ZnO Nanoparticles Induced Caspase-Dependent Apoptosis in Gingival Squamous Cell Carcinoma through Mitochondrial Dysfunction and p70S6K Signaling Pathway
Abstract
1. Introduction
2. Results
2.1. ZnO-NPs Inhibited Cell Growth of Gingival Squamous Cell Carcinomas
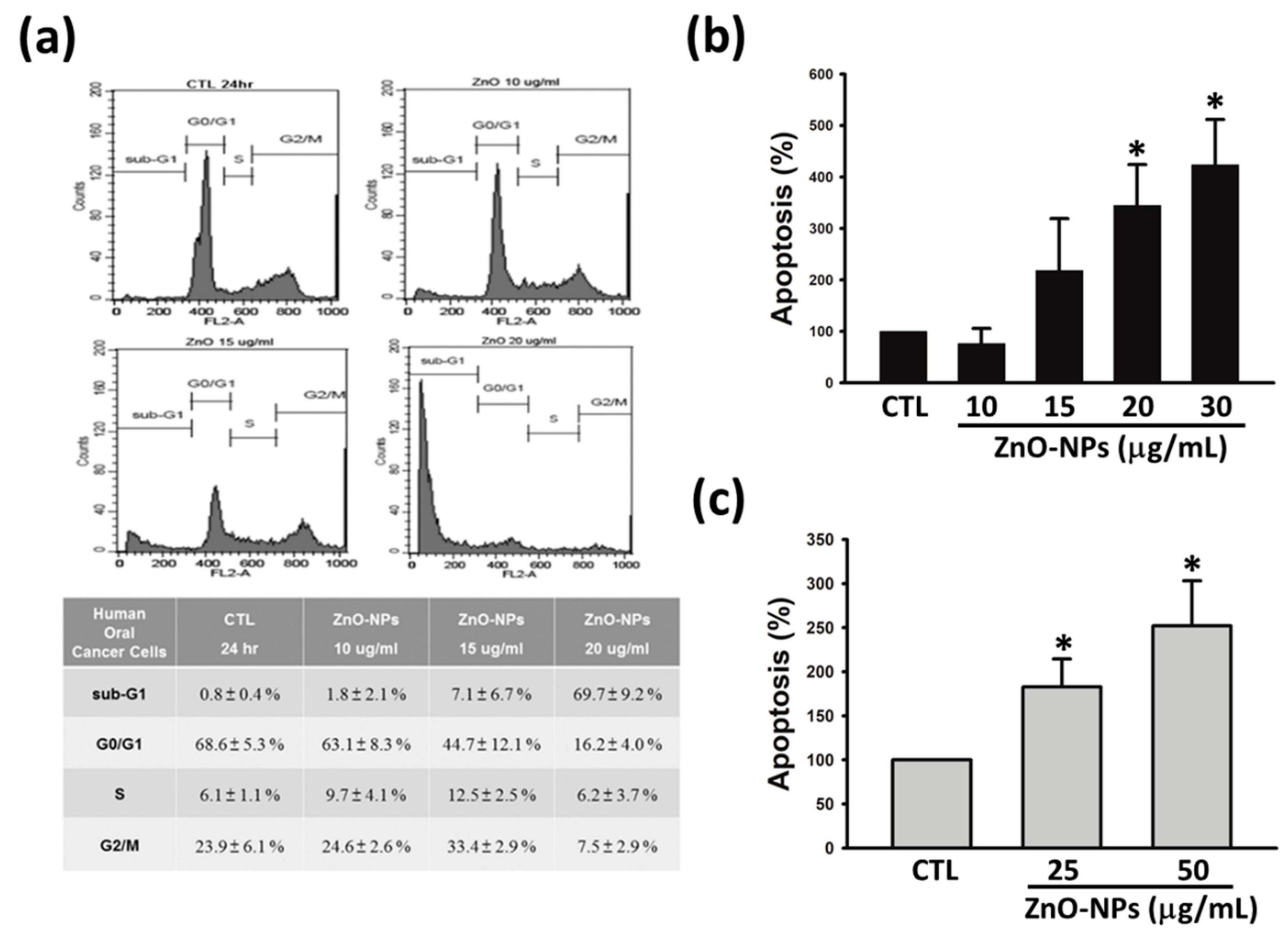
2.2. ZnO-NPs Caused Sub-G1 Arrest and Apoptosis in Gingival Squamous Cell Carcinomas
2.3. ZnO-NPs Stimulated ROS and Superoxide Generation in Gingival Squamous Cell Carcinomas
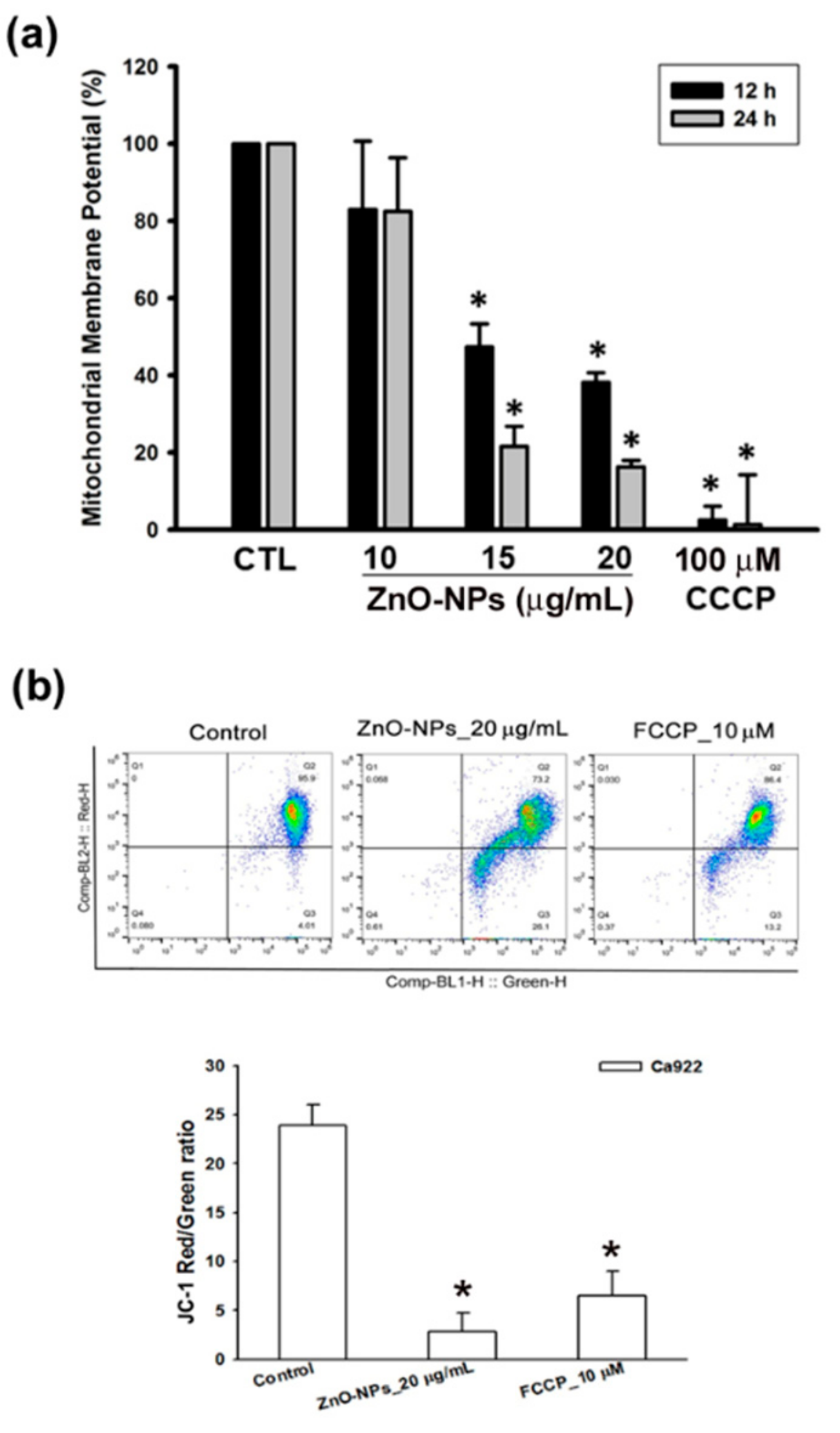
2.4. ZnO-NPs Triggered Mitochondrial Intrinsic Apoptosis in Gingival Squamous Cell Carcinomas
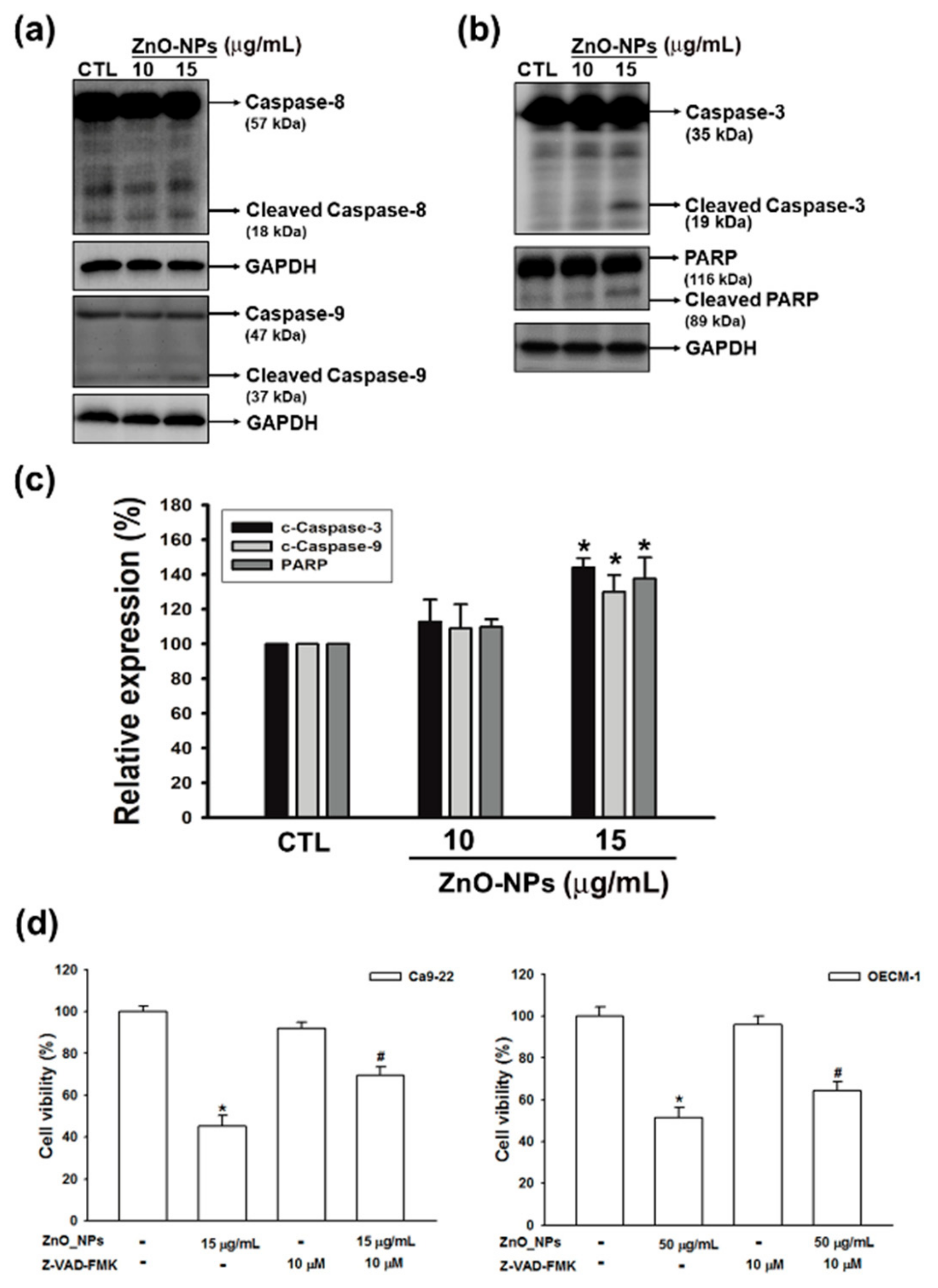
2.5. ZnO-NPs Induced Caspase-Dependent Apoptosis in Gingival Squamous Cell Carcinomas
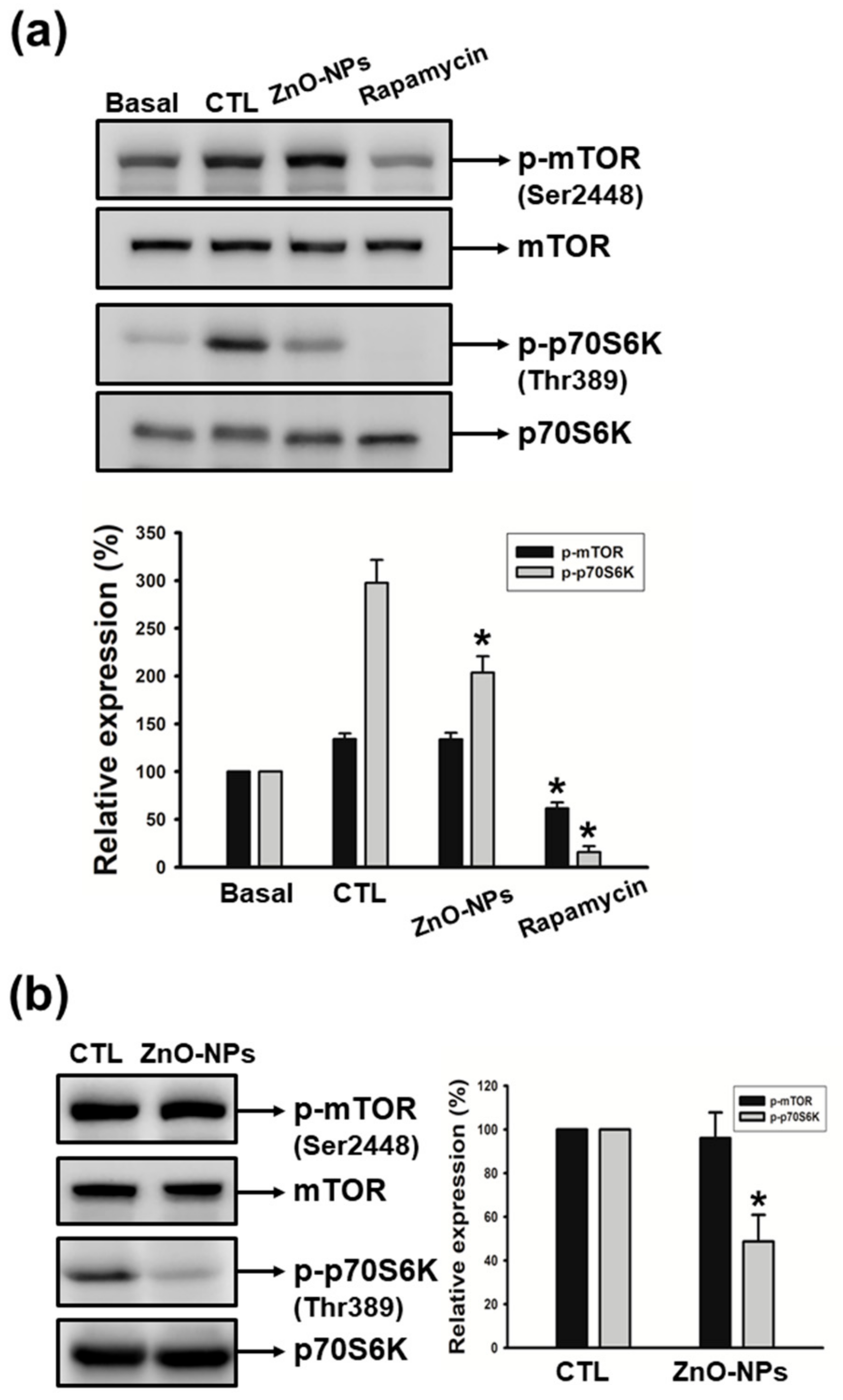
2.6. ZnO-NPs Inhibited p70S6K Signaling Pathway in Gingival Squamous Cell Carcinomas
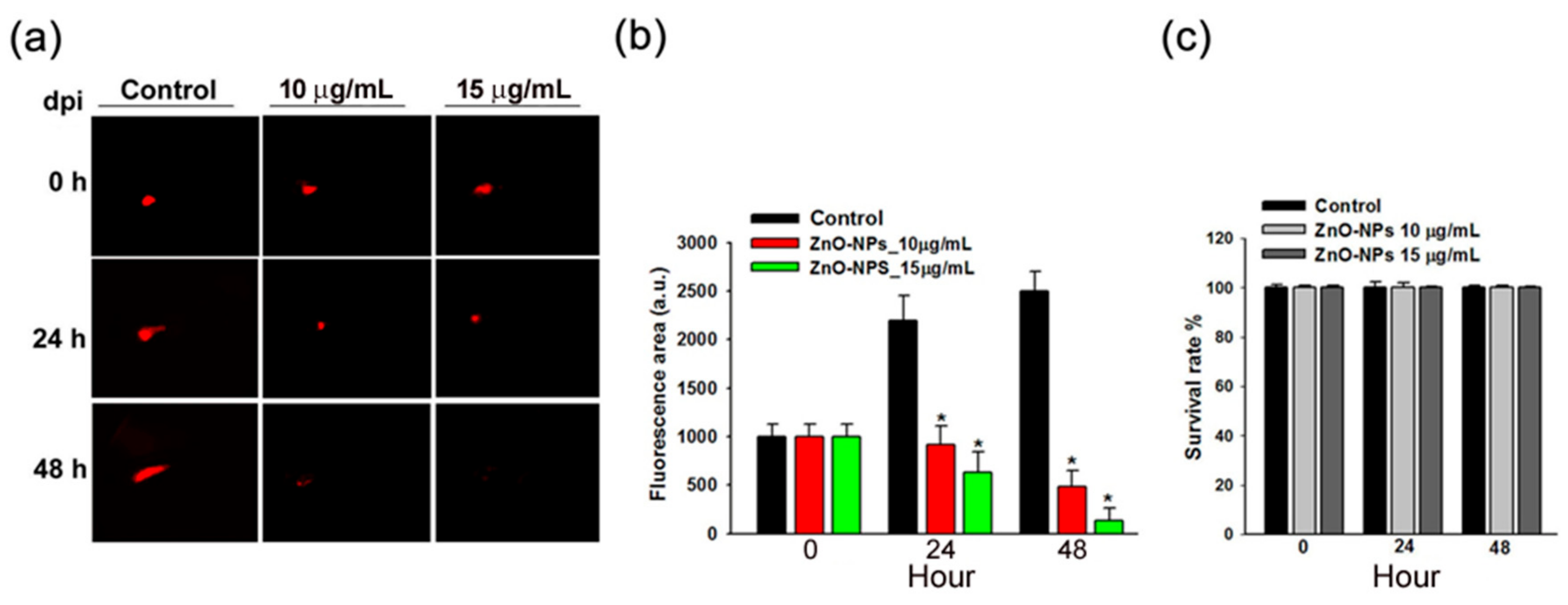
2.7. ZnO-NPs Impeded Tumor Growth of Gingival Squamous Cell Carcinomas in Vivo
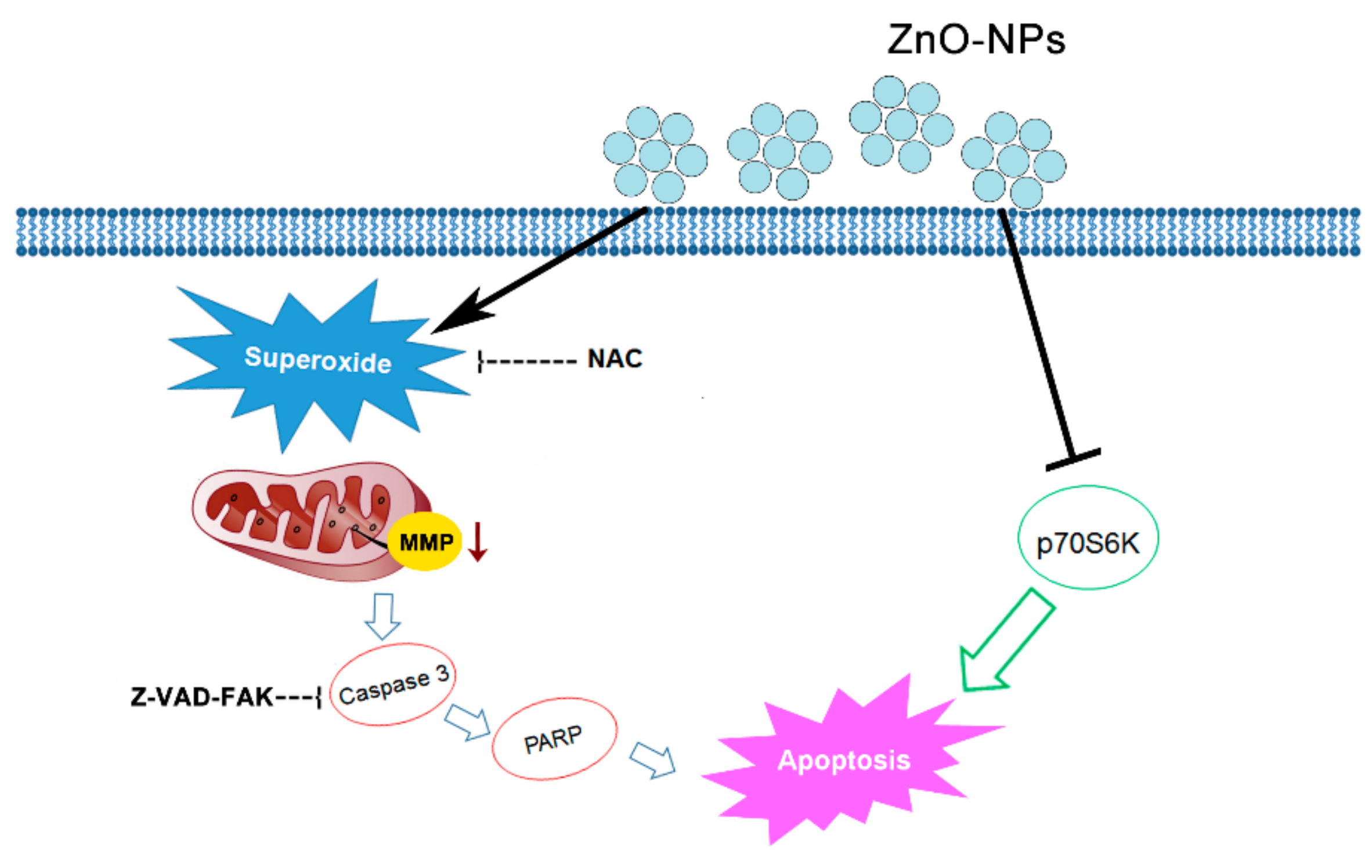
3. Discussion
4. Materials and Methods
4.1. Particle and Physicochemical Properties
4.2. Cell Culture
4.3. Cell Viability Assay
4.4. Cell Cycle Analysis
4.5. Measurement of ROS and Superoxide Production
4.6. Measurement of Intracellular ROS Content
4.7. Determination of Apoptosis
4.8. Mitochondrial Membrane Potential (MMP) Analysis
4.9. Western Blot Analysis
4.10. Detection of MMP By JC-1 Staining
4.11. Zebrafish Xenograft Assay
4.12. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ZnO-NPs | Zinc oxide nanoparticles |
| GSCC | Gingival squamous cell carcinoma |
| CCCP | Carbonyl cyanide m-chlorophenyl hydrazone |
| FCCP | Carbonyl cyanide-4-(trifluoromethoxy)phenylhydrazone |
| NAC | N-acetyl-L-cysteine |
| MMP | Mitochondrial membrane potential |
| PARP | Poly-(ADP-ribose) polymerase |
References
