The Polymorphic PolyQ Tail Protein of the Mediator Complex, Med15, Regulates the Variable Response to Diverse Stresses
Abstract
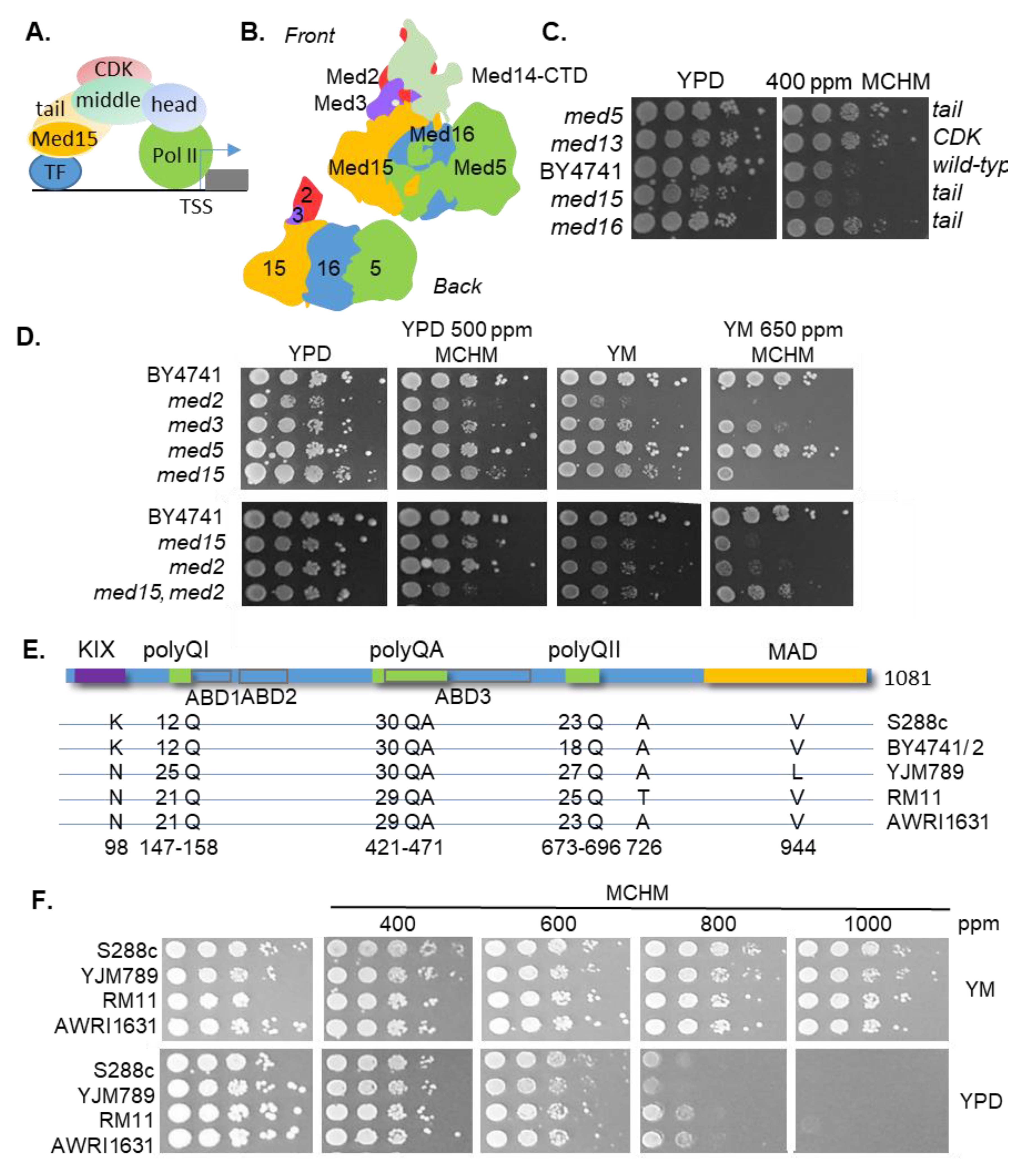
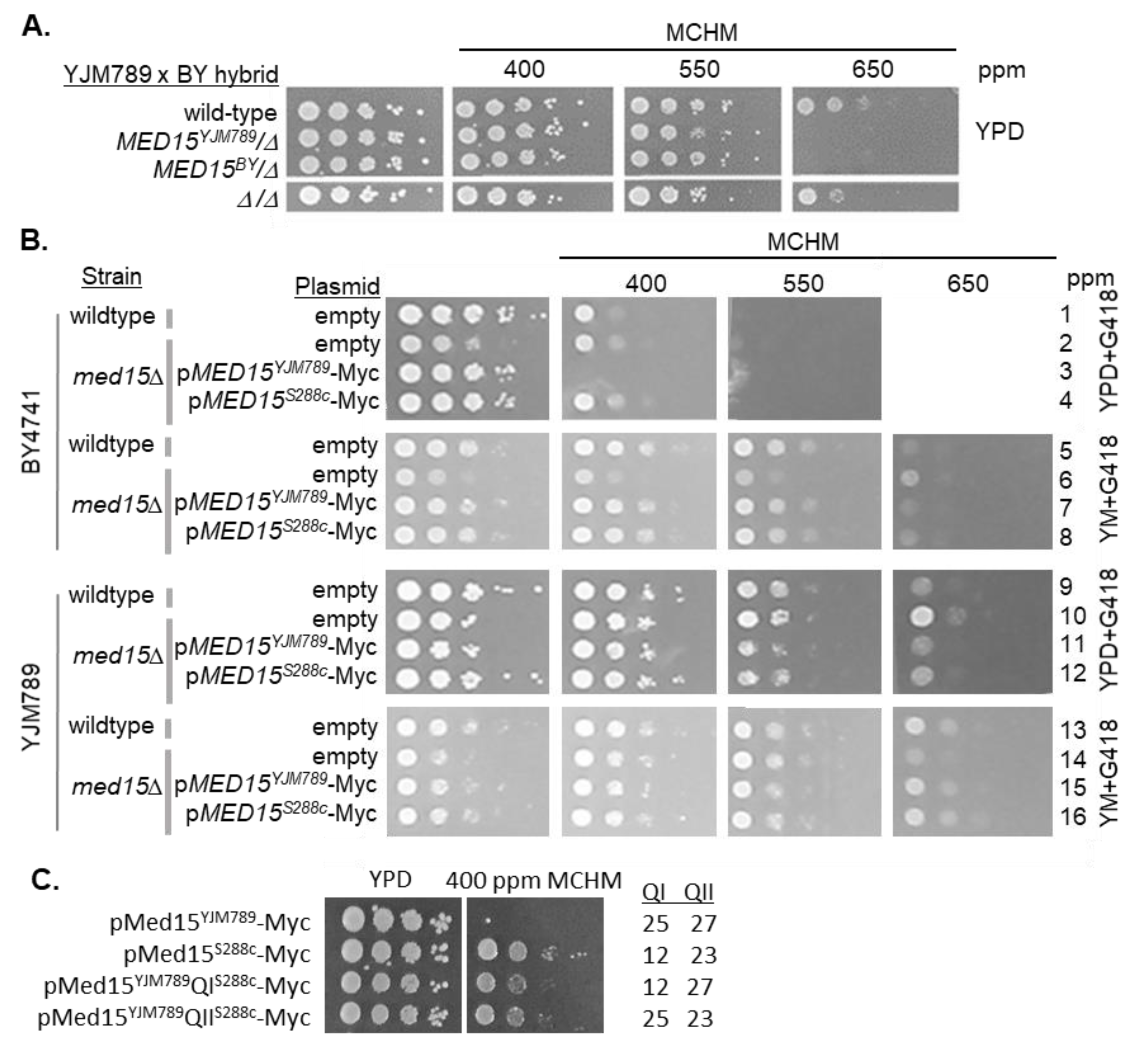
1. Introduction
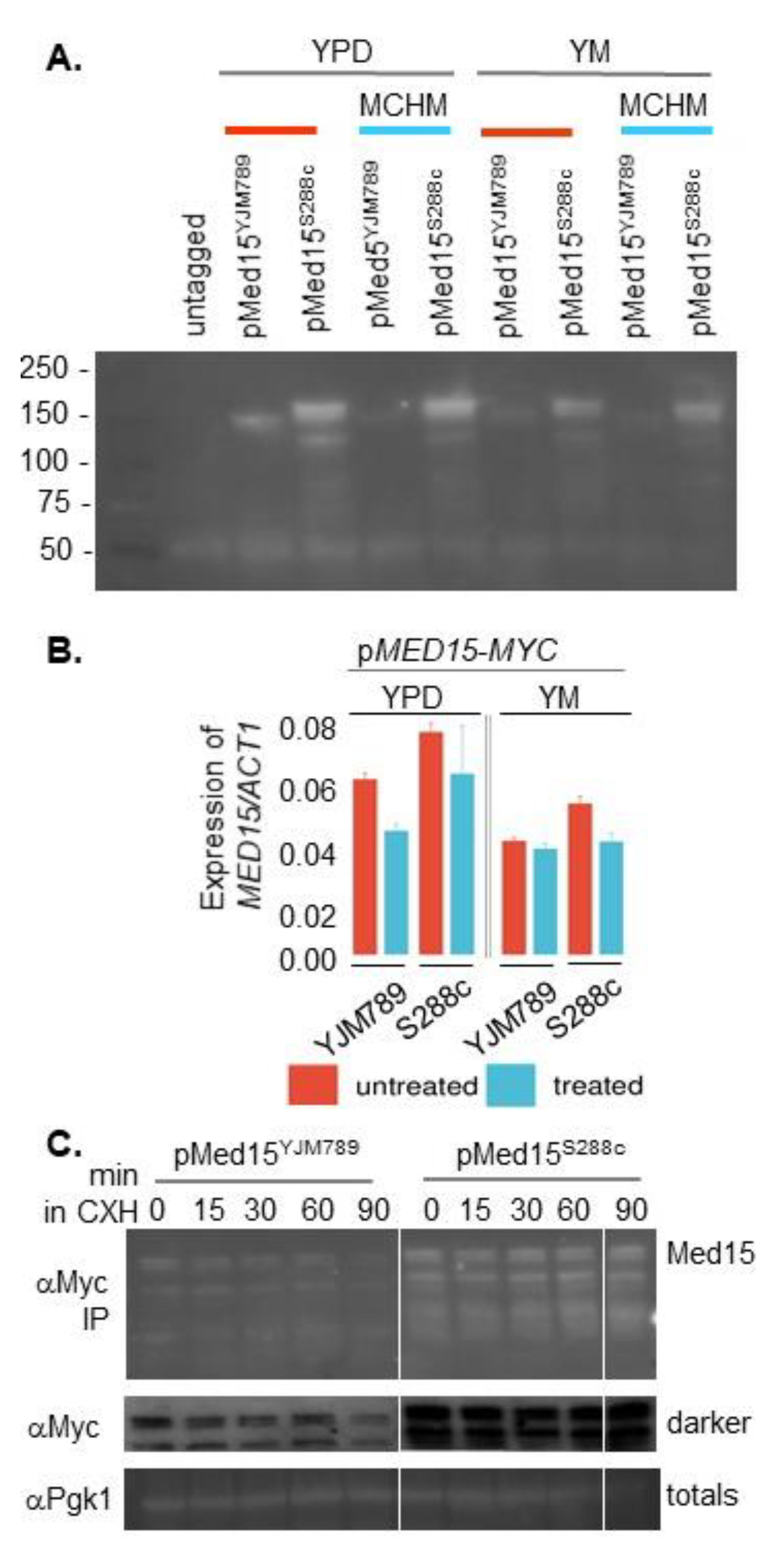
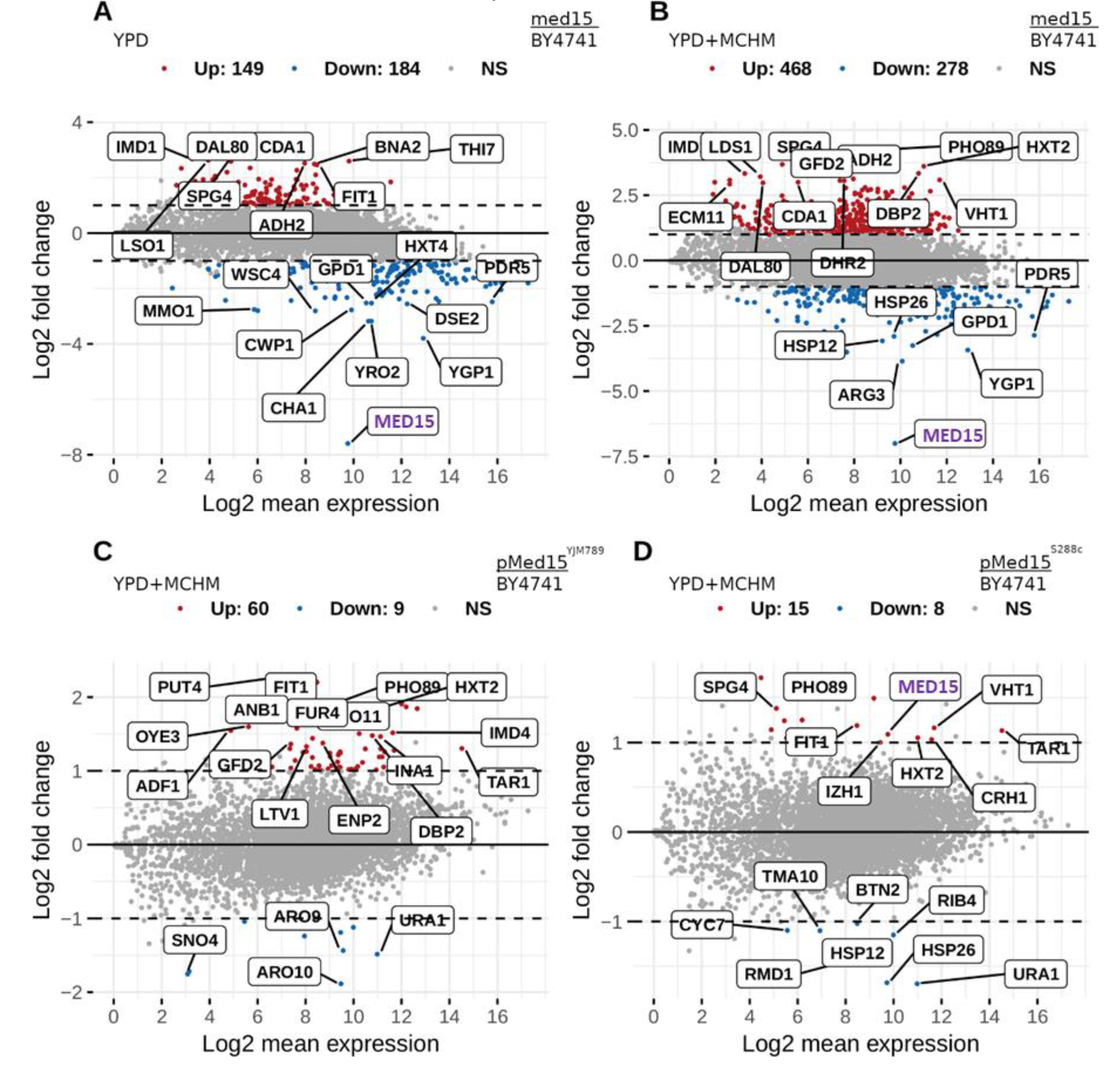
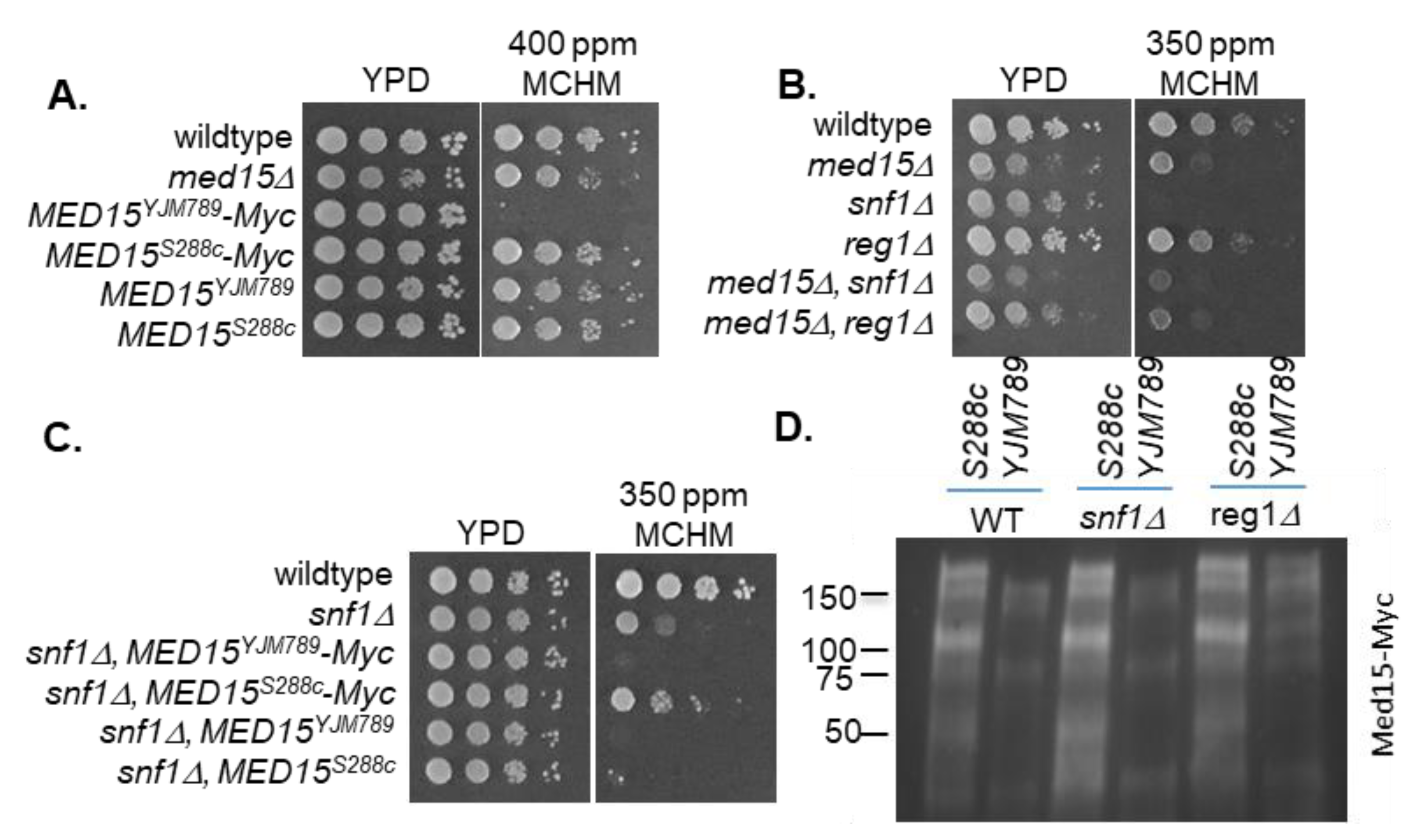
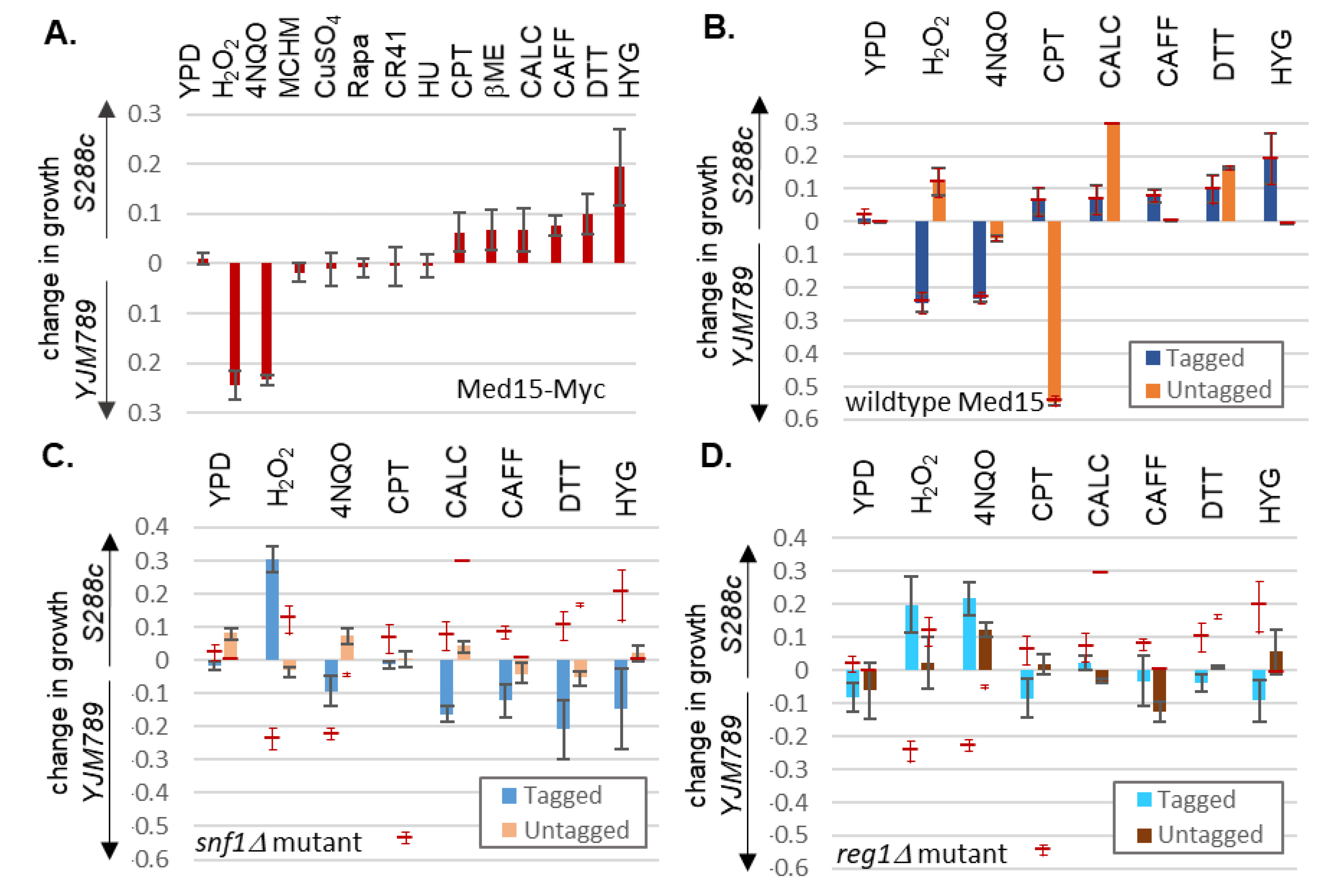
2. Results
3. Discussion
4. Materials and Methods
4.1. Strain Construction and Cloning
4.2. Growth Conditions
4.3. Transcriptomics
4.4. Western Blot
4.5. Flow Cytometry
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Verger, A.; Monté, D.; Villeret, V. Twenty years of Mediator complex structural studies. Biochem. Soc. Trans. 2019, 47, 399–410. [Google Scholar] [CrossRef]
- Tsai, K.L.; Tomomori-Sato, C.; Sato, S.; Conaway, R.C.; Conaway, J.W.; Asturias, F.J. Subunit architecture and functional modular rearrangements of the transcriptional mediator complex. Cell 2014, 157, 1430–1444. [Google Scholar] [CrossRef] [PubMed]
- Robinson, P.J.; Trnka, M.J.; Pellarin, R.; Greenberg, C.H.; Bushnell, D.A.; Davis, R.; Burlingame, A.L.; Sali, A.; Kornberg, R.D. Molecular architecture of the yeast Mediator complex. eLife 2015, 4, e08719. [Google Scholar] [CrossRef] [PubMed]
- Bourbon, H.-M. Comparative genomics supports a deep evolutionary origin for the large, four-module transcriptional mediator complex. Nucleic Acids Res. 2008, 36, 3993–4008. [Google Scholar] [CrossRef] [PubMed]
- Poss, Z.C.; Ebmeier, C.C.; Taatjes, D.J. The Mediator complex and transcription regulation. Crit. Rev. Biochem. Mol. Biol. 2013, 48, 575–608. [Google Scholar] [CrossRef] [PubMed]
