. J. 2003, 35, 211–213. [Google Scholar] [CrossRef] [PubMed]

| Tendon Injury | Age (Years) | DFTS Pathology | Total COMP Assay | Neoepitope Assay | Modified Neoepitope Assay |
|---|---|---|---|---|---|
| 1 | 11 | MF | x | x | |
| 2 | 9 | DDFT | x | x | |
| 3 | 13 | DDFT | x | x | |
| 4 | 7 | DDFT | x | x | |
| 5 | 19 | MF | x | x | |
| 6 | 7 | DDFT | x | x | x |
| 7 | 14 | DDFT | x | x | x |
| 8 | 10 | MF | x | x | x |
| 9 | 14 | MF | x | x | |
| 10 | 12 | MF | x | x | |
| 11 | 4 | DDFT | x | x | |
| 12 | ND | MF | x | x | x |
| 13 | 11 | DDFT | x | x | |
| 14 | 15 | MF | x | x | |
| 15 | ND | MF | x | x | x |
| 16 | 5 | DDFT (core) | x | x | x |
| 17 | 16 | SDFT | x | x | x |
| 18 | 12 | MF | x | x | |
| 19 | ND | DDFT | x | x | |
| 20 | ND | DDFT | x | x | |
| n | 20 | 13 | 14 | ||
| Controls | |||||
| 1 | Aged | Normal | x | x | |
| 2 | Middle | Normal | x | x | |
| 3 | Middle | Normal | x | x | |
| 4 | Middle | Normal | x | x | |
| 5 | Middle | Normal | x | x | |
| 6 | Middle | Normal | x | x | |
| 7 | Aged | Normal | x | x | |
| 8 | Aged | Normal | x | x | |
| 9 | 1 | Normal | x | x | |
| 10 | 3 | Normal | x | x | |
| 11 | Middle | Normal | x | x | |
| 12 | 4 | Normal | x | x | |
| 13 | ND | Normal | x | x | |
| n | 13 | 7 | 6 | ||
| Total Number of Samples | 33 | 20 | 20 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Smith, R.; Önnerfjord, P.; Holmgren, K.; di Grado, S.; Dudhia, J. Development of a Cartilage Oligomeric Matrix Protein Neo-Epitope Assay for the Detection of Intra-Thecal Tendon Disease. Int. J. Mol. Sci. 2020, 21, 2155. https://doi.org/10.3390/ijms21062155
Smith R, Önnerfjord P, Holmgren K, di Grado S, Dudhia J. Development of a Cartilage Oligomeric Matrix Protein Neo-Epitope Assay for the Detection of Intra-Thecal Tendon Disease. International Journal of Molecular Sciences. 2020; 21(6):2155. https://doi.org/10.3390/ijms21062155
Chicago/Turabian StyleSmith, Roger, Patrik Önnerfjord, Kristin Holmgren, Shacko di Grado, and Jayesh Dudhia. 2020. "Development of a Cartilage Oligomeric Matrix Protein Neo-Epitope Assay for the Detection of Intra-Thecal Tendon Disease" International Journal of Molecular Sciences 21, no. 6: 2155. https://doi.org/10.3390/ijms21062155
APA StyleSmith, R., Önnerfjord, P., Holmgren, K., di Grado, S., & Dudhia, J. (2020). Development of a Cartilage Oligomeric Matrix Protein Neo-Epitope Assay for the Detection of Intra-Thecal Tendon Disease. International Journal of Molecular Sciences, 21(6), 2155. https://doi.org/10.3390/ijms21062155





