Effect of Sonication on the Properties of Flaxseed Gum Films Incorporated with Carvacrol
Abstract
1. Introduction
2. Results and Discussion
2.1. Thickness
2.2. Tensile Strength (TS) and Elongation at Break (EB)Results
2.3. Barrier Properties against Water via Water Vapor Permeability (WVP) Tests
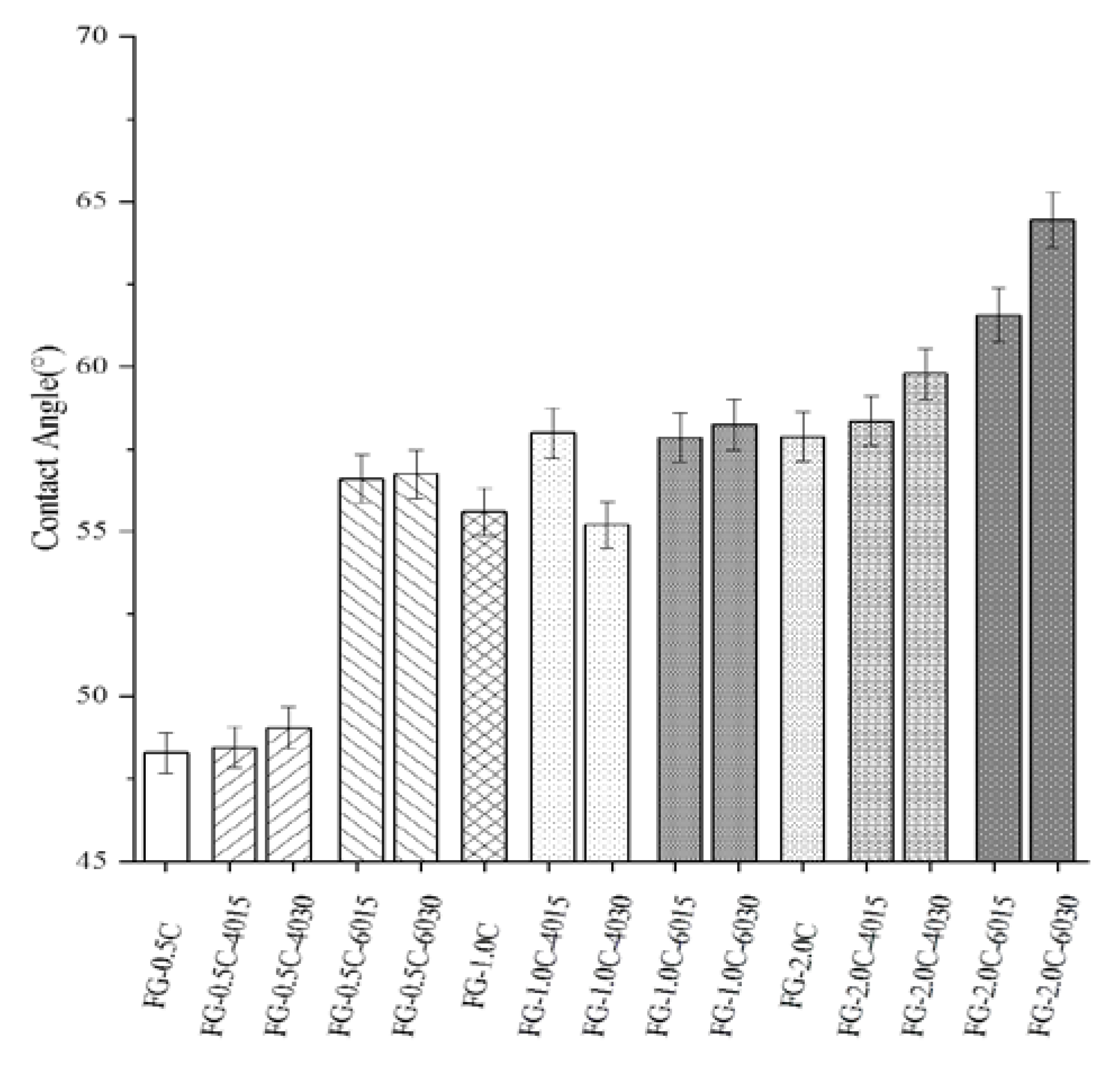
2.4. Surface Hydrophobicity via Contact Angle Measurements
2.5. Film Opacity
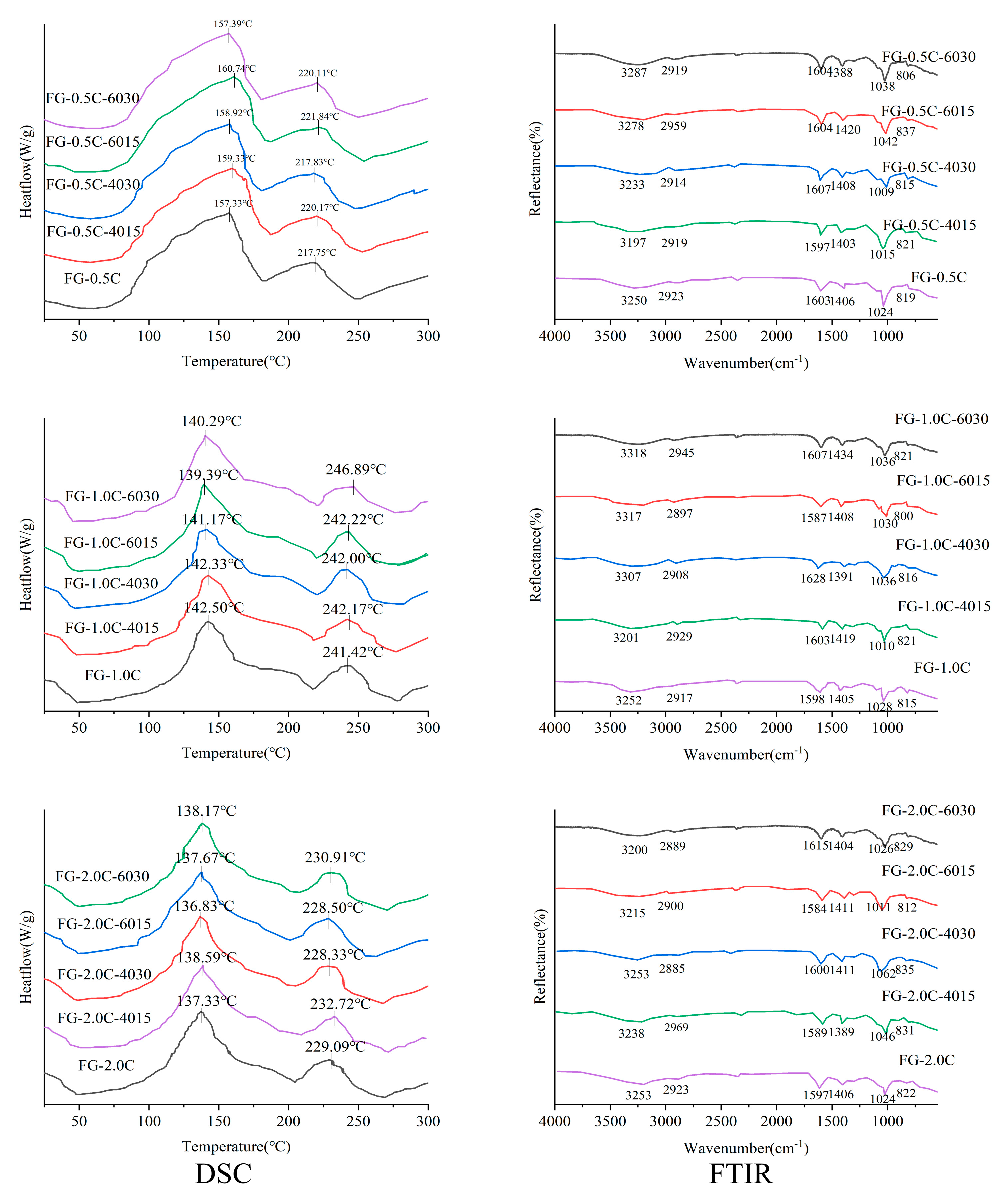
2.6. Film Thermal Stability via Differential Scanning Calorimetry (DSC) Analysis
2.7. Fourier Transform Infrared Spectroscopy (FTIR) Spectra
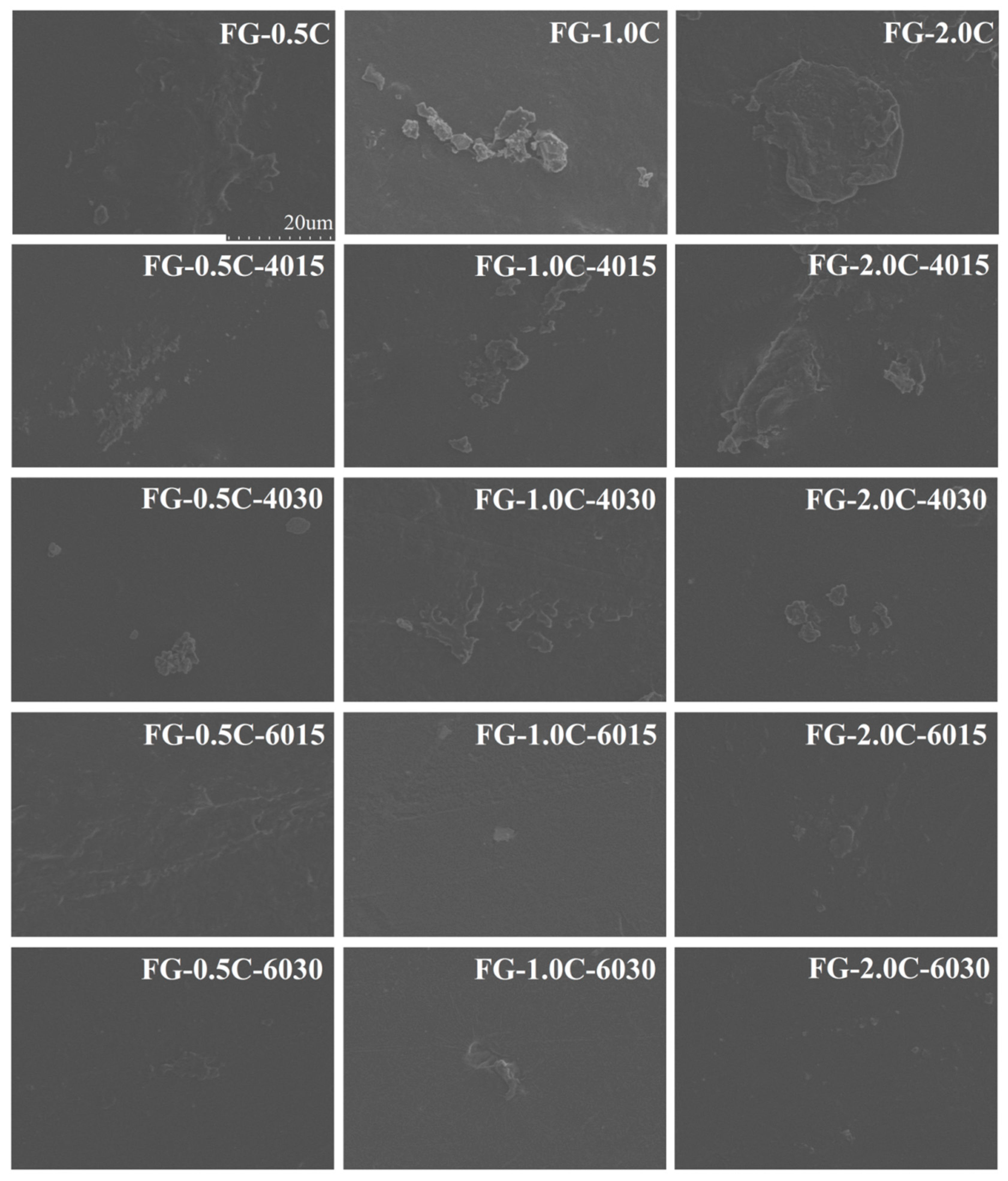
2.8. Film Morphology via SEM Observations
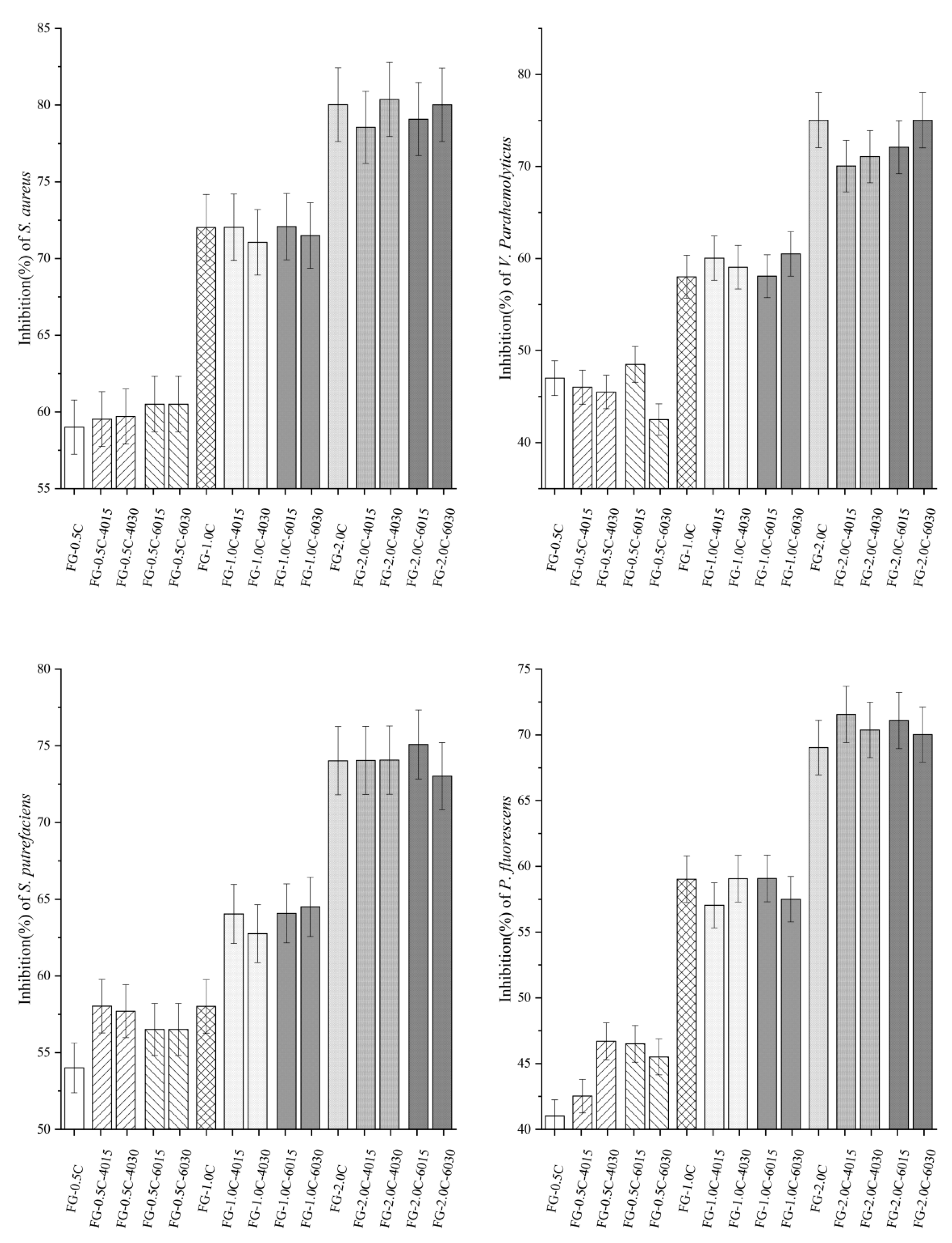
2.9. Antibacterial Activity
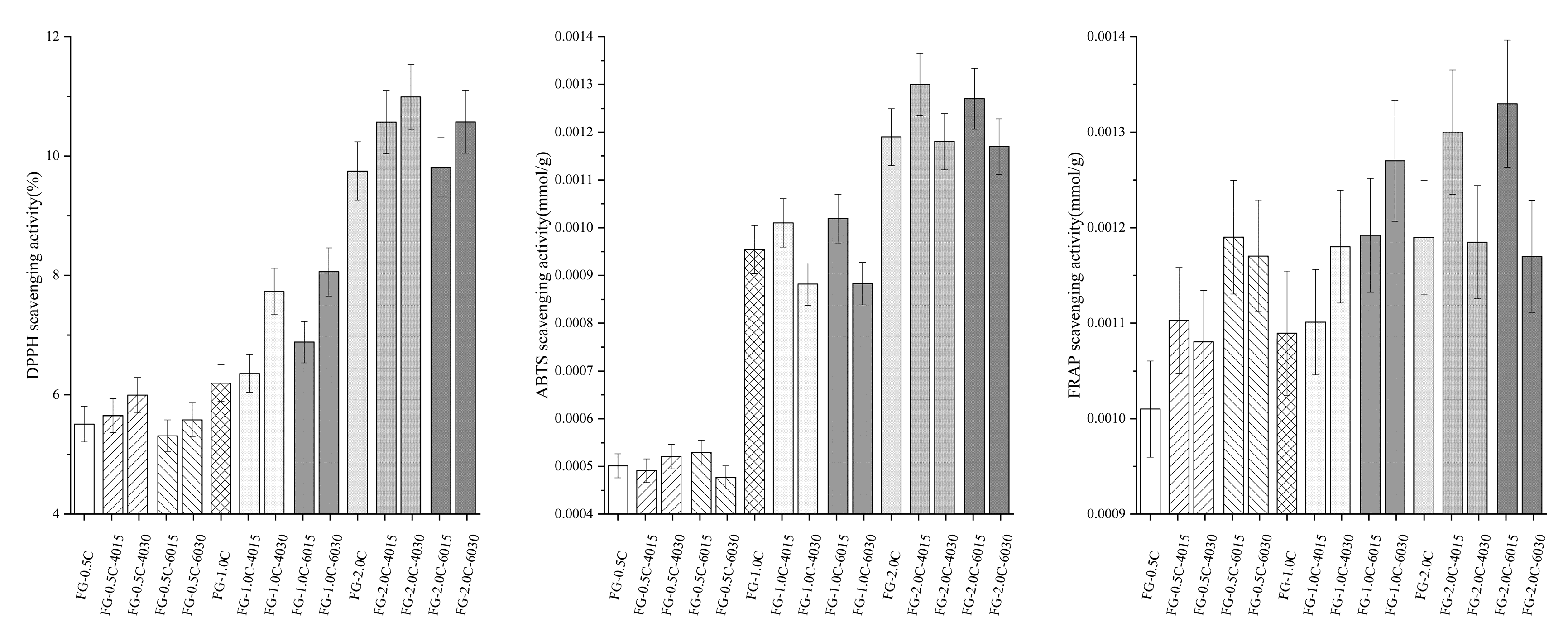
2.10. Antioxidant Activity
3. Materials and Methods
3.1. Preparation of the Active Films
3.2. Film Thickness
3.3. Mechanical Properties
3.4. Contact Angle Measurements
3.5. Film Opacity
3.6. WVP
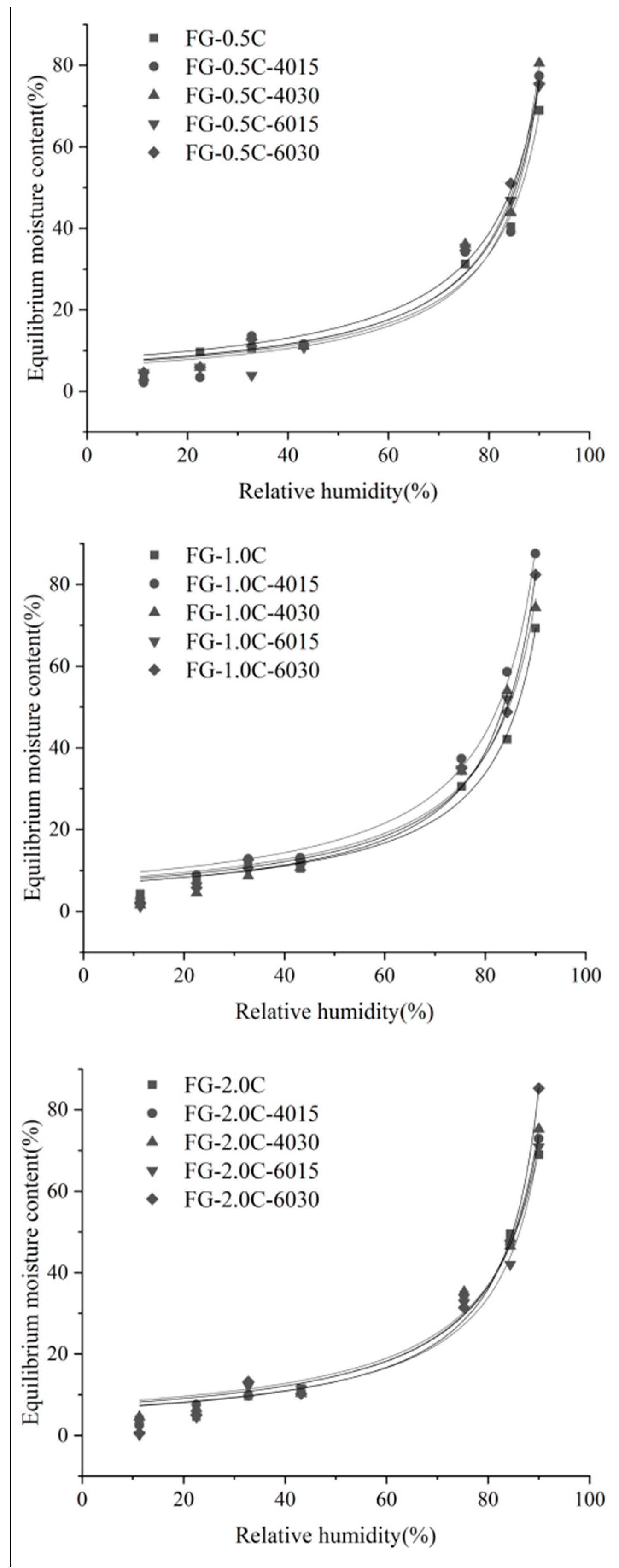
3.7. Water Sorption Isotherms
3.8. FTIR
3.9. DSC Determination
3.10. Antibacterial Activity
3.11. Antioxidant Activity
3.12. SEM Analysis
3.13. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hassan, B.; Chatha, S.A.S.; Hussain, A.I.; Zia, K.M.; Akhtar, N. Recent advances on polysaccharides, lipids and protein based edible films and coatings: A review. Int. J. Biol. Macromol. 2018, 109, 1095–1107. [Google Scholar] [CrossRef]
- Chen, H.H.; Xu, S.Y.; Wang, Z. Gelation properties of flaxseed gum. J. Food Eng. 2006, 77, 295–303. [Google Scholar] [CrossRef]
- Wang, Y.; Li, D.; Wang, L.; Yang, L.; Özkan, N. Dynamic mechanical properties of flaxseed gum based edible films. Carbohydr. Polym. 2011, 86, 499–504. [Google Scholar] [CrossRef]
- Cui, W.; Mazza, G.; Biliaderis, C.G. Chemical structure, molecular size distributions, and rheological properties of flaxseed gum. J. Agric. Food Chem. 1994, 42, 1891–1895. [Google Scholar] [CrossRef]
- Yong, W.; Wang, L.J.; Dong, L.; Özkan, N.; Xiao, D.C.; Mao, Z.H. Effect of flaxseed gum addition on rheological properties of native maize starch. J. Food Eng. 2008, 89, 87–92. [Google Scholar]
- Khalloufi, S.; Corredig, M.; Goff, H.D.; Alexander, M. Flaxseed gums and their adsorption on whey protein-stabilized oil-in-water emulsions. Food Hydrocoll. 2009, 23, 611–618. [Google Scholar] [CrossRef]
- Yousuf, B.; Srivastava, A.K. Flaxseed gum in combination with lemongrass essential oil as an effective edible coating for ready-to-eat pomegranate arils. Int. J. Biol. Macromol. 2017, 104, 1030–1038. [Google Scholar] [CrossRef] [PubMed]
