Functional Consequences of Low Activity of Transport System A for Neutral Amino Acids in Human Bone Marrow Mesenchymal Stem Cells
Abstract
1. Introduction
2. Results
2.1. MeAIB Does Not Inhibit Glutamine Transport in Human Mesenchymal Stem Cells
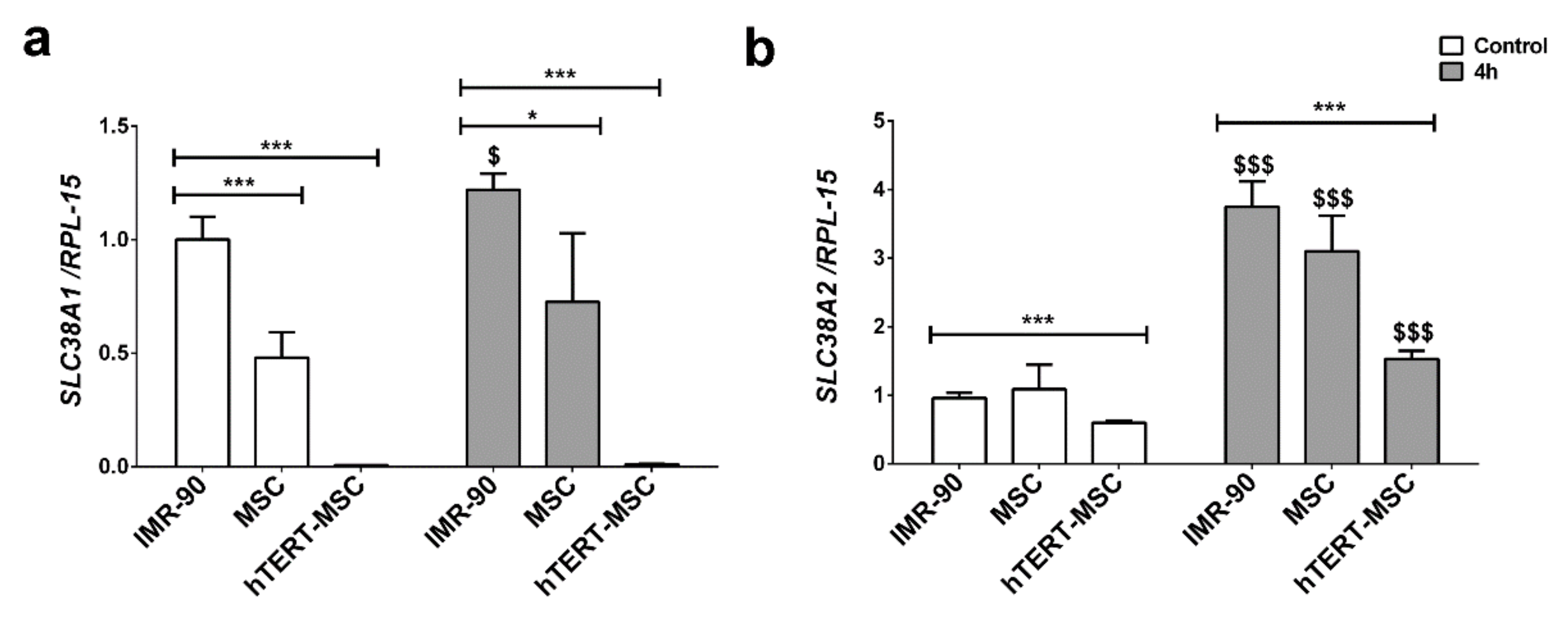
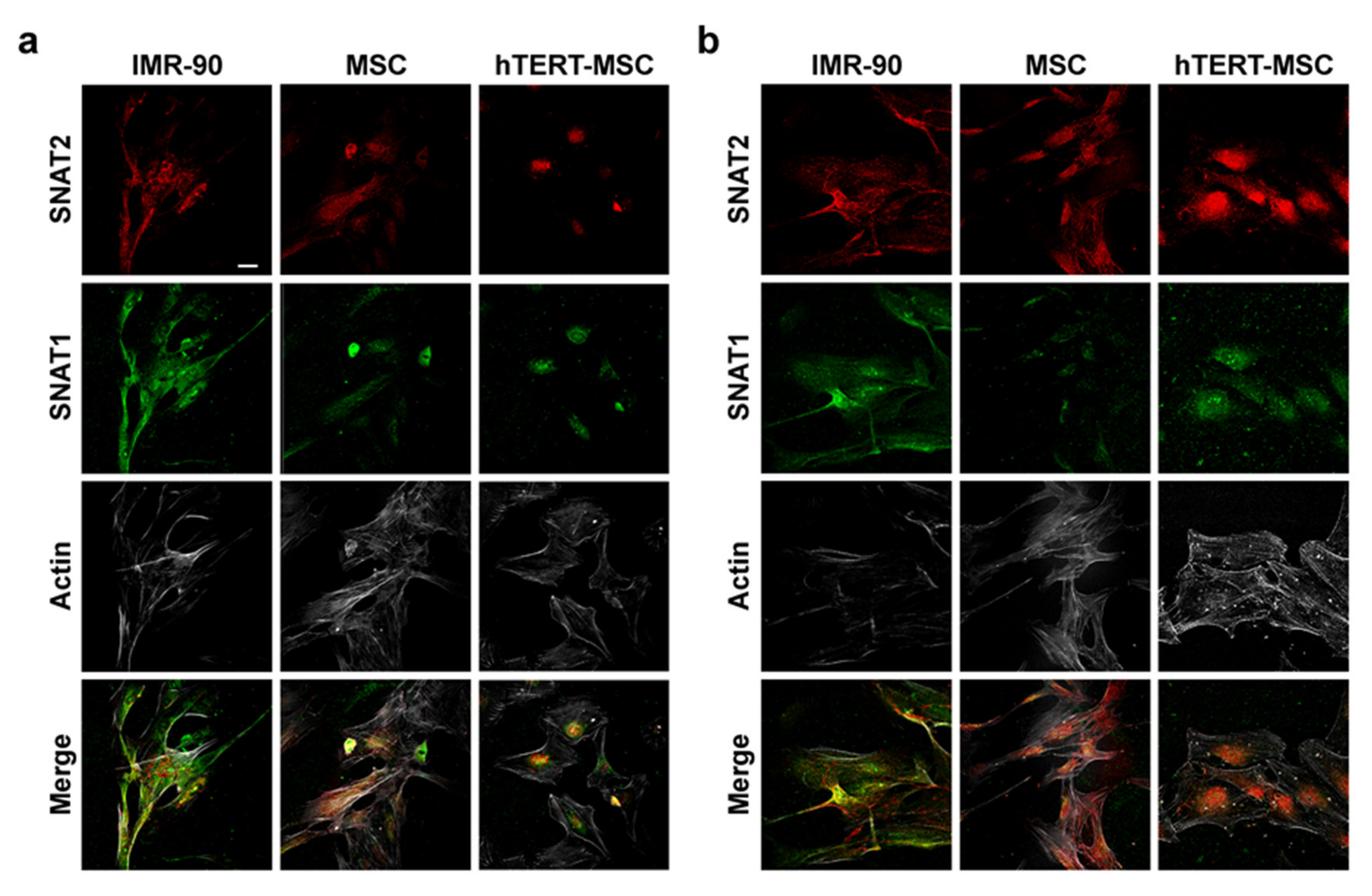
2.2. Mesenchymal Stem Cells Have a Low Expression of the System A Transporters
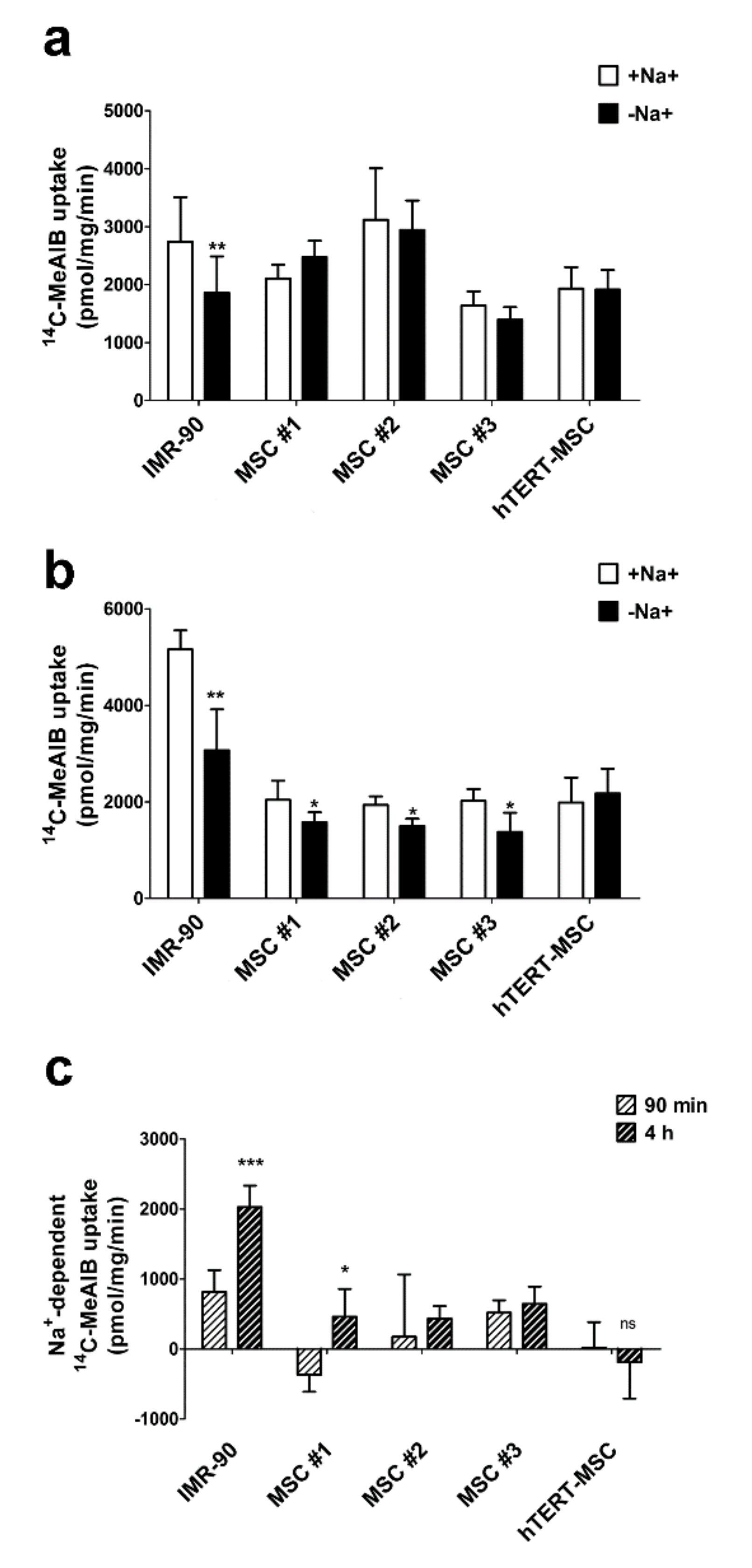
2.3. Mesenchymal Stem Cells Do Not Exhibit an Increase in System A Activity upon Amino Acid Starvation
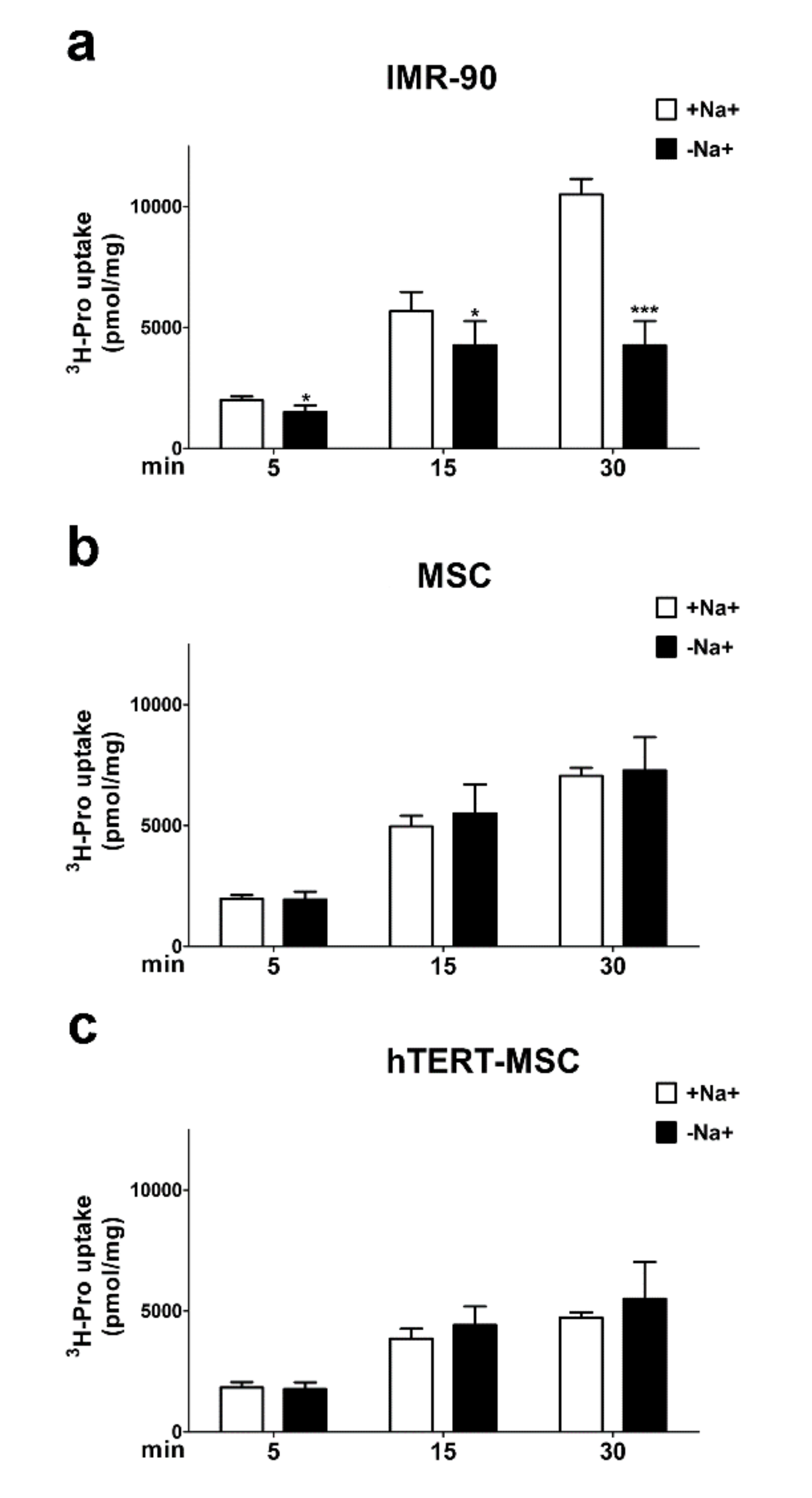
2.4. Mesenchymal Stem Cells Do Not Show a Sodium-Dependent Proline Accumulation
2.5. Mesenchymal Stem Cells Do Not Recover Cell Volume after Hypertonic Stress
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. RT-PCR Analysis
4.3. Amino Acid Uptake
4.4. Immunofluorescence
4.5. Intracellular Gln Content
4.6. Cell Volume
4.7. Statistical Analysis
4.8. Reagents
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pittenger, M.F.; Mackay, A.M.; Beck, S.C.; Jaiswal, R.K.; Douglas, R.; Mosca, J.D.; Moorman, M.A.; Simonetti, D.W.; Craig, S.; Marshak, D.R. Multilineage potential of adult human mesenchymal stem cells. Science 1999, 284, 143–147. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, J.T.; Mostacada, K.; de Lima, S.; Martinez, A.M. Bone marrow mesenchymal stem cell transplantation for improving nerve regeneration. Int. Rev. Neurobiol. 2013, 108, 59–77. [Google Scholar] [PubMed]
