Arginine Derivatives in Cerebrovascular Diseases: Mechanisms and Clinical Implications
Abstract
1. Precision Stroke Medicine: on Search for Novel Biomarkers
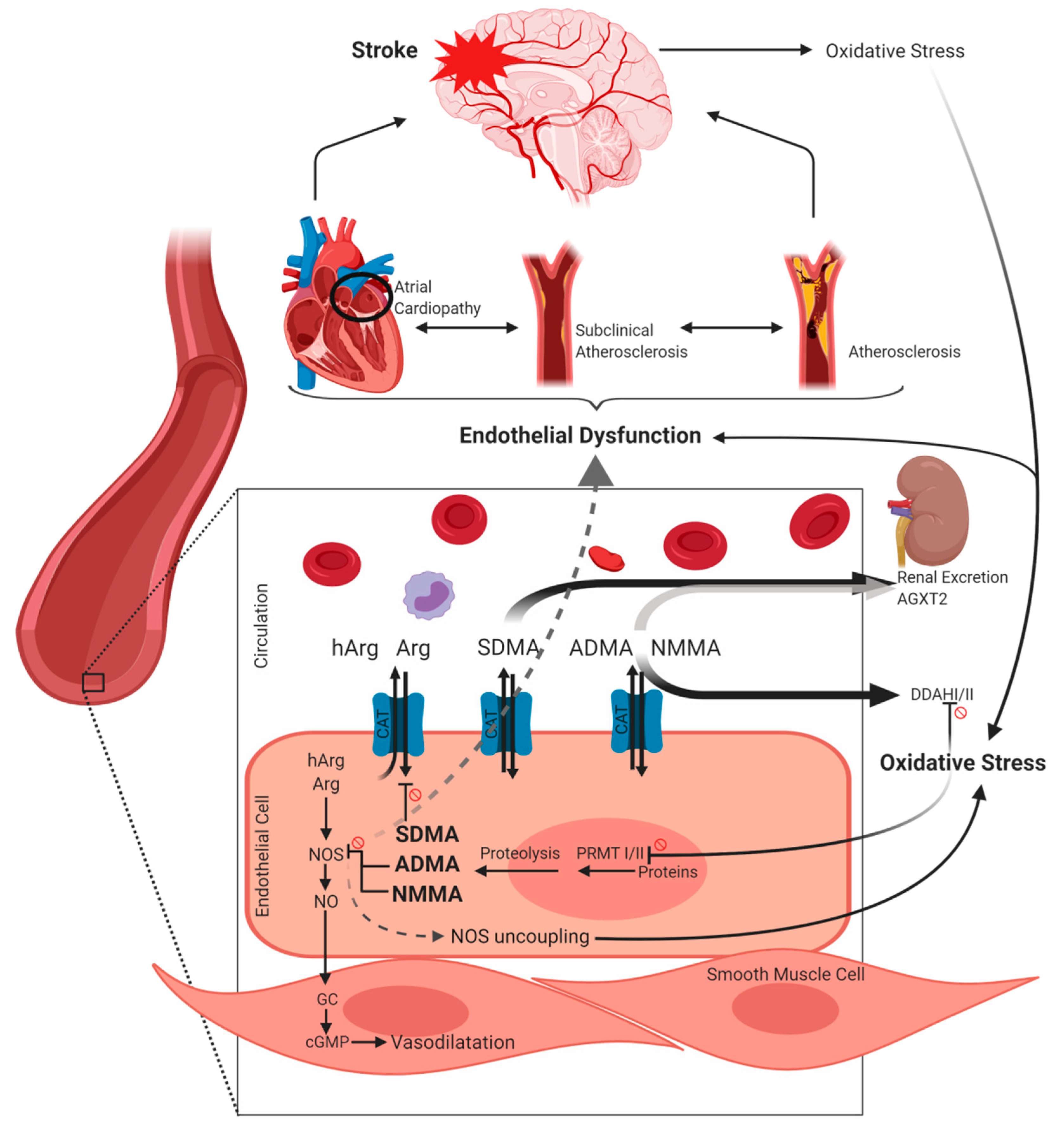
2. Metabolism of Arginine and its Derivatives
3. Arginine Derivatives as Markers of Cerebrovascular Risk and Mortality
3.1. The Relation of ADMA and SDMA to Atherosclerotic Disease
3.2. ADMA and SDMA in Relation to Vascular Risk Factors
3.3. Dimethylarginines Predict Morbidity and Mortality of Cerebrovascular Diseases
3.4. Homoarginine as Marker and Target in Cerebrovascular Diseases
4. Acute Response of Arginine Derivatives after Ischemic Stroke
Potential Mechanisms of Dimethylarginine Response after Ischemic Stroke
5. Arginine Derivatives as Markers of Stroke Etiology
5.1. Large Artery Atherosclerosis
5.2. Small Vessel Disease
5.3. Cardioembolic Stroke and Embolic Stroke of Undetermined Source
6. Conclusions
Funding
Conflicts of Interest
Abbreviations
| AE | adverse event |
| AGAT | arginine:glycine amidinotransferase |
| ADMA | asymmetric dimethylarginine |
| AF | atrial fibrillation |
| Arg | Arginine |
| CADASIL | Cerebral Autosomal Dominant Arteriopathy with Subcortical Infarcts and Leukoencephalopathy |
| CAT | cationic amino acid transporters |
| CES | cardioembolic stroke |
| cGMP | cyclic guanosine monophosphate |
| CIMT | carotid intima media thickness |
| CKD | chronic kidney disease |
| CRP | C-reactive protein |
| CVD | cardiovascular disease |
| DDAH | dimethylarginine dimethylaminohydrolase |
| eNOS | endothelial nitric oxide synthase |
| EPC | endothelial progenitor cells |
| ESUS | embolic stroke of undetermined source |
| hArg | Homoarginine |
| HC | healthy controls |
| HIF | hypoxia inducible factor |
| HIS | Hachinsky Ischemic Score |
| HPLC-FL | high performance liquid chromatography and fluorescence detection |
| HR | hazard ratio |
| ICA | internal carotid artery |
| IL | interleukin |
| IS | ischemic stroke |
| iNOS | inducible nitric oxide synthase |
| LAA | large artery atherosclerosis |
| MACE | major cardiovascular outcome event |
| MCAO | middle cerebral artery occlusion |
| MCP-1 | monocyte chemoattractant protein 1 |
| MTHFR | methylenetetrahydrofolate reductase |
| mRS | modified Rankin scale |
| NIHSS | National Institutes of Health stroke scale |
| NMMA | monomethylarginine |
| nNOS | neuronal nitric oxide synthase |
| NO | nitric oxide |
| NOS | nitric oxide synthase |
| NR | not reported |
| n.s. | not significant |
| OR | odds ratio |
| PRMT | protein arginine methyltransferases |
| ROS | radical oxygen species |
| RA | rheumatoid arthritis |
| rtPA | recombinant tissue-type plasminogen activator |
| SD | standard deviation |
| SDMA | symmetric dimethylarginine |
| SVD | small vessel disease |
| TIA | transient ischemic attack |
| TNFα | tumor necrosis factor alpha |
References
- Krishnamurthi, R.V.; Ikeda, T.; Feigin, V.L. Global, Regional and Country-Specific Burden of Ischaemic Stroke, Intracerebral Haemorrhage and Subarachnoid Haemorrhage: A Systematic Analysis of the Global Burden of Disease Study 2017. Neuroepidemiology 2020, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, L.B. Introduction for Focused Updates in Cerebrovascular Disease. Stroke 2020, STROKEAHA119024159. [Google Scholar] [CrossRef] [PubMed]
- GBD 2016 Lifetime Risk of Stroke Collaborators; Feigin, V.L.; Nguyen, G.; Cercy, K.; Johnson, C.O.; Alam, T.; Parmar, P.G.; Abajobir, A.A.; Abate, K.H.; Abd-Allah, F.; et al. Global, Regional, and Country-Specific Lifetime Risks of Stroke, 1990 and 2016. N. Engl. J. Med. 2018, 379, 2429–2437. [Google Scholar] [CrossRef]
- Hinman, J.D.; Rost, N.S.; Leung, T.W.; Montaner, J.; Muir, K.W.; Brown, S.; Arenillas, J.F.; Feldmann, E.; Liebeskind, D.S. Principles of Precision Medicine in Stroke. J. Neurol. Neurosurg. Psychiatry 2017, 88, 54–61. [Google Scholar] [CrossRef]
- Simpkins, A.N.; Janowski, M.; Oz, H.S.; Roberts, J.; Bix, G.; Dore, S.; Stowe, A.M. Biomarker Application for Precision Medicine in Stroke. Transl. Stroke Res. 2019. [Google Scholar] [CrossRef] [PubMed]
- Nitz, K.; Lacy, M.; Atzler, D. Amino Acids and their Metabolism in Atherosclerosis. Arterioscl. Thromb. Vasc. Biol. 2019, 39, 319–330. [Google Scholar] [CrossRef] [PubMed]
- Fulton, M.D.; Brown, T.; Zheng, Y.G. The Biological Axis of Protein Arginine Methylation and Asymmetric Dimethylarginine. Int. J. Mol. Sci. 2019, 20, 3322. [Google Scholar] [CrossRef] [PubMed]
- Förstermann, U.; Sessa, W.C. Nitric Oxide Synthases: Regulation and Function. Eur. Heart J. 2012, 33, 829. [Google Scholar] [CrossRef] [PubMed]
- Jarzebska, N.; Mangoni, A.A.; Martens-Lobenhoffer, J.; Bode-Böger, S.M.; Rodionov, R.N. The Second Life of Methylarginines as Cardiovascular Targets. Int. J. Mol. Sci. 2019, 20, 4592. [Google Scholar] [CrossRef]
- Zeiher, A.M.; Fissthaler, B.; Schray-Utz, B.; Busse, R. Nitric Oxide Modulates the Expression of Monocyte Chemoattractant Protein 1 in Cultured Human Endothelial Cells. Circ. Res. 1995, 76, 980–986. [Google Scholar] [CrossRef]
- Kubes, P.; Suzuki, M.; Granger, D.N. Nitric Oxide: An Endogenous Modulator of Leukocyte Adhesion. Proc. Natl. Acad. Sci. USA 1991, 88, 4651–4655. [Google Scholar] [CrossRef] [PubMed]
- Nakaki, T.; Nakayama, M.; Kato, R. Inhibition by Nitric Oxide and Nitric Oxide-Producing Vasodilators of DNA Synthesis in Vascular Smooth Muscle Cells. Eur. J. Pharmacol. 1990, 189, 347–353. [Google Scholar] [CrossRef]
- Steinert, J.R.; Chernova, T.; Forsythe, I.D. Nitric Oxide Signaling in Brain Function, Dysfunction, and Dementia. Neuroscientist 2010, 16, 435–452. [Google Scholar] [CrossRef]
- Choe, C.U.; Lewerenz, J.; Gerloff, C.; Magnus, T.; Donzelli, S. Nitroxyl in the Central Nervous System. Antioxid. Redox. Signal. 2011, 14, 1699–1711. [Google Scholar] [CrossRef] [PubMed]
- Antoniades, C.; Shirodaria, C.; Leeson, P.; Antonopoulos, A.; Warrick, N.; Van-Assche, T.; Cunnington, C.; Tousoulis, D.; Pillai, R.; Ratnatunga, C.; et al. Association of Plasma Asymmetrical Dimethylarginine (ADMA) with Elevated Vascular Superoxide Production and Endothelial Nitric Oxide Synthase Uncoupling: Implications for Endothelial Function in Human Atherosclerosis. Eur. Heart J. 2009, 30, 1142–1150. [Google Scholar] [CrossRef]
- Feliers, D.; Lee, D.; Gorin, Y.; Kasinath, B.S. Symmetric Dimethylarginine Alters Endothelial Nitric Oxide Activity in Glomerular Endothelial Cells. Cell Signal. 2015, 27, 1–5. [Google Scholar] [CrossRef]
- Kattoor, A.J.; Pothineni, N.V.K.; Palagiri, D.; Mehta, J.L. Oxidative Stress in Atherosclerosis. Curr. Atheroscler. Rep. 2017, 19, 42. [Google Scholar] [CrossRef]
- Chen, S.; Li, N.; Deb-Chatterji, M.; Dong, Q.; Kielstein, J.T.; Weissenborn, K.; Worthmann, H. Asymmetric Dimethyarginine as Marker and Mediator in Ischemic Stroke. Int. J. Mol. Sci. 2012, 13, 15983–16004. [Google Scholar] [CrossRef]
- Ogawa, T.; Kimoto, M.; Sasaoka, K. Purification and Properties of a New Enzyme, NG,NG-Dimethylarginine Dimethylaminohydrolase, from Rat Kidney. J. Biol. Chem. 1989. [Google Scholar]