- Shemesh, R.; Krepker, M.; Natan, M.; Danin-Poleg, Y.; Banin, E.; Kashi, Y.; Nitzan, N.; Vaxman, A.; Segal, E. Novel LDPE/halloysite nanotube films with sustained carvacrol release for broad-spectrum antimicrobial activity. RSC Adv. 2015, 5, 87108–87117. [Google Scholar] [CrossRef]
- Alves, V.L.; Rico, B.P.; Cruz, R.M.; Vicente, A.A.; Khmelinskii, I.; Vieira, M.C. Preparation and characterization of a chitosan film with grape seed extract-carvacrol microcapsules and its effect on the shelf-life of refrigerated Salmon (Salmo salar). LWT-Food Sci. Technol. 2018, 89, 525–534. [Google Scholar] [CrossRef]
- Wang, Y.; Li, D.; Wang, L.J.; Xue, J. Effects of high pressure homogenization on rheological properties of flaxseed gum. Carbohydr. Polym. 2011, 83, 489–494. [Google Scholar] [CrossRef]
- Borah, P.P.; Das, P.; Badwaik, L.S. Ultrasound treated potato peel and sweet lime pomace based biopolymer film development. Ultrason. Sonochem. 2017, 36, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, D.C.; Cunha, A.P.; Brito, E.S.; Azeredo, H.M.C.; Gallão, M.I. Mesquite seed gum and palm fruit oil emulsion edible films: Influence of oil content and sonication. Food Hydrocoll. 2016, 56, 227–235. [Google Scholar] [CrossRef]
- Acevedo-Fani, A.; Salvia-Trujillo, L.; Rojas-Graü, M.A.; Martín-Belloso, O. Edible films from essential-oil-loaded nanoemulsions: Physicochemical characterization and antimicrobial properties. Food Hydrocoll. 2015, 47, 168–177. [Google Scholar] [CrossRef]
- Klangmuang, P.; Sothornvit, R. Barrier properties, mechanical properties and antimicrobial activity of hydroxypropyl methylcellulose-based nanocomposite films incorporated with Thai essential oils. Food Hydrocoll. 2016, 61, 609–616. [Google Scholar] [CrossRef]
- Gul, O.; Saricaoglu, F.T.; Besir, A.; Atalar, I.; Yazici, F. Effect of ultrasound treatment on the properties of nano-emulsion films obtained from hazelnut meal protein and clove essential oil. Ultrason. Sonochem. 2018, 41, 466–474. [Google Scholar] [CrossRef]
- Bonilla, J.; Atarés, L.; Vargas, M.; Chiralt, A. Effect of essential oils and homogenization conditions on properties of chitosan-based films. Food Hydrocoll. 2012, 26, 9–16. [Google Scholar] [CrossRef]
- Kurek, M.; Guinault, A.; Voilley, A.; Galić, K.; Debeaufort, F. Effect of relative humidity on carvacrol release and permeation properties of chitosan based films and coatings. Food Chem. 2014, 144, 9–17. [Google Scholar] [CrossRef]
- Pérez-Gago, M.B.; Krochta, J.M. Lipid particle size effect on water vapor permeability and mechanical properties of whey protein/beeswax emulsion films. J. Agric. Food Chem. 2001, 49, 996–1002. [Google Scholar] [CrossRef]