- Karamini, A.; Bakopoulou, A.; Andreadis, D.; Gkiouras, K.; Kritis, A. Therapeutic potential of mesenchymal stromal stem cells in rheumatoid arthritis: A systematic review of in vivo studies. Stem Cell Rev. Rep. 2020. [Epub ahead of print]. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.; Cho, S.G. Overcoming immunoregulatory plasticity of mesenchymal stem cells for accelerated clinical applications. Int. J. Hematol. 2016, 103, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Naji, A.; Eitoku, M.; Favier, B.; Deschaseaux, F.; Rouas-Freiss, N.; Suganuma, N. Biological functions of mesenchymal stem cells and clinical implications. Cell Mol. Life Sci. 2019, 76, 3323–3348. [Google Scholar] [CrossRef]
- Yuan, X.; Logan, T.M.; Ma, T. Metabolism in human mesenchymal stromal cells: A missing link between hmsc biomanufacturing and therapy? Front. Immunol. 2019, 10, 977. [Google Scholar] [CrossRef]
- Iwamoto, S.; Mihara, K.; Downing, J.R.; Pui, C.H.; Campana, D. Mesenchymal cells regulate the response of acute lymphoblastic leukemia cells to asparaginase. J. Clin. Investig. 2007, 117, 1049–1057. [Google Scholar] [CrossRef]
- Ehsanipour, E.A.; Sheng, X.; Behan, J.W.; Wang, X.; Butturini, A.; Avramis, V.I.; Mittelman, S.D. Adipocytes cause leukemia cell resistance to l-asparaginase via release of glutamine. Cancer Res. 2013, 73, 2998–3006. [Google Scholar] [CrossRef]
- Chiu, M.; Taurino, G.; Bianchi, M.G.; Kilberg, M.S.; Bussolati, O. Asparagine synthetase in cancer: Beyond acute lymphoblastic leukemia. Front. Oncol. 2019, 9, 1480. [Google Scholar] [CrossRef]
- DeBerardinis, R.J.; Cheng, T. Q’s next: The diverse functions of glutamine in metabolism, cell biology and cancer. Oncogene 2010, 29, 313–324. [Google Scholar] [CrossRef]
- Bhutia, Y.D.; Ganapathy, V. Glutamine transporters in mammalian cells and their functions in physiology and cancer. Biochim. Biophys. Acta 2016, 1863, 2531–2539. [Google Scholar] [CrossRef] [PubMed]