- Miller, C.; Matic, I.; Maier, K.C.; Schwalb, B.; Roether, S.; Strasser, K.; Tresch, A.; Mann, M.; Cramer, P. Mediator phosphorylation prevents stress response transcription during non-stress conditions. J. Biol. Chem. 2012, 287, 44017–44026. [Google Scholar] [CrossRef] [PubMed]
- Korber, P.; Barbaric, S. The yeast PHO5 promoter: From single locus to systems biology of a paradigm for gene regulation through chromatin. Nucleic Acids Res. 2014, 42, 10888–10902. [Google Scholar] [CrossRef]
- Shao, D.; Creasy, C.L.; Bergman, L.W. A cysteine residue in helix II of the bHLH domain is essential for homodimerization of the yeast transcription factor Pho4p. Nucleic Acids Res. 1998, 26, 710–714. [Google Scholar] [CrossRef][Green Version]
- Shao, D.; Creasy, C.L.; Bergman, L.W. Interaction of Saccharomyces cerevisiae Pho2 with Pho4 increases the accessibility of the activation domain of Pho4. Mol. Gen. Genet. 1996, 251, 358–364. [Google Scholar]
- Herbig, E.; Warfield, L.; Fish, L.; Fishburn, J.; Knutson, B.A.; Moorefield, B.; Pacheco, D.; Hahn, S. Mechanism of Mediator recruitment by tandem Gcn4 activation domains and three Gal11 activator-binding domains. Mol. Cell. Biol. 2010, 30, 2376–2390. [Google Scholar] [CrossRef]
- Brzovic, P.S.; Heikaus, C.C.; Kisselev, L.; Vernon, R.; Herbig, E.; Pacheco, D.; Warfield, L.; Littlefield, P.; Baker, D.; Klevit, R.E.; et al. The acidic transcription activator Gcn4 binds the mediator subunit Gal11/Med15 using a simple protein interface forming a fuzzy complex. Mol. Cell 2011, 44, 942–953. [Google Scholar] [CrossRef] [PubMed]
