Improvement of Alcohol-Poisoning Symptoms in Mice by the Oral Administration of Live Lactobacillus plantarum SN13T Cells
Abstract
1. Introduction
2. Results
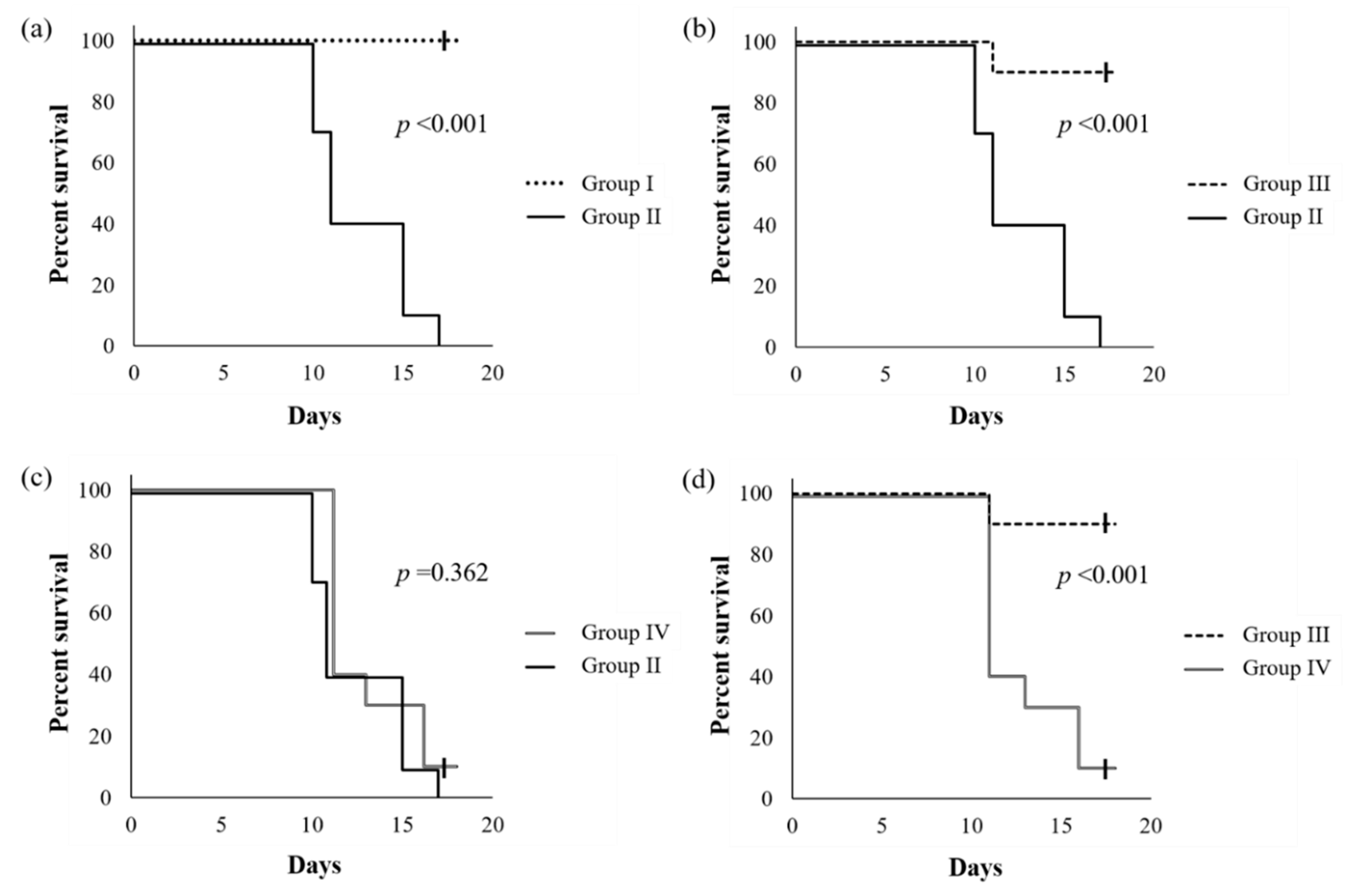
2.1. Health Benefit of Live SN13T Cells against the Alcohol-Poisoning Symptom in Mice
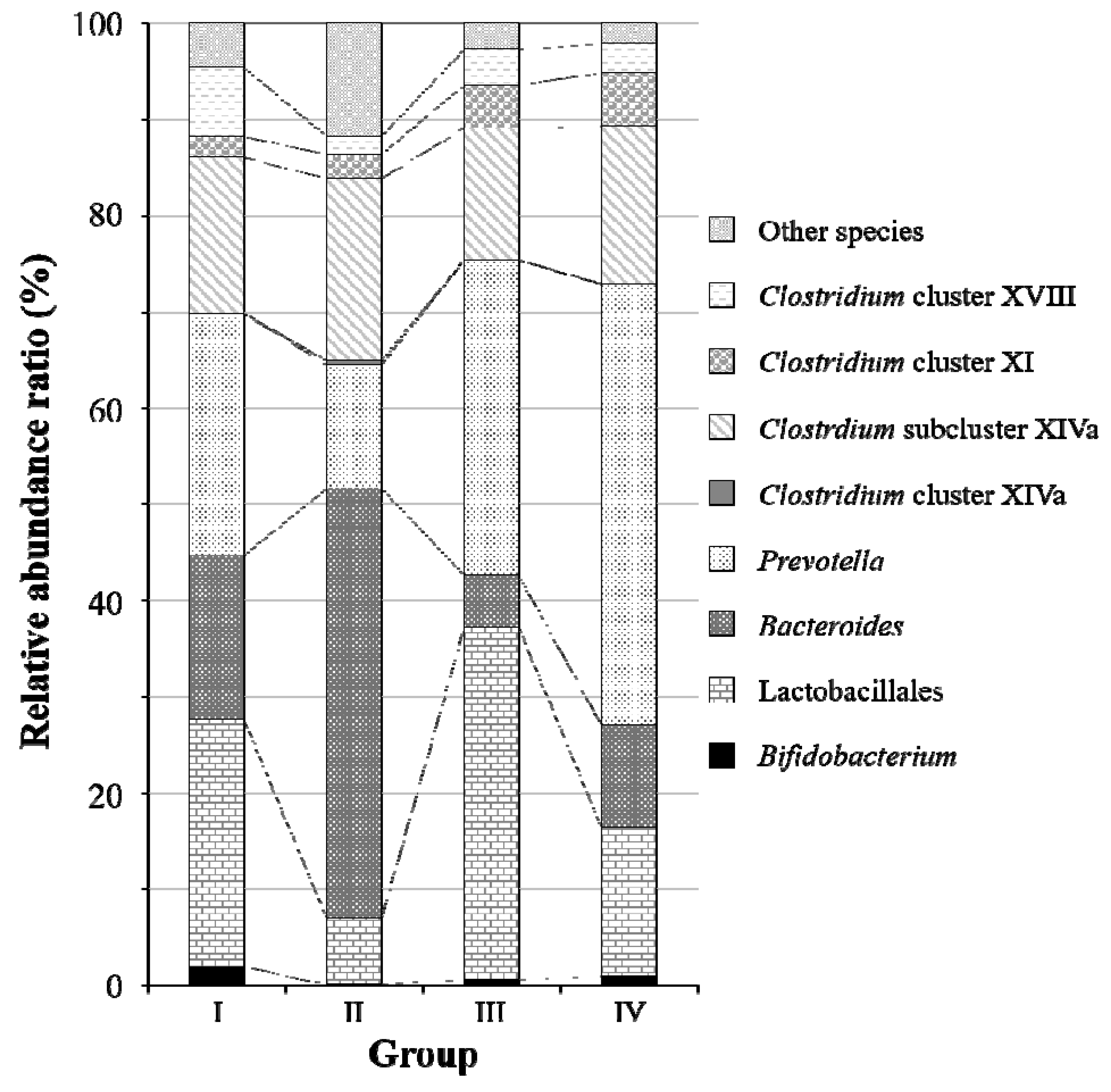
2.2. Fecal Microbiota Analysis Using the Terminal Restriction Fragment Length Polymorphism (T-RFLP) Method
2.3. Intestinal Microbiota Analysis
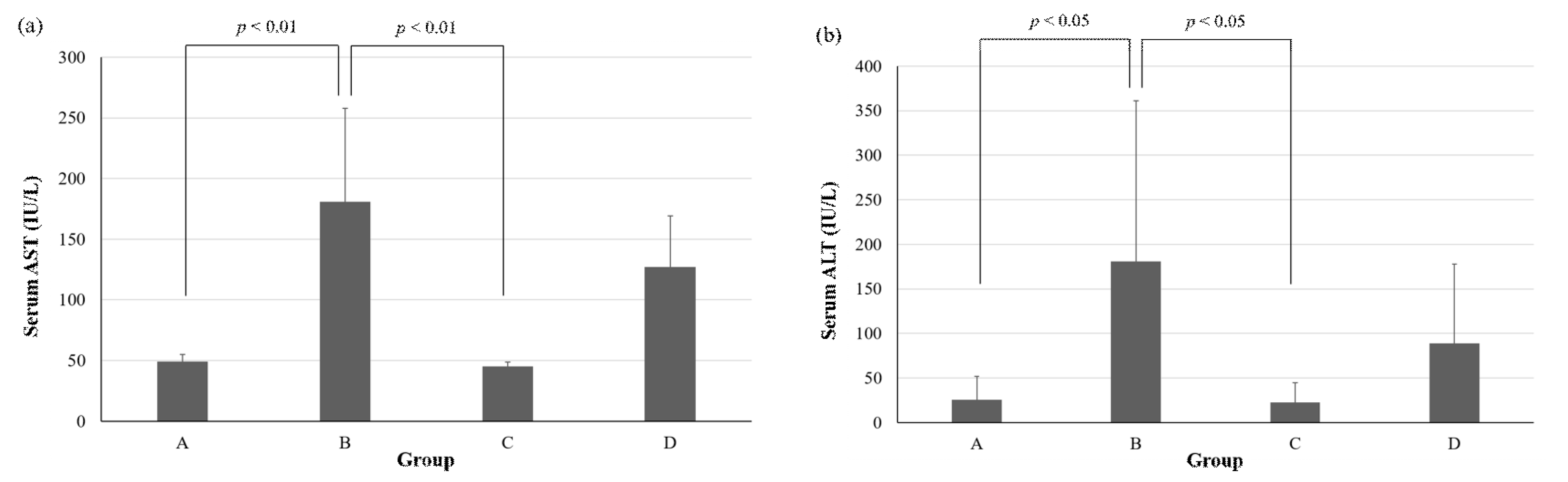
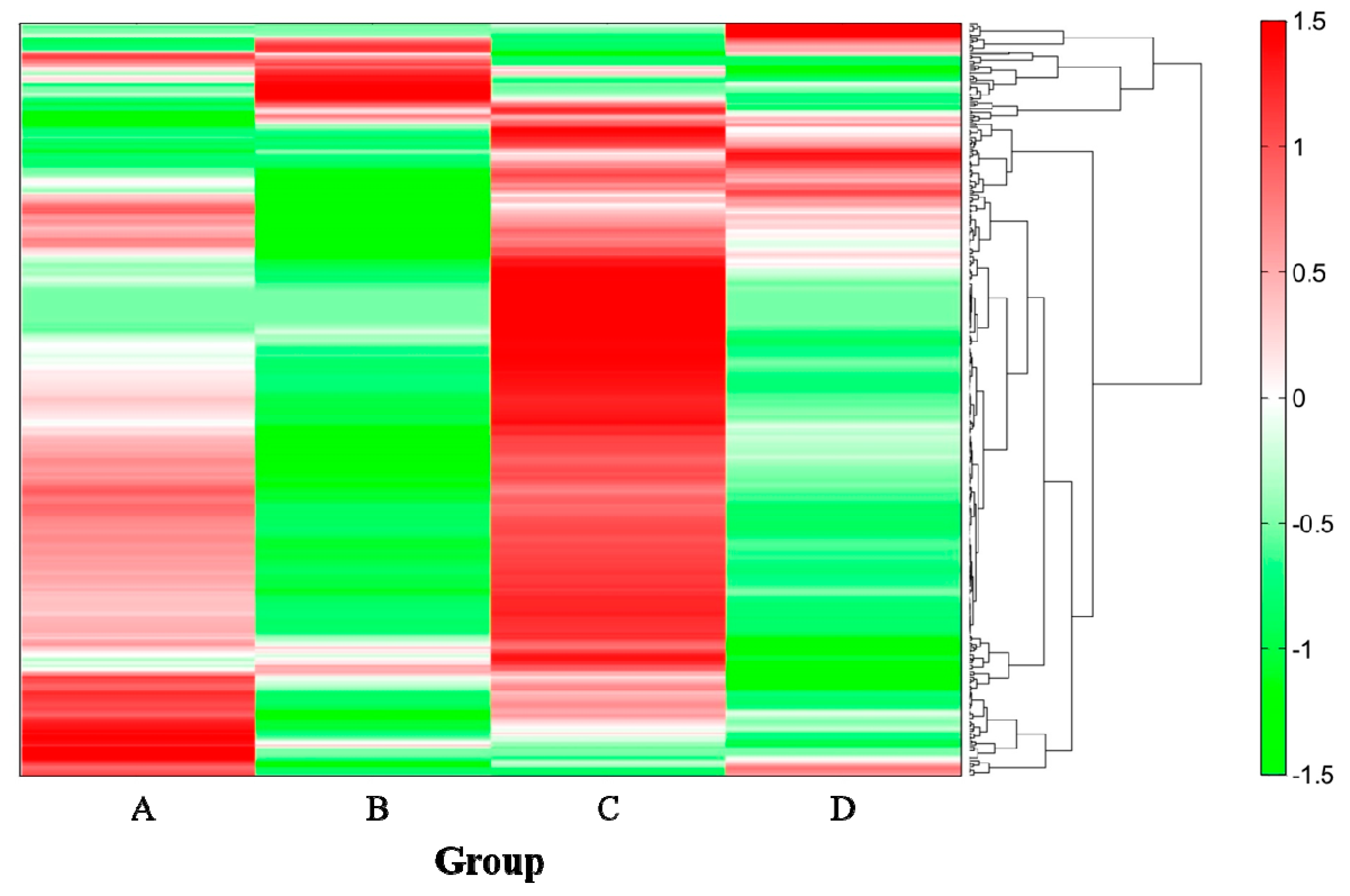
2.4. Analysis of Metabolites in the Cecum
3. Discussion
4. Materials and Methods
4.1. Culture Conditions of the LAB Strain
4.2. Preparation of Diet for Mice
4.3. Ethics Statement
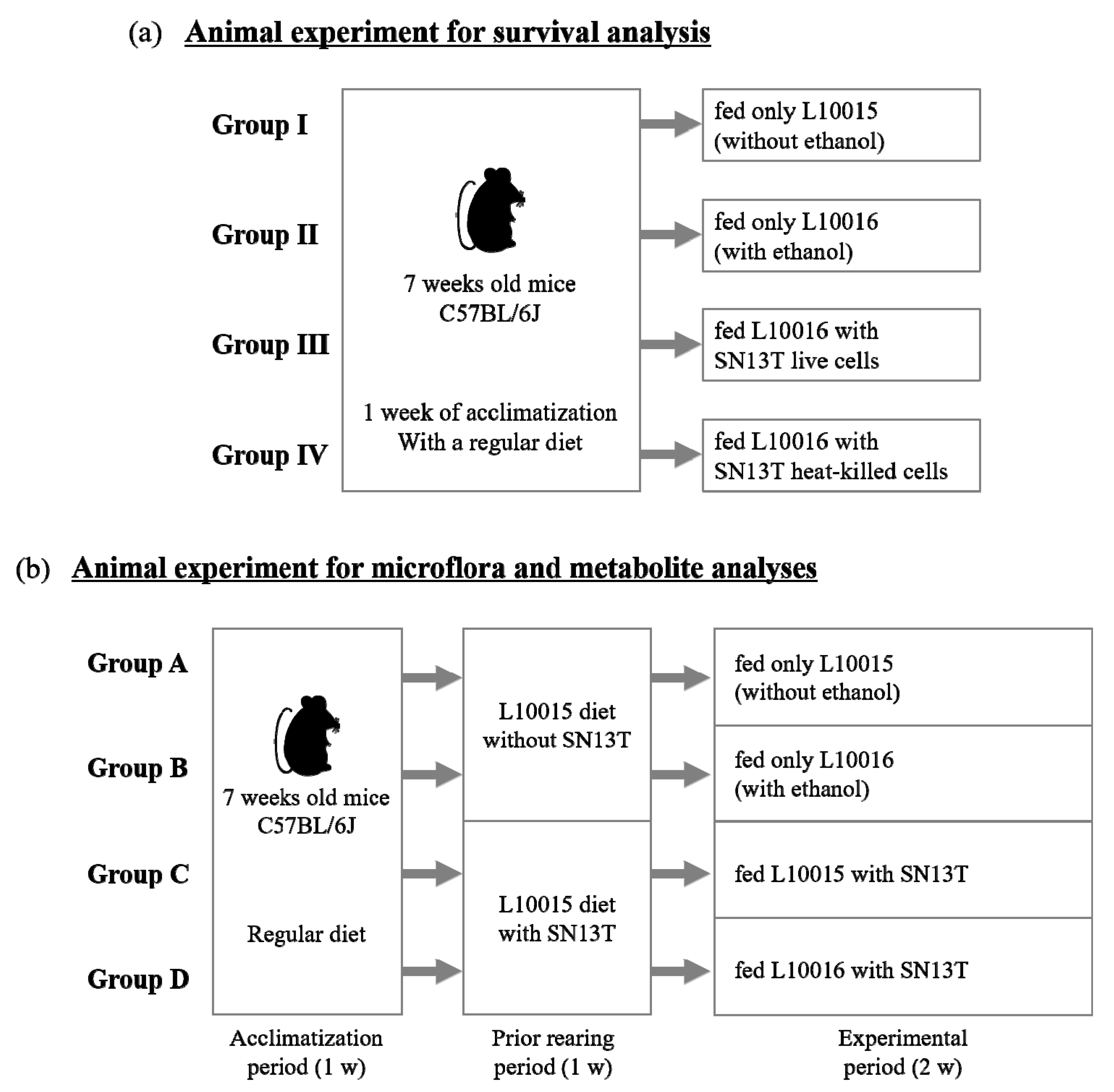
4.4. Animal Experiment to Evaluate Survival Rate
4.5. Animal Experiment to Analyze the Microbiota and Metabolites in the Intestine
4.6. Statistical Analyses
4.7. Analysis of the Fecal Microbiota Using the T-RFLP Method
4.8. Analysis of the Gut Microbiota via 16S rRNA Encoding Gene Sequencing
4.9. Sample Preparations for Metabolite Analysis of Bowel
4.10. Analytical Conditions for LC-TOFMS and CE-TOFMS
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ADH | alcohol dehydrogenase |
| ALT | alanine aminotransferase |
| AST | aspartate transaminase |
| CE | capillary electrophoresis |
| FAO | Food and Agriculture Organization of the United Nations |
| γ-GTP | γ-glutamyl transpeptidase |
| HCA | hierarchical cluster analysis |
| IBD | inflammatory bowel disease |
| IgA | immunoglobulin A |
| LAB | lactic acid bacteria |
| Lb. | Lactobacillus |
| LC | liquid chromatography |
| MRS | de Man, Rogosa, and Sharpe |
| ODS | octadecylsilyl |
| OTU | operational taxonomic unit |
| QIIME | Quantitative Insights into Microbial Ecology |
| rDNA | ribosomal DNA |
| rRNA | ribosomal RNA |
| SPF | specific pathogen free |
| TOF-MS | time-of-flight mass spectrometry |
| T-RFLP | terminal restriction fragment length polymorphism |
| UPGMA | unweighted pair-group method of analysis |
References
- Vallee, B.L. Alcohol in the western world. Sci. Am. 1998, 278, 80–85. [Google Scholar] [CrossRef]
- The Social Issues Research Centre (SIRC). Social and cultural aspects of drinking. In A Report to the European Commission; SIRC: Oxford, UK, 1998. [Google Scholar]
- Setshedi, M.; Wands, J.R.; de la Monte, S.M. Acetaldehyde adducts in alcoholic liver disease. Oxid. Med. Cell Longev. 2010, 3, 178–185. [Google Scholar] [CrossRef] [PubMed]