- Broer, S. The slc38 family of sodium-amino acid co-transporters. Pflüg. Arch. 2014, 466, 155–172. [Google Scholar] [CrossRef] [PubMed]
- Shotwell, M.A.; Jayme, D.W.; Kilberg, M.S.; Oxender, D.L. Neutral amino acid transport systems in chinese hamster ovary cells. J. Biol. Chem. 1981, 256, 5422–5427. [Google Scholar] [PubMed]
- Ericsson, A.; Hamark, B.; Jansson, N.; Johansson, B.R.; Powell, T.L.; Jansson, T. Hormonal regulation of glucose and system a amino acid transport in first trimester placental villous fragments. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2005, 288, R656–R662. [Google Scholar] [CrossRef] [PubMed]
- Christensen, H.N.; Oxender, D.L.; Liang, M.; Vatz, K.A. The use of n-methylation to direct route of mediated transport of amino acids. J. Biol. Chem. 1965, 240, 3609–3616. [Google Scholar]
- Mackenzie, B.; Erickson, J.D. Sodium-coupled neutral amino acid (system n/a) transporters of the slc38 gene family. Pflüg. Arch. 2004, 447, 784–795. [Google Scholar] [CrossRef]
- Takahashi, Y.; Nishimura, T.; Maruyama, T.; Tomi, M.; Nakashima, E. Contributions of system a subtypes to alpha-methylaminoisobutyric acid uptake by placental microvillous membranes of human and rat. Amino Acids 2017, 49, 795–803. [Google Scholar] [CrossRef]
- Foster, A.C.; Farnsworth, J.; Lind, G.E.; Li, Y.X.; Yang, J.Y.; Dang, V.; Penjwini, M.; Viswanath, V.; Staubli, U.; Kavanaugh, M.P. D-serine is a substrate for neutral amino acid transporters asct1/slc1a4 and asct2/slc1a5, and is transported by both subtypes in rat hippocampal astrocyte cultures. PLoS ONE 2016, 11, e0156551. [Google Scholar] [CrossRef]
