CXCL-8 in Preoperative Colorectal Cancer Patients: Significance for Diagnosis and Cancer Progression
Abstract
1. Introduction
2. Patients and Methods
Statistical Analysis
3. Results
3.1. Serum Levels of CXCL-8, CEA, and CRP in Colorectal Cancer Patients
3.2. Relationship Between Serum Levels of CXCL-8, CEA, and CRP in CRC Patients and Clinicopathological Features of CRC
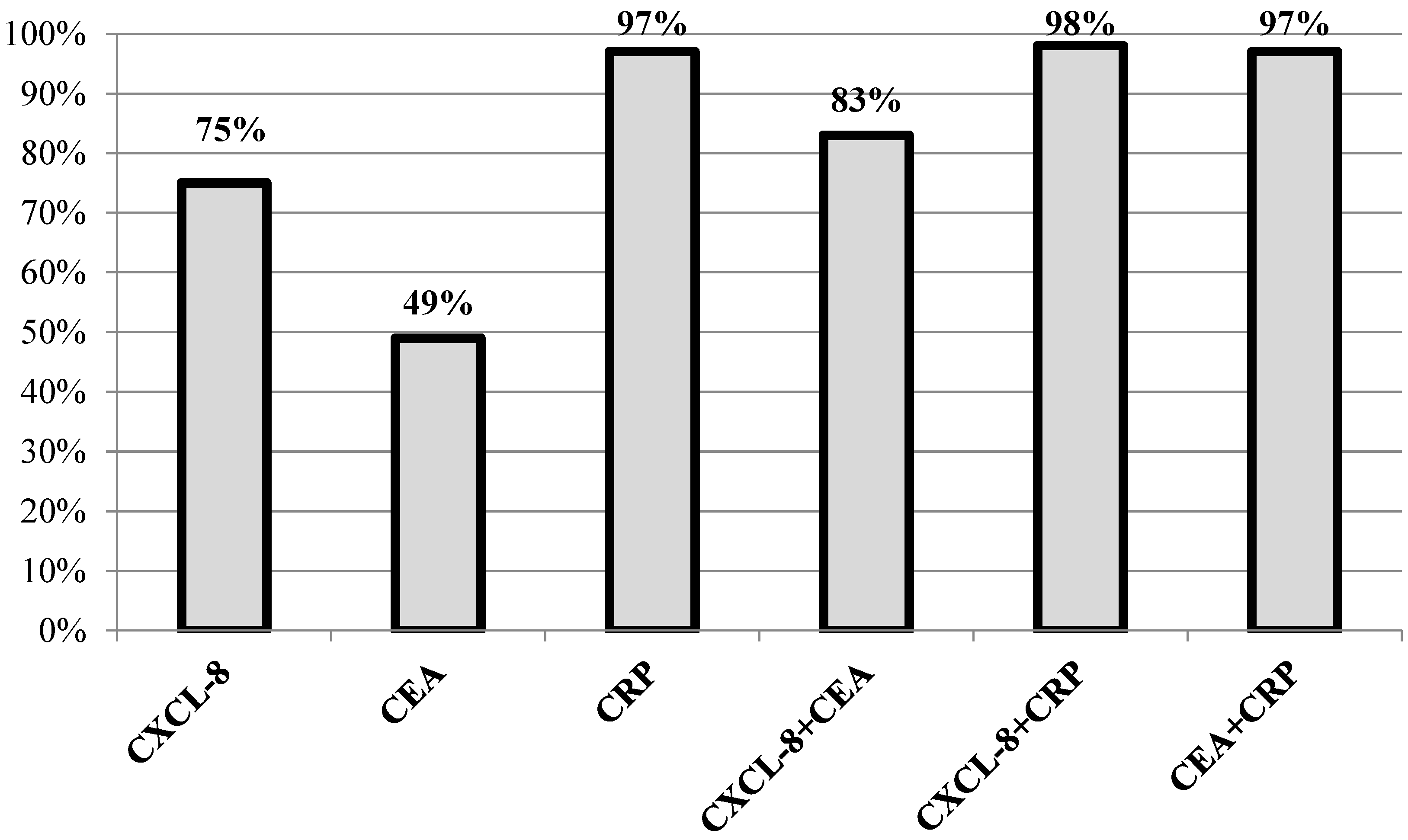
3.3. Diagnostic Usefulness of CXCL-8 in CRC Diagnosis
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Aran, V.; Victorino, A.P.; Thuler, L.C.; Gil Ferreira, C. Colorectal cancer: Epidemiology, disease mechanisms and interventions to reduce onset and mortality. Clin. Color. Cancer 2016, 15, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Rawla, P.; Sunkara, T.; Barsouk, A. Epidemiology of colorectal cancer: Incidence, mortality, survival, and risk factors. Gastroenterol. Rev. 2019, 14, 89–103. [Google Scholar] [CrossRef] [PubMed]
- Ferlay, J.; Soerjomataram, I.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Parkin, N.M.; Forman, D.; Bray, F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer 2014, 136, 359–386. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2019. CA Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef]
- Hadjipetrou, A.; Anyfantakis, D.; Galanakis, C.G.; Kastanakis, M.; Kastanakis, S. Colorectal cancer, screening and primary care: A mini literature review. World J. Gastroenterol. 2017, 23, 6049–6058. [Google Scholar] [CrossRef]
- Pérez-Escalante, E.; Cariño-Cortés, R.; Fernández-Martínez, E.; Ortiz, M.I.; Muñoz-Pérez, V.M.; Sánchez-Crisóstomo, I.; Jiménez-Ángeles, L.; Emmanuel, P.-E.; Raquel, C.-C.; Eduardo, F.-M.; et al. Colorectal cancer: Causes and evidence of chemopreventive treatments. Curr. Pharm. Biotechnol. 2019, 19, 1135–1155. [Google Scholar] [CrossRef]
- Fearon, E.R.; Vogelstein, B. A genetic model for colorectal tumorigenesis. Cell 1990, 61, 759–767. [Google Scholar] [CrossRef]
- Heras, S.C.-D.L.; Martínez-Balibrea, E. CXC family of chemokines as prognostic or predictive biomarkers and possible drug targets in colorectal cancer. World J. Gastroenterol. 2018, 24, 4738–4749. [Google Scholar] [CrossRef]
- Raman, D.; Baugher, P.J.; Thu, Y.M.; Richmond, A. Role of chemokines in tumor growth. Cancer Lett. 2007, 256, 137–165. [Google Scholar] [CrossRef]
- Miller, M.C.; Mayo, K.H. Chemokines from a structural perspective. Int. J. Mol. Sci. 2017, 18, 2088. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.-J.; Du, Y.; Zhao, X.; Ma, L.-Y.; Cao, G. Inflammation-related factors predicting prognosis of gastric cancer. World J. Gastroenterol. 2014, 20, 4586–4596. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.S.; Choi, I.; Ning, Y.; Kim, N.Y.; Khatchadourian, V.; Yang, D.; Chung, H.K.; Choi, D.; LaBonte, M.J.; Ladner, R.D.; et al. Interleukin-8 and its receptor CXCR2 in the tumour microenvironment promote colon cancer growth, progression and metastasis. Br. J. Cancer 2012, 106, 1833–1841. [Google Scholar] [CrossRef] [PubMed]