- Goud, E.; Malleedi, S.; Ramanathan, A.; Wong, G.R.; Hwei Ern, B.T.; Yean, G.Y.; Ann, H.H.; Syan, T.Y.; Zain, R.M. Association of Interleukin-10 Genotypes and Oral Cancer Susceptibility in Selected Malaysian Population: A Case- Control Study. Asian Pac. J. Cancer Prev. 2019, 20, 935–941. [Google Scholar] [CrossRef] [PubMed]
- Sagheb, K.; Kumar, V.; Rahimi-Nedjat, R.; Dollhausen, M.; Ziebart, T.; Al-Nawas, B.; Walter, C. Cervical Metastases Behavior of T1-2 Squamous Cell Carcinoma of the Tongue. J. Maxillofac. Oral. Surg. 2017, 16, 300–305. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.Y.; Wang, D.C.; Su, J.Z.; Jia, L.F.; Peng, X.; Yu, G.Y. Clinicopathological characteristics and outcomes of squamous cell carcinoma of the tongue in different age groups. Head Neck 2017, 39, 2276–2282. [Google Scholar] [CrossRef] [PubMed]
- Nassiri, A.M.; Campbell, B.R.; Mannion, K.; Sinard, R.J.; Netterville, J.L.; Rohde, S.L. Survival Outcomes in T4aN0M0 Mandibular Gingival Squamous Cell Carcinoma Treated with Surgery Alone. Otolaryngol. Head Neck Surg. 2019, 160, 870–875. [Google Scholar] [CrossRef]
- Ramesh, R.; Sadasivan, A. Oral squamous cell carcinoma masquerading as gingival overgrowth. Eur. J. Dent. 2017, 11, 390–394. [Google Scholar] [CrossRef]
- Bharanidharan, R.; Dineshkumar, T.; Raghavendhar, K.; Kumar, A.R. Squamous cell carcinoma of the gingiva: A diagnostic enigma. J. Oral. Maxillofac. Pathol. 2015, 19, 267. [Google Scholar] [CrossRef]
- Sun, W.; Bao, J.; Lin, W.; Gao, H.; Zhao, W.; Zhang, Q.; Leung, C.H.; Ma, D.L.; Lu, J.; Chen, X. 2-Methoxy-6-acetyl-7-methyljuglone (MAM), a natural naphthoquinone, induces NO-dependent apoptosis and necroptosis by H2O2-dependent JNK activation in cancer cells. Free Radic. Biol. & Med. 2016, 92, 61–77. [Google Scholar]
- Yu, C.I.; Chen, C.Y.; Liu, W.; Chang, P.C.; Huang, C.W.; Han, K.F.; Lin, I.P.; Lin, M.Y.; Lee, C.H. Sandensolide Induces Oxidative Stress-Mediated Apoptosis in Oral Cancer Cells and in Zebrafish Xenograft Model. Mar Drugs 2018, 16, 387. [Google Scholar] [CrossRef]
