Antisense Oligonucleotide in LNA-Gapmer Design Targeting TGFBR2—A Key Single Gene Target for Safe and Effective Inhibition of TGFβ Signaling
Abstract
1. Introduction
2. Results
2.1. Discovery Process
2.2. In Preparation to the First Experimental Round of Screening a Total of 118 Specific and Cross-Species Reactive Antisense Oligonucleotides Against TGFBR2 Were Designed In-Silico
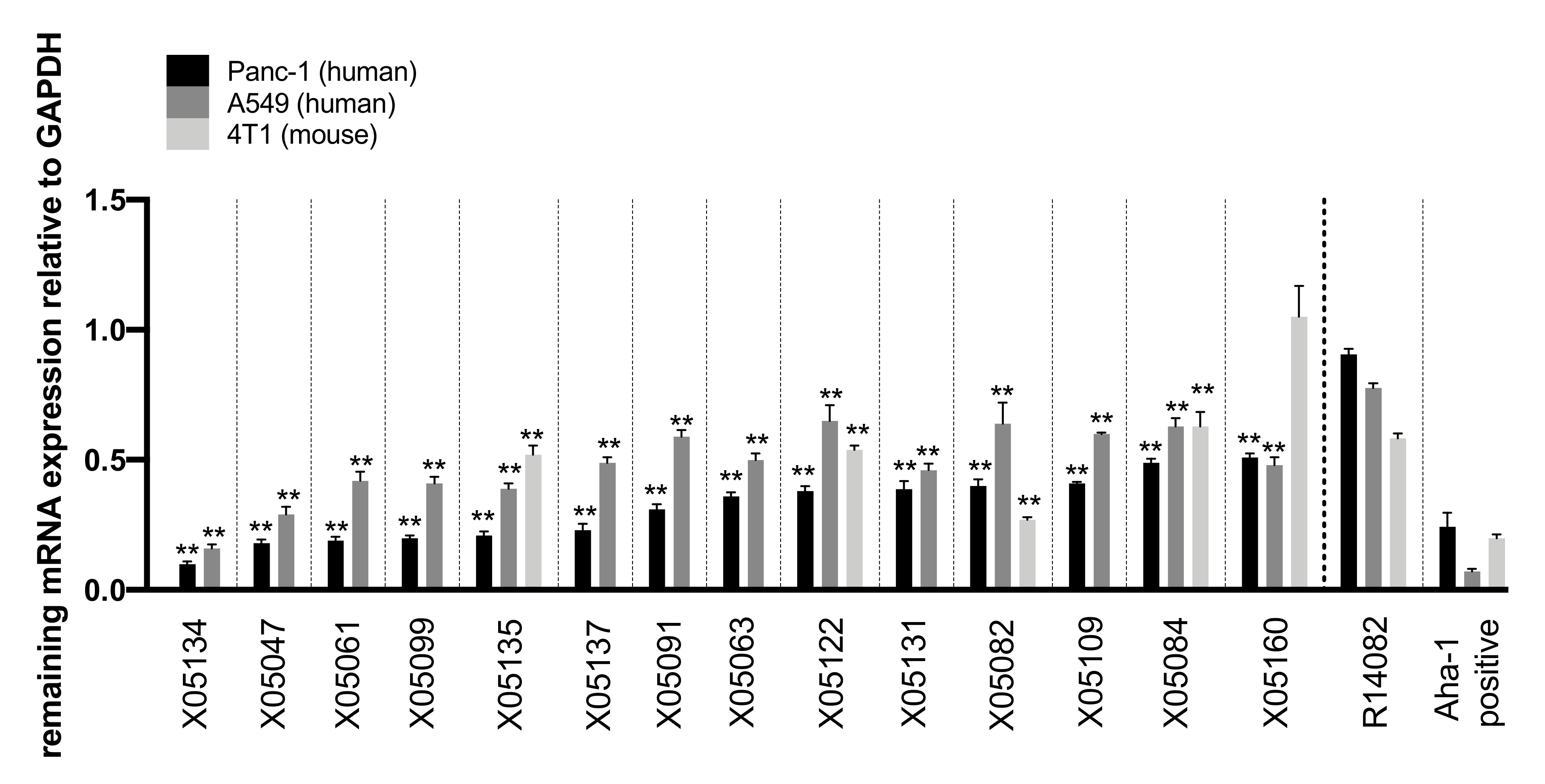
2.3. In-Vitro Screening Identified 14 Highly Active Antisense Oligonucleotides Against TGFBR2
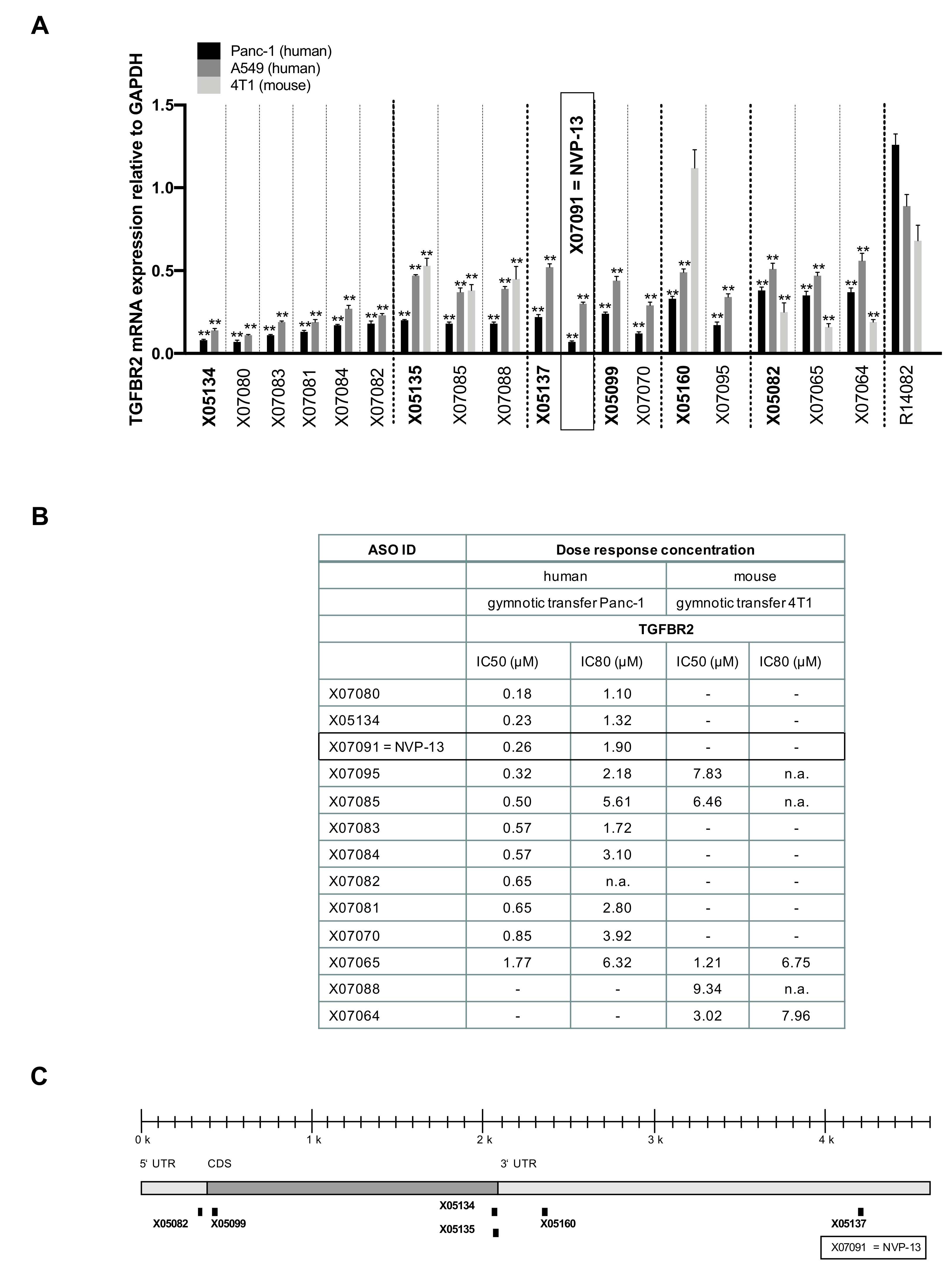
2.4. LNA Pattern Modifications Changed Activity of Antisense Oligonucleotides against TGFBR2
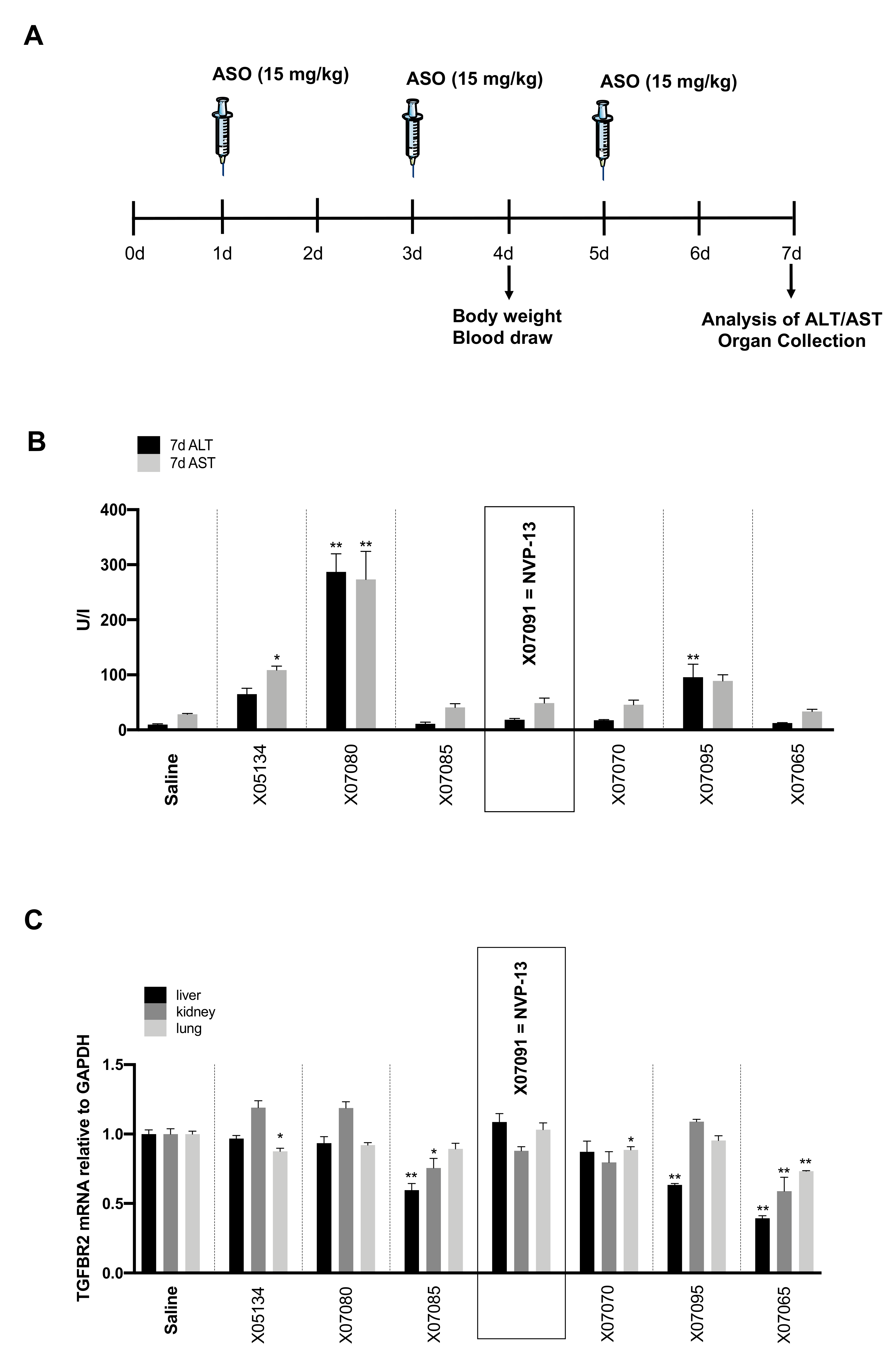
2.5. Early In-Vivo / In-Vitro Toxicity Assessment Indicates Probable Non-Toxic Candidates
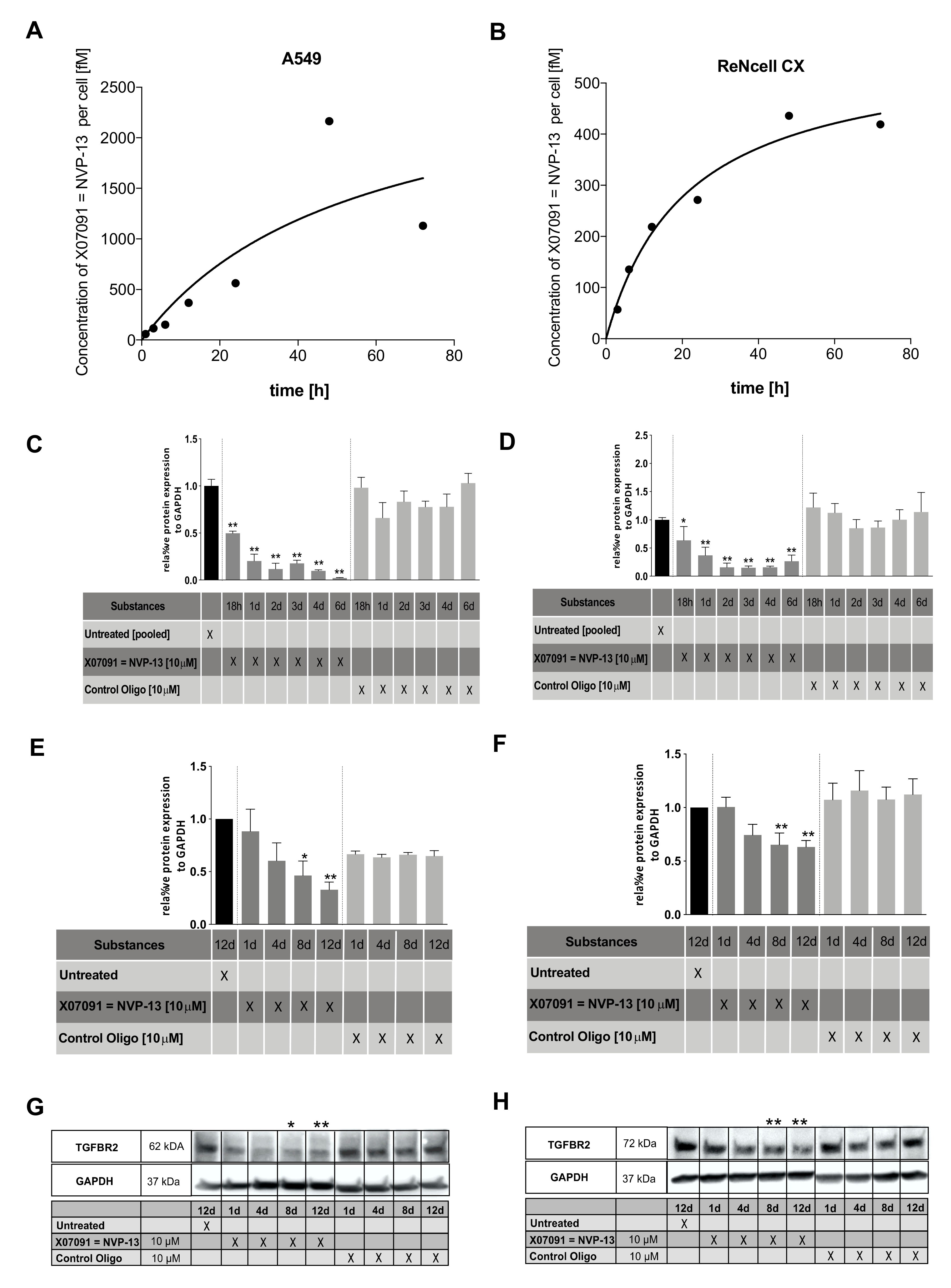
2.6. X07091 = NVP-13 Shows Time-Dependent Cellular Uptake, as well as TGFBR2 mRNA and Protein Downregulation
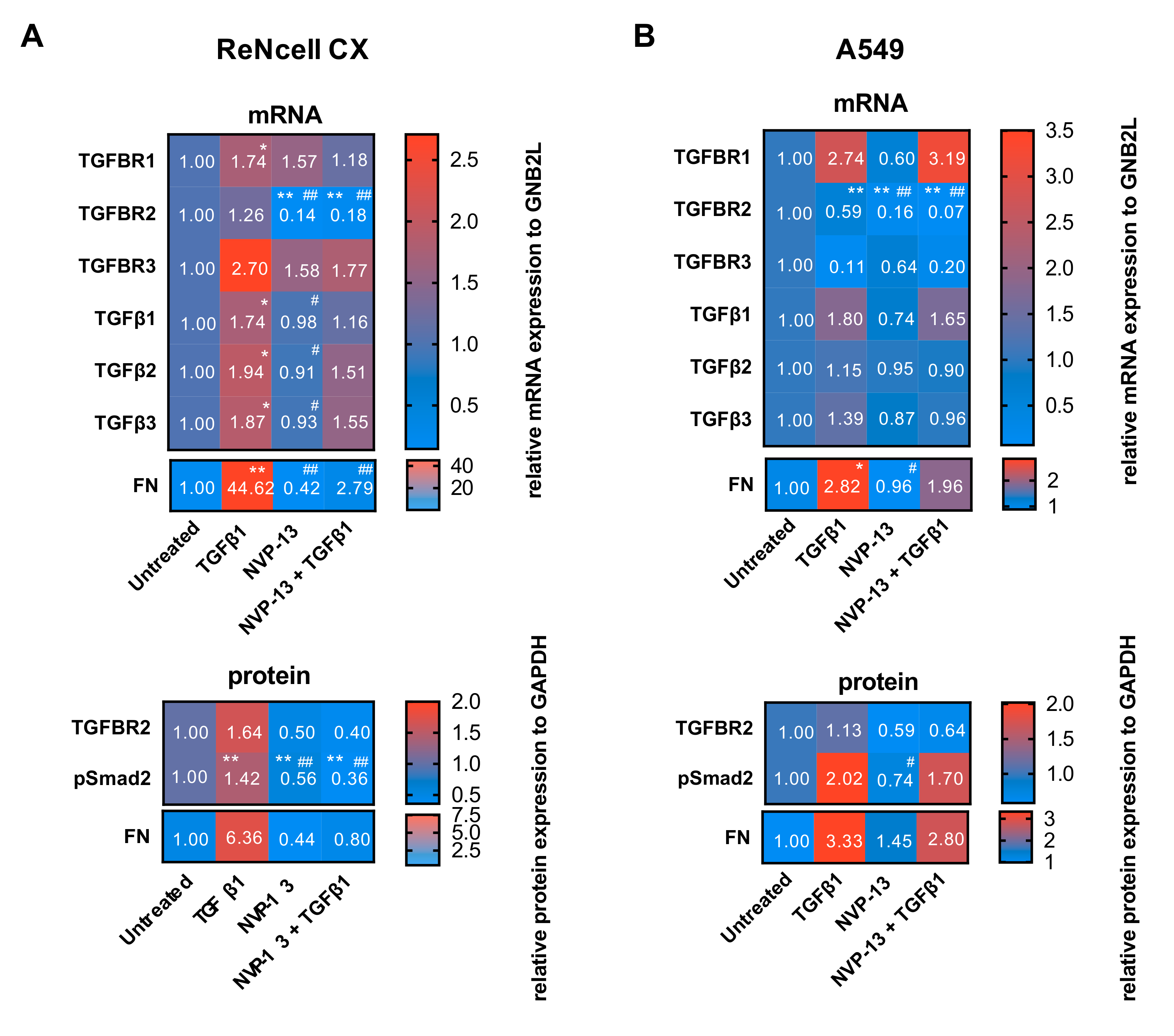
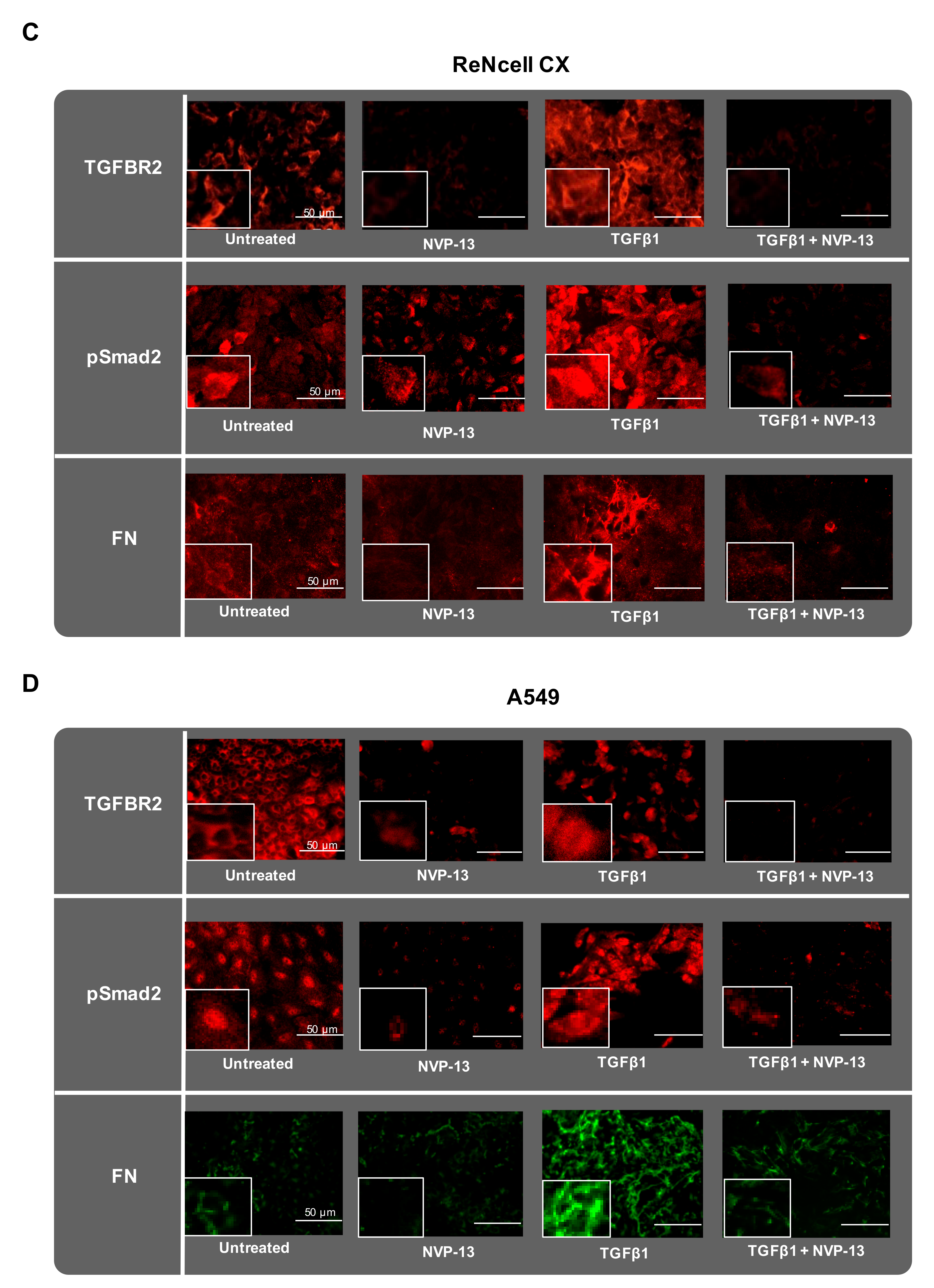
2.7. Gymnotic Transfer of NVP-13 in ReNcell CX® and A549 Cells is Efficacious even in Presence of TGFβ1
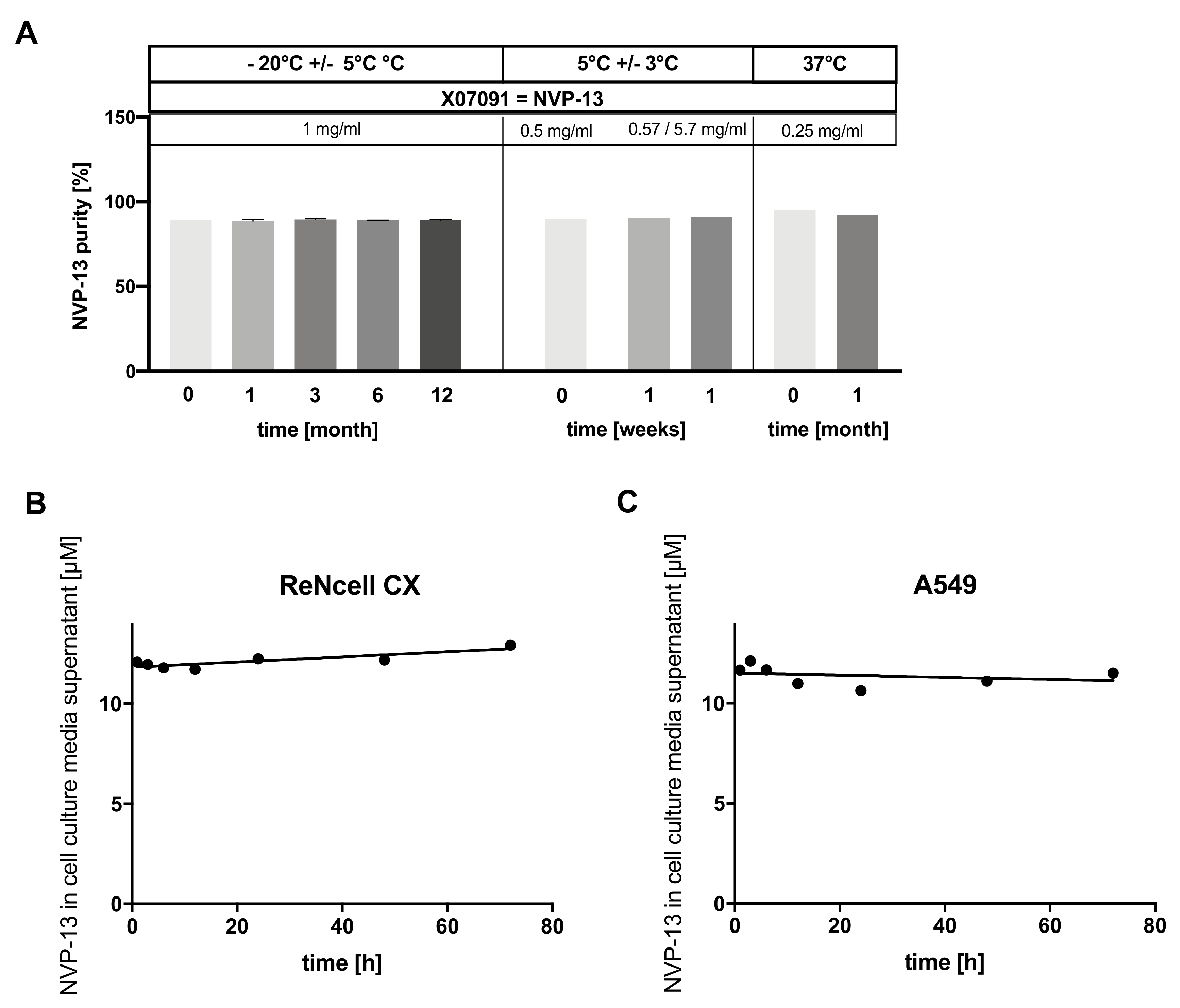
2.8. X07091 = NVP-13 Is Highly Stable in a Broad Range of Different Conditions
3. Discussion
4. Materials and Methods
4.1. TGFBR2 Antisense Oligonucleotide Design
4.2. Source of TGFBR2 Antisense Oligonucleotides
4.3. Cell Culture
4.4. Discovery Process
4.5. Dose-Response Analysis
4.6. Timeline Analysis
4.7. Gymnotic Transfer of ReNcell CX® and A549 Cells in Presence of TGFβ1
4.8. Determination of Cellular X07091 = NVP-13 Uptake
4.9. Branched DNA Assay (bDNA)
4.10. Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
4.11. Western Blot
4.12. Immunocytochemistry
4.13. Peripheral Blood Mononuclear Cell Assay (PBMC Assay)
4.14. In-Vivo Toxicity Study (Mouse Model)
4.15. A bioprobe against X07091 = NVP-13
4.16. Stability
4.17. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- DeVos, S.L.; Miller, T.M. Antisense Oligonucleotides: Treating Neurodegeneration at the Level of RNA. Neurotherapeutics 2013, 10, 486–497. [Google Scholar] [CrossRef]
- Evers, M.M.; Toonen, L.J.A.; van Roon-Mom, W.M.C. Antisense oligonucleotides in therapy for neurodegenerative disorders. Adv. Drug Deliv. Rev. 2015, 87, 90–103. [Google Scholar] [CrossRef]
- Geary, R.S.; Norris, D.; Yu, R.; Bennett, C.F. Pharmacokinetics, biodistribution and cell uptake of antisense oligonucleotides. Adv. Drug Deliv. Rev. 2015, 87, 46–51. [Google Scholar] [CrossRef] [PubMed]