- Otoni, C.G.; Pontes, S.F.O.; Medeiros, E.A.A.; Soares, N.D.F. Edible films from methylcellulose and nanoemulsions of clove bud (Syzygium aromaticum) and oregano (Origanum vulgare) essential oils as shelf life extenders for sliced bread. J. Agric. Food Chem. 2014, 62, 5214–5219. [Google Scholar] [CrossRef]
- Niknam, R.; Ghanbarzadeh, B.; Ayaseh, A.; Hamishehkar, H. Plantago major seed gum based biodegradable films: Effects of various plant oils on microstructure and physicochemical properties of emulsified films. Polym. Test. 2019, 77, 105868. [Google Scholar] [CrossRef]
- Abdollahi, M.; Damirchi, S.; Shafafi, M.; Rezaei, M.; Ariaii, P. Carboxymethyl cellulose-agar biocomposite film activated with summer savory essential oil as an antimicrobial agent. Int. J. Biol. Macromol. 2019, 126, 561–568. [Google Scholar] [CrossRef] [PubMed]
- Park, H.Y.; Kim, S.J.; Kim, K.M.; You, Y.S.; Kim, S.Y.; Han, J. Development of antioxidant packaging material by applying corn-zein to LLDPE film in combination with phenolic compounds. J. Food Sci. 2012, 77, E273–E279. [Google Scholar] [CrossRef] [PubMed]
- Jouki, M.; Mortazavi, S.A.; Yazdi, F.T.; Koocheki, A. Characterization of antioxidant-antibacterial quince seed mucilage films containing thyme essential oil. Carbohydr. Polym. 2014, 99, 537–546. [Google Scholar] [CrossRef] [PubMed]
- Kavoosi, G.; Dadfar, S.M.M.; Mohammadi Purfard, A.; Mehrabi, R. Antioxidant and antibacterial properties of gelatin films incorporated with carvacrol. J. Food Saf. 2013, 33, 423–432. [Google Scholar] [CrossRef]
- Hashemi, S.M.B.; Mousavi Khaneghah, A.; Ghaderi Ghahfarrokhi, M.; Eş, I. Basil-seed gum containing Origanum vulgare subsp. viride essential oil as edible coating for fresh cut apricots. Postharvest. Biol. Technol. 2017, 125, 26–34. [Google Scholar] [CrossRef]
- Perdones, Á.; Chiralt, A.; Vargas, M. Properties of film-forming dispersions and films based on chitosan containing basil or thyme essential oil. Food Hydrocoll. 2016, 57, 271–279. [Google Scholar] [CrossRef]
- Shojaee-Aliabadi, S.; Hosseini, H.; Mohammadifar, M.A.; Mohammadi, A.; Ghasemlou, M.; Ojagh, S.M.; Hosseini, S.M.; Khaksar, R. Characterization of antioxidant-antimicrobial κ-carrageenan films containing Satureja hortensis essential oil. Int. J. Biol. Macromol. 2013, 52, 116–124. [Google Scholar] [CrossRef]
- Cao, T.L.; Yang, S.Y.; Song, K.B.J. Development of burdock root inulin/chitosan blend films containing oregano and thyme essential oils. Int. J. Mol. Sci. 2018, 19, 131. [Google Scholar]
- Wang, Z.; Zhou, J.; Wang, X.X.; Zhang, N.; Sun, X.X.; Ma, Z.S. The effects of ultrasonic/microwave assisted treatment on the water vapor barrier properties of soybean protein isolate-based oleic acid/stearic acid blend edible films. Food Hydrocoll. 2014, 35, 51–58. [Google Scholar] [CrossRef]