- Ogawa, T.; Kimoto, M.; Watanabe, H.; Sasaoka, K. Metabolism of NG,NG- and NG,NG-Dimethylarginine in Rats. Arch. Biochem. Biophys. 1987, 252, 526–537. [Google Scholar] [CrossRef]
- Schwedhelm, E.; Böger, R.H. The Role of Asymmetric and Symmetric Dimethylarginines in Renal Disease. Nat. Rev. Nephrol. 2011, 7, 275–285. [Google Scholar] [CrossRef] [PubMed]
- Tsikas, D.D. Asymmetric Dimethylarginine (ADMA), Symmetric Dimethylarginine (SDMA) and Homoarginine (hArg): The ADMA, SDMA and hArg Paradoxes. Cardiovasc. Diabetol. 2018, 17, 1. [Google Scholar] [CrossRef] [PubMed]
- Davignon, J.; Ganz, P. Role of Endothelial Dysfunction in Atherosclerosis. Circulation 2004, 109, III27–III32. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.; Lee, S. Elevated Levels of Plasma Homocyst(E)Ine and Asymmetric Dimethylarginine in Elderly Patients with Stroke. Atherosclerosis 2001, 158, 425–430. [Google Scholar] [CrossRef]
- Wanby, P.; Teerlink, T.; Brudin, L.; Brattstrom, L.; Nilsson, I.; Palmqvist, P.; Carlsson, M. Asymmetric Dimethylarginine (ADMA) as a Risk Marker for Stroke and TIA in a Swedish Population. Atherosclerosis 2006, 185, 271–277. [Google Scholar] [CrossRef]
- Kielstein, J.T.; Impraim, B.; Simmel, S.; Bode-Böger, S.M.; Tsikas, D.; Frolich, J.C.; Hoeper, M.M.; Haller, H.; Fliser, D. Cardiovascular Effects of Systemic Nitric Oxide Synthase Inhibition with Asymmetrical Dimethylarginine in Humans. Circulation 2004, 109, 172–177. [Google Scholar] [CrossRef]
- Kielstein, J.T.; Donnerstag, F.; Gasper, S.; Menne, J.; Kielstein, A.; Martens-Lobenhoffer, J.; Scalera, F.; Cooke, J.P.; Fliser, D.; Bode-Böger, S.M. ADMA Increases Arterial Stiffness and Decreases Cerebral Blood Flow in Humans. Stroke 2006, 37, 2024–2029. [Google Scholar] [CrossRef]
- Baum, C.; Johannsen, S.S.; Zeller, T.; Atzler, D.; Ojeda, F.M.; Wild, P.S.; Sinning, C.R.; Lackner, K.J.; Gori, T.; Schwedhelm, E.; et al. ADMA and Arginine Derivatives in Relation to Non-Invasive Vascular Function in the General Population. Atherosclerosis 2016, 244, 149–156. [Google Scholar] [CrossRef]
- Smirnova, I.V.; Kajstura, M.; Sawamura, T.; Goligorsky, M.S. Asymmetric Dimethylarginine Upregulates LOX-1 in Activated Macrophages: Role in Foam Cell Formation. Am. J. Physiol. Heart Circ. Physiol. 2004, 287, 782. [Google Scholar] [CrossRef]
- Sun, L.; Zhang, T.; Yu, X.; Xin, W.; Lan, X.; Zhang, D.; Huang, C.; Du, G. Asymmetric Dimethylarginine Confers the Communication between Endothelial and Smooth Muscle Cells and Leads to VSMC Migration through p38 and ERK1/2 Signaling Cascade. FEBS Lett. 2011, 585, 2727–2734. [Google Scholar] [CrossRef]
- Yuan, Q.; Jiang, D.; Chen, Q.; Wang, S.; Xin, H.; Deng, H.; Li, Y. Role of Asymmetric Dimethylarginine in Homocysteine-Induced Apoptosis of Vascular Smooth Muscle Cells. Biochem. Biophys. Res. Commun. 2007, 356, 880–885. [Google Scholar] [CrossRef] [PubMed]
- Thum, T.; Tsikas, D.; Stein, S.; Schultheiss, M.; Eigenthaler, M.; Anker, S.D.; Poole-Wilson, P.A.; Ertl, G.; Bauersachs, J. Suppression of Endothelial Progenitor Cells in Human Coronary Artery Disease by the Endogenous Nitric Oxide Synthase Inhibitor Asymmetric Dimethylarginine. J. Am. Coll. Cardiol. 2005, 46, 1693–1701. [Google Scholar] [CrossRef] [PubMed]
- Geovanini, G.R.; Libby, P. Atherosclerosis and Inflammation: Overview and Updates. Clin. Sci. (Lond) 2018, 132, 1243–1252. [Google Scholar] [CrossRef] [PubMed]
- Antoniades, C.; Demosthenous, M.; Tousoulis, D.; Antonopoulos, A.S.; Vlachopoulos, C.; Toutouza, M.; Marinou, K.; Bakogiannis, C.; Mavragani, K.; Lazaros, G.; et al. Role of Asymmetrical Dimethylarginine in Inflammation-Induced Endothelial Dysfunction in Human Atherosclerosis. Hypertension 2011, 58, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Sandoo, A.; Dimitroulas, T.; Hodson, J.; Smith, J.P.; Douglas, K.M.; Kitas, G.D. Cumulative Inflammation Associates with Asymmetric Dimethylarginine in Rheumatoid Arthritis: A 6 Year Follow-Up Study. Rheumatology (Oxford) 2015, 54, 1145–1152. [Google Scholar] [CrossRef] [PubMed]
- Zafari, P.; Zarifian, A.; Alizadeh-Navaei, R.; Taghadosi, M.; Rafiei, A.; Samimi, Z.; Niksolat, F. Asymmetric and Symmetric Dimethylarginine Concentration as an Indicator of Cardiovascular Diseases in Rheumatoid Arthritis Patients: A Systematic Review and Meta-Analysis of Case-Control Studies. Clin. Rheumatol. 2020, 39, 127–134. [Google Scholar] [CrossRef]
- Dimitroulas, T.; Sandoo, A.; Kitas, G.D. Asymmetric Dimethylarginine as a Surrogate Marker of Endothelial Dysfunction and Cardiovascular Risk in Patients with Systemic Rheumatic Diseases. Int. J. Mol. Sci. 2012, 13, 12315–12335. [Google Scholar] [CrossRef]
- Dimitroulas, T.; Sandoo, A.; Hodson, J.; Smith, J.; Douglas, K.M.; Kitas, G.D. Associations between Asymmetric Dimethylarginine, Homocysteine, and the Methylenetetrahydrofolate Reductase (MTHFR) C677T Polymorphism (rs1801133) in Rheumatoid Arthritis. Scand. J. Rheumatol. 2016, 45, 267–273. [Google Scholar] [CrossRef]
- Naqvi, T.Z.; Lee, M. Carotid Intima-Media Thickness and Plaque in Cardiovascular Risk Assessment. JACC: Cardiovascular Imaging 2014, 7, 1025–1038. [Google Scholar] [CrossRef]
- Zsuga, J.; Török, J.; Magyar, M.T.; Valikovics, A.; Gesztelyi, R.; Kéki, S.; Csiba, L.; Zsuga, M.; Bereczki, D. Serum Asymmetric Dimethylarginine Negatively Correlates with Intima-Media Thickness in Early-Onset Atherosclerosis. CED 2007, 23, 388–394. [Google Scholar] [CrossRef]
- Bai, Y.; Sun, L.; Du, L.; Zhang, T.; Xin, W.; Lan, X.; Du, G. Association of Circulating Levels of Asymmetric Dimethylarginine (ADMA) with Carotid Intima-Media Thickness: Evidence from 6168 Participants. Ageing Res. Rev. 2013, 12, 699–707. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Xiong, R.; Feng, S.; Lu, X.; Li, H.; Wang, S. Association of Circulating Levels of ADMA with Carotid Intima-Media Thickness in Patients with CKD: A Systematic Review and Meta-Analysis. Kidney Blood Press. Res. 2018, 43, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Ozalper, V.; Kara, M.; Tanoglu, A.; Cetındaglı, I.; Ozturker, C.; Hancerlı, Y.; Hıra, S.; Kara, K.; Beyazıt, Y.; Yazgan, Y. Evaluation of Endothelial Dysfunction in Patients with Familial Mediterranean Fever: The Relationship between the Levels of Asymmetric Dimethylarginine and Endocan with Carotid Intima-Media Thickness and Endothelium-Dependent Vasodilation. Clin. Rheumatol. 2017, 36, 2071–2077. [Google Scholar] [CrossRef] [PubMed]
- Ilisson, J.; Zagura, M.; Zilmer, K.; Salum, E.; Heilman, K.; Piir, A.; Tillmann, V.; Kals, J.; Zilmer, M.; Pruunsild, C. Increased Carotid Artery Intima-Media Thickness and Myeloperoxidase Level in Children with Newly Diagnosed Juvenile Idiopathic Arthritis. Arthritis Res. Ther. 2015, 17, 180. [Google Scholar] [CrossRef]
- Mels, C.M.C.; Schutte, A.E.; Huisman, H.W.; Smith, W.; Kruger, R.; van Rooyen, J.M.; Schwedhelm, E.; Atzler, D.; Böger, R.H.; Malan, N.T.; et al. Asymmetric Dimethylarginine and Symmetric Dimethylarginine Prospectively Relates to Carotid Wall Thickening in Black Men: The SABPA Study. Amino Acids 2017, 49, 1843–1853. [Google Scholar] [CrossRef]