- Scoles, D.R.; Pulst, S.M. Oligonucleotide therapeutics in neurodegenerative diseases. RNA Biol. 2018, 15, 707–714. [Google Scholar] [CrossRef] [PubMed]
- Wurster, C.D.; Ludolph, A.C. Antisense oligonucleotides in neurological disorders. Ther. Adv. Neurol. Disord. 2018, 11, 175628641877693. [Google Scholar] [CrossRef] [PubMed]
- Scoles, D.R.; Minikel, E.V.; Pulst, S.M. Antisense oligonucleotides. Neurol. Genet. 2019, 5, e323. [Google Scholar] [CrossRef]
- Smith, C.I.E.; Zain, R. Therapeutic Oligonucleotides: State of the Art. Annu. Rev. Pharmacol. Toxicol. 2019, 59, 605–630. [Google Scholar] [CrossRef]
- Neil, E.E.; Bisaccia, E.K. Nusinersen: A Novel Antisense Oligonucleotide for the Treatment of Spinal Muscular Atrophy. J. Pediatr. Pharmacol. Ther. 2019, 24, 194–203. [Google Scholar] [CrossRef]
- Wurster, C.D.; Ludolph, A.C. Nusinersen for spinal muscular atrophy. Ther. Adv. Neurol. Disord. 2018, 11, 175628561875445. [Google Scholar] [CrossRef]
- Wood, M.J.A.; Talbot, K.; Bowerman, M. Spinal muscular atrophy: Antisense oligonucleotide therapy opens the door to an integrated therapeutic landscape. Hum. Mol. Genet. 2017, 26, R151–R159. [Google Scholar] [CrossRef]
- Darras, B.T.; Chiriboga, C.A.; Iannaccone, S.T.; Swoboda, K.J.; Montes, J.; Mignon, L.; Xia, S.; Bennett, C.F.; Bishop, K.M.; Shefner, J.M.; et al. Nusinersen in later-onset spinal muscular atrophy. Neurology 2019, 92, e2492–e2506. [Google Scholar] [CrossRef] [PubMed]
- Aigner, L.; Bogdahn, U. TGF-beta in neural stem cells and in tumors of the central nervous system. Cell Tissue Res. 2007, 331, 225–241. [Google Scholar] [CrossRef] [PubMed]
- Kashima, R.; Hata, A. The role of TGF-β superfamily signaling in neurological disorders. Acta Biochim. Biophys. Sin. 2017, 50, 106–120. [Google Scholar] [CrossRef] [PubMed]
- Buckwalter, M.S.; Wyss-Coray, T. Modelling neuroinflammatory phenotypes in vivo. J. Neuroinflamm 2004, 1, 10–12. [Google Scholar] [CrossRef][Green Version]
- Endo, F.; Yamanaka, K. Astrocytic TGF-β1: Detrimental factor in ALS. Oncotarget 2015, 6, 15728–15729. [Google Scholar] [CrossRef]
- Peters, S.; Zitzelsperger, E.; Kuespert, S.; Iberl, S.; Heydn, R.; Johannesen, S.; Petri, S.; Aigner, L.; Thal, D.R.; Hermann, A.; et al. The TGF-β System As a Potential Pathogenic Player in Disease Modulation of Amyotrophic Lateral Sclerosis. Front. Neurol. 2017, 8, 669. [Google Scholar] [CrossRef]