- National Institute on Alcohol Abuse and Alcoholism (NIAAA). Alcohol metabolism: An update. In Alcohol Alert, No. 58; NIAAA: Rockville, MD, USA, 2002. [Google Scholar]
- Rasineni, K.; Casey, C.A. Molecular mechanism of alcoholic fatty liver. Indian J. Pharmacol. 2012, 44, 299–303. [Google Scholar] [CrossRef]
- National Institute on Alcohol Abuse and Alcoholism (NIAAA). Alcohol and Nutrition. In Alcohol Alert, No. 22; NIAAA: Rockville, MD, USA, 1993. [Google Scholar]
- Schuppan, D.; Afdhal, N.H. Liver cirrhosis. Lancet 2008, 8, 838–851. [Google Scholar] [CrossRef]
- Sanders, M.E. Probiotics: Definition, source, selection, and uses. Clin. Infect. Dis. 2008, 46, S58–S61. [Google Scholar] [CrossRef] [PubMed]
- Adolfsson, O.; Meydani, S.N.; Russell, R.M. Yogurt and gut function. Am. J. Clin. Nutr. 2004, 80, 245–256. [Google Scholar] [CrossRef]
- Cross, M.L.; Stevenson, L.M.; Gill, H.S. Anti-allergy properties of fermented foods: An important immunoregulatory mechanism of lactic acid bacteria? Int. Immunopharmacol. 2001, 1, 891–901. [Google Scholar] [CrossRef]
- Heyman, M. Effect of lactic acid bacteria on diarrheal diseases. J. Am. Coll. Nutr. 2000, 19, 37S–146S. [Google Scholar] [CrossRef]
- Meydani, S.N.; Ha, W.K. Immunologic effects of yogurt. Am. J. Clin. Nutr. 2000, 71, 861–872. [Google Scholar] [CrossRef]
- Parvez, S.; Malik, K.A.; Ah Kang, S.; Kim, H.Y. Probiotics and their fermented food products are beneficial for health. J. Appl. Microbiol. 2006, 100, 1171–1185. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, N.; Xi, A.; Ahmed, Z.; Zhang, B.; Bai, X. Effects of Lactobacillus plantarum MA2 isolated from Tibet kefir on lipid metabolism and intestinal microflora of rats fed on high-cholesterol diet. Appl. Microbiol. Biotechnol. 2009, 84, 341–347. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.D.; Kang, J.H.; Lee, M.S. Characterization of Lactobacillus plantarum PH04, a potential probiotic bacterium with cholesterol-lowering effects. Int. J. Food Microbiol. 2007, 113, 358–361. [Google Scholar] [CrossRef] [PubMed]
- Liong, M.T.; Shah, N.P. Effects of a Lactobacillus casei synbiotic on serum lipoprotein, intestinal microflora, and organic acids in rats. J. Dairy Sci. 2006, 89, 1390–1399. [Google Scholar] [CrossRef]
- Jin, H.; Higashikawa, F.; Noda, M.; Zhao, X.; Matoba, Y.; Kumagai, T.; Sugiyama, M. Establishment of an in vitro Peyer’s patch cell culture system correlative to in vivo study using intestine and screening of lactic acid bacteria enhancing intestinal immunity. Biol. Pharm. Bull. 2010, 33, 289–293. [Google Scholar] [CrossRef][Green Version]