- Bussolati, O.; Rotoli, B.M.; Laris, P.C.; Dall’Asta, V.; Gazzola, G.C. The preferential interaction of l-threonine with transport system asc in cultured human fibroblasts. Biochim. Biophys. Acta 1991, 1070, 305–312. [Google Scholar] [CrossRef]
- Chaudhry, F.A.; Reimer, R.J.; Krizaj, D.; Barber, D.; Storm-Mathisen, J.; Copenhagen, D.R.; Edwards, R.H. Molecular analysis of system n suggests novel physiological roles in nitrogen metabolism and synaptic transmission. Cell 1999, 99, 769–780. [Google Scholar] [CrossRef]
- Rae, C.; Hare, N.; Bubb, W.A.; McEwan, S.R.; Broer, A.; McQuillan, J.A.; Balcar, V.J.; Conigrave, A.D.; Broer, S. Inhibition of glutamine transport depletes glutamate and gaba neurotransmitter pools: Further evidence for metabolic compartmentation. J. Neurochem. 2003, 85, 503–514. [Google Scholar] [CrossRef] [PubMed]
- Chiu, M.; Bussolati, O. Department of Medicine and Surgery, University of Parma: Parma, Italy. 2020; Manuscript in preparation. [Google Scholar]
- Franchi-Gazzola, R.; Gaccioli, F.; Bevilacqua, E.; Visigalli, R.; Dall’Asta, V.; Sala, R.; Varoqui, H.; Erickson, J.D.; Gazzola, G.C.; Bussolati, O. The synthesis of snat2 transporters is required for the hypertonic stimulation of system a transport activity. Biochim. Biophys. Acta 2004, 1667, 157–166. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Takanaga, H.; Mackenzie, B.; Suzuki, Y.; Hediger, M.A. Identification of mammalian proline transporter sit1 (slc6a20) with characteristics of classical system imino. J. Biol. Chem. 2005, 280, 8974–8984. [Google Scholar] [CrossRef] [PubMed]