- Ashokkumar, M.; Sunartio, D.; Kentish, S.; Mawson, R.; Simons, L.; Vilkhu, K.; Versteeg, C.J. Modification of food ingredients by ultrasound to improve functionality: A preliminary study on a model system. Innov. Food Sci. Emerg. 2008, 9, 155–160. [Google Scholar] [CrossRef]
- Mu, C.; Guo, J.; Li, X.; Lin, W.; Li, D. Preparation and properties of dialdehyde carboxymethyl cellulose crosslinked gelatin edible films. Food Hydrocoll. 2012, 27, 22–29. [Google Scholar] [CrossRef]
- Atef, M.; Rezaei, M.; Behrooz, R. Characterization of physical, mechanical, and antibacterial properties of agar-cellulose bionanocomposite films incorporated with savory essential oil. Food Hydrocoll. 2015, 45, 150–157. [Google Scholar] [CrossRef]
- Estrada-Fernández, A.G.; Román-Guerrero, A.; Jiménez-Alvarado, R.; Lobato-Calleros, C.; Alvarez-Ramirez, J.; Vernon-Carter, E.J. Stabilization of oil-in-water-in-oil (O1/W/O2) Pickering double emulsions by soluble and insoluble whey protein concentrate-gum Arabic complexes used as inner and outer interfaces. J. Food Eng. 2018, 221, 35–44. [Google Scholar] [CrossRef]
- Dhumal, C.V.; Ahmed, J.; Bandara, N.; Sarkar, P. Improvement of antimicrobial activity of sago starch/guar gum bi-phasic edible films by incorporating carvacrol and citral. Food Packag. Shelf 2019, 21, 100380. [Google Scholar] [CrossRef]
- Nisar, T.; Wang, Z.C.; Yang, X.; Tian, Y.; Iqbal, M.; Guo, Y. Characterization of citrus pectin films integrated with clove bud essential oil: Physical, thermal, barrier, antioxidant and antibacterial properties. Int. J. Biol. Macromol. 2018, 106, 670–680. [Google Scholar] [CrossRef] [PubMed]
- Espitia, P.J.P.; Avena-Bustillos, R.J.; Du, W.X.; Teófilo, R.F.; Soares, N.F.F.; McHugh, T.H. Optimal antimicrobial formulation and physical–mechanical properties of edible films based on açaí and pectin for food preservation. Food Packag. Shelf Life 2014, 2, 38–49. [Google Scholar] [CrossRef]
- Gursoy, M.; Sargin, I.; Mujtaba, M.; Akyuz, B.; Ilk, S.; Akyuz, L.; Kaya, M.; Cakmak, Y.S.; Salaberria, A.M.; Labidi, J.; et al. False flax (Camelina sativa) seed oil as suitable ingredient for the enhancement of physicochemical and biological properties of chitosan films. Int. J. Biol. Macromol. 2018, 114, 1224–1232. [Google Scholar] [CrossRef]
- Cao, T.L.; Song, K.B. Active gum karaya/Cloisite Na+ nanocomposite films containing cinnamaldehyde. Food Hydrocoll. 2019, 89, 453–460. [Google Scholar] [CrossRef]
- Hadad, S.; Goli, S.A.H. Fabrication and characterization of electrospun nanofibers using flaxseed (Linum usitatissimum) mucilage. Int. J. Biol. Macromol. 2018, 114, 408–414. [Google Scholar] [CrossRef]