- Dekker, E.; Rex, D.K. Advances in CRC prevention: Screening and surveillance. Gastroenterology 2018, 154, 1970–1984. [Google Scholar] [CrossRef] [PubMed]
- Łukaszewicz-Zając, M.; Mroczko, B.; Kozłowski, M.; Szmitkowski, M. The serum concentrations of chemokine CXCL12 and its specific receptor CXCR4 in patients with esophageal cancer. Dis. Markers 2016, 2016, 1–7. [Google Scholar] [CrossRef][Green Version]
- Łukaszewicz-Zając, M.; Pączek, S.; Muszyński, P.; Kozłowski, M.; Mroczko, B. Comparison between clinical significance of serum CXCL-8 and classical tumor markers in oesophageal cancer (OC) patients. Clin. Exp. Med. 2019, 19, 191–199. [Google Scholar] [CrossRef]
- Łukaszewicz-Zając, M.; Muszyński, P.; Kozłowski, M.; Kulczynska-Przybik, A.; Szmitkowski, M.; Mroczko, B. Serum concentrations of receptor for interleukin 8 in patients with esophageal cancer. Pol. Arch. Med. Wewn. 2016, 126, 854–861. [Google Scholar] [CrossRef]
- Litman-Zawadzka, A.; Łukaszewicz-Zając, M.; Gryko, M.; Kulczyńska-Przybik, A.; Mroczko, B. Serum chemokine CXCL-8 as a better biomarker for diagnosis and prediction of pancreatic cancer than its specific receptor CXCR-2, CRP and classical tumor markers (CA 19-9 and CEA). Pol. Arch. Intern. Med. 2018, 128, 524–531. [Google Scholar] [CrossRef]
- Guzik-Makaruk, E.M.; Plywaczewski, E.W.; Mroczko, P.; Olesiuk-Okomska, M.; Kulczyńska-Przybik, A. Consent to medical procedures of patients with neurodegenerative diseases: A comparative study of legal regulations in selected European countries and in the United States. J. Alzheimer’s Dis. 2018, 63, 53–67. [Google Scholar] [CrossRef]
- Guzik-Makaruk, E.M.; Pływaczewski, E.W.; Laskowska, K.; Filipkowski, W.; Jurgielewicz-Delegacz, E.; Mroczko, P. A comparative analysis of the treatment of decision-making by or for patients with neurodegenerative diseases in four legal jurisdictions. J. Alzheimer’s Dis. 2019, 70, 1–10. [Google Scholar] [CrossRef]
- Garborg, K. Colorectal cancer screening. Surg. Clin. N. Am. 2015, 95, 979–989. [Google Scholar] [CrossRef]
- Karin, N. Chemokines and cancer: New immune checkpoints for cancer therapy. Curr. Opin. Immunol. 2018, 51, 140–145. [Google Scholar] [CrossRef] [PubMed]
