Association between Pericytes in Intraplaque Neovessels and Magnetic Resonance Angiography Findings
Abstract
1. Introduction
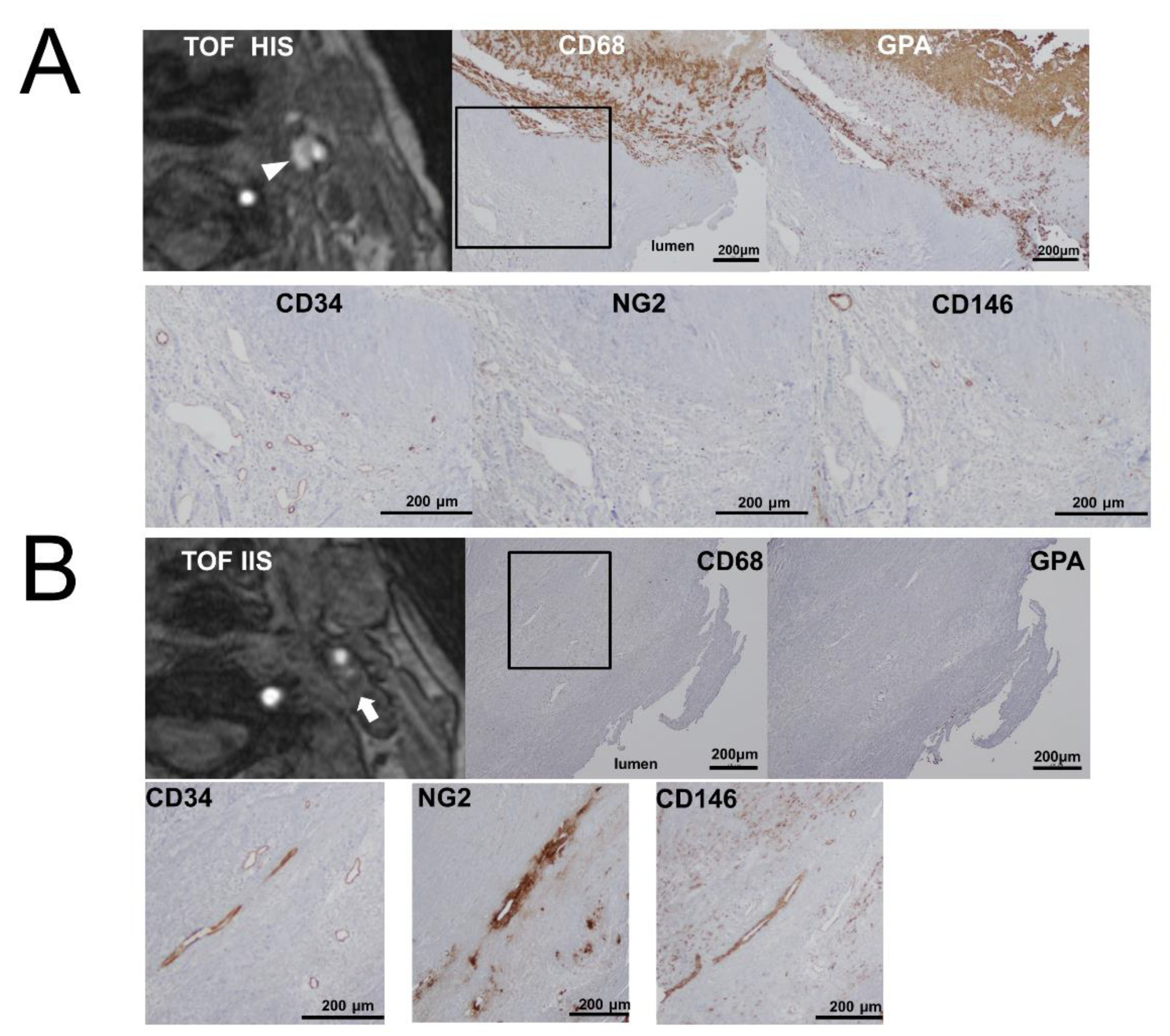
2. Results
3. Discussion
4. Materials and Methods
4.1. Patients and Study Design
4.2. Preoperative Plaque Imaging and Image Analysis
4.3. Immunohistochemical Analysis
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| CD | Cluster of differentiation |
| CEA | Carotid endarterectomy |
| GPA | glycophorin A |
| IPH | intraplaque hemorrhage |
| NG2 | Neuron-glial antigen 2 |
References
- McCarthy, M.J.; Loftus, I.; Thompson, M.; Jones, L.; London, N.J.; Bell, P.R.; Naylor, R.; Brindle, N. Angiogenesis and the atherosclerotic carotid plaque: An association between symptomatology and plaque morphology. J. Vasc. Surg. 1999, 30, 261–268. [Google Scholar] [CrossRef]
- Mofidi, R.; Crotty, T.B.; McCarthy, P.; Sheehan, S.J.; Mehigan, D.; Keaveny, T.V. Association between plaque instability, angiogenesis and symptomatic carotid occlusive disease. BJS 2001, 88, 945–950. [Google Scholar] [CrossRef]
- Hennerici, M.G. The unstable plaque. Cerebrovasc. Dis. 2004, 17 (Suppl. 3), 17–22. [Google Scholar] [CrossRef]
- Kumamoto, M.; Nakashima, Y.; Sueishi, K. Intimal neovascularization in human coronary atherosclerosis: Its origin and pathophysiological significance. Hum. Pathol. 1995, 26, 450–456. [Google Scholar] [CrossRef]
- Oord, S.V.D.; Akkus, Z.; Renaud, G.; Bosch, J.G.; Van Der Steen, A.F.W.; Sijbrands, E.; Schinkel, A.F. Assessment of carotid atherosclerosis, intraplaque neovascularization, and plaque ulceration using quantitative contrast-enhanced ultrasound in asymptomatic patients with diabetes mellitus. Eur. Hear. J. Cardiovasc. Imaging 2014, 15, 1213–1218. [Google Scholar] [CrossRef] [PubMed]
- Vrijenhoek, J.E.P.; Ruijter, H.M.D.; De Borst, G.J.; De Kleijn, D.P.V.; De Vries, J.-P.P.; Bots, M.L.; Van De Weg, S.M.; Vink, A.; Moll, F.L.; Pasterkamp, G.; et al. Sex Is Associated With the Presence of Atherosclerotic Plaque Hemorrhage and Modifies the Relation Between Plaque Hemorrhage and Cardiovascular Outcome. Stroke 2013, 44, 3318–3323. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Ogata, A.; Masuoka, J.; Mizokami, T.; Wakamiya, T.; Nakahara, Y.; Inoue, K.; Shimokawa, S.; Yoshioka, F.; Momozaki, N.; et al. Possible involvement of pericytes in intraplaque hemorrhage of carotid artery stenosis. J. Neurosurg. 2019, 130, 1971–1977. [Google Scholar] [CrossRef] [PubMed]
- Davaine, J.-M.; Quillard, T.; Brion, R.; Laperine, O.; Guyomarch, B.; Merlini, T.; Chatelais, M.; Guilbaud, F.; Brennan, M.Á.; Charrier, C.; et al. Osteoprotegerin, Pericytes and Bone-Like Vascular Calcification Are Associated with Carotid Plaque Stability. PLoS ONE 2014, 9, e107642. [Google Scholar] [CrossRef] [PubMed]