- Liang, S.; Liao, W.; Ma, X.; Li, X.; Wang, Y. H2O2 oxidative preparation, characterization and antiradical activity of a novel oligosaccharide derived from flaxseed gum. Food Chem. 2017, 230, 135–144. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, S.; Lan, W.; Qin, W. Development of ultrasound treated polyvinyl alcohol/tea polyphenol composite films and their physicochemical properties. Ultrason. Sonochem. 2019, 51, 386–394. [Google Scholar] [CrossRef] [PubMed]
- Baek, S.K.; Kim, S.; Song, K. Characterization of Ecklonia cava alginate films containing cinnamon essential oils. Int. J. Mol. Sci. 2018, 19, 3545. [Google Scholar] [CrossRef] [PubMed]
- Kaya, M.; Ravikumar, P.; Ilk, S.; Mujtaba, M.; Akyuz, L.; Labidi, J.; Salaberria, A.M.; Cakmak, Y.S.; Erkul, S.K. Production and characterization of chitosan based edible films from Berberis crataegina’s fruit extract and seed oil. Innov. Food Sci. Emerg. 2018, 45, 287–297. [Google Scholar] [CrossRef]
- Hasheminya, S.-M.; Mokarram, R.R.; Ghanbarzadeh, B.; Hamishekar, H.; Kafil, H.S.; Dehghannya, J. Development and characterization of biocomposite films made from kefiran, carboxymethyl cellulose and Satureja Khuzestanica essential oil. Food Chem. 2019, 289, 443–452. [Google Scholar] [CrossRef]
- Zhong, T.; Huang, R.; Sui, S.; Lian, Z.; Sun, X.; Wan, A.; Li, H. Effects of ultrasound treatment on lipid self-association and properties of methylcellulose/stearic acid blending films. Carbohydr. Polym. 2015, 131, 415–423. [Google Scholar] [CrossRef]
- Marcuzzo, E.; Peressini, D.; Debeaufort, F.; Sensidoni, A. Effect of ultrasound treatment on properties of gluten-based film. Innov. Food Sci. Emerg. 2010, 11, 451–457. [Google Scholar] [CrossRef]
- Chiumarelli, M.; Hubinger, M.D. Evaluation of edible films and coatings formulated with cassava starch, glycerol, carnauba wax and stearic acid. Food Hydrocoll. 2014, 38, 20–27. [Google Scholar] [CrossRef]
- Windiasti, G.; Feng, J.; Ma, L.; Hu, Y.; Hakeem, M.J.; Amoako, K.; Delaquis, P.; Lu, X. Investigating the synergistic antimicrobial effect of carvacrol and zinc oxide nanoparticles against Campylobacter jejuni. Food Control 2019, 96, 39–46. [Google Scholar] [CrossRef]
- Kachur, K.; Suntres, Z. The antibacterial properties of phenolic isomers, carvacrol and thymol. Crit. Rev. Food Sci. Nutr. 2019, 1–12. [Google Scholar] [CrossRef]
- Lanne, A.B.M.; Goode, A.; Prattley, C.; Kumari, D.; Drasbek, M.R.; Williams, P.; Conde-Álvarez, R.; Moriyón, I.; Bonev, B.B. Molecular recognition of lipopolysaccharide by the lantibiotic nisin. Biochim. Biophys. Acta Biomembr. 2019, 1861, 83–92. [Google Scholar] [CrossRef]