- Bie, Y.; Ge, W.; Yang, Z.; Cheng, X.; Zhao, Z.; Li, S.; Wang, W.; Wang, Y.; Zhao, X.; Yin, Z.; et al. The crucial role of CXCL8 and its receptors in colorectal liver metastasis. Dis. Markers 2019, 2019, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Łukaszewicz-Zając, M.; Gryko, M.; Pączek, S.; Szmitkowski, M.; Kędra, B.; Mroczko, B. Matrix metalloproteinase 2 (MMP-2) and its tissue inhibitor 2 (TIMP-2) in pancreatic cancer (PC). Oncotarget 2019, 10, 395–403. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Łukaszewicz-Zając, M.; Szmitkowski, M.; Litman-Zawadzka, A.; Mroczko, B. Matrix metalloproteinases and their tissue inhibitors in comparison to other inflammatory proteins in gastric cancer (GC). Cancer Investig. 2016, 34, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Sgourakis, G.; Papapanagiotou, A.; Kontovounisios, C.; Karamouzis, M.V.; Dedemadi, G.; Goumas, C.; Karaliotas, C.; Papavassiliou, A.G. The combined use of serum neurotensin and IL-8 as screening markers for colorectal cancer. Tumor Boil. 2014, 35, 5993–6002. [Google Scholar] [CrossRef] [PubMed]
- Oladipo, O.; Conlon, S.; O’Grady, A.; Purcell, C.; Wilson, C.; Maxwell, P.J.; Johnston, P.G.; Stevenson, M.; Kay, E.W.; Wilson, R.H.; et al. The expression and prognostic impact of CXC-chemokines in stage II and III colorectal cancer epithelial and stromal tissue. Br. J. Cancer 2011, 104, 480–487. [Google Scholar] [CrossRef]
- Rubie, C.; Frick, V.O.; Pfeil, S.; Wagner, M.; Kollmar, O.; Kopp, B.; Gräber, S.; Rau, B.M.; Schilling, M.K. Correlation of IL-8 with induction, progression and metastatic potential of colorectal cancer. World J. Gastroenterol. 2007, 13, 4996–5002. [Google Scholar] [CrossRef]
- Kaminska, J.; Nowacki, M.; Kowalska, M.; Rysinska, A.; Chwalinski, M.; Fuksiewicz, M.; Michalski, W.; Chechlinska, M. Clinical significance of serum cytokine measurements in untreated colorectal cancer patients: Soluble tumor necrosis factor receptor type I—An independent prognostic factor. Tumor Boil. 2005, 26, 186–194. [Google Scholar] [CrossRef]
- Ueda, T.; Shimada, E.; Urakawa, T. Serum levels of cytokines in patients with colorectal cancer: Possible involvement of interleukin-6 and interleukin-8 in hematogenous metastasis. J. Gastroenterol. 1994, 29, 423–429. [Google Scholar] [CrossRef]
- Csiszár, A.; Szentes, T.; Haraszti, B.; Balázs, A.; Petrányi, G.G.; Pócsik, E. The pattern of cytokine gene expression in human colorectal carcinoma. Pathol. Oncol. Res. 2004, 10, 109–116. [Google Scholar] [CrossRef]
- Erreni, M.; Bianchi, P.; Laghi, L.; Mirolo, M.; Fabbri, M.; Locati, M.; Mantovani, A.; Allavena, P. Chapter 5 expression of chemokines and chemokine receptors in human colon cancer. Methods Enzymol. 2009, 460, 105–121. [Google Scholar] [PubMed]
- Baier, P.K.; Eggstein, S.; Wolff-Vorbeck, G.; Baumgartner, U.; Hopt, U.T. Chemokines in human colorectal carcinoma. Anticancer. Res. 2005, 25, 3581–3584. [Google Scholar] [PubMed]