- Grosse, G.M.; Biber, S.; Sieweke, J.T.; Martens-Lobenhoffer, J.; Gabriel, M.M.; Putzer, A.S.; Hasse, I.; van Gemmeren, T.; Schuppner, R.; Worthmann, H.; et al. Plasma Dimethylarginine Levels and Carotid Intima-Media Thickness are Related to Atrial Fibrillation in Patients with Embolic Stroke. Int. J. Mol. Sci. 2019, 20, 730. [Google Scholar] [CrossRef]
- Furuki, K.; Adachi, H.; Enomoto, M.; Otsuka, M.; Fukami, A.; Kumagae, S.; Matsuoka, H.; Nanjo, Y.; Kakuma, T.; Imaizumi, T. Plasma Level of Asymmetric Dimethylarginine (ADMA) as a Predictor of Carotid Intima-Media Thickness Progression: Six-Year Prospective Study using Carotid Ultrasonography. Hypertens. Res. 2008, 31, 1185–1189. [Google Scholar] [CrossRef]
- Furuki, K.; Adachi, H.; Matsuoka, H.; Enomoto, M.; Satoh, A.; Hino, A.; Hirai, Y.; Imaizumi, T. Plasma Levels of Asymmetric Dimethylarginine (ADMA) are Related to Intima-Media Thickness of the Carotid Artery: An Epidemiological Study. Atherosclerosis 2007, 191, 206–210. [Google Scholar] [CrossRef]
- Dimitroulas, T.; Hodson, J.; Sandoo, A.; Smith, J.; Kitas, G.D. Endothelial Injury in Rheumatoid Arthritis: A Crosstalk between Dimethylarginines and Systemic Inflammation. Arthritis Res. Ther. 2017, 19, 32. [Google Scholar] [CrossRef]
- Riccioni, G.; Scotti, L.; D’Orazio, N.; Gallina, S.; Speziale, G.; Speranza, L.; Bucciarelli, T. ADMA/SDMA in Elderly Subjects with Asymptomatic Carotid Atherosclerosis: Values and Site-Specific Association. Int. J. Mol. Sci. 2014, 15, 6391–6398. [Google Scholar] [CrossRef]
- Xia, W.; Xu, L.; Xu, W.; Wang, X.; Yao, Y. Asymmetric Dimethylarginine is Associated with Carotid Atherosclerosis in Patients with Essential Hypertension. Clin. Exp. Hypertens. 2015, 37, 393–397. [Google Scholar] [CrossRef] [PubMed]
- Maas, R.; Xanthakis, V.; Polak, J.F.; Schwedhelm, E.; Sullivan, L.M.; Benndorf, R.; Schulze, F.; Vasan, R.S.; Wolf, P.A.; Böger, R.H.; et al. Association of the Endogenous Nitric Oxide Synthase Inhibitor ADMA with Carotid Artery Intimal Media Thickness in the Framingham Heart Study Offspring Cohort. Stroke 2009, 40, 2715. [Google Scholar] [CrossRef] [PubMed]
- Notsu, Y.; Yano, S.; Shibata, H.; Nagai, A.; Nabika, T. Plasma Arginine/ADMA Ratio as a Sensitive Risk Marker for Atherosclerosis: Shimane CoHRE Study. Atherosclerosis 2015, 239, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Vladimirova-Kitova, L.; Terzieva, D.; Marinov, B. Intima-Media Thickness and Flow-Mediated Vasodilation in Asymptomatic Subjects with Newly Detected Severe Hypercholesterolemia. Echocardiography 2009, 26, 1060–1068. [Google Scholar] [CrossRef] [PubMed]
- Vladimirova-Kitova, L.; Deneva, T.; Marinov, B. Predictors of the Intima-Media Thickness of Carotid Artery in Asymptomatic Newly Detected Severe Hypercholesterolemic Patients. Clin. Physiol. Funct. Imaging 2010, 30, 250–259. [Google Scholar] [CrossRef] [PubMed]
- Bahls, M.; Friedrich, N.; Atzler, D.; Felix, S.B.; Nauck, M.A.; Böger, R.H.; Volzke, H.; Schwedhelm, E.; Dorr, M. L-Arginine and SDMA Serum Concentrations are Associated with Subclinical Atherosclerosis in the Study of Health in Pomerania (SHIP). PLoS ONE 2015, 10, e0131293. [Google Scholar] [CrossRef] [PubMed]
- Mels, C.M.; Loots, I.; Schwedhelm, E.; Atzler, D.; Böger, R.H.; Schutte, A.E. Nitric oxide synthesis capacity, ambulatory blood pressure and end organ damage in a black and white population: the SABPA study. Amino Acids 2016, 48, 801–810. [Google Scholar] [CrossRef]
- Haghikia, A.; Yanchev, G.R.; Kayacelebi, A.A.; Hanff, E.; Bledau, N.; Widera, C.; Sonnenschein, K.; Haghikia, A.; Weissenborn, K.; Bauersachs, J.; et al. The Role of L-Arginine/L-Homoarginine/Nitric Oxide Pathway for Aortic Distensibility and Intima-Media Thickness in Stroke Patients. Amino Acids 2017, 49, 1111–1121. [Google Scholar] [CrossRef]
- Boehme, A.K.; Esenwa, C.; Elkind, M.S.V. Stroke Risk Factors, Genetics, and Prevention. Circ. Res. 2017, 120, 472. [Google Scholar] [CrossRef]
- Tain, Y.; Huang, L. Restoration of Asymmetric Dimethylarginine–Nitric Oxide Balance to Prevent the Development of Hypertension. Int. J. Mol. Sci. 2014, 15, 11773–11782. [Google Scholar] [CrossRef]
- Inan, B.; Ates, I.; Ozkayar, N.; Kundi, H.; Topcuoglu, C.; Dede, F.; Sennaroglu, E. Are Increased Oxidative Stress and Asymmetric Dimethylarginine Levels Associated with Masked Hypertension? Clin. Exp. Hypertens. 2016, 38, 294–298. [Google Scholar] [CrossRef] [PubMed]
- Taner, A.; Unlu, A.; Kayrak, M.; Tekinalp, M.; Ayhan, S.S.; Arıbaş, A.; Erdem, S.S. The Value of Serum Asymmetric Dimethylarginine Levels for the Determination of Masked Hypertension in Patients with Diabetes Mellitus. Atherosclerosis 2013, 228, 432–437. [Google Scholar] [CrossRef] [PubMed]
- Zwan, L.v.d.; Davids, M.; Scheffer, P.; Dekker, J.; Stehouwer, C.; Teerlink, T. L-Homoarginine and L-Arginine are Antagonistically Related to Blood Pressure in an Elderly Population: The Hoorn Study. J. Hypertens. 2013, 31, 1114–1123. [Google Scholar] [CrossRef] [PubMed]
- Veldink, H.; Faulhaber-Walter, R.; Park, J.; Martens-Lobenhoffer, J.; Bode-Böger, S.; Schuett, H.; Haghikia, A.; Hilfiker-Kleiner, D.; Kielstein, J.T. Effects of Chronic SDMA Infusion on Glomerular Filtration Rate, Blood Pressure, Myocardial Function and Renal Histology in C57BL6/J Mice. Nephrol. Dial. Transplant. 2013, 28, 1434–1439. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Böger, R.H.; Bode-Böger, S.M.; Szuba, A.; Tsao, P.S.; Chan, J.R.; Tangphao, O.; Blaschke, T.F.; Cooke, J.P. Asymmetric Dimethylarginine (ADMA): A Novel Risk Factor for Endothelial Dysfunction: Its Role in Hypercholesterolemia. Circulation 1998, 98, 1842–1847. [Google Scholar] [CrossRef]
- Böger, R.; Sydow, K.; Borlak, J.; Thum, T.; Lenzen, H.; Schubert, B.; Tsikas, D.; Bode-Böger, S. LDL Cholesterol Upregulates Synthesis of Asymmetrical Dimethylarginine in Human Endothelial Cells: Involvement of S-Adenosylmethionine–Dependent Methyltransferases. Circ. Res. 2000, 87, 99–105. [Google Scholar] [CrossRef]
- Brinkmann, S.J.; Worner, E.A.; Buijs, N.; Richir, M.; Cynober, L.; van Leeuwen, P.A.; Couderc, R. The Arginine/ADMA Ratio is Related to the Prevention of Atherosclerotic Plaques in Hypercholesterolemic Rabbits when Giving a Combined Therapy with Atorvastatine and Arginine. Int. J. Mol. Sci. 2015, 16, 12230–12242. [Google Scholar] [CrossRef]
- Zewinger, S.; Kleber, M.E.; Rohrer, L.; Lehmann, M.; Triem, S.; Jennings, R.T.; Petrakis, I.; Dressel, A.; Lepper, P.M.; Scharnagl, H.; et al. Symmetric Dimethylarginine, High-Density Lipoproteins and Cardiovascular Disease. Eur. Heart J. 2017, 38, 1597–1607. [Google Scholar] [CrossRef]
- Kasner, S.E.; Lavados, P.; Sharma, M.; Wang, Y.; Wang, Y.; Davalos, A.; Shamalov, N.; Cunha, L.; Lindgren, A.; Mikulik, R.; et al. Characterization of Patients with Embolic Strokes of Undetermined Source in the NAVIGATE ESUS Randomized Trial. J. Stroke Cerebrovasc. Dis. 2018. [Google Scholar] [CrossRef]
- Ntaios, G.; Vemmos, K.; Lip, G.Y.; Koroboki, E.; Manios, E.; Vemmou, A.; Rodriguez-Campello, A.; Cuadrado-Godia, E.; Giralt-Steinhauer, E.; Arnao, V.; et al. Risk Stratification for Recurrence and Mortality in Embolic Stroke of Undetermined Source. Stroke 2016, 47, 2278–2285. [Google Scholar] [CrossRef]