- Zhao, X.; Higashikawa, F.; Noda, M.; Kawamura, Y.; Matoba, Y.; Kumagai, T.; Sugiyama, M. The obesity and fatty liver are reduced by plant-derived Pediococcus pentosaceus LP28 in high fat diet-induced obese mice. PLoS ONE 2012, 7, e30696. [Google Scholar] [CrossRef]
- Higashikawa, F.; Noda, M.; Awaya, T.; Danshiitsoodol, N.; Matoba, Y.; Kumagai, T.; Sugiyama, M. Antiobesity effect of Pediococcus pentosaceus LP28 on overweight subjects: A randomized, double-blind, placebo-controlled clinical trial. Eur. J. Clin. Nutr. 2016, 70, 582–587. [Google Scholar] [CrossRef]
- Noda, M.; Sultana, N.; Hayashi, I.; Fukamachi, M.; Sugiyama, M. Exopolysaccharide produced by Lactobacillus paracasei IJH-SONE68 prevents and improves the picryl chloride-induced contact dermatitis. Molecules 2019, 24, 2970. [Google Scholar] [CrossRef]
- Higashikawa, F.; Noda, M.; Awaya, T.; Nomura, K.; Oku, H.; Sugiyama, M. Improvement of constipation and liver function by plant-derived lactic acid bacteria: A double-blind, randomized trial. Nutrition 2010, 26, 367–374. [Google Scholar] [CrossRef]
- Chadha, K.C.; Stadler, I.; Albini, B.; Nakeeb, S.M.; Thacore, H.R. Effect of alcohol on spleen cells and their functions in C57BL/6 mice. Alcohol 1991, 8, 481–485. [Google Scholar] [CrossRef]
- Phillips, T.J.; Crabbe, J.C.; Metten, P.; Belknap, J.K. Localization of genes affecting alcohol drinking in mice. Alcohol Clin. Exp. Res. 1994, 18, 931–941. [Google Scholar] [CrossRef]
- Belknap, J.K.; Mogil, J.S.; Helms, M.L.; Richards, S.P.; O’Toole, L.A.; Bergeson, S.E.; Buck, K.J. Localization to chromosome 10 of a locus influencing morphine analgesia in crosses derived from C57BL/6 and DBA/2 strains. Life Sci. 1995, 57, PL117–PL124. [Google Scholar] [CrossRef]
- Grahame, N.J.; Phillips, T.J.; Burkhart-Kasch, S.; Cunningham, C.L. Intravenous cocaine self-administration in the C57BL/6J mouse. Pharmacol. Biochem. Behav. 1995, 51, 827–834. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations (FAO) and World Health Organization (WHO). Toxicological evaluation of some extraction solvents and certain other substances. In FAO Nutrition Meetings Report Series No. 48A. 216; Ethanol; WHO/FOOD ADD/70.39; FAO/WHO: Geneva, Switzerland, 1970. [Google Scholar]
- Canesso, M.C.C.; Lacerda, N.L.; Ferreira, C.M.; Gonçalves, J.L.; Almeida, D.; Gamba, C.; Cassali, G.; Pedroso, S.H.; Moreira, C.; Martins, F.S.; et al. Comparing the effects of acute alcohol consumption in germ-free and conventional mice: The role of the gut microbiota. BMC Microbiol. 2014, 14, 240. [Google Scholar]