- Ramos, M.; Jiménez, A.; Peltzer, M.; Garrigós, M.C. Characterization and antimicrobial activity studies of polypropylene films with carvacrol and thymol for active packaging. J. Food Eng. 2012, 109, 513–519. [Google Scholar] [CrossRef]
- Fang, S.; Zhou, Q.; Hu, Y.; Liu, F.; Mei, J.; Xie, J. Antimicrobial carvacrol incorporated in flaxseed gum-sodium alginate active films to improve the quality attributes of Chinese sea bass (Lateolabrax maculatus) during cold storage. Molecules 2019, 24, 3292. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Wang, R.; Kang, X.; Cui, B.; Yu, B. Effects of ultrasonic treatment on amylose-lipid complex formation and properties of sweet potato starch-based films. Ultrason. Sonochem. 2018, 44, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimi, B.; Mohammadi, R.; Rouhi, M.; Mortazavian, A.M.; Shojaeealiabadi, S.; Koushki, M.R. Survival of probiotic bacteria in carboxymethyl cellulose-based edible film and assessment of quality parameters. LWT-Food Sci. Technol. 2017, 87, 54–60. [Google Scholar] [CrossRef]
- Guo, C.; Zhang, B.; Zhao, J. Dispersion process and effect of oleic acid on properties of cellulose sulfate- oleic acid composite film. Materials 2015, 8, 2346–2360. [Google Scholar]
- González Sandoval, D.C.; Luna Sosa, B.; Martínez-Ávila, G.C.G.; Rodríguez Fuentes, H.; Avendaño Abarca, V.H.; Rojas, R. Formulation and characterization of edible films based on organic mucilage from Mexican opuntia ficus-indica. Coatings 2019, 9, 506. [Google Scholar] [CrossRef]
- Slavutsky, A.M.; Bertuzzi, M.A. Improvement of water barrier properties of starch films by lipid nanolamination. Food Packag. Shelf Life 2016, 7, 41–46. [Google Scholar] [CrossRef]
- Augusto, A.; Dias, J.; Campos, M.; Alves, N.; Pedrosa, R.; Silva, S. Influence of codiumtomentosumextract in the properties of alginate and chitosan edible films. Foods 2018, 7, 53. [Google Scholar] [CrossRef]
- Aguilar-Sánchez, R.; Munguía-Pérez, R.; Reyes-Jurado, F.; Navarro-Cruz, A.R.; Cid-Pérez, T.S.; Hernández-Carranza, P.; Beristain-Bauza, S.d.C.; Ochoa-Velasco, C.E.; Avila-Sosa, R. Structural, physical, and antifungal characterization of starch edible films added with nanocomposites and mexicanoregano (Lippiaber landieri Schauer) essential oil. Molecules 2019, 24, 2340. [Google Scholar] [CrossRef]
- Govindaswamy, R.; Robinson, J.S.; Geevaretnam, J.; Pandurengan, P. Physico-functional and anti-oxidative properties of carp swim bladder gelatin and brown seaweed fucoidan based edible films. J. Packag. Technol. Res. 2018, 2, 77–89. [Google Scholar] [CrossRef]