- Cordts, K.; Grzybowski, R.; Lezius, S.; Luneburg, N.; Atzler, D.; Neu, A.; Hornig, S.; Böger, R.H.; Gerloff, C.; Magnus, T.; et al. Guanidino Compound Ratios are Associated with Stroke Etiology, Internal Carotid Artery Stenosis and CHA2DS2-VASc Score in Three Cross-Sectional Studies. J. Neurol. Sci. 2018, 397, 156–161. [Google Scholar] [CrossRef] [PubMed]
- Busse, R.; Lückhoff, A.; Bassenge, E. Endothelium-Derived Relaxant Factor Inhibits Platelet Activation. Naunyn Schmiedebergs Arch. Pharmacol. 1987, 336, 566–571. [Google Scholar] [CrossRef] [PubMed]
- Alheid, U.; Frölich, J.C.; Förstermann, U. Endothelium-Derived Relaxing Factor from Cultured Human Endothelial Cells Inhibits Aggregation of Human Platelets. Thromb. Res. 1987, 47, 561–571. [Google Scholar] [CrossRef]
- De Meirelles, L.R.; Mendes-Ribeiro, A.C.; Santoro, M.M.; Mendes, M.A.; Da Silva, M.N.; Mann, G.E.; Brunini, T.M. Inhibitory Effects of Endogenous L-Arginine Analogues on Nitric Oxide Synthesis in Platelets: Role in Platelet Hyperaggregability in Hypertension. Clin. Exp. Pharmacol. Physiol. 2007, 34, 1267–1271. [Google Scholar] [CrossRef] [PubMed]
- Brunini, T.T. Inhibition of L-Arginine Transport in Platelets by Asymmetric Dimethylarginine and N-Monomethyl-L-Arginine: Effects of Arterial Hypertension. Clin. Exp. Pharmacol. Physiol. 2004, 31, 738–740. [Google Scholar] [CrossRef]
- Willeit, P.; Freitag, D.F.; Laukkanen, J.A.; Chowdhury, S.; Gobin, R.; Mayr, M.; Di Angelantonio, E.; Chowdhury, R. Asymmetric Dimethylarginine and Cardiovascular Risk: Systematic Review and Meta-Analysis of 22 Prospective Studies. J. Am. Heart Assoc. 2015, 4, e001833. [Google Scholar] [CrossRef]
- Emrich, I.E.; Zawada, A.M.; Martens-Lobenhoffer, J.; Fliser, D.; Wagenpfeil, S.; Heine, G.H.; Bode-Böger, S.M. Symmetric Dimethylarginine (SDMA) Outperforms Asymmetric Dimethylarginine (ADMA) and Other Methylarginines as Predictor of Renal and Cardiovascular Outcome in Non-Dialysis Chronic Kidney Disease. Clin. Res. Cardiol. 2018, 107, 201–213. [Google Scholar] [CrossRef]
- Zobel, E.H.; von Scholten, B.J.; Reinhard, H.; Persson, F.; Teerlink, T.; Hansen, T.W.; Parving, H.H.; Jacobsen, P.K.; Rossing, P. Symmetric and Asymmetric Dimethylarginine as Risk Markers of Cardiovascular Disease, all-Cause Mortality and Deterioration in Kidney Function in Persons with Type 2 Diabetes and Microalbuminuria. Cardiovasc. Diabetol. 2017, 16, 88. [Google Scholar] [CrossRef]
- Horowitz, J.D.; De Caterina, R.; Heresztyn, T.; Alexander, J.H.; Andersson, U.; Lopes, R.D.; Steg, P.G.; Hylek, E.M.; Mohan, P.; Hanna, M.; et al. Asymmetric and Symmetric Dimethylarginine Predict Outcomes in Patients with Atrial Fibrillation: An ARISTOTLE Substudy. J. Am. Coll. Cardiol. 2018, 72, 721–733. [Google Scholar] [CrossRef]
- Schlesinger, S.; Sonntag, S.R.; Lieb, W.; Maas, R. Asymmetric and Symmetric Dimethylarginine as Risk Markers for Total Mortality and Cardiovascular Outcomes: A Systematic Review and Meta-Analysis of Prospective Studies. PLoS ONE 2016, 11, e0165811. [Google Scholar] [CrossRef]
- Major, R.W.; Cheng, M.R.I.; Grant, R.A.; Shantikumar, S.; Xu, G.; Oozeerally, I.; Brunskill, N.J.; Gray, L.J. Cardiovascular Disease Risk Factors in Chronic Kidney Disease: A Systematic Review and Meta-Analysis. PLoS ONE 2018, 13, e0192895. [Google Scholar] [CrossRef] [PubMed]
- Bode-Böger, S.M.; Scalera, F.; Kielstein, J.T.; Martens-Lobenhoffer, J.; Breithardt, G.; Fobker, M.; Reinecke, H. Symmetrical Dimethylarginine: A New Combined Parameter for Renal Function and Extent of Coronary Artery Disease. J. Am. Soc. Nephrol. 2006, 17, 1128–1134. [Google Scholar] [CrossRef] [PubMed]
- Schulze, F.; Carter, A.M.; Schwedhelm, E.; Ajjan, R.; Maas, R.; von Holten, R.A.; Atzler, D.; Grant, P.J.; Böger, R.H. Symmetric Dimethylarginine Predicts all-Cause Mortality Following Ischemic Stroke. Atherosclerosis 2010, 208, 518–523. [Google Scholar] [CrossRef] [PubMed]
- Luneburg, N.; von Holten, R.A.; Topper, R.F.; Schwedhelm, E.; Maas, R.; Böger, R.H. Symmetric Dimethylarginine is a Marker of Detrimental Outcome in the Acute Phase After Ischaemic Stroke: Role of Renal Function. Clin. Sci. (Lond) 2012, 122, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Brouns, R.; Marescau, B.; Possemiers, I.; Sheorajpanday, R.; De Deyn, P.P. Dimethylarginine Levels in Cerebrospinal Fluid of Hyperacute Ischemic Stroke Patients are Associated with Stroke Severity. Neurochem. Res. 2009, 34, 1642–1649. [Google Scholar] [CrossRef] [PubMed]
- Worthmann, H.; Chen, S.; Martens-Lobenhoffer, J.; Li, N.; Deb, M.; Tryc, A.B.; Goldbecker, A.; Dong, Q.; Kielstein, J.T.; Bode-Böger, S.M.; et al. High Plasma Dimethylarginine Levels are Associated with Adverse Clinical Outcome After Stroke. J. Atheroscler. Thromb. 2011, 18, 753–761. [Google Scholar] [CrossRef]
- Rueda-Clausen, C.F.; Córdoba-Porras, A.; Bedoya, G.; Silva, F.A.; Zarruk, J.G.; López-Jaramillo, P.; Villa, L.A. Increased Plasma Levels of Total Homocysteine but Not Asymmetric Dimethylarginine in Hispanic Subjects with Ischemic Stroke FREC-VI Sub-Study. Eur. J. Neurol. 2012, 19, 417–425. [Google Scholar] [CrossRef]
- Molnar, T.; Pusch, G.; Papp, V.; Feher, G.; Szapary, L.; Biri, B.; Nagy, L.; Keki, S.; Illes, Z. The L-Arginine Pathway in Acute Ischemic Stroke and Severe Carotid Stenosis: Temporal Profiles and Association with Biomarkers and Outcome. J. Stroke Cerebrovasc. Dis. 2014, 23, 2206–2214. [Google Scholar] [CrossRef]
- Marz, W.; Meinitzer, A.; Drechsler, C.; Pilz, S.; Krane, V.; Kleber, M.E.; Fischer, J.; Winkelmann, B.R.; Bohm, B.O.; Ritz, E.; et al. Homoarginine, Cardiovascular Risk, and Mortality. Circulation 2010, 122, 967–975. [Google Scholar] [CrossRef]
- Atzler, D.; Schwedhelm, E.; Choe, C.U. L-Homoarginine and Cardiovascular Disease. Curr. Opin. Clin. Nutr. Metab. Care 2015, 18, 83–88. [Google Scholar] [CrossRef]
- Choe, C.; Atzler, D.; Wild, P.S.; Carter, A.M.; Böger, R.H.; Ojeda, F.; Simova, O.; Stockebrand, M.; Lackner, K.; Nabuurs, C.; et al. Homoarginine Levels are Regulated by L-Arginine:Glycine Amidinotransferase and Affect Stroke Outcome: Results from Human and Murine Studies. Circulation 2013, 128, 1451–1461. [Google Scholar] [CrossRef] [PubMed]
- Pilz, S.; Teerlink, T.; Scheffer, P.G.; Meinitzer, A.; Rutters, F.; Tomaschitz, A.; Drechsler, C.; Kienreich, K.; Nijpels, G.; Stehouwer, C.D.A.; et al. Homoarginine and Mortality in an Older Population: The Hoorn Study. Eur. J. Clin. Investig. 2014, 44, 200–208. [Google Scholar] [CrossRef] [PubMed]
- Atzler, D.; Gore, M.O.; Ayers, C.R.; Choe, C.U.; Böger, R.H.; de Lemos, J.A.; McGuire, D.K.; Schwedhelm, E. Homoarginine and Cardiovascular Outcome in the Population-Based Dallas Heart Study. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 2501–2507. [Google Scholar] [CrossRef] [PubMed]
- Balasubramanian, R.; Paynter, N.P.; Giulianini, F.; Manson, J.E.; Zhao, Y.; Chen, J.C.; Vitolins, M.Z.; Albert, C.A.; Clish, C.; Rexrode, K.M. Metabolomic Profiles Associated with all-Cause Mortality in the Women’s Health Initiative. Int. J. Epidemiol. 2019. [Google Scholar] [CrossRef] [PubMed]