- Lee, C.H.; Huang, C.W.; Chang, P.C.; Shiau, J.P.; Lin, I.P.; Lin, M.Y.; Lai, C.C.; Chen, C.Y. Reactive oxygen species mediate the chemopreventive effects of syringin in breast cancer cells. Phytomedicine 2019, 61, 152844. [Google Scholar] [CrossRef]
- Chen, P.; Stone, J.; Sullivan, G.; Drisko, J.A.; Chen, Q. Anti-cancer effect of pharmacologic ascorbate and its interaction with supplementary parenteral glutathione in preclinical cancer models. Free Radic. Biol. Med. 2011, 51, 681–687. [Google Scholar]
- Cho, H.D.; Lee, J.H.; Moon, K.D.; Park, K.H.; Lee, M.K.; Seo, K.I. Auriculasin-induced ROS causes prostate cancer cell death via induction of apoptosis. Food Chem. Toxicol. 2018, 111, 660–669. [Google Scholar] [CrossRef] [PubMed]
- Memarzadeh, K.; Sharili, A.S.; Huang, J.; Rawlinson, S.C.; Allaker, R.P. Nanoparticulate zinc oxide as a coating material for orthopedic and dental implants. J. Biomed. Mater. Res. A 2015, 103, 981–989. [Google Scholar] [CrossRef]
- Javidi, M.; Zarei, M.; Naghavi, N.; Mortazavi, M.; Nejat, A.H. Zinc oxide nano-particles as sealer in endodontics and its sealing ability. Contemp. Clin. Dent. 2014, 5, 20–24. [Google Scholar] [PubMed]
- Osmond, M.J.; McCall, M.J. Zinc oxide nanoparticles in modern sunscreens: An analysis of potential exposure and hazard. Nanotoxicology 2010, 4, 15–41. [Google Scholar] [CrossRef]
- Hassan, H.F.; Mansour, A.M.; Abo-Youssef, A.M.; Elsadek, B.E.; Messiha, B.A. Zinc oxide nanoparticles as a novel anticancer approach; in vitro and in vivo evidence. Clin. Exp. Pharmacol. Physiol. 2017, 44, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Gehrke, T.; Scherzad, A.; Ickrath, P.; Schendzielorz, P.; Hagen, R.; Kleinsasser, N.; Hackenberg, S. Zinc oxide nanoparticles antagonize the effect of Cetuximab on head and neck squamous cell carcinoma in vitro. Cancer Biol. Ther. 2017, 18, 513–518. [Google Scholar] [CrossRef]