- Díaz, O.; Ferreiro, T.; Rodríguez-Otero, J.L.; Cobos, Á. Characterization of chickpea (Cicer arietinum L.) flour films: effects of pH and plasticizer concentration. Int. J. Mol. Sci. 2019, 20, 1246. [Google Scholar] [CrossRef] [PubMed]
- Polumackanycz, M.; Sledzinski, T.; Goyke, E.; Wesolowski, M.; Viapiana, A. A comparative study on the phenolic composition and biological activities of Morus alba L. commercial samples. Molecules 2019, 24, 3082. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.; Li, L.; Wang, Q.; Wu, G.; Liu, C.; Jiang, B.; Xu, J. Effect of antioxidant and antimicrobial coating based on whey protein nanofibrils with TiO2 nanotubes on the quality and shelf life of chilled meat. Int. J. Mol. Sci. 2019, 20, 1184. [Google Scholar] [CrossRef] [PubMed]
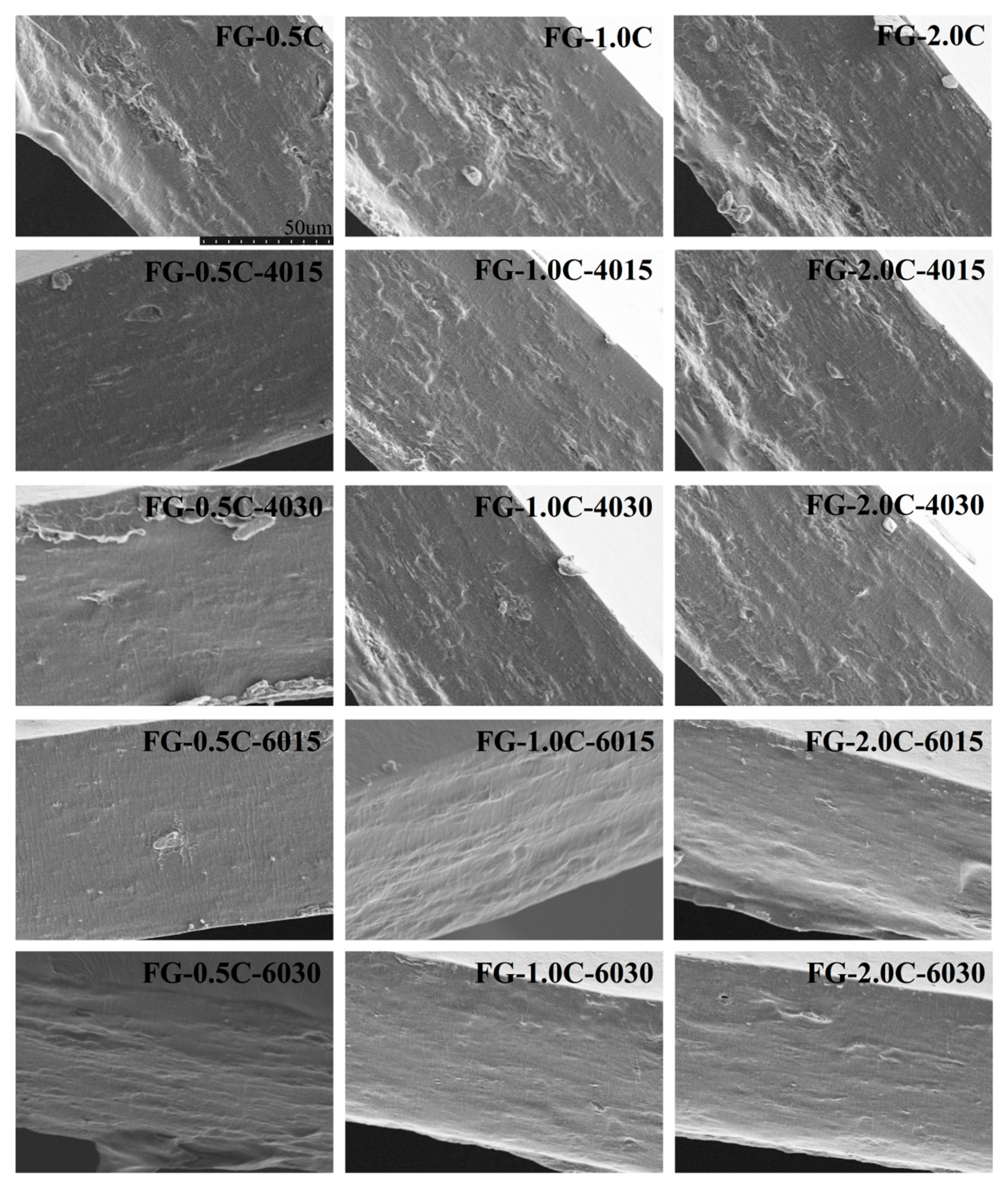
| Number | Sample | Carvacrol Content (Mass Fraction, %) | Power (W/cm2) | Time (min) |
|---|---|---|---|---|
| 1 | FG-0.5C | 0.05 | - | - |
| 2 | FG-0.5C-4015 | 0.05 | 400 | 15 |
| 3 | FG-0.5C-4030 | 0.05 | 400 | 30 |
| 4 | FG-0.5C-6015 | 0.05 | 600 | 15 |
| 5 | FG-0.5C-6030 | 0.05 | 600 | 30 |
| 6 | FG-1.0C | 0.1 | - | - |
| 7 | FG-1.0C-4015 | 0.1 | 400 | 15 |
| 8 | FG-1.0C-4030 | 0.1 | 400 | 30 |
| 9 | FG-1.0C-6015 | 0.1 | 600 | 15 |
| 10 | FG-1.0C-6030 | 0.1 | 600 | 30 |
| 11 | FG-2.0C | 0.2 | - | - |
| 12 | FG-2.0C-4015 | 0.2 | 400 | 15 |
| 13 | FG-2.0C-4030 | 0.2 | 400 | 30 |
| 14 | FG-2.0C-6015 | 0.2 | 600 | 15 |
| 15 | FG-2.0C-6030 | 0.2 | 600 | 30 |
| Films | Thickness (mm) | Tensile Test | WVP (g H2O m−2 s−1 MPa−1) | |
|---|---|---|---|---|
| TS (MPa) | EB (%) | |||
| FG-0.5C | 0.081 ± 0.002 | 25.46 ± 1.81 | 3.60 ± 0.09 | 1.90 ± 0.16 |
| FG-0.5C-4015 | 0.080 ± 0.001 | 25.22 ± 1.98 | 3.79 ± 0.16 | 2.72 ± 0.13 |
| FG-0.5C-4030 | 0.081 ± 0.001 | 32.29 ± 1.33 | 4.04 ± 0.07 | 1.10 ± 0.21 |
| FG-0.5C-6015 | 0.083 ± 0.002 | 30.09 ± 1.24 | 4.19 ± 0.30 | 2.74 ± 0.33 |
| FG-0.5C-6030 | 0.083 ± 0.002 | 33.40 ± 1.68 | 4.46 ± 0.40 | 1.57 ± 0.11 |
| FG-1.0C | 0.084 ± 0.001 | 22.81 ± 0.93 | 2.69 ± 0.28 | 2.31 ± 0.21 |
| FG-1.0C-4015 | 0.085 ± 0.001 | 23.60 ± 1.50 | 3.22 ± 0.33 | 2.46 ± 0.11 |
| FG-1.0C-4030 | 0.081 ± 0.002 | 24.32 ± 1.40 | 3.30 ± 0.05 | 1.80 ± 0.25 |
| FG-1.0C-6015 | 0.081 ± 0.001 | 27.04 ± 1.79 | 3.54 ± 0.05 | 2.27 ± 0.29 |
| FG-1.0C-6030 | 0.081 ± 0.001 | 29.16 ± 0.85 | 3.84 ± 0.19 | 1.99 ± 0.22 |
| FG-2.0C | 0.085 ± 0.002 | 18.45 ± 1.22 | 2.60 ± 0.23 | 2.51 ± 0.39 |
| FG-2.0C-4015 | 0.085 ± 0.002 | 21.81 ± 1.13 | 2.66 ± 0.20 | 1.79 ± 0.33 |
| FG-2.0C-4030 | 0.082 ± 0.002 | 23.08 ± 1.50 | 3.02 ± 0.21 | 2.07 ± 0.19 |
| FG-2.0C-6015 | 0.084 ± 0.001 | 25.32 ± 0.92 | 2.88 ± 0.05 | 1.31 ± 0.14 |