- Rao, R. Endotoxemia and gut barrier dysfunction in alcoholic liver disease. Hepatology 2009, 50, 638–644. [Google Scholar] [CrossRef]
- Purohit, V.; Bode, J.C.; Bode, C.; Brenner, D.A.; Choudhry, M.A.; Hamilton, F.; Kang, Y.J.; Keshavarzian, A.; Rao, R.; Sartor, R.B.; et al. Alcohol, intestinal bacterial growth, intestinal permeability to endotoxin, and medical consequences: Summary of a symposium. Alcohol 2008, 42, 349–361. [Google Scholar] [CrossRef]
- Forsyth, C.B.; Voigt, R.M.; Keshavarzian, A. Intestinal CYP2E1: A mediator of alcohol-induced gut leakiness. Redox Biol. 2014, 3, 40–46. [Google Scholar] [CrossRef]
- Yoseph, B.P.; Breed, E.; Overgaard, C.E.; Ward, C.J.; Liang, Z.; Wagener, M.E.; Lexcen, D.R.; Lusczek, E.R.; Beilman, G.J.; Burd, E.M.; et al. Chronic alcohol ingestion increases mortality and organ injury in a murine model of septic peritonitis. PLoS ONE 2013, 8, e62792. [Google Scholar] [CrossRef] [PubMed]
- Bollinger, R.R.; Barbas, A.S.; Bush, E.L.; Lin, S.S.; Parker, W. Biofilms in the large bowel suggest an apparent function of the human vermiform appendix. J. Theor. Biol. 2007, 249, 826–831. [Google Scholar] [CrossRef]
- Sanders, N.L.; Bollinger, R.R.; Lee, R.; Thomas, S.; Parker, W. Appendectomy and Clostridium difficile colitis: Relationships revealed by clinical observations and immunology. World J. Gastroenterol. 2013, 19, 5607–5614. [Google Scholar] [CrossRef]
- Masahata, K.; Umemoto, E.; Kayama, H.; Kotani, M.; Nakamura, S.; Kurakawa, T.; Kikuta, J.; Gotoh, K.; Motooka, D.; Sato, S.; et al. Generation of colonic IgA-secreting cells in the caecal patch. Nat. Commun. 2014, 5, 3704. [Google Scholar] [CrossRef] [PubMed]
- Smith, H.F.; Parker, W.; Kotzé, S.H.; Laurine, M. Morphological evolution of the mammalian cecum and cecal appendix. Comptes Rendus Palevol 2017, 16, 39–57. [Google Scholar] [CrossRef]
- Collado, M.C.; Derrien, M.; Isolauri, E.; de Vos, W.M.; Salminen, S. Intestinal integrity and Akkermansia muciniphila, a mucin-degrading member of the intestinal microbiota present in infants, adults, and the elderly. Appl. Environ. Microbiol. 2007, 73, 7767–7770. [Google Scholar] [CrossRef]
- Derrien, M.; Collado, M.C.; Ben-Amor, K.; Salminen, S.; de Vos, W.M. The mucin degrader Akkermansia muciniphila is an abundant resident of the human intestinal tract. Appl. Environ. Microbiol. 2008, 74, 1646–1648. [Google Scholar] [CrossRef] [PubMed]