- Yin, H.; Casey, P.S.; McCall, M.J.; Fenech, M. Size-dependent cytotoxicity and genotoxicity of ZnO particles to human lymphoblastoid (WIL2-NS) cells. Environ. Mol. Mutagen. 2015, 56, 767–776. [Google Scholar] [CrossRef]
- Wang, J.; Lee, J.S.; Kim, D.; Zhu, L. Exploration of Zinc Oxide Nanoparticles as a Multitarget and Multifunctional Anticancer Nanomedicine. ACS Appl. Mater. Interfaces 2017, 9, 39971–39984. [Google Scholar] [CrossRef]
- An, W.; Lai, H.; Zhang, Y.; Liu, M.; Lin, X.; Cao, S. Apoptotic Pathway as the Therapeutic Target for Anticancer Traditional Chinese Medicines. Front. Pharmacol. 2019, 10, 758. [Google Scholar] [CrossRef]
- Wiesmann, N.; Kluenker, M.; Demuth, P.; Brenner, W.; Tremel, W.; Brieger, J. Zinc overload mediated by zinc oxide nanoparticles as innovative anti-tumor agent. J. Trace Elem. Med. Biol. 2019, 51, 226–234. [Google Scholar] [CrossRef]
- Wingett, D.; Louka, P.; Anders, C.B.; Zhang, J.; Punnoose, A. A role of ZnO nanoparticle electrostatic properties in cancer cell cytotoxicity. Nanotechnol. Sci. Appl. 2016, 9, 29–45. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, M.J.; Ahamed, M.; Kumar, S.; Khan, M.M.; Ahmad, J.; Alrokayan, S.A. Zinc oxide nanoparticles selectively induce apoptosis in human cancer cells through reactive oxygen species. Int. J. Nanomedicine 2012, 7, 845–857. [Google Scholar]
- Olsson, M.; Zhivotovsky, B. Caspases and cancer. Cell Death Differ. 2011, 18, 1441–1449. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, S.; John, S.; Sapra, L.; Sharma, S.C.; Das, S.N. Targeted disruption of PI3K/Akt/mTOR signaling pathway, via PI3K inhibitors, promotes growth inhibitory effects in oral cancer cells. Cancer Chemother. Pharmacol. 2019, 83, 451–461. [Google Scholar] [CrossRef] [PubMed]
- Tan, F.H.; Bai, Y.; Saintigny, P.; Darido, C. mTOR Signalling in Head and Neck Cancer: Heads Up. Cells 2019, 8, 333. [Google Scholar] [CrossRef]
- El-Shorbagy, H.M.; Eissa, S.M.; Sabet, S.; El-Ghor, A.A. Apoptosis and oxidative stress as relevant mechanisms of antitumor activity and genotoxicity of ZnO-NPs alone and in combination with N-acetyl cysteine in tumor-bearing mice. Int. J. Nanomedicine 2019, 14, 3911–3928. [Google Scholar] [CrossRef]