- Zinellu, A.; Paliogiannis, P.; Carru, C.; Mangoni, A.A. Homoarginine and all-Cause Mortality: A Systematic Review and Meta-Analysis. Eur. J. Clin. Investig. 2018, 48, e12960. [Google Scholar] [CrossRef] [PubMed]
- Drechsler, C.; Meinitzer, A.; Pilz, S.; Krane, V.; Tomaschitz, A.; Ritz, E.; März, W.; Wanner, C. Homoarginine, Heart Failure, and Sudden Cardiac Death in Haemodialysis Patients. Eur. J. Heart Fail. 2011, 13, 852–859. [Google Scholar] [CrossRef]
- Pilz, S.; Tomaschitz, A.; Meinitzer, A.; Drechsler, C.; Ritz, E.; Krane, V.; Wanner, C.; Bohm, B.O.; Marz, W. Low Serum Homoarginine is a Novel Risk Factor for Fatal Strokes in Patients Undergoing Coronary Angiography. Stroke 2011, 42, 1132–1134. [Google Scholar] [CrossRef]
- Choe, C.; Lezius, S.; Cordts, K.; Gerloff, C.; Böger, R.H.; Schwedhelm, E.; Grant, P.J. Low Homoarginine/SDMA Ratio is Associated with Poor Short- and Long-Term Outcome After Stroke in Two Prospective Studies. Neurol. Sci. 2020, 41, 149–153. [Google Scholar] [CrossRef]
- Rodionov, R.N.; Oppici, E.; Martens-Lobenhoffer, J.; Jarzebska, N.; Brilloff, S.; Burdin, D.; Demyanov, A.; Kolouschek, A.; Leiper, J.; Maas, R.; et al. A Novel Pathway for Metabolism of the Cardiovascular Risk Factor Homoarginine by Alanine:Glyoxylate Aminotransferase 2. Sci. Rep. 2016, 6, 35277. [Google Scholar] [CrossRef]
- Hrabák, A.; Bajor, T.; Temesi, A. Comparison of Substrate and Inhibitor Specificity of Arginase and Nitric Oxide (NO) Synthase for Arginine Analogues and Related Compounds in Murine and Rat Macrophages. Biochem. Biophys. Res. Commun. 1994, 198, 206–212. [Google Scholar] [CrossRef]
- Tommasi, S.; Elliot, D.J.; Da Boit, M.; Gray, S.R.; Lewis, B.C.; Mangoni, A.A. Homoarginine and Inhibition of Human Arginase Activity: Kinetic Characterization and Biological Relevance. Sci. Rep. 2018, 8, 3697. [Google Scholar] [CrossRef]
- Faller, K.M.E.; Atzler, D.; McAndrew, D.J.; Zervou, S.; Whittington, H.J.; Simon, J.N.; Aksentijevic, D.; Ten Hove, M.; Choe, C.U.; Isbrandt, D.; et al. Impaired Cardiac Contractile Function in Arginine:Glycine Amidinotransferase Knockout Mice Devoid of Creatine is Rescued by Homoarginine but Not Creatine. Cardiovasc. Res. 2018, 114, 417–430. [Google Scholar] [CrossRef]
- Wetzel, M.D.; Gao, T.; Venkatachalam, M.; Morris, S.M.; Awad, A.S. L-Homoarginine Supplementation Prevents Diabetic Kidney Damage. Physiol. Rep. 2019, 7, e14235. [Google Scholar] [CrossRef]
- Rodionov, R.N.; Begmatov, H.; Jarzebska, N.; Patel, K.; Mills, M.T.; Ghani, Z.; Khakshour, D.; Tamboli, P.; Patel, M.N.; Abdalla, M.; et al. Homoarginine Supplementation Prevents Left Ventricular Dilatation and Preserves Systolic Function in a Model of Coronary Artery Disease. J. Am. Heart Assoc. 2019, 8, e012486. [Google Scholar] [CrossRef] [PubMed]
- Dellera, F.; Ganzetti, G.S.; Froio, A.; Manzini, S.; Busnelli, M.; Meinitzer, A.; Sirtori, C.R.; Chiesa, G.; Parolini, C. L-Homoarginine Administration Reduces Neointimal Hyperplasia in Balloon-Injured Rat Carotids. Thromb. Haemost. 2016, 116, 400–402. [Google Scholar] [CrossRef] [PubMed]
- Atzler, D.; McAndrew, D.J.; Cordts, K.; Schneider, J.E.; Zervou, S.; Schwedhelm, E.; Neubauer, S.; Lygate, C.A. Dietary Supplementation with Homoarginine Preserves Cardiac Function in a Murine Model of Post-Myocardial Infarction Heart Failure. Circulation 2017, 135, 400–402. [Google Scholar] [CrossRef]
- Karetnikova, E.S.; Jarzebska, N.; Markov, A.G.; Weiss, N.; Lentz, S.R.; Rodionov, R.N. Is Homoarginine a Protective Cardiovascular Risk Factor? Arterioscler. Thromb. Vasc. Biol. 2019, 39, 869–875. [Google Scholar] [CrossRef]
- Atzler, D.; Schonhoff, M.; Cordts, K.; Ortland, I.; Hoppe, J.; Hummel, F.C.; Gerloff, C.; Jaehde, U.; Jagodzinski, A.; Böger, R.H.; et al. Oral Supplementation with L-Homoarginine in Young Volunteers. Br. J. Clin. Pharmacol. 2016, 82, 1477–1485. [Google Scholar] [CrossRef] [PubMed]
- Schonhoff, M.; Weineck, G.; Hoppe, J.; Hornig, S.; Cordts, K.; Atzler, D.; Gerloff, C.; Böger, R.; Neu, A.; Schwedhelm, E.; et al. Cognitive Performance of 20 Healthy Humans Supplemented with L-Homoarginine for 4weeks. J. Clin. Neurosci. 2018, 50, 237–241. [Google Scholar] [CrossRef]
- Molnar, T.; Pusch, G.; Nagy, L.; Keki, S.; Berki, T.; Illes, Z. Correlation of the L-Arginine Pathway with Thrombo-Inflammation may Contribute to the Outcome of Acute Ischemic Stroke. J. Stroke Cerebrovasc. Dis. 2016, 25, 2055–2060. [Google Scholar] [CrossRef]
- Iapichino, G.; Umbrello, M.; Albicini, M.; Spanu, P.; Bellani, G.; Polli, F.; Pavlovic, R.; Cugno, M.; Fermo, I.; Paroni, R. Time Course of Endogenous Nitric Oxide Inhibitors in Severe Sepsis in Humans. Minerva Anestesiol. 2010, 76, 325–333. [Google Scholar] [PubMed]
- Winkler, M.S.; Nierhaus, A.; Rosler, G.; Lezius, S.; Harlandt, O.; Schwedhelm, E.; Böger, R.H.; Kluge, S. Symmetrical (SDMA) and Asymmetrical Dimethylarginine (ADMA) in Sepsis: High Plasma Levels as Combined Risk Markers for Sepsis Survival. Crit. Care 2018, 22, 216. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Fan, D.; Zhang, N. The Relationship between Serum Asymmetric Dimethylarginine and ABCD2 Score in Transient Ischemic Attack Patients. Zhonghua Nei Ke Za Zhi 2014, 53, 876–879. [Google Scholar] [PubMed]
- Ercan, M.; Mungan, S.; Guzel, I.; Celik, H.T.; Bal, C.; Abusoglu, S.; Akbulut, D.; Oguz, E.F.; Yilmaz, F.M. Serum Asymmetric Dimethylarginine and Nitric Oxide Levels in Turkish Patients with Acute Ischemic Stroke. Adv. Clin. Exp. Med. 2019, 28, 693–698. [Google Scholar] [CrossRef]
- Chen, S.; Martens-Lobenhoffer, J.; Weissenborn, K.; Kielstein, J.; Lichtinghagen, R.; Deb, M.; Li, N.; Tryc, A.; Goldbecker, A.; Dong, Q.; et al. Association of Dimethylarginines and Mediators of Inflammation After Acute Ischemic Stroke. J. Neuroinflamm. 2012, 9, 251. [Google Scholar] [CrossRef]
- Ziegler, N.L.; Sieweke, J.T.; Biber, S.; Gabriel, M.M.; Schuppner, R.; Worthmann, H.; Martens-Lobenhoffer, J.; Lichtinghagen, R.; Bode-Böger, S.M.; Bavendiek, U.; et al. Markers of Endothelial Pathology to Support Detection of Atrial Fibrillation in Embolic Stroke of Undetermined Source. Sci. Rep. 2019, 9, 19424–19429. [Google Scholar] [CrossRef]
- Iadecola, C.; Pelligrino, D.A.; Moskowitz, M.A.; Lassen, N.A. Nitric Oxide Synthase Inhibition and Cerebrovascular Regulation. J. Cereb. Blood Flow Metab. 1994, 14, 175–192. [Google Scholar] [CrossRef]
- Faraci, F.M.; Brian, J.E.; Heistad, D.D. Response of Cerebral Blood Vessels to an Endogenous Inhibitor of Nitric Oxide Synthase. Am. J. Physiol. 1995, 269, H1522–H1527. [Google Scholar] [CrossRef]
- Dayoub, H.; Rodionov, R.N.; Lynch, C.; Cooke, J.P.; Arning, E.; Bottiglieri, T.; Lentz, S.R.; Faraci, F.M. Overexpression of Dimethylarginine Dimethylaminohydrolase Inhibits Asymmetric Dimethylarginine-Induced Endothelial Dysfunction in the Cerebral Circulation. Stroke 2008, 39, 180–184. [Google Scholar] [CrossRef]
- Segarra, G.; Medina, P.; Ballester, R.M.; Lluch, P.; Aldasoro, M.; Vila, J.M.; Lluch, S.; Pelligrino, D.A. Effects of some Guanidino Compounds on Human Cerebral Arteries. Stroke 1999, 30, 2206–2211. [Google Scholar] [CrossRef]