- Dall’Asta, V.; Rossi, P.A.; Bussolati, O.; Gazzola, G.C. Response of human fibroblasts to hypertonic stress. Cell shrinkage is counteracted by an enhanced active transport of neutral amino acids. J. Biol. Chem. 1994, 269, 10485–10491. [Google Scholar]
- Breborowicz, A.; Polubinska, A.; Oreopoulos, D.G. Changes in volume of peritoneal mesothelial cells exposed to osmotic stress. Perit. Dial. Int. 1999, 19, 119–123. [Google Scholar] [CrossRef]
- Dall’Asta, V.; Bussolati, O.; Sala, R.; Parolari, A.; Alamanni, F.; Biglioli, P.; Gazzola, G.C. Amino acids are compatible osmolytes for volume recovery after hypertonic shrinkage in vascular endothelial cells. Am. J. Physiol. 1999, 276, C865–C872. [Google Scholar] [CrossRef]
- Maallem, S.; Mutin, M.; Gonzalez-Gonzalez, I.M.; Zafra, F.; Tappaz, M.L. Selective tonicity-induced expression of the neutral amino-acid transporter snat2 in oligodendrocytes in rat brain following systemic hypertonicity. Neuroscience 2008, 153, 95–107. [Google Scholar] [CrossRef]
- Franchi-Gazzola, R.; Visigalli, R.; Dall’Asta, V.; Sala, R.; Woo, S.K.; Kwon, H.M.; Gazzola, G.C.; Bussolati, O. Amino acid depletion activates tonebp and sodium-coupled inositol transport. Am. J. Physiol. Cell Physiol. 2001, 280, C1465–C1474. [Google Scholar] [CrossRef]
- Dall’asta, V.; Franchi-Gazzola, R.; Bussolati, O.; Sala, R.; Rotoli, B.M.; Rossi, P.A.; Uggeri, J.; Belletti, S.; Visigalli, R.; Gazzola, G.C. Emerging roles for sodium dependent amino acid transport in mesenchymal cells. Amino Acids 1996, 11, 117–133. [Google Scholar]
- Nicklin, P.; Bergman, P.; Zhang, B.; Triantafellow, E.; Wang, H.; Nyfeler, B.; Yang, H.; Hild, M.; Kung, C.; Wilson, C.; et al. Bidirectional transport of amino acids regulates mtor and autophagy. Cell 2009, 136, 521–534. [Google Scholar] [CrossRef]