- Moos, P.J.; Chung, K.; Woessner, D.; Honeggar, M.; Cutler, N.S.; Veranth, J.M. ZnO particulate matter requires cell contact for toxicity in human colon cancer cells. Chem. Res. Toxicol. 2010, 23, 733–739. [Google Scholar] [CrossRef]
- Bisht, G.; Rayamajhi, S. ZnO Nanoparticles: A Promising Anticancer Agent. Nanobiomedicine 2016, 3, 9. [Google Scholar] [CrossRef]
- Carmody, R.J.; Cotter, T.G. Signalling apoptosis: A radical approach. Redox Rep. 2001, 6, 77–90. [Google Scholar] [CrossRef]
- Li, D.; Ueta, E.; Kimura, T.; Yamamoto, T.; Osaki, T. Reactive oxygen species (ROS) control the expression of Bcl-2 family proteins by regulating their phosphorylation and ubiquitination. Cancer Sci. 2004, 95, 644–650. [Google Scholar] [CrossRef]
- Wang, T.L.; Ouyang, C.S.; Lin, L.Z. beta-Asarone suppresses Wnt/beta-catenin signaling to reduce viability, inhibit migration/invasion/adhesion and induce mitochondria-related apoptosis in lung cancer cells. Biomed. Pharmacother. 2018, 106, 821–830. [Google Scholar] [CrossRef] [PubMed]
- Mohammadinejad, R.; Moosavi, M.A.; Tavakol, S.; Vardar, D.O.; Hosseini, A.; Rahmati, M.; Dini, L.; Hussain, S.; Mandegary, A.; Klionsky, D.J. Necrotic, apoptotic and autophagic cell fates triggered by nanoparticles. Autophagy 2019, 15, 4–33. [Google Scholar] [CrossRef] [PubMed]
- De Berardis, B.; Civitelli, G.; Condello, M.; Lista, P.; Pozzi, R.; Arancia, G.; Meschini, S. Exposure to ZnO nanoparticles induces oxidative stress and cytotoxicity in human colon carcinoma cells. Toxicol. Appl. Pharmacol. 2010, 246, 116–127. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Ghosh, S.; Chandna, S. Evidence for microRNA-31 dependent Bim-Bax interaction preceding mitochondrial Bax translocation during radiation-induced apoptosis. Sci. Rep. 2015, 5, 15923. [Google Scholar] [CrossRef] [PubMed]