| Colorectal Cancer Group | Number of Patients | |
|---|---|---|
| Gender | Male | 30 |
| Female | 29 | |
| TNM stage | I + II | 25 |
| III | 23 | |
| IV | 11 | |
| Depth of tumor invasion (T-factor) | T1 + T2 | 6 |
| T3 | 47 | |
| T4 | 6 | |
| Lymph node metastases (N-factor) | N0 | 26 |
| N1 + N2 | 33 | |
| Distant metastases (M-factor) | M0 | 47 |
| M1 | 12 | |
| CXCL-8 [pg/mL] | CRP [mg/L] | CEA [ng/mL] | ||
|---|---|---|---|---|
| Control group n = 46 | Minimum | 0.000 | 0.50 | 0.2 |
| Median | 8.61450 | 1.2150 | 1.050 | |
| Maximum | 72.312 | 4.54 | 5.0 | |
| Colorectal cancer group n = 59 | Minimum | 0.000 | 0.50 | 0.4 |
| Median | 22.18600 | 3.2000 | 8.800 | |
| Maximum | 1540.375 | 1500.00 | 202.3 | |
| p (Mann–Whitney test) | p < 0.001 | p < 0.001 | p < 0.001 | |
| Colorectal Cancer Group | CXCL-8 (pg/mL) | CRP (mg/L) | CEA (ng/mL) | ||
|---|---|---|---|---|---|
| TNM (Tumor stage) | 1 + 2 n = 25 | Min | 0.000 | 0.4 | 0.50 |
| Me | 25.43300 | 9.200 | 1.6900 | ||
| Max | 174.082 | 159.2 | 118.68 | ||
| 3 n = 23 | Min | 0.000 | 1.1 | 0.69 | |
| Me | 14.84200 | 5.000 | 3.0700 | ||
| Max | 401.986 | 65.2 | 540.17 | ||
| 4 n = 11 | Min | 15.703 | 3.4 | 1.99 | |
| Me | 39.68100 | 53.700 | 60.3000 | ||
| Max | 1540.375 | 202.3 | 1500.00 | ||
| Kruskal–Wallis test (p) | 0.029 | 0.013 | 0.001 | ||
| T-factor (The depth of tumor invasion) | T1 n = 1 | Min | 27.255 | 2.2 | 3.56 |
| Me | 27.25500 | 2.200 | 3.5600 | ||
| Max | 27.255 | 2.2 | 3.56 | ||
| T2 n = 5 | Min | 16.686 | 0.4 | 0.89 | |
| Me | 19.29700 | 13.600 | 1.1900 | ||
| Max | 92.600 | 57.9 | 18.92 | ||
| T3 n = 47 | Min | 0.000 | 1.1 | 0.50 | |
| Me | 18.73300 | 6.100 | 3.9100 | ||
| Max | 1540.375 | 202.3 | 1500.00 | ||
| T4 n = 6 | Min | 14.842 | 5.3 | 1.05 | |
| Me | 29.49200 | 32.600 | 5.9500 | ||
| Max | 351.515 | 82.1 | 1500.00 | ||
| Kruskal–Wallis test (p) | 0.690 | 0.245 | 0.515 | ||
| N-factor (The presence of lymph node metastases) | N0 n = 26 | Min | 0.000 | 0.4 | 0.50 |
| Me | 20.76900 | 11.100 | 1.7100 | ||
| Max | 174.082 | 159.2 | 118.68 | ||
| N1 + N2 n = 33 | Min | 0.000 | 1.100 | 0.69 | |
| Me | 27.593 | 8.200 | 4.820 | ||
| Max | 1540.375 | 202.3 | 1500.00 | ||
| Mann–Whitney test (p) | 0.652 | 0.737 | 0.005 | ||
| M-factor (The presence of distant metastases) | M0 n = 47 | Min | 0.000 | 0.4 | 0.50 |
| Me | 17.98700 | 5.300 | 2.5900 | ||
| Max | 270.582 | 159.2 | 540.17 | ||
| M1 n = 12 | Min | 15.703 | 3.4 | 1.99 | |
| Me | 58.78200 | 38.900 | 65.9850 | ||
| Max | 1540.375 | 202.3 | 1500.00 | ||
| Mann–Whitney test (p) | 0.004 | 0.009 | p < 0.05 | ||