- Alberti, S.; Halfmann, R.; King, O.; Kapila, A.; Lindquist, S. A systematic survey identifies prions and illuminates sequence features of prionogenic proteins. Cell 2009, 137, 146–158. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Chen, L.; Carlsten, J.O.P.; Liu, Q.; Yang, J.; Liu, B.; Gustafsson, C.M. Mediator tail subunits can form amyloid-like aggregates in vivo and affect stress response in yeast. Nucleic Acids Res. 2015, 43, 7306–7314. [Google Scholar] [CrossRef] [PubMed]
- Baxendale, S.; MacDonald, M.E.; Mott, R.; Francis, F.; Lin, C.; Kirby, S.F.; James, M.; Zehetner, G.; Hummerich, H.; Valdes, J. A cosmid contig and high resolution restriction map of the 2 megabase region containing the Huntington’s disease gene. Nat. Genet. 1993, 4, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Landles, C.; Bates, G.P. Huntingtin and the molecular pathogenesis of Huntington’s disease. EMBO Rep. 2004, 5, 958–963. [Google Scholar] [CrossRef] [PubMed]
- Krobitsch, S.; Lindquist, S. Aggregation of huntingtin in yeast varies with the length of the polyglutamine expansion and the expression of chaperone proteins. Proc. Natl. Acad. Sci. USA 1999, 97, 1589–1594. [Google Scholar] [CrossRef]
- Gillies, A.T.; Taylor, R.; Gestwicki, J.E. Synthetic lethal interactions in yeast reveal functional roles of J protein co-chaperones. Mol. Biosyst. 2012, 8, 2901–2908. [Google Scholar] [CrossRef]
- Hines, J.K.; Li, X.; Du, Z.; Higurashi, T.; Li, L.; Craig, E.A. [SWI], the prion formed by the chromatin remodeling factor Swi1, is highly sensitive to alterations in Hsp70 chaperone system activity. PLoS Genet. 2011, 7, e1001309. [Google Scholar] [CrossRef]
- Tuttle, L.M.; Pacheco, D.; Warfield, L.; Luo, J.; Ranish, J.; Hahn, S.; Klevit, R.E. Gcn4-Mediator Specificity Is Mediated by a Large and Dynamic Fuzzy Protein-Protein Complex. Cell Rep. 2018, 22, 3251–3264. [Google Scholar] [CrossRef]