- Jia, L.; Patwari, Y.; Srinivasula, S.M.; Newland, A.C.; Fernandes-Alnemri, T.; Alnemri, E.S.; Kelsey, S.M. Bax translocation is crucial for the sensitivity of leukaemic cells to etoposide-induced apoptosis. Oncogene 2001, 20, 4817–4826. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Park, M.T.; Kang, J.A.; Choi, J.A.; Kang, C.M.; Kim, T.H.; Bae, S.; Kang, S.; Kim, S.; Choi, W.I.; Cho, C.K.; et al. Phytosphingosine induces apoptotic cell death via caspase 8 activation and Bax translocation in human cancer cells. Clin. Cancer Res. 2003, 9, 878–885. [Google Scholar]
- Buranrat, B.; Bootha, S. Antiproliferative and antimigratory activities of bisphosphonates in human breast cancer cell line MCF-7. Oncol. Lett. 2019, 18, 1246–1258. [Google Scholar] [CrossRef]
- Day, T.A.; Shirai, K.; O’Brien, P.E.; Matheus, M.G.; Godwin, K.; Sood, A.J.; Kompelli, A.; Vick, J.A.; Martin, D.; Vitale-Cross, L.; et al. Inhibition of mTOR Signaling and Clinical Activity of Rapamycin in Head and Neck Cancer in a Window of Opportunity Trial. Clin. Cancer Res. 2019, 25, 1156–1164. [Google Scholar] [CrossRef]
- Varghese, E.; Samuel, S.M.; Sadiq, Z.; Kubatka, P.; Liskova, A.; Benacka, J.; Pazinka, P.; Kruzliak, P.; Busselberg, D. Anti-Cancer Agents in Proliferation and Cell Death: The Calcium Connection. Int. J. Mol. Sci. 2019, 20, 3017. [Google Scholar] [CrossRef]
- Wei, X.; Luo, L.; Chen, J. Roles of mTOR Signaling in Tissue Regeneration. Cells 2019, 8, 1075. [Google Scholar] [CrossRef] [PubMed]
- Feigin, M.E.; Akshinthala, S.D.; Araki, K.; Rosenberg, A.Z.; Muthuswamy, L.B.; Martin, B.; Lehmann, B.D.; Berman, H.K.; Pietenpol, J.A.; Cardiff, R.D.; et al. Mislocalization of the cell polarity protein scribble promotes mammary tumorigenesis and is associated with basal breast cancer. Cancer Res. 2014, 74, 3180–3194. [Google Scholar] [CrossRef] [PubMed]
- Vivanco, I.; Sawyers, C.L. The phosphatidylinositol 3-Kinase AKT pathway in human cancer. Nat. Rev. Cancer 2002, 2, 489–501. [Google Scholar] [CrossRef] [PubMed]