- Schuppner, R.; Dirks, M.; Grosse, G.M.; Bockmann, M.; Goetz, F.; Pasedag, T.; Bode-Böger, S.M.; Martens-Lobenhoffer, J.; Budde, U.; Lanfermann, H.; et al. ADAMTS-13 Activity Predicts Outcome in Acute Ischaemic Stroke Patients Undergoing Endovascular Treatment. Thromb. Haemost. 2018, 118, 758–767. [Google Scholar] [PubMed]
- Worthmann, H.; Martens-Lobenhoffer, J.; Joumaah, M.; Li, N.; Lichtinghagen, R.; Hecker, H.; Kielstein, J.T.; Ehrenreich, H.; Bode-Böger, S.M.; Weissenborn, K. Asymmetric Dimethylarginine in Response to Recombinant Tissue-Type Plasminogen Activator and Erythropoietin in Acute Stroke. Stroke 2013, 44, 2128–2133. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Willmot, M.; Gray, L.; Gibson, C.; Murphy, S.; Bath, P.M. A Systematic Review of Nitric Oxide Donors and L-Arginine in Experimental Stroke; Effects on Infarct Size and Cerebral Blood Flow. Nitric Oxide 2005, 12, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Harston, G.W.; Sutherland, B.A.; Kennedy, J.; Buchan, A.M. The Contribution of L-Arginine to the Neurotoxicity of Recombinant Tissue Plasminogen Activator Following Cerebral Ischemia: A Review of rtPA Neurotoxicity. J. Cereb. Blood Flow Metab. 2010, 30, 1804–1816. [Google Scholar] [CrossRef]
- Putzer, A.S.; Worthmann, H.; Grosse, G.M.; Goetz, F.; Martens-Lobenhoffer, J.; Dirks, M.; Kielstein, J.T.; Lichtinghagen, R.; Budde, U.; Bode-Böger, S.M.; et al. ADAMTS13 Activity is Associated with Early Neurological Improvement in Acute Ischemic Stroke Patients Treated with Intravenous Thrombolysis. J. Thromb. Thrombolysis 2019. [Google Scholar] [CrossRef]
- Sydow, K.; Munzel, T. ADMA and Oxidative Stress. Atheroscler. Suppl. 2003, 4, 41–51. [Google Scholar] [CrossRef]
- Lee, D.Y.; Teyssier, C.; Strahl, B.D.; Stallcup, M.R. Role of Protein Methylation in Regulation of Transcription. Endocr. Rev. 2005, 26, 147–170. [Google Scholar] [CrossRef]
- Wells, S.M.; Holian, A. Asymmetric Dimethylarginine Induces Oxidative and Nitrosative Stress in Murine Lung Epithelial Cells. Am. J. Respir. Cell Mol. Biol. 2007, 36, 520–528. [Google Scholar] [CrossRef]
- Daiber, A.; Xia, N.; Steven, S.; Oelze, M.; Hanf, A.; Kroller-Schon, S.; Munzel, T.; Li, H. New Therapeutic Implications of Endothelial Nitric Oxide Synthase (eNOS) Function/Dysfunction in Cardiovascular Disease. Int. J. Mol. Sci. 2019, 20, 187. [Google Scholar] [CrossRef]
- Rashid, P.A.; Whitehurst, A.; Lawson, N.; Bath, P.M. Plasma Nitric Oxide (Nitrate/Nitrite) Levels in Acute Stroke and their Relationship with Severity and Outcome. J. Stroke Cerebrovasc. Dis. 2003, 12, 82–87. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Huang, P.L.; Panahian, N.; Dalkara, T.; Fishman, M.C.; Moskowitz, M.A. Effects of Cerebral Ischemia in Mice Deficient in Neuronal Nitric Oxide Synthase. Science 1994, 265, 1883–1885. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Haensel, C.; Araki, E.; Ross, M.E.; Iadecola, C. Gene-Dosing Effect and Persistence of Reduction in Ischemic Brain Injury in Mice Lacking Inducible Nitric Oxide Synthase. Brain Res. 2000, 872, 215–218. [Google Scholar] [CrossRef]
- Samdani, A.F.; Dawson, T.M.; Dawson, V.L. Nitric Oxide Synthase in Models of Focal Ischemia. Stroke 1997, 28, 1283–1288. [Google Scholar] [CrossRef] [PubMed]
- Murphy, S.; Gibson, C.L. Nitric Oxide, Ischaemia and Brain Inflammation. Biochem. Soc. Trans. 2007, 35, 1133–1137. [Google Scholar] [CrossRef] [PubMed]
- Fickling, S.A.; Holden, D.P.; Cartwright, J.E.; Nussey, S.S.; Vallance, P.; Whitley, G.S. Regulation of Macrophage Nitric Oxide Synthesis by Endothelial Cells: A Role for NG,NG-Dimethylarginine. Acta Physiol. Scand. 1999, 167, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Tsikas, D. Does the Inhibitory Action of Asymmetric Dimethylarginine (ADMA) on the Endothelial Nitric Oxide Synthase Activity Explain its Importance in the Cardiovascular System? the ADMA Paradox. J. Controvers. Biomed. Res. 2017, 3, 16–22. [Google Scholar] [CrossRef][Green Version]
- Leypoldt, F.; Choe, C.U.; Gelderblom, M.; von Leitner, E.C.; Atzler, D.; Schwedhelm, E.; Gerloff, C.; Sydow, K.; Böger, R.H.; Magnus, T. Dimethylarginine Dimethylaminohydrolase-1 Transgenic Mice are Not Protected from Ischemic Stroke. PLoS ONE 2009, 4, e7337. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhou, Y.; Ma, X.; Liu, X.; Zhao, Y.; Liu, X. DDAH-1 Via HIF-1 Target Genes Improves Cerebral Ischemic Tolerance After Hypoxic Preconditioning and Middle Cerebral Artery Occlusion-Reperfusion. Nitric Oxide 2020, 95, 17–28. [Google Scholar] [CrossRef]
- Zhang, G.G.; Bai, Y.P.; Chen, M.F.; Shi, R.Z.; Jiang, D.J.; Fu, Q.M.; Tan, G.S.; Li, Y.J. Asymmetric Dimethylarginine Induces TNF-Alpha Production Via ROS/NF-kappaB Dependent Pathway in Human Monocytic Cells and the Inhibitory Effect of Reinioside C. Vascul. Pharmacol. 2008, 48, 115–121. [Google Scholar] [CrossRef]
- Jiang, J.L.; Wang, S.; Li, N.S.; Zhang, X.H.; Deng, H.W.; Li, Y.J. The Inhibitory Effect of Simvastatin on the ADMA-Induced Inflammatory Reaction is Mediated by MAPK Pathways in Endothelial Cells. Biochem. Cell Biol. 2007, 85, 66–77. [Google Scholar] [CrossRef]
- Von Leitner, E.C.; Klinke, A.; Atzler, D.; Slocum, J.L.; Lund, N.; Kielstein, J.T.; Maas, R.; Schmidt-Haupt, R.; Pekarova, M.; Hellwinkel, O.; et al. Pathogenic Cycle between the Endogenous Nitric Oxide Synthase Inhibitor Asymmetrical Dimethylarginine and the Leukocyte-Derived Hemoprotein Myeloperoxidase. Circulation 2011, 124, 2735–2745. [Google Scholar] [CrossRef] [PubMed]
- Zoccali, C.; Maas, R.; Cutrupi, S.; Pizzini, P.; Finocchiaro, P.; Cambareri, F.; Panuccio, V.; Martorano, C.; Schulze, F.; Enia, G.; et al. Asymmetric Dimethyl-Arginine (ADMA) Response to Inflammation in Acute Infections. Nephrol. Dial. Transplant. 2007, 22, 801–806. [Google Scholar] [CrossRef]
- Closs, E.I.; Basha, F.Z.; Habermeier, A.; Forstermann, U. Interference of L-Arginine Analogues with L-Arginine Transport Mediated by the Y+ Carrier hCAT-2B. Nitric Oxide 1997, 1, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Tojo, A.; Welch, W.J.; Bremer, V.; Kimoto, M.; Kimura, K.; Omata, M.; Ogawa, T.; Vallance, P.; Wilcox, C.S. Colocalization of Demethylating Enzymes and NOS and Functional Effects of Methylarginines in Rat Kidney. Kidney Int. 1997, 52, 1593–1601. [Google Scholar] [CrossRef] [PubMed]
- Schepers, E.; Glorieux, G.; Dhondt, A.; Leybaert, L.; Vanholder, R. Role of Symmetric Dimethylarginine in Vascular Damage by Increasing ROS Via Store-Operated Calcium Influx in Monocytes. Nephrol. Dial. Transplant. 2009, 24, 1429–1435. [Google Scholar] [CrossRef] [PubMed]
- Schepers, E.; Barreto, D.; Liabeuf, S.; Glorieux, G.; Eloot, S.; Barreto, F.; Massy, Z.; Vanholder, R. Symmetric Dimethylarginine as a Proinflammatory Agent in Chronic Kidney Disease. Clin. J. Am. Soc. Nephrol. 2011, 6, 2374–2383. [Google Scholar] [CrossRef] [PubMed]