- Armulik, A.; Genové, G.; Betsholtz, C.; Keller, A. Pericytes: Developmental, Physiological, and Pathological Perspectives, Problems, and Promises. Dev. Cell 2011, 21, 193–215. [Google Scholar] [CrossRef]
- Takaya, N.; Yuan, C.; Chu, B.; Saam, T.; Polissar, N.L.; Jarvik, G.P.; Isaac, C.; McDonough, J.; Natiello, C.; Small, R.; et al. Presence of Intraplaque Hemorrhage Stimulates Progression of Carotid Atherosclerotic Plaques. Circulation 2005, 111, 2768–2775. [Google Scholar] [CrossRef]
- Ogata, A.; Kawashima, M.; Wakamiya, T.; Nishihara, M.; Masuoka, J.; Nakahara, Y.; Ebashi, R.; Inoue, K.; Takase, Y.; Irie, H.; et al. Carotid artery stenosis with a high-intensity signal plaque on time-of-flight magnetic resonance angiography and association with evidence of intraplaque hypoxia. J. Neurosurg. 2016, 126, 1873–1878. [Google Scholar] [CrossRef] [PubMed]
- Orekhov, A.N.; Bobryshev, Y.V.; Chistiakov, D.A. The complexity of cell composition of the intima of large arteries: Focus on pericyte-like cells. Cardiovasc. Res. 2014, 103, 438–451. [Google Scholar] [CrossRef] [PubMed]
- Esposito-Bauer, L.; Saam, T.; Ghodrati, I.; Pelisek, J.; Heider, P.; Bauer, M.; Wolf, P.; Bockelbrink, A.; Feurer, R.; Sepp, D.; et al. MRI Plaque Imaging Detects Carotid Plaques with a High Risk for Future Cerebrovascular Events in Asymptomatic Patients. PLoS ONE 2013, 8, e67927. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, S.; Yamada, K.; Kawasaki, M.; Asano, T.; Kanematsu, M.; Takamatsu, M.; Hara, A.; Iwama, T. High-Intensity Signal on Time-of-Flight Magnetic Resonance Angiography Indicates Carotid Plaques at High Risk for Cerebral Embolism During Stenting. Stroke 2011, 42, 3132–3137. [Google Scholar] [CrossRef]
- Omote, Y.; Deguchi, K.; Kono, S.; Liu, N.; Liu, W.; Kurata, T.; Yamashita, T.; Ikeda, Y.; Abe, K. Neurovascular protection of cilostazol in stroke-prone spontaneous hypertensive rats associated with angiogenesis and pericyte proliferation. J. Neurosci. Res. 2013, 92, 369–374. [Google Scholar] [CrossRef]
- Takagi, T.; Imai, T.; Mishiro, K.; Ishisaka, M.; Tsujimoto, M.; Ito, H.; Nagashima, K.; Matsukawa, H.; Tsuruma, K.; Shimazawa, M.; et al. Cilostazol ameliorates collagenase-induced cerebral hemorrhage by protecting the blood–brain barrier. Br. J. Pharmacol. 2016, 37, 123–139. [Google Scholar] [CrossRef]
- Abbott, A. Medical (Nonsurgical) Intervention Alone Is Now Best for Prevention of Stroke Associated With Asymptomatic Severe Carotid Stenosis. Stroke 2009, 40, e573–e583. [Google Scholar] [CrossRef]