- Timmerman, L.A.; Holton, T.; Yuneva, M.; Louie, R.J.; Padro, M.; Daemen, A.; Hu, M.; Chan, D.A.; Ethier, S.P.; van ‘t Veer, L.J.; et al. Glutamine sensitivity analysis identifies the xct antiporter as a common triple-negative breast tumor therapeutic target. Cancer Cell 2013, 24, 450–465. [Google Scholar] [CrossRef] [PubMed]
- Franchi-Gazzola, R.; Dall’Asta, V.; Sala, R.; Visigalli, R.; Bevilacqua, E.; Gaccioli, F.; Gazzola, G.C.; Bussolati, O. The role of the neutral amino acid transporter snat2 in cell volume regulation. Acta Physiol. 2006, 187, 273–283. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, T.M.; Cwiklinski, E.; Shah, D.S.; Stretton, C.; Hyde, R.; Taylor, P.M.; Hundal, H.S. Effects of sodium and amino acid substrate availability upon the expression and stability of the snat2 (slc38a2) amino acid transporter. Front. Pharmacol. 2018, 9, 63. [Google Scholar] [CrossRef] [PubMed]
- Gaccioli, F.; Huang, C.C.; Wang, C.; Bevilacqua, E.; Franchi-Gazzola, R.; Gazzola, G.C.; Bussolati, O.; Snider, M.D.; Hatzoglou, M. Amino acid starvation induces the snat2 neutral amino acid transporter by a mechanism that involves eukaryotic initiation factor 2alpha phosphorylation and cap-independent translation. J. Biol. Chem. 2006, 281, 17929–17940. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, S.; Park, S.H.; Patel, A.; Carn, J.; Lee, K.; Kaplan, D.L. Hypoxia and amino acid supplementation synergistically promote the osteogenesis of human mesenchymal stem cells on silk protein scaffolds. Tissue Eng. Part A 2010, 16, 3623–3634. [Google Scholar] [CrossRef] [PubMed]