- Scherbakov, N.; Sandek, A.; Martens-Lobenhoffer, J.; Kung, T.; Turhan, G.; Liman, T.; Ebinger, M.; von Haehling, S.; Bode-Böger, S.M.; Endres, M.; et al. Endothelial Dysfunction of the Peripheral Vascular Bed in the Acute Phase After Ischemic Stroke. Cerebrovasc. Dis. 2012, 33, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Chung, J.W.; Oh, M.J.; Cho, Y.H.; Moon, G.J.; Kim, G.M.; Chung, C.S.; Lee, K.H.; Bang, O.Y. Distinct Roles of Endothelial Dysfunction and Inflammation in Intracranial Atherosclerotic Stroke. Eur. Neurol. 2017, 77, 211–219. [Google Scholar] [CrossRef]
- Zinellu, A.A. Carotid Restenosis is Associated with Plasma ADMA Concentrations in Carotid Endarterectomy Patients. Clin. Chem. Lab. Med. 2011, 49, 897–901. [Google Scholar] [CrossRef]
- Tsuda, K. Asymmetric Dimethylarginine and Hypertension in Cerebral Small Vessel Disease. Stroke 2007, 38, e48. [Google Scholar] [CrossRef]
- Janes, F.; Cifù, A.; Pessa, M.E.; Domenis, R.; Gigli, G.L.; Sanvilli, N.; Nilo, A.; Garbo, R.; Curcio, F.; Giacomello, R.; et al. ADMA as a Possible Marker of Endothelial Damage. A Study in Young Asymptomatic Patients with Cerebral Small Vessel Disease. Sci. Rep. 2019, 9, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Fan, Y.; Mu, L.; Ma, L.; Song, Z.; Zhang, Y. S100B and ADMA in Cerebral Small Vessel Disease and Cognitive Dysfunction. J. Neurol. Sci. 2015, 354, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Rufa, A.; Blardi, P.; De Lalla, A.; Cevenini, G.; De Stefano, N.; Zicari, E.; Auteri, A.; Federico, A.; Dotti, M.T. Plasma Levels of Asymmetric Dimethylarginine in Cerebral Autosomal Dominant Arteriopathy with Subcortical Infarct and Leukoencephalopathy. Cerebrovasc. Dis. 2008, 26, 636–640. [Google Scholar] [CrossRef] [PubMed]
- Fleszar, M.G.; Wiśniewski, J.; Zboch, M.; Diakowska, D.; Gamian, A.; Krzystek-Korpacka, M. Targeted Metabolomic Analysis of Nitric Oxide/L-Arginine Pathway Metabolites in Dementia: Association with Pathology, Severity, and Structural Brain Changes. Sci. Rep. 2019, 9, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Hart, R.G.; Diener, H.C.; Coutts, S.B.; Easton, J.D.; Granger, C.B.; O’Donnell, M.J.; Sacco, R.L.; Connolly, S.J. Cryptogenic Stroke/ESUS International Working Group. Embolic Strokes of Undetermined Source: The Case for a New Clinical Construct. Lancet Neurol. 2014, 13, 429–438. [Google Scholar] [CrossRef]
- Perera, K.S.; Vanassche, T.; Bosch, J.; Giruparajah, M.; Swaminathan, B.; Mattina, K.R.; Berkowitz, S.D.; Arauz, A.; O’Donnell, M.J.; Ameriso, S.F.; et al. Embolic Strokes of Undetermined Source: Prevalence and Patient Features in the ESUS Global Registry. Int. J. Stroke 2016, 11, 526–533. [Google Scholar] [CrossRef] [PubMed]
- Diener, H.C.; Sacco, R.L.; Easton, J.D.; Granger, C.B.; Bernstein, R.A.; Uchiyama, S.; Kreuzer, J.; Cronin, L.; Cotton, D.; Grauer, C.; et al. Dabigatran for Prevention of Stroke After Embolic Stroke of Undetermined Source. N. Engl. J. Med. 2019, 380, 1906–1917. [Google Scholar] [CrossRef]
- Hart, R.G.; Sharma, M.; Mundl, H.; Shoamanesh, A.; Kasner, S.E.; Berkowitz, S.D.; Pare, G.; Kirsch, B.; Pogue, J.; Pater, C.; et al. Rivaroxaban for Secondary Stroke Prevention in Patients with Embolic Strokes of Undetermined Source: Design of the NAVIGATE ESUS Randomized Trial. Eur. Stroke J. 2016, 3, 146–154. [Google Scholar] [CrossRef]
- Elkind, M.S.V. Atrial Cardiopathy and Stroke Prevention. Curr. Cardiol. Rep. 2018, 20, 103. [Google Scholar] [CrossRef]
- Yaghi, S.; Kamel, H.; Elkind, M.S.V. Atrial Cardiopathy: A Mechanism of Cryptogenic Stroke. Expert Rev. Cardiovasc. Ther. 2017, 15, 591–599. [Google Scholar]
- Kamel, H.; Bartz, T.M.; Elkind, M.S.V.; Okin, P.M.; Thacker, E.L.; Patton, K.K.; Stein, P.K.; deFilippi, C.R.; Gottesman, R.F.; Heckbert, S.R.; et al. Atrial Cardiopathy and the Risk of Ischemic Stroke in the CHS (Cardiovascular Health Study). Stroke 2018, 49, 980–986. [Google Scholar] [CrossRef] [PubMed]
- Cai, H.; Li, Z.; Goette, A.; Mera, F.; Honeycutt, C.; Feterik, K.; Wilcox, J.N.; Dudley, S.C., Jr.; Harrison, D.G.; Langberg, J. Downregulation of Endocardial Nitric Oxide Synthase Expression and Nitric Oxide Production in Atrial Fibrillation: Potential Mechanisms for Atrial Thrombosis and Stroke. Circulation 2002, 2854–2858. [Google Scholar] [CrossRef] [PubMed]
- Guazzi, M.; Belletti, S.; Lenatti, L.; Bianco, E.; Guazzi, M.D. Effects of Cardioversion of Atrial Fibrillation on Endothelial Function in Hypertension Or Diabetes. Eur. J. Clin. Investig. 2007, 37, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Wijesurendra, R.S.; Casadei, B. Atrial Fibrillation: Effects Beyond the Atrium? Cardiovasc. Res. 2015, 105, 238–247. [Google Scholar] [CrossRef] [PubMed]
- Heeringa, J.; Kuip, D.; Hofman, A.; Kors, J.; Rooij, F.; Lip, G.; Witteman, J. Subclinical Atherosclerosis and Risk of Atrial Fibrillation: The Rotterdam Study. Arch. Intern. Med. 2007, 167, 382–387. [Google Scholar] [CrossRef] [PubMed]
- Bonilla, I.M.; Sridhar, A.; Györke, S.; Cardounel, A.J.; Carnes, C.A. Nitric Oxide Synthases and Atrial Fibrillation. Front. Physiol. 2012, 3, 105. [Google Scholar] [CrossRef]
- Willeit, K.; Kiechl, S. Atherosclerosis and Atrial Fibrillation---Two Closely Intertwined Diseases. Atherosclerosis 2014, 233, 679–681. [Google Scholar] [CrossRef]
- Kim, S.J.; Choisy, S.C.; Barman, P.; Zhang, H.; Hancox, J.C.; Jones, S.A.; James, A.F. Atrial Remodeling and the Substrate for Atrial Fibrillation in Rat Hearts with Elevated Afterload. Circ. Arrhythm. Electrophysiol. 2011, 4, 761–769. [Google Scholar] [CrossRef]
- Stamboul, K.; Lorin, J.; Lorgis, L.; Guenancia, C.; Beer, J.; Touzery, C.; Rochette, L.; Vergely, C.; Cottin, Y.; Zeller, M. Atrial Fibrillation is Associated with a Marker of Endothelial Function and Oxidative Stress in Patients with Acute Myocardial Infarction. PLoS ONE 2015, 10, e0131439. [Google Scholar] [CrossRef]
- Lim, H.S.; Willoughby, S.R.; Schultz, C.; Alasady, M.; Rangnekar, G.; Dang, J.; Gan, C.; Lau, D.H.; Roberts-Thomson, K.C.; Young, G.D.; et al. Thrombogenic Risk in Patients With Atrial Fibrillation: Importance of Comorbid Conditions and Intracardiac Changes. JACC. Clin. Electrophysiol. 2015, 1, 210–217. [Google Scholar] [CrossRef]
- Csecsei, P.; Varnai, R.; Nagy, L.; Keki, S.; Molnar, T.; Illes, Z.; Farkas, N.; Szapary, L. L-Arginine Pathway Metabolites can Discriminate Paroxysmal from Permanent Atrial Fibrillation in Acute Ischemic Stroke. Ideggyogy Sz. 2019, 72, 79–88. [Google Scholar] [CrossRef]
- Schnabel, R.B.; Maas, R.; Wang, N.; Yin, X.; Larson, M.G.; Levy, D.; Ellinor, P.T.; Lubitz, S.A.; McManus, D.D.; Magnani, J.W.; et al. Asymmetric Dimethylarginine, Related Arginine Derivatives, and Incident Atrial Fibrillation. Am. Heart J. 2016, 176, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Ramuschkat, M.; Appelbaum, S.; Atzler, D.; Zeller, T.; Bauer, C.; Ojeda, F.M.; Sinning, C.R.; Hoffmann, B.; Lackner, K.J.; Böger, R.H.; et al. ADMA, Subclinical Changes and Atrial Fibrillation in the General Population. Int. J. Cardiol. 2016, 203, 640–646. [Google Scholar] [CrossRef] [PubMed]