- Everard, A.; Belzer, C.; Geurts, L.; Ouwerkerk, J.P.; Druart, C.; Bindels, L.B.; Guiot, Y.; Derrien, M.; Muccioli, G.G.; Delzenne, N.M.; et al. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc. Natl. Acad. Sci. USA 2013, 110, 9066–9071. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Lin, S.; Vanhoutte, P.M.; Woo, C.W.; Xu, A. Akkermansia muciniphila protects against atherosclerosis by preventing metabolic endotoxemia-induced inflammation in Apoe-/-mice. Circulation 2016, 133, 2434–2446. [Google Scholar] [CrossRef] [PubMed]
- Van Passel, M.W.; Kant, R.; Zoetendal, E.G.; Plugge, C.M.; Derrien, M.; Malfatti, S.A.; Chain, P.S.; Woyke, T.; Palva, A.; de Vos, W.M.; et al. The genome of Akkermansia muciniphila, a dedicated intestinal mucin degrader, and its use in exploring intestinal metagenomes. PLoS ONE 2011, 6, e16876. [Google Scholar] [CrossRef] [PubMed]
- Grander, C.; Adolph, T.E.; Wieser, V.; Lowe, P.; Wrzosek, L.; Gyongyosi, B.; Ward, D.V.; Grabherr, F.; Gerner, R.R.; Pfister, A.; et al. Recovery of ethanol-induced Akkermansia muciniphila depletion ameliorates alcoholic liver disease. Gut 2018, 67, 891–901. [Google Scholar] [CrossRef]
- Castro-Mejía, J.; Jakesevic, M.; Krych, Ł.; Nielsen, D.S.; Hansen, L.H.; Sondergaard, B.C.; Kvist, P.H.; Hansen, A.K.; Holm, T.L. Treatment with a monoclonal anti-IL-12p40 antibody induces substantial gut microbiota changes in an experimental colitis model. Gastroenterol. Res. Pract. 2016, 2016, 4953120. [Google Scholar] [CrossRef]
- Shimokawa, C.; Obi, S.; Shibata, M.; Olia, A.; Imai, T.; Suzue, K.; Hisaeda, H. Suppression of obesity by an intestinal helminth through interactions with intestinal microbiota. Infect. Immun. 2019, 87, e00042-19. [Google Scholar] [CrossRef]
- Liang, S.; Wu, X.; Jin, F. Gut-brain psychology: Rethinking psychology from the microbiota-gut-brain axis. Front. Integr. Neurosci. 2018, 12, 33. [Google Scholar] [CrossRef]
- Liu, W.; Marsh, T.L.; Cheng, H.; Forney, L.J. Characterization of microbial diversity by determining terminal restriction fragment length polymorphism of genes encoding 16S rRNA. Appl. Environ. Microbiol. 1997, 63, 4516–4522. [Google Scholar] [CrossRef] [PubMed]
- Nagashima, K.; Hisada, T.; Sato, M.; Mochizuki, J. Application of new primer-enzyme combinations to terminal restriction fragment length polymorphism profiling of bacterial populations in human feces. Appl. Environ. Microbiol. 2003, 69, 1251–1262. [Google Scholar] [CrossRef] [PubMed]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Pena, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef] [PubMed]
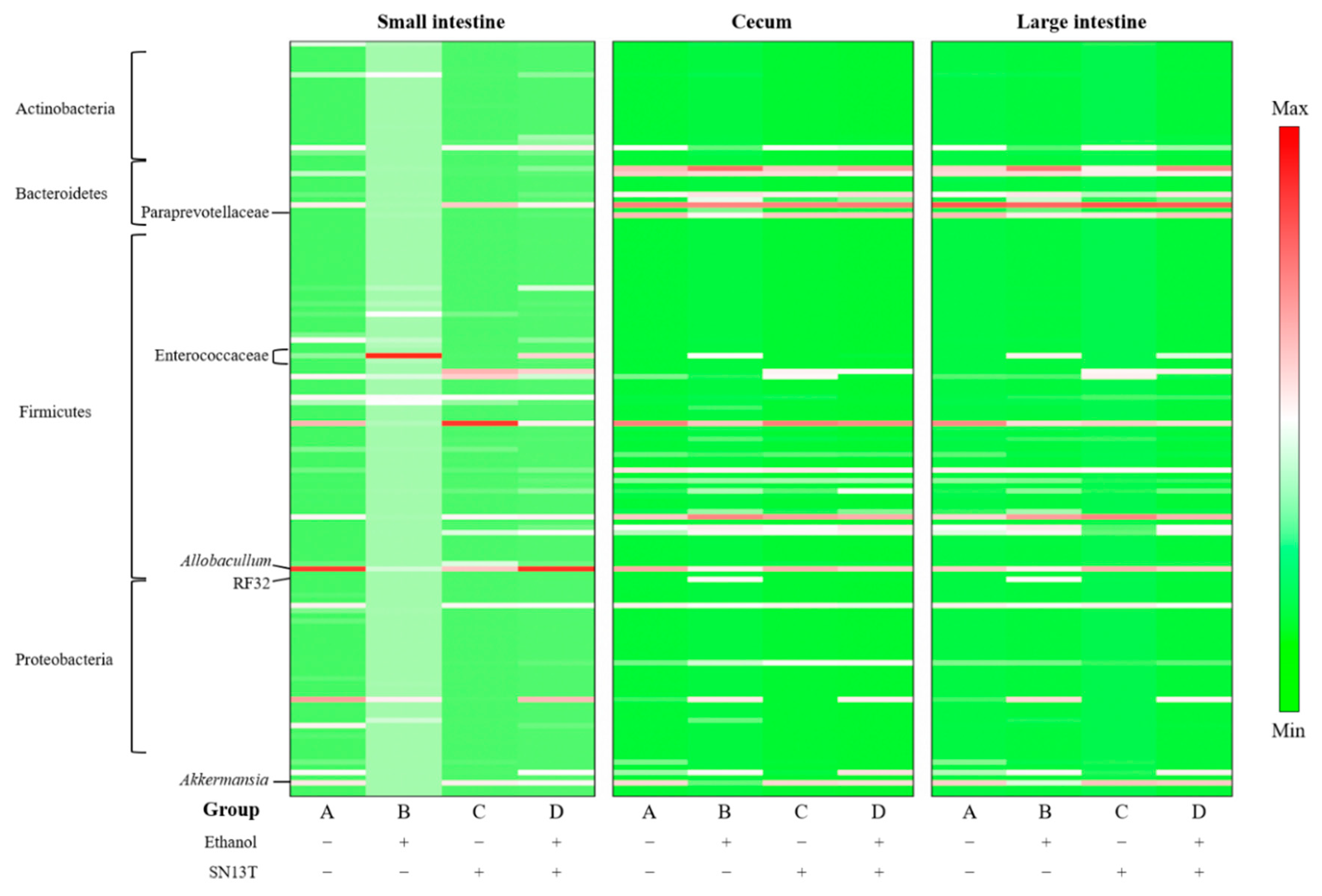