- Wachs, F.-P.; Winner, B.; Couillard-Despres, S.; Schiller, T.; Aigner, R.; Winkler, J.; Bogdahn, U.; Aigner, L. Transforming growth factor-beta1 is a negative modulator of adult neurogenesis. J. Neuropathol. Exp. Neurol. 2006, 65, 358–370. [Google Scholar] [CrossRef]
- Khalil, N.; O’Connor, R.N.; Unruh, H.W.; Warren, P.W.; Flanders, K.C.; Kemp, A.; Bereznay, O.H.; Greenberg, A.H. Increased production and immunohistochemical localization of transforming growth factor-beta in idiopathic pulmonary fibrosis. Am. J. Respir. Cell Mol. Biol. 1991, 5, 155–162. [Google Scholar] [CrossRef]
- Alvarez, M.A.; Freitas, J.P.; Mazher Hussain, S.; Glazer, E.S. TGF-β Inhibitors in Metastatic Pancreatic Ductal Adenocarcinoma. J. Gastrointest Cancer 2019, 50, 207–213. [Google Scholar] [CrossRef]
- Batlle, E.; Massagué, J. Transforming Growth Factor-β Signaling in Immunity and Cancer. Immunity 2019, 50, 924–940. [Google Scholar] [CrossRef]
- Ganesh, K.; Massagué, J. TGF-β Inhibition and Immunotherapy: Checkmate. Immunity 2018, 48, 626–628. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Zheng, L.; Yuan, Q.; Zhen, G.; Crane, J.L.; Zhou, X.; Cao, X. Transforming growth factor-β in stem cells and tissue homeostasis. Bone Res. 2018, 6, 2–31. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.; Chen, Y.-G. Regulation of TGF-β receptor activity. Cell Biosci. 2012, 2, 9. [Google Scholar] [CrossRef] [PubMed]
- Qiong, W.; Xiaofeng, G.; Junfang, W. Transforming growth factor-β1 (TGF-β1) induces mouse precartilaginous stem cell differentiation through TGFRII-CK1ε-β-catenin signalling. Int J. Exp. Path. 2018, 99, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Massagué, J.; Xi, Q. TGF-β control of stem cell differentiation genes. FEBS Lett. 2012, 586, 1953–1958. [Google Scholar] [CrossRef] [PubMed]
- Zi, Z.; Chapnick, D.A.; Liu, X. Dynamics of TGF-β/Smad signaling. FEBS Lett. 2012, 586, 1921–1928. [Google Scholar] [CrossRef]
- Roberts, A.B.; Wakefield, L.M. The two faces of transforming growth factor beta in carcinogenesis. Proc. Natl. Acad. Sci. USA 2003, 100, 8621–8623. [Google Scholar] [CrossRef]
- Orum, H.; Wengel, J. Locked nucleic acids: A promising molecular family for gene-function analysis and antisense drug development. Curr. Opin. Mol. Ther. 2001, 3, 239–243. [Google Scholar]
- Braasch, D.A.; Corey, D.R. Locked nucleic acid (LNA): Fine-tuning the recognition of DNA and RNA. Chem. Biol. 2001, 8, 1–7. [Google Scholar] [CrossRef]