- Pacheco, D.; Warfield, L.; Brajcich, M.; Robbins, H.; Luo, J.; Ranish, J.; Hahn, S. Transcription Activation Domains of the Yeast Factors Met4 and Ino2: Tandem Activation Domains with Properties Similar to the Yeast Gcn4 Activator. Mol. Cell. Biol. 2018, 38, e00038-18. [Google Scholar] [CrossRef]
- Warfield, L.; Tuttle, L.M.; Pacheco, D.; Klevit, R.E.; Hahn, S. A sequence-specific transcription activator motif and powerful synthetic variants that bind Mediator using a fuzzy protein interface. Proc. Natl. Acad. Sci. USA 2014, 111, E3506–E3513. [Google Scholar] [CrossRef] [PubMed]
- Boija, A.; Klein, I.A.; Sabari, B.R.; Dall’Agnese, A.; Coffey, E.L.; Zamudio, A.V.; Li, C.H.; Shrinivas, K.; Manteiga, J.C.; Hannett, N.M.; et al. Transcription Factors Activate Genes through the Phase-Separation Capacity of Their Activation Domains. Cell 2018, 175, 1842–1855.e16. [Google Scholar] [CrossRef] [PubMed]
- Hahn, S. Phase Separation, Protein Disorder, and Enhancer Function. Cell 2018, 175, 1723–1725. [Google Scholar] [CrossRef] [PubMed]
- Alberti, S.; Gladfelter, A.; Mittag, T. Considerations and Challenges in Studying Liquid-Liquid Phase Separation and Biomolecular Condensates. Cell 2019, 176, 419–434. [Google Scholar] [CrossRef]
- Hsu, J.; Del Rosario, M.C.; Thomasson, E.; Bixler, D.; Haddy, L.; Duncan, M.A. Hospital Impact after a Chemical Spill That Compromised the Potable Water Supply: West Virginia, January 2014. Disaster Med. Public Health Prep. 2017, 11, 621–624. [Google Scholar] [CrossRef]
- Thomasson, E.D.; Scharman, E.; Fechter-Leggett, E.; Bixler, D.; Ibrahim, S.; Duncan, M.A.; Hsu, J.; Scott, M.; Wilson, S.; Haddy, L.; et al. Acute health effects after the Elk River chemical spill, West Virginia, January 2014. Public Health Rep. 2017, 132, 196–202. [Google Scholar] [CrossRef]