- Sattler, K.; Behnes, M.; Barth, C.; Wenke, A.; Sartorius, B.; El-Battrawy, I.; Mashayekhi, K.; Kuschyk, J.; Hoffmann, U.; Papavasiliu, T.; et al. Occlusion of Left Atrial Appendage Affects Metabolomic Profile: Focus on Glycolysis, Tricarboxylic Acid and Urea Metabolism. Metabolomics 2017, 13, 127. [Google Scholar] [CrossRef] [PubMed]
- Seppala, I.; Kleber, M.E.; Bevan, S.; Lyytikainen, L.P.; Oksala, N.; Hernesniemi, J.A.; Makela, K.M.; Rothwell, P.M.; Sudlow, C.; Dichgans, M.; et al. Associations of Functional Alanine-Glyoxylate Aminotransferase 2 Gene Variants with Atrial Fibrillation and Ischemic Stroke. Sci. Rep. 2016, 6, 23207. [Google Scholar] [CrossRef]
- Lüneburg, N.; Lieb, W.; Zeller, T.; Chen, M.; Maas, R.; Carter, A.M.; Xanthakis, V.; Glazer, N.L.; Schwedhelm, E.; Seshadri, S.; et al. Genome-Wide Association Study of L-Arginine and Dimethylarginines Reveals Novel Metabolic Pathway for Symmetric Dimethylarginine. Circ. Cardiovasc. Genet. 2014, 7, 864–872. [Google Scholar] [CrossRef]
- Seppälä, I.; Kleber, M.E.; Lyytikäinen, L.; Hernesniemi, J.A.; Mäkelä, K.; Oksala, N.; Laaksonen, R.; Pilz, S.; Tomaschitz, A.; Silbernagel, G.; et al. Genome-Wide Association Study on Dimethylarginines Reveals Novel AGXT2 Variants Associated with Heart Rate Variability but Not with overall Mortality. Eur. Heart J. 2014, 35, 524–531. [Google Scholar] [CrossRef]
- Oliva-Damaso, E.; Oliva-Damaso, N.; Rodriguez-Esparragon, F.; Payan, J.; Baamonde-Laborda, E.; Gonzalez-Cabrera, F.; Santana-Estupiñan, R.; Rodriguez-Perez, J.C. Asymmetric (ADMA) and Symmetric (SDMA) Dimethylarginines in Chronic Kidney Disease: A Clinical Approach. Int. J. Mol. Sci. 2019, 20, 3668. [Google Scholar] [CrossRef]
- Lau, Y.C.; Proietti, M.; Guiducci, E.; Blann, A.D.; Lip, G.Y.H. Atrial Fibrillation and Thromboembolism in Patients with Chronic Kidney Disease. J. Am. Coll. Cardiol. 2016, 68, 1452–1464. [Google Scholar] [CrossRef]
- Caplin, B.; Wang, Z.; Slaviero, A.; Tomlinson, J.; Dowsett, L.; Delahaye, M.; Salama, A.; Wheeler, D.C.; Leiper, J. Alanine-Glyoxylate Aminotransferase-2 Metabolizes Endogenous Methylarginines, Regulates NO, and Controls Blood Pressure. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 2892–2900. [Google Scholar] [CrossRef]
- Cooke, J.P.; Sukhovershin, R.A. Novel Markers for Adverse Events in Atrial Fibrillation. J. Am. Coll. Cardiol. 2018, 72, 734–737. [Google Scholar] [CrossRef] [PubMed]
| Reference | Sample Size (Population) | Biomarker Investigated | Comparison/Outcome | Adjusted HR/OR |
|---|---|---|---|---|
| Yoo et al. 2001 [24] | 87 (IS patients and HC) | ADMA cutoff: 1.43 µmol/l | IS versus HC | OR: 6.05 (95% CI: 2.77–13.3) |
| Wanby et al. 2006 [25] | 119 (IS patients and HC) | highest versus. lowest Arg/ADMA quartile | IS versus HC | OR: 0.28 (95% CI 0.11–0.72) |
| 89 (TIA patients and HC) | highest versus lowest ADMA quartile | TIA versus HC | OR: 13.1 (95% CI: 2.91–58.6) | |
| Brouns et al. 2009 [85] | 91 (IS patients and HC) | CSF ADMA | IS versus HC | NR |
| 45 (TIA patients and HC) | CSF ADMA | TIA versus HC | NR | |
| Schulze et al. 2010 [83] | 394 (IS patients) | ADMA | all-cause mortality | n.s. |
| Worthmann et al. 2011 [86] | 67 (IS patients) | ADMA | clinical outcome | OR: 7.19 (95% CI: 1.73–29.82) |
| Rueda-Clausen et al. 2012 [87] | 476 (IS patients and HC) | ADMA | IS versus HC | n.s. |
| Lüneburg et al. 2012 [84] | 137 (IS patients) | ADMA | AE | n.s. |
| Molnar et al. 2014 [88] | 55 (IS patients) | ADMA | all-cause mortality | n.s. |
| Willeit et al. 2015 [76] | 8016 (IS patients and HC; meta-analysis) | highest vs. lower ADMA tertiles | IS versus HC | RR: 1.60 (95% CI: 1.33–1.91) |
| Emrich et al. 2018 [77] | 528 (CKD patients) | ADMA | MACE | n.s. |
| Horowitz et al. 2018 [79] | 4978 (anticoagulated AF patients) | ADMA | stroke/systemic embolism | HR: 1.19 (95% CI: 1.02–1.39) |
| cardiovascular mortality | HR: 1.31 (95% CI: 1.18–1.46) | |||
| 4966 (anticoagulated AF patients) | ADMA | major bleeding | HR: 1.19 (95% CI: 1.07–1.34) |
| Reference. | Sample Size (Population) | Biomarker Investigated | Comparison/Outcome | Adjusted HR/OR |
|---|---|---|---|---|
| Brouns et al. 2009 [85] | 91 (IS patients and HC) | CSF SDMA | IS versus HC | NR |
| 45 (TIA patients and HC) | CSF SDMA | TIA versus. HC | NR | |
| Schulze et al. 2010 [83] | 394 (IS patients) | highest versus lowest SDMA quartile | all-cause mortality | HR: 2.99 (95% CI: 1.64, 5.44) |
| Worthmann et al. 2011 [86] | 67 (IS patients) | SDMA | clinical outcome | OR: 7.16 (95% CI: 1.67–30.69) |
| Lüneburg et al. 2012 [84] | 137 (IS patients) | SDMA | AE | n.s. |
| Molnar et al. 2014 [88] | 55 (IS patients) | SDMA | all-cause mortality | n.s. |
| Willeit et al. 2015 [76] | 3132 (IS patients and HC; meta-analysis) | highest versus lower SDMA tertiles | IS versus HC | n.s. |
| Emrich et al. 2018 [77] | 528 (CKD patients) | highest versus lowest SDMA tertile | MACE | HR: 2.678 (95% CI: 1.261–5.684) |
| Horowitz et al. 2018 [79] | 4978 (anticoagulated AF patients) | SDMA | stroke/systemic embolism | n.s. |
| cardiovascular death | HR: 1.40 (95% CI: 1.25–1.56) | |||
| 4966 (anticoagulated AF patients) | SDMA | major bleeding | HR: 1.41 (95% CI: 1.27–1.57) |
| Reference | Sample Size (Population) | Biomarker Investigated | Comparison/Outcome | Adjusted HR/OR |
|---|---|---|---|---|
| Choe et al. 2013 [91] | 389 (IS patients) | 1-SD increase in log hArg | all-cause mortality | HR: 0.79 (95% CI: 0.64–0.96) |
| 135 (IS patients) | 1-SD increase in log hArg | AE | HR: 0.69 (95% CI: 0.50–0.94) | |
| Pilz et al. 2014 [92] | 606 (population based) | lowest versus higher hArg quartiles | cardiovascular mortality | HR: 4.20 (95% CI: 2·03–8·69) |
| Cordts et al. 2019 [71] | 803 (IS patients) | hArg/ADMA hArg/SDMA | LAA/CES versus SVD/other | OR: 1.52 (95% CI: 1.12–2.06) OR: 2.01 (95% CI: 1.35–3.00 |
| hArg/ADMA hArg/SDMA | ICA stenosis versus no ICA stenosis | OR: 0.73 (95% CI: 0.55–0.97) OR: 0.69 (95% CI: 0.50–0.94) | ||
| Choe et al. 2020 [98] | 394 (IS patients) | hArg/ADMA hArg/SDMA | all-cause mortality | HR: 0.75 (95% CI: 0.62–0.92) HR: 0.68 (95% CI: 0.54–0.85) |
| 135 (IS patients) | hArg/SDMA | AE | HR: 0.73 (95% CI 0.57–0.92) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grosse, G.M.; Schwedhelm, E.; Worthmann, H.; Choe, C.-u. Arginine Derivatives in Cerebrovascular Diseases: Mechanisms and Clinical Implications. Int. J. Mol. Sci. 2020, 21, 1798. https://doi.org/10.3390/ijms21051798
Grosse GM, Schwedhelm E, Worthmann H, Choe C-u. Arginine Derivatives in Cerebrovascular Diseases: Mechanisms and Clinical Implications. International Journal of Molecular Sciences. 2020; 21(5):1798. https://doi.org/10.3390/ijms21051798
Chicago/Turabian StyleGrosse, Gerrit M., Edzard Schwedhelm, Hans Worthmann, and Chi-un Choe. 2020. "Arginine Derivatives in Cerebrovascular Diseases: Mechanisms and Clinical Implications" International Journal of Molecular Sciences 21, no. 5: 1798. https://doi.org/10.3390/ijms21051798
APA StyleGrosse, G. M., Schwedhelm, E., Worthmann, H., & Choe, C.-u. (2020). Arginine Derivatives in Cerebrovascular Diseases: Mechanisms and Clinical Implications. International Journal of Molecular Sciences, 21(5), 1798. https://doi.org/10.3390/ijms21051798