- Bondensgaard, K.; Petersen, M.; Singh, S.K.; Rajwanshi, V.K.; Kumar, R.; Wengel, J.; Jacobsen, J.P. Structural studies of LNA:RNA duplexes by NMR: Conformations and implications for RNase H activity. Chemistry 2000, 6, 2687–2695. [Google Scholar] [CrossRef]
- Kurreck, J. Antisense technologies. Improvement through novel chemical modifications. Eur J. Biochem. 2003, 270, 1628–1644. [Google Scholar] [CrossRef] [PubMed]
- Shen, W.; De Hoyos, C.L.; Migawa, M.T.; Vickers, T.A.; Sun, H.; Low, A.; Bell, T.A.; Rahdar, M.; Mukhopadhyay, S.; Hart, C.E.; et al. Chemical modification of PS-ASO therapeutics reduces cellular protein-binding and improves the therapeutic index. Nat. Biotechnol. 2019, 37, 640–650. [Google Scholar] [CrossRef] [PubMed]
- Rigo, F.; Chun, S.J.; Norris, D.A.; Hung, G.; Lee, S.; Matson, J.; Fey, R.A.; Gaus, H.; Hua, Y.; Grundy, J.S.; et al. Pharmacology of a Central Nervous System Delivered 2′- O-Methoxyethyl–Modified Survival of Motor Neuron Splicing Oligonucleotide in Mice and Nonhuman Primates. J. Pharmacol. Exp. Ther. 2014, 350, 46–55. [Google Scholar] [CrossRef] [PubMed]
- Hua, Y.; Vickers, T.A.; Okunola, H.L.; Bennett, C.F.; Krainer, A.R. Antisense Masking of an hnRNP A1/A2 Intronic Splicing Silencer Corrects SMN2 Splicing in Transgenic Mice. Am. J. Hum. Genet. 2008, 82, 834–848. [Google Scholar] [CrossRef] [PubMed]
- Chiriboga, C.A.; Swoboda, K.J.; Darras, B.T.; Iannaccone, S.T.; Montes, J.; De Vivo, D.C.; Norris, D.A.; Bennett, C.F.; Bishop, K.M. Results from a phase 1 study of nusinersen (ISIS-SMN Rx) in children with spinal muscular atrophy. Neurology 2016, 86, 890–897. [Google Scholar] [CrossRef] [PubMed]
- Stein, C.A.; Hansen, J.B.; Lai, J.; Wu, S.; Voskresenskiy, A.; Benimetskaya, L.; Worm, J.; Hedtjärn, M.; Souleimanian, N.; Miller, P.; et al. Efficient gene silencing by delivery of locked nucleic acid antisense oligonucleotides, unassisted by transfection reagents. Nucleic Acids Res. 2009, 38, e3. [Google Scholar] [CrossRef] [PubMed]
- Sleigh, J.N.; Rossor, A.M.; Fellows, A.D.; Tosolini, A.P.; Schiavo, G. Axonal transport and neurological disease. Nat. Rev. Neurol. 2019, 23, 1–13. [Google Scholar] [CrossRef]
- Heindryckx, F.; Li, J.-P. Role of proteoglycans in neuro-inflammation and central nervous system fibrosis. Matrix Biol. 2018, 68, 589–601. [Google Scholar] [CrossRef]