- Pupo, A.; Ayers, M.C.; Sherman, Z.N.; Vance, R.J.; Cumming, J.R.; Gallagher, J.E.G.G. MCHM Acts as a Hydrotrope, Altering the Balance of Metals in Yeast. Biol. Trace Elem. Res. 2019, 1–12. [Google Scholar] [CrossRef]
- Patel, A.; Malinovska, L.; Saha, S.; Wang, J.; Alberti, S.; Krishnan, Y.; Hyman, A.A. ATP as a biological hydrotrope. Science 2017, 356, 753–756. [Google Scholar] [CrossRef]
- Hayes, M.H.; Peuchen, E.H.; Dovichi, N.J.; Weeks, D.L. Dual roles for ATP in the regulation of phase separated protein aggregates in Xenopus oocyte nucleoli. eLife 2018, 7, e35224. [Google Scholar] [CrossRef]
- Kang, J.; Lim, L.; Song, J. ATP enhances at low concentrations but dissolves at high concentrations liquid-liquid phase separation (LLPS) of ALS/FTD-causing FUS. Biochem. Biophys. Res. Commun. 2018, 504, 545–551. [Google Scholar] [CrossRef]
- Lin, Y.; Protter, D.S.W.; Rosen, M.K.; Parker, R. Formation and Maturation of Phase-Separated Liquid Droplets by RNA-Binding Proteins. Mol. Cell 2015, 60, 208–219. [Google Scholar] [CrossRef] [PubMed]
- Pupo, A.; Ku, K.M.; Gallagher, J.E.G. Effects of MCHM on yeast metabolism. PLoS ONE 2019, 14, e0223909. [Google Scholar] [CrossRef] [PubMed]
- Hedbacker, K.; Carlson, M. SNF1/AMPK pathways in yeast. Front. Biosci. 2008, 13, 2408–2420. [Google Scholar] [CrossRef]
- Simpson-Lavy, K.; Kupiec, M. A reversible liquid drop aggregation controls glucose response in yeast. Curr. Genet. 2018, 64, 785–788. [Google Scholar] [CrossRef]
- Schmit, M. (University of Pittsburgh, Pittsburgh, PA, USA). Personal communication, 2019.