- Jiang, K.; Chen, H.; Tang, K.; Guan, W.; Zhou, H.; Guo, X.; Chen, Z.; Ye, Z.; Xu, H. Puerarin inhibits bladder cancer cell proliferation through the mTOR/p70S6K signaling pathway. Oncol. Lett. 2018, 15, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, X.; Jia, Y.; Liu, Y. Effects of bufalin on the mTOR/p70S6K pathway and apoptosis in esophageal squamous cell carcinoma in nude mice. Int. J. Mol. Med. 2017, 40, 357–366. [Google Scholar] [CrossRef]
- Zhou, H.; Zhao, H.; Liu, H.; Xu, X.; Dong, X.; Zhao, E. Influence of carboplatin on the proliferation and apoptosis of ovarian cancer cells through mTOR/p70s6k signaling pathway. J BUON 2018, 23, 1732–1738. [Google Scholar]
- Qiu, Z.X.; Sun, R.F.; Mo, X.M.; Li, W.M. The p70S6K Specific Inhibitor PF-4708671 Impedes Non-Small Cell Lung Cancer Growth. PLoS ONE 2016, 11, e0147185. [Google Scholar] [CrossRef]
- Volarevic, S.; Thomas, G. Role of S6 phosphorylation and S6 kinase in cell growth. Prog. Nucleic Acid Res. Mol. Biol. 2001, 65, 101–127. [Google Scholar]
- Wu, D.; Cheng, J.; Sun, G.; Wu, S.; Li, M.; Gao, Z.; Zhai, S.; Li, P.; Su, D.; Wang, X. p70S6K promotes IL-6-induced epithelial-mesenchymal transition and metastasis of head and neck squamous cell carcinoma. Oncotarget 2016, 7, 36539–36550. [Google Scholar] [CrossRef]
- Chien, M.H.; Lee, T.H.; Lee, W.J.; Yeh, Y.H.; Li, T.K.; Wang, P.C.; Chen, J.J.; Chow, J.M.; Lin, Y.W.; Hsiao, M.; et al. Trichodermin induces c-Jun N-terminal kinase-dependent apoptosis caused by mitotic arrest and DNA damage in human p53-mutated pancreatic cancer cells and xenografts. Cancer Lett. 2017, 388, 249–261. [Google Scholar] [CrossRef]
- Wang, G.S.; Shen, Y.S.; Chou, W.Y.; Tang, C.H.; Yeh, H.I.; Wang, L.Y.; Yen, J.Y.; Huang, T.Y.; Liu, S.C.; Yang, C.Y.; et al. Senescence Induces Dysfunctions in Endothelial Progenitor Cells and Osteoblasts by Interfering Translational Machinery and Bioenergetic Homeostasis. Int. J. Mol. Sci. 2018, 19, 1997. [Google Scholar] [CrossRef]