- Tom, V.J.; Doller, C.M.; Malouf, A.T.; Silver, J. Astrocyte-associated fibronectin is critical for axonal regeneration in adult white matter. J. Neurosci. 2004, 24, 9282–9290. [Google Scholar] [CrossRef]
- Györfi, A.H.; Matei, A.E.; Distler, J.H.W. Targeting TGF-β signaling for the treatment of fibrosis. Matrix Biol. 2018, 68, 8–27. [Google Scholar] [CrossRef]
- Saito, A.; Horie, M.; Nagase, T. TGF-β Signaling in Lung Health and Disease. IJMS 2018, 19, 2460. [Google Scholar] [CrossRef] [PubMed]
- Hinck, A.P. Structural studies of the TGF-βs and their receptors-insights into evolution of the TGF-β superfamily. FEBS Lett. 2012, 586, 1860–1870. [Google Scholar] [CrossRef] [PubMed]
- Akhurst, R.J.; Hata, A. Targeting the TGFβ signalling pathway in disease. Nat. Rev. Drug Discov. 2012, 11, 790–811. [Google Scholar] [CrossRef] [PubMed]
- Akhurst, R.J. Targeting TGF-β Signaling for Therapeutic Gain. Cold Spring Harb. Perspect Biol. 2017, 9, a022301. [Google Scholar] [CrossRef] [PubMed]
- Yingling, J.M.; Blanchard, K.L.; Sawyer, J.S. Development of TGF-β signalling inhibitors for cancer therapy. Nat. Rev. Drug Discov. 2004, 3, 1011–1022. [Google Scholar] [CrossRef] [PubMed]
- Haque, S.; Morris, J.C. Transforming growth factor-β: A therapeutic target for cancer. Hum. Vaccines Immunother. 2017, 13, 1741–1750. [Google Scholar] [CrossRef]
- Tsukazaki, T.; Chiang, T.A.; Davison, A.F.; Attisano, L.; Wrana, J.L. SARA, a FYVE domain protein that recruits Smad2 to the TGFbeta receptor. Cell 1998, 95, 779–791. [Google Scholar] [CrossRef]
- Tang, B.; Vu, M.; Booker, T.; Santner, S.J.; Miller, F.R.; Anver, M.R.; Wakefield, L.M. TGF-beta switches from tumor suppressor to prometastatic factor in a model of breast cancer progression. J. Clin. Invest. 2003, 112, 1116–1124. [Google Scholar] [CrossRef]
- Jahangirian, H.; Kalantari, K.; Izadiyan, Z.; Rafiee-Moghaddam, R.; Shameli, K.; Webster, T.J. A review of small molecules and drug delivery applications using gold and iron nanoparticles. Int. J. Nanomed. 2019, 14, 1633–1657. [Google Scholar] [CrossRef]
- Massagué, J. TGF-beta signal transduction. Annu. Rev. Biochem. 1998, 67, 753–791. [Google Scholar] [CrossRef]
- Kandasamy, M.; Lehner, B.; Kraus, S.; Sander, P.R.; Marschallinger, J.; Rivera, F.J.; Trümbach, D.; Ueberham, U.; Reitsamer, H.A.; Strauss, O.; et al. TGF-beta signalling in the adult neurogenic niche promotes stem cell quiescence as well as generation of new neurons. J. Cell. Mol. Med. 2014, 18, 1444–1459. [Google Scholar] [CrossRef] [PubMed]