- Uthe, H.; Vanselow, J.T.; Schlosser, A. Proteomic Analysis of the Mediator Complex Interactome in Saccharomyces cerevisiae. Sci. Rep. 2017, 7, 43584. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, J.E.G.; Zheng, W.; Rong, X.; Miranda, N.; Lin, Z.; Dunn, B.; Zhao, H.; Snyder, M.P. Divergence in a master variator generates distinct phenotypes and transcriptional responses. Genes Dev. 2014, 28, 409–421. [Google Scholar] [CrossRef][Green Version]
- Rong-Mullins, X.X.; Ayers, M.C.; Summers, M.; Gallagher, J.E.G. Transcriptional Profiling of Saccharomyces cerevisiae Reveals the Impact of Variation of a Single Transcription Factor on Differential Gene Expression in 4NQO, Fermentable, and Nonfermentable Carbon Sources. G3 Genes Genomes Genet. 2018, 8, 607–619. [Google Scholar] [CrossRef]
- Cooper, D.G.; Fassler, J.S. Med15: Glutamine-Rich Mediator Subunit with Potential for Plasticity. Trends Biochem. Sci. 2019, 9, 737–751. [Google Scholar] [CrossRef]
- Michelitsch, M.D.; Weissman, J.S. A census of glutamine/asparagine-rich regions: Implications for their conserved function and the prediction of novel prions. Proc. Natl. Acad. Sci. USA 2000, 97, 11910–11915. [Google Scholar] [CrossRef]
- Kachroo, A.H.; Laurent, J.M.; Yellman, C.M.; Meyer, A.G.; Wilke, C.O.; Marcotte, E.M. Systematic humanization of yeast genes reveals conserved functions and genetic modularity. Science 2015, 348, 921–925. [Google Scholar] [CrossRef] [PubMed]
- Albuquerque, C.P.; Smolka, M.B.; Payne, S.H.; Bafna, V.; Eng, J.; Zhou, H. A multidimensional chromatography technology for in-depth phosphoproteome analysis. Mol. Cell. Proteom. 2008, 7, 1389–1396. [Google Scholar] [CrossRef] [PubMed]
- Holt, L.J.; Tuch, B.B.; Villén, J.; Johnson, A.D.; Gygi, S.P.; Morgan, D.O. Global analysis of Cdk1 substrate phosphorylation sites provides insights into evolution. Science 2009, 325, 1682–1686. [Google Scholar] [CrossRef] [PubMed]
- Soulard, A.; Cremonesi, A.; Moes, S.; Schütz, F.; Jenö, P.; Hall, M.N. The Rapamycin-sensitive Phosphoproteome Reveals That TOR Controls Protein Kinase A Toward Some But Not All Substrates. Mol. Biol. Cell 2010, 21, 3475–3486. [Google Scholar] [CrossRef] [PubMed]
- Swaney, D.L.; Beltrao, P.; Starita, L.; Guo, A.; Rush, J.; Fields, S.; Krogan, N.J.; Villén, J.; Villen, J. Global analysis of phosphorylation and ubiquitylation cross-talk in protein degradation. Nat. Methods 2013, 10, 676–682. [Google Scholar] [CrossRef]
- Jedidi, I.; Zhang, F.; Qiu, H.; Stahl, S.J.; Palmer, I.; Kaufman, J.D.; Nadaud, P.S.; Mukherjee, S.; Wingfield, P.T.; Jaroniec, C.P.; et al. Activator Gcn4 employs multiple segments of Med15/Gal11, including the KIX domain, to recruit mediator to target genes in vivo. J. Biol. Chem. 2010, 285, 2438–2455. [Google Scholar] [CrossRef]
- Ansari, S.A.; Ganapathi, M.; Benschop, J.J.; Holstege, F.C.P.; Wade, J.T.; Morse, R.H. Distinct role of Mediator tail module in regulation of SAGA-dependent, TATA-containing genes in yeast. EMBO J. 2011, 31, 44–57. [Google Scholar] [CrossRef]
- Larsson, M.; Uvell, H.; Sandstrom, J.; Ryden, P.; Selth, L.A.; Bjorklund, S. Functional studies of the yeast med5, med15 and med16 mediator tail subunits. PLoS ONE 2013, 8, e73137. [Google Scholar] [CrossRef]
- Huisinga, K.L.; Pugh, B.F. A genome-wide housekeeping role for TFIID and a highly regulated stress-related role for SAGA in Saccharomyces cerevisiae. Mol. Cell 2004, 13, 573–585. [Google Scholar] [CrossRef]
- Jeronimo, C.; Robert, F. Kin28 regulates the transient association of Mediator with core promoters. Nat. Struct. Mol. Biol. 2014, 21, 449–455. [Google Scholar] [CrossRef]
- Dunn, B.; Stanford University, Stanford, CA, USA; Srivas, R.; Stanford University, Stanford, CA, USA; Kawli, T.; Stanford University, Stanford, CA, USA; Jiang, L.; Stanford University, Stanford, CA, USA; Li, E.; Stanford University, Stanford, CA, USA; Choe, K.; Stanford University, Stanford, CA, USA; Gallagher, J.E.G.; West Virginia University, Morgantown, WV, USA; Snyder, M.P.; Stanford University, Stanford, CA, USA. Integrative analysis of variation in gene expression regulation networks among diverse strains of S. cerevisiae. Unpublished work. 2018. [Google Scholar]
- Island, M.D.; Perry, J.R.; Naider, F.; Becker, J.M. Isolation and characterization of S. cerevisiae mutants deficient in amino acid-inducible peptide transport. Curr. Genet. 1991, 20, 457–463. [Google Scholar] [CrossRef] [PubMed]
- Vandenbol, M.; Jauniaux, J.C.; Grenson, M. Nucleotide sequence of the Saccharomyces cerevisiae PUT4 proline-permease-encoding gene: Similarities between CAN1, HIP1 and PUT4 permeases. Gene 1989, 83, 153–159. [Google Scholar] [CrossRef]
- Mara, P.; Fragiadakis, G.S.; Gkountromichos, F.; Alexandraki, D. The pleiotropic effects of the glutamate dehydrogenase (GDH) pathway in Saccharomyces cerevisiae. Microb. Cell Fact. 2018, 17, 170. [Google Scholar] [CrossRef]
- Villers, J.; Savocco, J.; Szopinska, A.; Degand, H.; Nootens, S.; Morsomme, P. Study of the Plasma Membrane Proteome Dynamics Reveals Novel Targets of the Nitrogen Regulation in Yeast. Mol. Cell. Proteom. 2017, 16, 1652–1668. [Google Scholar] [CrossRef]