| Part of Intestine/Alteration | Phylum | Class | Order | Family | Genus |
|---|---|---|---|---|---|
| Small intestine | |||||
| Increased 1 | Epsilonproteobacteria *,3 | Bacillales | Propionibacteriaceae * | Propionibacterium * | |
| Lactobacillales * | Paraprevotellaceae * | Staphylococcus * | |||
| Campylobacterales * | Staphylococcaceae * | Facklamia * | |||
| Enterococcaceae * | Enterococcus | ||||
| Campylobacteraceae * | Arcobacter * | ||||
| Proteus * | |||||
| Decreased 2 | Bacteroidetes * | Coriobacteriia * | Coriobacteriales * | Coriobacteriaceae * | Adlercreutzia * |
| Cyanobacteria | Bacteroidiia * | Bacteroidales * | Porphyromonadaceae * | Parabacteroides * | |
| Proteobacteria * | Chloroplast | Streptophyta | S24-7 * | Lactobacillus | |
| TM7 * | Clostridia * | Clostridiales * | Lactobacillaceae * | Clostridium | |
| Tenericutes * | Erysipelotrichi * | Erysipelotrichales * | Clostridiaceae | Coprococcus * | |
| Verrucomicrobia * | Alphaproteobacteria * | Rhizobiales | Lachnospiraceae * | Allobaculum * | |
| Betaproteobacteria * | Burkholderiales * | Ruminococcaceae * | Sutterella * | ||
| Deltaproteobacteria * | Desulfovibrionales * | Erysipelotrichaceae * | Bilophila * | ||
| TM7-3 * | Spirobacillales | Methylobacteriaceae | Akkermansia * | ||
| Mollicutes * | Pseudomonadales * | Alcaligenaceae * | |||
| Verrucomicrobiae * | CW040 * | Desulfovibrionaceae * | |||
| RF39 * | F16 * | ||||
| Verrucomicrobiales * | Verrucomicrobiaceae * | ||||
| Cecum | |||||
| Increased | Gammaproteobacteria | Bacillales | Bifidobacteriaceae | Bifidobacterium | |
| Turicibacterales * | Staphylococcaceae * | Jeotgalicoccus * | |||
| RF32 * | Enterococcaceae * | Staphylococcus * | |||
| Rhodocyclales | Turicibacteraceae * | Enterococcus | |||
| Enterobacteriales | Oxalobacteraceae * | Turicibacter * | |||
| Rhodocyclaceae | Anaerotruncus * | ||||
| Enterobacteriaceae | Anaerovorax | ||||
| Peptoniphilus * | |||||
| Ralstonia * | |||||
| Zoogloea * | |||||
| Proteus * | |||||
| Decreased | Verrucomicrobia | Verrucomicrobiae * | Rhizobiales | Prevotellaceae * | Clostridium * |
| Verrucomicrobiales * | Bradyrhizobiaceae | Akkermansia * | |||
| Verrucomicrobiaceae * | |||||
| Large intestine | |||||
| Increased | Alphaproteobacteria * | Bifidobacteriales | Bifidobacteriaceae | Bifidobacterium | |
| Gammaproteobacteria | Bacillales * | Staphylococcaceae * | AF12 | ||
| Turicibacterales * | Enterococcaceae * | Jeotgalicoccus | |||
| Enterobacteriales | Turicibacteraceae * | Staphylococcus * | |||
| Tissierellaceae * | Facklamia * | ||||