- Senatorov, V.V., Jr.; Friedman, A.R.; Milikovsky, D.Z.; Ofer, J.; Saar-Ashkenazy, R.; Charbash, A.; Jahan, N.; Chin, G.; Mihaly, E.; Lin, J.M.; et al. Blood-brain barrier dysfunction in aging induces hyperactivation of TGFβ signaling and chronic yet reversible neural dysfunction. Sci. Transl. Med. 2019, 11, eaaw8283. [Google Scholar] [CrossRef] [PubMed]
- Hinz, B. The extracellular matrix and transforming growth factor-β1: Tale of a strained relationship. Matrix Biol. 2015, 47, 54–65. [Google Scholar] [CrossRef] [PubMed]
- Jain, R.K. Molecular regulation of vessel maturation. Nat. Med. 2003, 9, 685–693. [Google Scholar] [CrossRef] [PubMed]
- Junglas, B.; Kuespert, S.; Seleem, A.A.; Struller, T.; Ullmann, S.; Bösl, M.; Bosserhoff, A.; Köstler, J.; Wagner, R.; Tamm, E.R.; et al. Connective tissue growth factor causes glaucoma by modifying the actin cytoskeleton of the trabecular meshwork. Am. J. Pathol. 2012, 180, 2386–2403. [Google Scholar] [CrossRef] [PubMed]
- Petersen, M.; Thorikay, M.; Deckers, M.; van Dinther, M.; Grygielko, E.T.; Gellibert, F.; de Gouville, A.C.; Huet, S.; Ten Dijke, P.; Laping, N.J. Oral administration of GW788388, an inhibitor of TGF-β type I and II receptor kinases, decreases renal fibrosis. Kidney Int. 2008, 73, 705–715. [Google Scholar] [CrossRef] [PubMed]
- Azuma, A. Pirfenidone treatment of idiopathic pulmonary fibrosis. Ther. Adv. Respir. 2012, 6, 107–114. [Google Scholar] [CrossRef]
- Tolcher, A.W.; Berlin, J.D.; Cosaert, J.; Kauh, J.; Chan, E.; Piha-Paul, S.A.; Amaya, A.; Tang, S.; Driscoll, K.; Kimbung, R.; et al. A phase 1 study of anti-TGFβ receptor type-II monoclonal antibody LY3022859 in patients with advanced solid tumors. Cancer Chemother Pharmacol. 2017, 79, 673–680. [Google Scholar] [CrossRef]
- Hagedorn, P.H.; Persson, R.; Funder, E.D.; Albæk, N.; Diemer, S.L.; Hansen, D.J.; Møller, M.R.; Papargyri, N.; Christiansen, H.; Hansen, B.R.; et al. Locked nucleic acid: Modality, diversity, and drug discovery. Drug Discov. Today 2018, 23, 101–114. [Google Scholar] [CrossRef]
- Crooke, S.T.; Wang, S.; Vickers, T.A.; Shen, W.; Liang, X.-H. Cellular uptake and trafficking of antisense oligonucleotides. Nat. Biotechnol. 2017, 35, 230–237. [Google Scholar] [CrossRef]
- Juliano, R.L. The delivery of therapeutic oligonucleotides. Nucleic Acids Res. 2016, 44, 6518–6548. [Google Scholar] [CrossRef] [PubMed]