- Liem, M.I.; Schreuder, F.H.; Van Dijk, A.C.; De Rotte, A.A.J.; Truijman, M.T.; Daemen, M.J.; Van Der Steen, A.F.; Hendrikse, J.; Nederveen, A.J.; Van Der Lugt, A.; et al. Use of Antiplatelet Agents Is Associated With Intraplaque Hemorrhage on Carotid Magnetic Resonance Imaging. Stroke 2015, 46, 3411–3415. [Google Scholar] [CrossRef]
- Makihara, N.; Arimura, K.; Ago, T.; Tachibana, M.; Nishimura, A.; Nakamura, K.; Matsuo, R.; Wakisaka, Y.; Kuroda, J.; Sugimori, H.; et al. Involvement of platelet-derived growth factor receptor beta in fibrosis through extracellular matrix protein production after ischemic stroke. Exp. Neurol. 2015, 264, 127–134. [Google Scholar] [CrossRef]
- Qian, Y.-N.; Luo, Y.-T.; Duan, H.; Feng, L.-Q.; Bi, Q.; Wang, Y.; Yan, X.-Y. Adhesion Molecule CD146 and its Soluble Form Correlate Well with Carotid Atherosclerosis and Plaque Instability. CNS Neurosci. Ther. 2014, 20, 438–445. [Google Scholar] [CrossRef]
- Von Elm, E.; Altman, U.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: Guidelines for Reporting Observational Studies. PLoS Med. 2007, 4, e296. [Google Scholar] [CrossRef] [PubMed]
- Altaf, N.; MacSweeney, S.T.; Gladman, J.R.F.; Auer, D.P. Carotid Intraplaque Hemorrhage Predicts Recurrent Symptoms in Patients with High-Grade Carotid Stenosis. Stroke 2007, 38, 1633–1635. [Google Scholar] [CrossRef] [PubMed]
- Hall, A.P. Review of the Pericyte during Angiogenesis and its Role in Cancer and Diabetic Retinopathy. Toxicol. Pathol. 2006, 34, 763–775. [Google Scholar] [CrossRef]
- Olson, F.J.; Strömberg, S.; Hjelmgren, O.; Kjelldahl, J.; Fagerberg, B.; Bergström, G. Increased vascularization of shoulder regions of carotid atherosclerotic plaques from patients with diabetes. J. Vasc. Surg. 2011, 54, 1324–1331.e5. [Google Scholar] [CrossRef] [PubMed]
- Jeziorska, M.; Woolley, D.E. Local neovascularization and cellular composition within vulnerable regions of atherosclerotic plaques of human carotid arteries. J. Pathol. 1999, 188, 189–196. [Google Scholar] [CrossRef]
- Kolodgie, F.D.; Gold, H.K.; Burke, A.P.; Fowler, D.; Kruth, H.S.; Weber, D.K.; Farb, A.; Guerrero, L.; Hayase, M.; Kutys, R.; et al. Intraplaque Hemorrhage and Progression of Coronary Atheroma. N. Engl. J. Med. 2003, 349, 2316–2325. [Google Scholar] [CrossRef]


| Variable | TOF-HIS (28 Lesions) | TOF-IIS (21 Lesions) | p-Value |
|---|---|---|---|
| Age (y) | 72.8 ± 1.4 | 65.3 ± 2.7 | 0.013 |
| Male sex (%) | 28 (100) | 18 (85) | NS |
| Rate of stenosis (%) | 70.8 ± 2.7 | 78.7 ± 3.3 | NS |
| Symptomatic lesions (%) | 23 (82) | 16 (76) | NS |