- Comes, S.; Gagliardi, M.; Laprano, N.; Fico, A.; Cimmino, A.; Palamidessi, A.; De Cesare, D.; De Falco, S.; Angelini, C.; Scita, G.; et al. L-proline induces a mesenchymal-like invasive program in embryonic stem cells by remodeling h3k9 and h3k36 methylation. Stem Cell Rep. 2013, 1, 307–321. [Google Scholar] [CrossRef]
- D’Aniello, C.; Fico, A.; Casalino, L.; Guardiola, O.; Di Napoli, G.; Cermola, F.; De Cesare, D.; Tate, R.; Cobellis, G.; Patriarca, E.J.; et al. A novel autoregulatory loop between the gcn2-atf4 pathway and (l)-proline [corrected] metabolism controls stem cell identity. Cell Death Differ. 2015, 22, 1094–1105. [Google Scholar] [CrossRef]
- Yi, X.; Liu, J.; Wu, P.; Gong, Y.; Xu, X.; Li, W. The whole transcriptional profiling of cellular metabolism during adipogenesis from hmscs. J. Cell. Physiol. 2020, 235, 349–363. [Google Scholar] [CrossRef]
- Meier, C.; Camargo, S.M.; Hunziker, S.; Moehrlen, U.; Gros, S.J.; Bode, P.; Leu, S.; Meuli, M.; Holland-Cunz, S.; Verrey, F.; et al. Intestinal imino transporter sit1 is not expressed in human newborns. Am. J. Physiol. Gastrointest. Liver Physiol. 2018, 315, G887–G895. [Google Scholar] [CrossRef]
- Andre, V.; Longoni, D.; Bresolin, S.; Cappuzzello, C.; Dander, E.; Galbiati, M.; Bugarin, C.; Di Meglio, A.; Nicolis, E.; Maserati, E.; et al. Mesenchymal stem cells from shwachman-diamond syndrome patients display normal functions and do not contribute to hematological defects. Blood Cancer J. 2012, 2, e94. [Google Scholar] [CrossRef]
- Bustin, S.A. Absolute quantification of mrna using real-time reverse transcription polymerase chain reaction assays. J. Mol. Endocrinol. 2000, 25, 169–193. [Google Scholar] [CrossRef] [PubMed]