- Peters, S.; Küspert, S.; Wirkert, E.; Heydn, R.; Jurek, B.; Johannesen, S.; Hsam, O.; Korte, S.; Ludwig, F.; Mecklenburg, L.; et al. Modulating the neurogenic niche to recondition the central nervous system; Nature Publishing Group: London, UK, 2020. [Google Scholar]
| Specific and X-Reactive Candidates | SUM | X05177 and Derivatives | |||
|---|---|---|---|---|---|
| Human and NHP | Human, NHP and Rodent | Human, NHP and Rodent | |||
| Number of Candidates | Number of Candidates | ||||
| 17mer | 13 | 9 | 22 | 17mer | 1 |
| 16mer | 19 | 7 | 26 | 16mer | 2 |
| 15mer | 15 | 3** | 18 | 15mer | - |
| 14mer | 7 | 9 | 16 | 14mer | 2** |
| 13mer | 17 | 4 | 21 | 13mer | - |
| 12mer | 9 | 1 | 10 | 12mer | - |
| Σ | 80 | 33 | 113 | Σ | 5 |
| ID | Length | Position | 5′-3′ Sequence |
|---|---|---|---|
| X05134 | 16 | 2064 | GbsTbsAbsdGsdTsdGsdTsdTsdTsdAsdGsdGsdGsAbsGbsCb |
| X07080 | 16 | 2064 | GbsTbsAbsGbsdTsdGsdTsdTsdTsdAsdGsdGsdGsAbsGbsCb |
| X07083 | 16 | 2064 | GbsTbsAbsdGsdTsdGsdTsdTsdTsdAsdGsdGsdGsdAsGbsCb |
| X07081 | 16 | 2064 | GbsTbsAbsdGsdTsdGsdTsdTsdTsdAsdGsdGsGbsAbsGbsCb |
| X07084 | 16 | 2064 | GbsTbsdAsdGsdTsdGsdTsdTsdTsdAsdGsdGsdGsAbsGbsCb |
| X07082 | 16 | 2064 | GbsTbsAbsGbsdTsdGsdTsdTsdTsdAsdGsdGsGbsAbsGbsCb |
| X05135 | 16 | 2072 | GbsCbsTbsdAsdTsdTsdTsdGsdGsdTsdAsdGsdTsGbsTbsTb |
| X07085 | 16 | 2072 | GbsCbsTbsAbsdTsdTsdTsdGsdGsdTsdAsdGsdTsGbsTbsTb |
| X07088 | 16 | 2072 | GbsCbsTbsdAsdTsdTsdTsdGsdGsdTsdAsdGsdTsdGsTbsTb |
| X05137 | 16 | 4217 | CbsAbsTbsdGsdAsdAsdTsdGsdGsdAsdCsdCsdAsGbsTbsAb |
| X07091 = NVP-13 | 16 | 4217 | CbsAbsTbsdGsdAsdAsdTsdGsdGsdAsdCsdCsAbsGbsTbsAb |
| X05099 | 15 | 429 | CbsGbsAbsdTsdAsdCsdGsdCsdGsdTsdCsdCsAbsCbsAb |
| X07070 | 15 | 429 | CbsGbsAbsTbsdAsdCsdGsdCsdGsdTsdCsdCsAbsCbsAb |
| X05160 | 17 | 2355 | CbsAbsGbsGbsdCsdAsdTsdTsdAsdAsdTsdAsdAsAbsGbsTbsGb |
| X07095 | 17 | 2355 | CbsAbsGbsdGsdCsdAsdTsdTsdAsdAsdTsdAsdAsdAsGbsTbsGb |
| X05082 | 14 | 355 | CbsTbsCbsdGsdTsdCsdAsdTsdAsdGsdAsCbsCbsGb |
| X07065 | 14 | 355 | CbsTbsdCsdGsdTsdCsdAsdTsdAsdGsdAsCbsCbsGb |
| X07064 | 14 | 355 | CbsTbsCbsdGsdTsdCsdAsdTsdAsdGsdAsdCsCbsGb |
| Molecular Formula (Sodium Salt) | C164H183O83N64S15P15Na15 |
|---|---|
| Molecular weight | 5365.3 Da (free acid) / 5693.94 Da (sodium salt) |
| Description | White to off-white powder, odourless |
| Stereochemistry | NVP-13 is a mixture of 215 stereoisomers |
| Hygroscopicity | The drug substance is hygroscopic |
| Crystalline form | Amorphous |