| FG-2.0C-6030 | 0.083 ± 0.002 | 27.08 ± 1.10 | 3.06 ± 0.18 | 1.67 ± 0.29 |
| Films | W0 (%) | C | k | R2 |
|---|---|---|---|---|
| FG-0.5C | 7.2524 | 15.0117 | 0.0099 | 0.9894 |
| FG-0.5C-4015 | 9.9610 | 4.0095 | 0.0098 | 0.9939 |
| FG-0.5C-4030 | 7.9505 | 5.9960 | 0.0100 | 0.9831 |
| FG-0.5C-6015 | 10.2233 | 1.9750 | 0.0097 | 0.9918 |
| FG-0.5C-6030 | 10.3772 | 3.3244 | 0.0096 | 0.9948 |
| FG-1.0C | 7.0905 | 27.2029 | 0.0100 | 0.9954 |
| FG-1.0C-4015 | 10.4380 | 5.2432 | 0.0098 | 0.9986 |
| FG-1.0C-4030 | 13.2619 | 1.4699 | 0.0094 | 0.9994 |
| FG-1.0C-6015 | 11.2298 | 3.0018 | 0.0095 | 0.9951 |
| FG-1.0C-6030 | 8.5154 | 8.8591 | 0.0100 | 0.9826 |
| FG-2.0C | −11.8895 | 1.7437 | −0.0126 | 0.9989 |
| FG-2.0C-4015 | 9.1218 | 5.4526 | 0.0097 | 0.9945 |
| FG-2.0C-4030 | 8.7280 | 5.8071 | 0.0098 | 0.9936 |
| FG-2.0C-6015 | −8.1933 | 1.2650 | −0.037 | 0.9856 |
| FG-2.0C-6030 | 12.4246 | 1.8996 | 0.0094 | 0.9978 |
| Films | Thickness(mm) | Opacity(mm−1) |
|---|---|---|
| FG-0.5C | 0.081 ± 0.002 | 0.925 ± 0.007 |
| FG-0.5C-4015 | 0.080 ± 0.001 | 0.981 ± 0.008 |
| FG-0.5C-4030 | 0.081 ± 0.001 | 0.975 ± 0.020 |
| FG-0.5C-6015 | 0.083 ± 0.002 | 0.946 ± 0.005 |
| FG-0.5C-6030 | 0.083 ± 0.002 | 0.953 ± 0.005 |
| FG-1.0C | 0.084 ± 0.001 | 0.866 ± 0.013 |
| FG-1.0C-4015 | 0.085 ± 0.001 | 0.879 ± 0.017 |
| FG-1.0C-4030 | 0.081 ± 0.002 | 0.929 ± 0.009 |
| FG-1.0C-6015 | 0.081 ± 0.001 | 0.922 ± 0.019 |
| FG-1.0C-6030 | 0.081 ± 0.001 | 0.933 ± 0.017 |
| FG-2.0C | 0.085 ± 0.002 | 0.821 ± 0.010 |
| FG-2.0C-4015 | 0.085 ± 0.002 | 0.852 ± 0.018 |
| FG-2.0C-4030 | 0.082 ± 0.002 | 0.884 ± 0.010 |
| FG-2.0C-6015 | 0.084 ± 0.001 | 0.866 ± 0.019 |
| FG-2.0C-6030 | 0.083 ± 0.002 | 0.880 ± 0.012 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fang, S.; Qiu, W.; Mei, J.; Xie, J. Effect of Sonication on the Properties of Flaxseed Gum Films Incorporated with Carvacrol. Int. J. Mol. Sci. 2020, 21, 1637. https://doi.org/10.3390/ijms21051637
Fang S, Qiu W, Mei J, Xie J. Effect of Sonication on the Properties of Flaxseed Gum Films Incorporated with Carvacrol. International Journal of Molecular Sciences. 2020; 21(5):1637. https://doi.org/10.3390/ijms21051637
Chicago/Turabian StyleFang, Shiyuan, Weiqiang Qiu, Jun Mei, and Jing Xie. 2020. "Effect of Sonication on the Properties of Flaxseed Gum Films Incorporated with Carvacrol" International Journal of Molecular Sciences 21, no. 5: 1637. https://doi.org/10.3390/ijms21051637
APA StyleFang, S., Qiu, W., Mei, J., & Xie, J. (2020). Effect of Sonication on the Properties of Flaxseed Gum Films Incorporated with Carvacrol. International Journal of Molecular Sciences, 21(5), 1637. https://doi.org/10.3390/ijms21051637