- Chiu, M.; Taurino, G.; Bianchi, M.G.; Ottaviani, L.; Andreoli, R.; Ciociola, T.; Lagrasta, C.A.M.; Tardito, S.; Bussolati, O. Oligodendroglioma cells lack glutamine synthetase and are auxotrophic for glutamine, but do not depend on glutamine anaplerosis for growth. Int. J. Mol. Sci. 2018, 19, 1099. [Google Scholar] [CrossRef] [PubMed]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chiu, M.; Taurino, G.; Bianchi, M.G.; Dander, E.; Fallati, A.; Giuliani, N.; D’Amico, G.; Bussolati, O. Functional Consequences of Low Activity of Transport System A for Neutral Amino Acids in Human Bone Marrow Mesenchymal Stem Cells. Int. J. Mol. Sci. 2020, 21, 1899. https://doi.org/10.3390/ijms21051899
Chiu M, Taurino G, Bianchi MG, Dander E, Fallati A, Giuliani N, D’Amico G, Bussolati O. Functional Consequences of Low Activity of Transport System A for Neutral Amino Acids in Human Bone Marrow Mesenchymal Stem Cells. International Journal of Molecular Sciences. 2020; 21(5):1899. https://doi.org/10.3390/ijms21051899
Chicago/Turabian StyleChiu, Martina, Giuseppe Taurino, Massimiliano G. Bianchi, Erica Dander, Alessandra Fallati, Nicola Giuliani, Giovanna D’Amico, and Ovidio Bussolati. 2020. "Functional Consequences of Low Activity of Transport System A for Neutral Amino Acids in Human Bone Marrow Mesenchymal Stem Cells" International Journal of Molecular Sciences 21, no. 5: 1899. https://doi.org/10.3390/ijms21051899
APA StyleChiu, M., Taurino, G., Bianchi, M. G., Dander, E., Fallati, A., Giuliani, N., D’Amico, G., & Bussolati, O. (2020). Functional Consequences of Low Activity of Transport System A for Neutral Amino Acids in Human Bone Marrow Mesenchymal Stem Cells. International Journal of Molecular Sciences, 21(5), 1899. https://doi.org/10.3390/ijms21051899