- Caplan, A.J.; Douglas, M.G. Characterization of YDJI: A yeast homologue of the bacterial dnaJ protein. J. Cell Biol. 1991, 114, 609–621. [Google Scholar] [CrossRef]
- Ayers, M.C.; West Virginia Univeristy, Morgantown, WV, USA; Sherman, Z.N.; West Virginia University, Morgantown, WV, USA; Gallagher, J.E.G.; West Virginia University, Morgantown, WV, USA. Stress Responses and Nutrient Starvation in MCHM Treated Yeast. Unpublished work. 2020. [Google Scholar]
- Gallagher, D.L.; Phetxumphou, K.; Smiley, E.; Dietrich, A.M. Tale of Two Isomers: Complexities of Human Odor Perception for cis - and trans -4-Methylcyclohexane Methanol from the Chemical Spill in West Virginia. Environ. Sci. Technol. 2015, 49, 1319–1327. [Google Scholar] [CrossRef]
- Hyman, A.A.; Weber, C.A.; Ulicher, F.J. Downloaded from www.annualreviews.org Access provided by West Virginia University on 05/17/19. For personal use only. Annu. Rev. Cell Dev. Biol. 2014, 30, 39–58. [Google Scholar] [CrossRef]
- Qiu, H.; Chereji, R.V.; Hu, C.; Cole, H.A.; Rawal, Y.; Clark, D.J.; Hinnebusch, A.G. Genome-wide cooperation by HAT Gcn5, remodeler SWI/SNF, and chaperone Ydj1 in promoter nucleosome eviction and transcriptional activation. Genome Res. 2016, 26, 211–225. [Google Scholar] [CrossRef]
- Summers, D.W.; Douglas, P.M.; Ren, H.-Y.; Cyr, D.M. The type I Hsp40 Ydj1 utilizes a farnesyl moiety and zinc finger-like region to suppress prion toxicity. J. Biol. Chem. 2009, 284, 3628–3639. [Google Scholar] [CrossRef] [PubMed]
- Muchowski, P.J.; Schaffar, G.; Sittler, A.; Wanker, E.E.; Hayer-Hartl, M.K.; Hartl, F.U. Hsp70 and hsp40 chaperones can inhibit self-assembly of polyglutamine proteins into amyloid-like fibrils. Proc. Natl. Acad. Sci. USA 2000, 97, 7841–7846. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Weisenhorn, E.; MacDiarmid, C.W.; Andreini, C.; Bucci, M.; Taggart, J.; Banci, L.; Russell, J.; Coon, J.J.; Eide, D.J. The cellular economy of the Saccharomyces cerevisiae zinc proteome. Metallomics 2018, 10, 1755–1776. [Google Scholar] [CrossRef] [PubMed]
- Eastman Crude MCHM Studies. Available online: http://www.eastman.com/Pages/Eastman-Crude-MCHM-Studies.aspx (accessed on 17 August 2014).
- Horzmann, K.A.; de Perre, C.; Lee, L.S.; Whelton, A.J.; Freeman, J.L. Comparative analytical and toxicological assessment of methylcyclohexanemethanol (MCHM) mixtures associated with the Elk River chemical spill. Chemosphere 2017, 188, 599–607. [Google Scholar] [CrossRef]
- Han, A.A.; Fabyanic, E.B.; Miller, J.V.; Prediger, M.S.; Prince, N.; Mouch, J.A.; Boyd, J. In vitro cytotoxicity assessment of a West Virginia chemical spill mixture involving 4-methylcyclohexanemethanol and propylene glycol phenyl ether. Environ. Monit. Assess. 2017, 189, 190. [Google Scholar] [CrossRef]
- Phetxumphou, K.; Dietrich, A.M.; Shanaiah, N.; Smiley, E.; Gallagher, D.L. Subtleties of human exposure and response to chemical mixtures from spills. Environ. Pollut. 2016, 214, 618–626. [Google Scholar] [CrossRef]
- Gwinn, W.M.; Bousquet, R.W.; Perry, C.E.; Urbano, N.C.; Auerbach, S.S. NTP Research Report on the Preliminary Evaluation of 4-Methylcyclohexylmethanol in an In Vitro Human Airway Model; National Toxicology Program, 2018. Available online: https://www.ncbi.nlm.nih.gov/books/NBK535764/ (accessed on 1 December 2018).
- Weidhaas, J.; Lin, L.-S.; Buzby, K.; Li, X. Biodegradation of MCHM and PPH in River Microcosms and Activated Sludge. J. Environ. Eng. 2016, 142, 04016056. [Google Scholar] [CrossRef]
- Paustenbach, D.J.; Winans, B.; Novick, R.M.; Green, S.M. The toxicity of crude 4-methylcyclohexanemethanol (MCHM): Review of experimental data and results of predictive models for its constituents and a putative metabolite. Crit. Rev. Toxicol. 2015, 45, 1–55. [Google Scholar] [CrossRef]
- Cozzarelli, I.M.; Akob, D.M.; Baedecker, M.J.; Spencer, T.; Jaeschke, J.; Dunlap, D.S.; Mumford, A.C.; Poret-Peterson, A.T.; Chambers, D.B. Degradation of Crude 4-MCHM (4-Methylcyclohexanemethanol) in Sediments from Elk River, West Virginia. Environ. Sci. Technol. 2017, 51, 12139–12145. [Google Scholar] [CrossRef]
- Lan, J.; Hu, M.; Gao, C.; Alshawabkeh, A.; Gu, A.Z. Toxicity Assessment of 4-Methyl-1-cyclohexanemethanol and Its Metabolites in Response to a Recent Chemical Spill in West Virginia, USA. Environ. Sci. Technol. 2015, 49, 6284–6293. [Google Scholar] [CrossRef] [PubMed]
- Cui, D.; Mebel, A.M.; Arroyo-Mora, L.E.; Holness, H.; Furton, K.G.; O’Shea, K. Kinetic, product, and computational studies of the ultrasonic induced degradation of 4-methylcyclohexanemethanol (MCHM). Water Res. 2017, 126, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Martinez, P.; Persson, B.L. Identification, cloning and characterization of a derepressible Na+-coupled phosphate transporter in Saccharomyces cerevisiae. Mol. Gen. Genet. 1998, 258, 628–638. [Google Scholar] [CrossRef] [PubMed]
- Bun-Ya, M.; Nishimura, M.; Harashima, S.; Oshima, Y. The PHO84 gene of Saccharomyces cerevisiae encodes an inorganic phosphate transporter. Mol. Cell. Biol. 1991, 11, 3229–3238. [Google Scholar] [CrossRef] [PubMed]