| Enterobacteriaceae | Enterococcus * | ||||
| Turicibacter * | |||||
| Anaerotruncus * | |||||
| Finegoldia | |||||
| Enterobacter | |||||
| Proteus * | |||||
| Decreased | Verrucomicrobia | Verrucomicrobiae * | Verrucomicrobiales * | Prevotellaceae * | Anaerofustis |
| Eubacteriaceae | Akkermansia * | ||||
| Verrucomicrobiaceae * |
| Compound Name | Group (Ethanol/SN13T) | |||
|---|---|---|---|---|
| A (−/−) | B (+/−) | C (−/+) | D (+/+) | |
| Pyruvic acid | 0.133 | 0.913 | 0.372 | −1.418 |
| Propionic acid | 0.141 | 1.139 | 0.017 | −1.297 |
| 1,3-Diaminopropane | −0.338 | 1.197 | 0.307 | −1.166 |
| Stearoyl ethanolamide | −0.461 | 1.238 | 0.302 | −1.079 |
| 5α-Pregnan-3α-ol-20-one | 0.190 | 1.333 | −0.642 | −0.880 |
| Cadaverine | 0.218 | 1.326 | −0.772 | −0.772 |
| Isobutyric acid, Butyric acid | 0.018 | 1.397 | −0.859 | −0.557 |
| N-Acetylglutamic acid | −0.943 | 1.386 | −0.434 | −0.009 |
| o-Hydroxybenzoic acid | −0.610 | 1.490 | −0.541 | −0.339 |
| Tyramine | −0.500 | 1.500 | −0.500 | −0.500 |
| N1,N8-Diacetylspermidine | −0.195 | 1.440 | −0.383 | −0.861 |
| Isovaleric acid, Valeric acid | −0.356 | 1.466 | −0.323 | −0.787 |
| Isopropanolamine | −0.642 | 1.455 | −0.141 | −0.672 |
| 2-Hydroxy-4-methylvaleric acid | −0.748 | 1.367 | 0.126 | −0.745 |
| 2-Oxoglutaric acid | −0.878 | 1.189 | 0.464 | −0.775 |
| Glycine (Gly) | 0.635 | −1.190 | 0.994 | −0.439 |
| Alanine (Ala) | 0.511 | −0.990 | 1.146 | −0.667 |
| Valine (Val) | 0.683 | −1.098 | 0.996 | −0.581 |
| Leucine (Leu) | 0.573 | −1.045 | 1.093 | −0.621 |
| Isoleucine (Ilo) | 0.755 | −1.053 | 0.948 | −0.649 |
| Methionine (Met) | 0.603 | −0.990 | 1.083 | −0.696 |
| Proline (Pro) | 0.546 | −1.223 | 1.037 | −0.360 |
| Phenylalanine (Phe) | 0.566 | −1.016 | 1.104 | −0.655 |
| Tryptophan (Try) | 0.569 | −0.929 | 1.115 | −0.755 |
| Serine (Ser) | 0.602 | −1.158 | 1.034 | −0.478 |
| Threonine (Thr) | 0.520 | −1.097 | 1.113 | −0.537 |
| Tyrosine (Tyr) | 0.616 | −1.068 | 1.056 | −0.604 |
| Cystine | 1.113 | −0.984 | 0.562 | −0.691 |
| Arginine (Arg) | 0.544 | −0.972 | 1.127 | −0.699 |
| Histidine (His) | 0.570 | −0.891 | 1.117 | −0.796 |
| Lysine (Lys) | 0.530 | −1.003 | 1.131 | −0.659 |
| Asparagine (Asn) | 0.383 | −0.839 | 1.239 | −0.783 |
| Glutamine (Gln) | 0.353 | −0.886 | 1.253 | −0.720 |
| Asparatic acid (Asp) | 0.600 | −1.177 | 1.026 | −0.449 |
| Glutamic acid (Glu) | 0.701 | −1.059 | 0.993 | −0.634 |
| Conditions | Positive Mode | Negative Mode |
|---|---|---|
| LC-TOFMS conditions | ||
| LC system | Agilent 1200 series RRLC system SL | |
| Column | ODS column, 2 × 50 mm, 2 μm | |