| T-factor | N-factor | M-factor | TNM | G-factor | Age | CXCL-8 | CEA | CRP | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| CRC group | T-factor | r | 1.00 | 0.25 | 0.28 | 0.35 | 0.01 | −0.12 | 0.08 | 0.18 | 0.24 |
| p | 0.052 | 0.032 | 0.007 | 0.951 | 0.385 | 0.541 | 0.173 | 0.071 | |||
| N-factor | r | 0.25 | 1.00 | 0.35 | 0.75 | −0.15 | 0.07 | 0.14 | 0.42 | 0.03 | |
| p | 0.052 | 0.007 | 0.000 | 0.258 | 0.604 | 0.303 | 0.001 | 0.833 | |||
| M-factor | r | 0.28 | 0.35 | 1.00 | 0.72 | −0.09 | 0.14 | 0.38 | 0.50 | 0.34 | |
| p | 0.032 | 0.007 | 0.000 | 0.496 | 0.300 | 0.003 | 0.000 | 0.008 | |||
| TNM | r | 0.35 | 0.75 | 0.72 | 1.00 | −0.10 | 0.06 | 0.15 | 0.45 | 0.14 | |
| p | 0.007 | 0.000 | 0.000 | 0.458 | 0.646 | 0.260 | 0.000 | 0.304 | |||
| G-factor | r | 0.01 | −0.15 | −0.09 | −0.10 | 1.00 | 0.13 | −0.06 | 0.05 | −0.01 | |
| p | 0.951 | 0.258 | 0.496 | 0.458 | 0.350 | 0.647 | 0.731 | 0.968 | |||
| Age | r | −0.12 | 0.07 | 0.14 | 0.06 | 0.13 | 1.00 | 0.09 | 0.12 | 0.05 | |
| p | 0.385 | 0.604 | 0.300 | 0.646 | 0.350 | 0.478 | 0.376 | 0.707 | |||
| CXCL-8 | r | 0.08 | 0.14 | 0.38 | 0.15 | −0.06 | 0.09 | 1.00 | 0.31 | 0.57 | |
| p | 0.541 | 0.303 | 0.003 | 0.260 | 0.647 | 0.478 | 0.019 | 0.000 | |||
| CEA | r | 0.18 | 0.42 | 0.50 | 0.45 | 0.05 | 0.12 | 0.31 | 1.00 | 0.20 | |
| p | 0.173 | 0.001 | 0.000 | 0.000 | 0.731 | 0.376 | 0.019 | 0.125 | |||
| CRP | r | 0.24 | 0.03 | 0.34 | 0.14 | -0.01 | 0.05 | 0.57 | 0.20 | 1.00 | |
| p | 0.071 | 0.833 | 0.008 | 0.304 | 0.968 | 0.707 | 0.000 | 0.125 | |||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pączek, S.; Łukaszewicz-Zając, M.; Gryko, M.; Mroczko, P.; Kulczyńska-Przybik, A.; Mroczko, B. CXCL-8 in Preoperative Colorectal Cancer Patients: Significance for Diagnosis and Cancer Progression. Int. J. Mol. Sci. 2020, 21, 2040. https://doi.org/10.3390/ijms21062040
Pączek S, Łukaszewicz-Zając M, Gryko M, Mroczko P, Kulczyńska-Przybik A, Mroczko B. CXCL-8 in Preoperative Colorectal Cancer Patients: Significance for Diagnosis and Cancer Progression. International Journal of Molecular Sciences. 2020; 21(6):2040. https://doi.org/10.3390/ijms21062040
Chicago/Turabian StylePączek, Sara, Marta Łukaszewicz-Zając, Mariusz Gryko, Piotr Mroczko, Agnieszka Kulczyńska-Przybik, and Barbara Mroczko. 2020. "CXCL-8 in Preoperative Colorectal Cancer Patients: Significance for Diagnosis and Cancer Progression" International Journal of Molecular Sciences 21, no. 6: 2040. https://doi.org/10.3390/ijms21062040
APA StylePączek, S., Łukaszewicz-Zając, M., Gryko, M., Mroczko, P., Kulczyńska-Przybik, A., & Mroczko, B. (2020). CXCL-8 in Preoperative Colorectal Cancer Patients: Significance for Diagnosis and Cancer Progression. International Journal of Molecular Sciences, 21(6), 2040. https://doi.org/10.3390/ijms21062040