| pH | The pH value of a 5 mg/mL solution of NVP-13 in - aCSF is approximately 7.1 - water for injection (WfI) is approximately 7.2, - isotonic sterile saline (0.9% NaCl) is approx. 6.5. |
| Solubility | The compound is a sodium salt that is soluble in aqueous solution. The solubility is ≥ 30 mg/mL |
| A549 cells | |||
|---|---|---|---|
| Primary Antibody | Dilution | Company | Order Number |
| FN (rabbit) | 1:50 | Proteintech (St. Leon-Rot, Germany) | 15613-1-AP |
| pSmad2 (rabbit) | 1:50 | Cell Signaling (Danvers, MA, USA) | cs3104s |
| TGF-βRII (rabbit) | 1:50 | Millipore (Darmstadt, Germany) | 06-227 |
| Secondary Antibody | Dilution | Company | Order Number |
| Cy3 goat-anti-rabbit | 1:1000 | Life Technologies (Darmstadt, Germany) | A10520 |
| Alexa Fluor 488 | 1:1500 | Life Technologies (Darmstadt, Germany) | A21441 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuespert, S.; Heydn, R.; Peters, S.; Wirkert, E.; Meyer, A.-L.; Siebörger, M.; Johannesen, S.; Aigner, L.; Bogdahn, U.; Bruun, T.-H. Antisense Oligonucleotide in LNA-Gapmer Design Targeting TGFBR2—A Key Single Gene Target for Safe and Effective Inhibition of TGFβ Signaling. Int. J. Mol. Sci. 2020, 21, 1952. https://doi.org/10.3390/ijms21061952
Kuespert S, Heydn R, Peters S, Wirkert E, Meyer A-L, Siebörger M, Johannesen S, Aigner L, Bogdahn U, Bruun T-H. Antisense Oligonucleotide in LNA-Gapmer Design Targeting TGFBR2—A Key Single Gene Target for Safe and Effective Inhibition of TGFβ Signaling. International Journal of Molecular Sciences. 2020; 21(6):1952. https://doi.org/10.3390/ijms21061952
Chicago/Turabian StyleKuespert, Sabrina, Rosmarie Heydn, Sebastian Peters, Eva Wirkert, Anne-Louise Meyer, Mareile Siebörger, Siw Johannesen, Ludwig Aigner, Ulrich Bogdahn, and Tim-Henrik Bruun. 2020. "Antisense Oligonucleotide in LNA-Gapmer Design Targeting TGFBR2—A Key Single Gene Target for Safe and Effective Inhibition of TGFβ Signaling" International Journal of Molecular Sciences 21, no. 6: 1952. https://doi.org/10.3390/ijms21061952
APA StyleKuespert, S., Heydn, R., Peters, S., Wirkert, E., Meyer, A.-L., Siebörger, M., Johannesen, S., Aigner, L., Bogdahn, U., & Bruun, T.-H. (2020). Antisense Oligonucleotide in LNA-Gapmer Design Targeting TGFBR2—A Key Single Gene Target for Safe and Effective Inhibition of TGFβ Signaling. International Journal of Molecular Sciences, 21(6), 1952. https://doi.org/10.3390/ijms21061952