| MS system | Agilent LC/MSD TOF | |
| Column temperature | 40 °C | |
| Mobile phase | A: H2O/0.1% HCOOH | |
| B: Isopropano:Acetonitrile:H2O (63:30:5)/0.1% HCOOH, 2 mM HCOONH4 | ||
| Flow rate | 0.3 mL/min | |
| Run time | 20 min | |
| Post time | 7.5 min | |
| Gradient condition | 0–0.5 min: B 1%, 0.5–13.5 min: B 1%–100%, 13.5–20 min: B 100% | |
| MS ionization mode | ESI positive | ESI negative |
| MS nebulizer pressure | 40 psi | |
| MS dry gas flow | 10 L/min | |
| MS dry gas temperature | 350 °C | |
| MS capillary voltage | 3500 V | |
| MS scan range | m/z 100–1700 | |
| Sample injection volume | 1 μL | |
| CE-TOFMS conditions | ||
| CE system | Agilent CE-TOFMS system | |
| Capillary | Fused silica capillary id 50 μm × 80 cm | |
| Run buffer | Cation Buffer Soln. (p/n: H3301–1001) | Anion Buffer Soln. (p/n: H3302–1021) |
| Rinse buffer | Cation Buffer Soln. (p/n: H3301–1001) | Anion Buffer Soln. (p/n: H3302–1021) |
| Sample injection | Pressure injection 50 mbar, 10 s | Pressure injection 50 mbar, 25 s |
| CE voltage | Positive, 27 kV | Positive, 30 kV |
| MS ionization | ESI positive | ESI negative |
| MS capillary voltage | 4000 V | 3500 V |
| MS scan range | m/z 50–1000 | |
| Sheath liquid | HMT Sheath Liquid (p/n: H3301–1020) | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Noda, M.; Maruyama, M.; Danshiitsoodol, N.; Higashikawa, F.; Sugiyama, M. Improvement of Alcohol-Poisoning Symptoms in Mice by the Oral Administration of Live Lactobacillus plantarum SN13T Cells. Int. J. Mol. Sci. 2020, 21, 1896. https://doi.org/10.3390/ijms21051896
Noda M, Maruyama M, Danshiitsoodol N, Higashikawa F, Sugiyama M. Improvement of Alcohol-Poisoning Symptoms in Mice by the Oral Administration of Live Lactobacillus plantarum SN13T Cells. International Journal of Molecular Sciences. 2020; 21(5):1896. https://doi.org/10.3390/ijms21051896
Chicago/Turabian StyleNoda, Masafumi, Masafumi Maruyama, Narandalai Danshiitsoodol, Fumiko Higashikawa, and Masanori Sugiyama. 2020. "Improvement of Alcohol-Poisoning Symptoms in Mice by the Oral Administration of Live Lactobacillus plantarum SN13T Cells" International Journal of Molecular Sciences 21, no. 5: 1896. https://doi.org/10.3390/ijms21051896
APA StyleNoda, M., Maruyama, M., Danshiitsoodol, N., Higashikawa, F., & Sugiyama, M. (2020). Improvement of Alcohol-Poisoning Symptoms in Mice by the Oral Administration of Live Lactobacillus plantarum SN13T Cells. International Journal of Molecular Sciences, 21(5), 1896. https://doi.org/10.3390/ijms21051896