| Hypertension (%) | 18 (64) | 18 (86) | NS |
| Dyslipidemia (%) | 21 (57) | 8 (42) | NS |
| Diabetes mellitus (%) | 12 (43) | 10 (48) | NS |
| Coronary artery disease (%) | 11 (39) | 6 (28) | NS |
| Smoking (%) | 15 (53) | 13 (62) | NS |
| Urgent CEA (%) | 8 (29) | 3 (14) | NS |
| MRI-DWI high intensity (%) | 2 (7) | 3 (14) | NS |
| Restenosis (%) | 2 (7) | 1 (5) | NS |
| Variable | TOF-HIS (28 Lesions) | TOF-IIS (21 Lesions) | p-Value |
|---|---|---|---|
| Area of positive stain | |||
| Glycophorin A (%) | 32.8 ± 2.7 | 8.3 ± 1.7 | <0.0001 |
| CD68 (%) | 5.1 ± 0.7 | 2.2 ± 0.6 | 0.0039 |
| Mean number of neovessels | |||
| CD34 | 10.7 ± 1.0 | 10.6 ± 1.3 | NS |
| NG2 | 5.0 ± 0.4 | 15.5 ± 2.3 | <0.0001 |
| CD146 | 5.9 ± 0.8 | 13.9 ± 1.9 | <0.0001 |
| Variable | Observer | Mean Number of Neovessels | p-Value a | Interobserver Variability (Paired t-Test p-Value) | |
|---|---|---|---|---|---|
| TOF HIS | TOF IIS | ||||
| CD34 | Observer 1 | 11.2 ± 1.3 | 10.3 ± 1.5 | 0.64 | 0.36 |
| Observer 2 | 10.1 ± 1.2 | 11.0 ± 1.9 | 0.69 | ||
| NG2 | Observer 1 | 4.3 ± 0.3 | 18.5 ± 3.0 | <0.0001 | 0.11 |
| Observer 2 | 5.7 ± 0.7 | 12.6 ± 1.8 | 0.0003 | ||
| CD146 | Observer 1 | 5.5 ± 1.1 | 10.7 ± 2.1 | 0.023 | 0.16 |
| Observer 2 | 6.2 ± 0.8 | 17.1 ± 2.9 | 0.0002 | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ogata, A.; Wakamiya, T.; Nishihara, M.; Tanaka, T.; Mizokami, T.; Masuoka, J.; Momozaki, N.; Sakata, S.; Irie, H.; Abe, T. Association between Pericytes in Intraplaque Neovessels and Magnetic Resonance Angiography Findings. Int. J. Mol. Sci. 2020, 21, 1980. https://doi.org/10.3390/ijms21061980
Ogata A, Wakamiya T, Nishihara M, Tanaka T, Mizokami T, Masuoka J, Momozaki N, Sakata S, Irie H, Abe T. Association between Pericytes in Intraplaque Neovessels and Magnetic Resonance Angiography Findings. International Journal of Molecular Sciences. 2020; 21(6):1980. https://doi.org/10.3390/ijms21061980
Chicago/Turabian StyleOgata, Atsushi, Tomihiro Wakamiya, Masashi Nishihara, Tatsuya Tanaka, Taichiro Mizokami, Jun Masuoka, Nobuaki Momozaki, Shuji Sakata, Hiroyuki Irie, and Tatsuya Abe. 2020. "Association between Pericytes in Intraplaque Neovessels and Magnetic Resonance Angiography Findings" International Journal of Molecular Sciences 21, no. 6: 1980. https://doi.org/10.3390/ijms21061980
APA StyleOgata, A., Wakamiya, T., Nishihara, M., Tanaka, T., Mizokami, T., Masuoka, J., Momozaki, N., Sakata, S., Irie, H., & Abe, T. (2020). Association between Pericytes in Intraplaque Neovessels and Magnetic Resonance Angiography Findings. International Journal of Molecular Sciences, 21(6), 1980. https://doi.org/10.3390/ijms21061980