- Serra-Cardona, A.; Petrezselyova, S.; Canadell, D.; Ramos, J.; Arino, J. Coregulated Expression of the Na+/Phosphate Pho89 Transporter and Ena1 Na+-ATPase Allows Their Functional Coupling under High-pH Stress. Mol. Cell. Biol. 2014, 34, 4420–4435. [Google Scholar] [CrossRef]
- Zhang, F.; Sumibcay, L.; Hinnebusch, A.G.; Swanson, M.J. A triad of subunits from the Gal11/tail domain of Srb mediator is an in vivo target of transcriptional activator Gcn4p. Mol. Cell. Biol. 2004, 24, 6871–6886. [Google Scholar] [CrossRef]
- Evan, G.I.; Lewis, G.K.; Ramsay, G.; Bishop, J.M. Isolation of monoclonal antibodies specific for human c-myc proto-oncogene product. Mol. Cell. Biol. 1985, 5, 3610–3616. [Google Scholar] [CrossRef]
- Bähler, J.; Wu, J.-Q.; Longtine, M.S.; Shah, N.G.; Mckenzie III, A.; Steever, A.B.; Wach, A.; Philippsen, P.; Pringle, J.R. Heterologous modules for efficient and versatile PCR-based gene targeting inSchizosaccharomyces pombe. Yeast 1998, 14, 943–951. [Google Scholar] [CrossRef]
- Anandhakumar, J.; Moustafa, Y.W.; Chowdhary, S.; Kainth, A.S.; Gross, D.S. Evidence for Multiple Mediator Complexes in Yeast Independently Recruited by Activated Heat Shock Factor. Mol. Cell. Biol. 2016, 36, 1943–1960. [Google Scholar] [CrossRef]
- He, Q.; Battistella, L.; Morse, R.H. Mediator requirement downstream of chromatin remodeling during transcriptional activation of CHA1 in yeast. J. Biol. Chem. 2008, 283, 5276–5286. [Google Scholar] [CrossRef]
- Song, G.; Dickins, B.J.; Demeter, J.; Engel, S.; Gallagher, J.; Choe Barbara., K.D.; Cherry, J.M. AGAPE (Automated Genome Analysis PipelinE) for pan-genome analysis of Saccharomyces cerevisiae. PLoS ONE 2015, 10, e0120671. [Google Scholar] [CrossRef]
- Rong-Mullins, X.; Winans, M.J.; Lee, J.B.; Lonergan, Z.R.; Pilolli, V.A.; Weatherly, L.M.; Carmenzind, T.W.; Jiang, L.; Cumming, J.R.; Oporto, G.S.; et al. Proteomic and genetic analysis of the response of S. cerevisiae to soluble copper leads to improvement of the antimicrobial function of cellulosic copper nanoparticles. Metallomics 2017, 9, 1304–1315. [Google Scholar] [CrossRef]
- Giaever, G.; Chu, A.M.; Ni, L.; Connelly, C.; Riles, L.; Veronneau, S.; Dow, S.; Lucau-Danila, A.; Anderson, K.; Andre, B.; et al. Functional profiling of the Saccharomyces cerevisiae genome. Nature 2002, 418, 387–391. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, J.E.G.; Baserga, S.J. Two-hybrid Mpp10p interaction-defective Imp4 proteins are not interaction defective in vivo but do confer specific pre-rRNA processing defects in Saccharomyces cerevisiae. Nucleic Acids Res. 2004, 32, 1404–1413. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Wang, L.-G.; Han, Y.; He, Q.-Y. clusterProfiler: An R Package for Comparing Biological Themes Among Gene Clusters. Omi. A J. Integr. Biol. 2012, 16, 284–287. [Google Scholar] [CrossRef] [PubMed]
- Sabari, B.R.; Dall’Agnese, A.; Boija, A.; Klein, I.A.; Coffey, E.L.; Shrinivas, K.; Abraham, B.J.; Hannett, N.M.; Zamudio, A.V.; Manteiga, J.C.; et al. Coactivator condensation at super-enhancers links phase separation and gene control. Science 2018, 361, eaar3958. [Google Scholar] [CrossRef]
- Boehning, M.; Dugast-Darzacq, C.; Rankovic, M.; Hansen, A.S.; Yu, T.; Marie-Nelly, H.; McSwiggen, D.T.; Kokic, G.; Dailey, G.M.; Cramer, P.; et al. RNA polymerase II clustering through carboxy-terminal domain phase separation. Nat. Struct. Mol. Biol. 2018, 25, 833–840. [Google Scholar] [CrossRef]
- Cho, W.-K.; Spille, J.-H.; Hecht, M.; Lee, C.; Li, C.; Grube, V.; Cisse, I.I. Mediator and RNA polymerase II clusters associate in transcription-dependent condensates. Science 2018, 361, 412–415. [Google Scholar] [CrossRef]
- Read, T.; Richmond, P.A.; Dowell, R.D.; Félix, M.; Bentley, D.; Chakravarti, A. A trans-acting Variant within the Transcription Factor RIM101 Interacts with Genetic Background to Determine its Regulatory Capacity. PLoS Genet. 2016, 12, e1005746. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gallagher, J.E.G.; Ser, S.L.; Ayers, M.C.; Nassif, C.; Pupo, A. The Polymorphic PolyQ Tail Protein of the Mediator Complex, Med15, Regulates the Variable Response to Diverse Stresses. Int. J. Mol. Sci. 2020, 21, 1894. https://doi.org/10.3390/ijms21051894
Gallagher JEG, Ser SL, Ayers MC, Nassif C, Pupo A. The Polymorphic PolyQ Tail Protein of the Mediator Complex, Med15, Regulates the Variable Response to Diverse Stresses. International Journal of Molecular Sciences. 2020; 21(5):1894. https://doi.org/10.3390/ijms21051894
Chicago/Turabian StyleGallagher, Jennifer E.G., Suk Lan Ser, Michael C. Ayers, Casey Nassif, and Amaury Pupo. 2020. "The Polymorphic PolyQ Tail Protein of the Mediator Complex, Med15, Regulates the Variable Response to Diverse Stresses" International Journal of Molecular Sciences 21, no. 5: 1894. https://doi.org/10.3390/ijms21051894
APA StyleGallagher, J. E. G., Ser, S. L., Ayers, M. C., Nassif, C., & Pupo, A. (2020). The Polymorphic PolyQ Tail Protein of the Mediator Complex, Med15, Regulates the Variable Response to Diverse Stresses. International Journal of Molecular Sciences, 21(5), 1894. https://doi.org/10.3390/ijms21051894