- Tai, H.C.; Lee, T.H.; Tang, C.H.; Chen, L.P.; Chen, W.C.; Lee, M.S.; Chen, P.C.; Lin, C.Y.; Chi, C.W.; Chen, Y.J.; et al. Phomaketide A Inhibits Lymphangiogenesis in Human Lymphatic Endothelial Cells. Mar. Drugs 2019, 17, 215. [Google Scholar] [CrossRef] [PubMed]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.-W.; Lee, C.-H.; Lin, M.-S.; Chi, C.-W.; Chen, Y.-J.; Wang, G.-S.; Liao, K.-W.; Chiu, L.-P.; Wu, S.-H.; Huang, D.-M.; et al. ZnO Nanoparticles Induced Caspase-Dependent Apoptosis in Gingival Squamous Cell Carcinoma through Mitochondrial Dysfunction and p70S6K Signaling Pathway. Int. J. Mol. Sci. 2020, 21, 1612. https://doi.org/10.3390/ijms21051612
Wang S-W, Lee C-H, Lin M-S, Chi C-W, Chen Y-J, Wang G-S, Liao K-W, Chiu L-P, Wu S-H, Huang D-M, et al. ZnO Nanoparticles Induced Caspase-Dependent Apoptosis in Gingival Squamous Cell Carcinoma through Mitochondrial Dysfunction and p70S6K Signaling Pathway. International Journal of Molecular Sciences. 2020; 21(5):1612. https://doi.org/10.3390/ijms21051612
Chicago/Turabian StyleWang, Shih-Wei, Chien-Hsing Lee, Ming-Shen Lin, Chih-Wen Chi, Yu-Jen Chen, Guo-Shou Wang, Kuang-Wen Liao, Li-Pin Chiu, Shu-Hui Wu, Dong-Ming Huang, and et al. 2020. "ZnO Nanoparticles Induced Caspase-Dependent Apoptosis in Gingival Squamous Cell Carcinoma through Mitochondrial Dysfunction and p70S6K Signaling Pathway" International Journal of Molecular Sciences 21, no. 5: 1612. https://doi.org/10.3390/ijms21051612
APA StyleWang, S.-W., Lee, C.-H., Lin, M.-S., Chi, C.-W., Chen, Y.-J., Wang, G.-S., Liao, K.-W., Chiu, L.-P., Wu, S.-H., Huang, D.-M., Chen, L., & Shen, Y.-S. (2020). ZnO Nanoparticles Induced Caspase-Dependent Apoptosis in Gingival Squamous Cell Carcinoma through Mitochondrial Dysfunction and p70S6K Signaling Pathway. International Journal of Molecular Sciences, 21(5), 1612. https://doi.org/10.3390/ijms21051612





