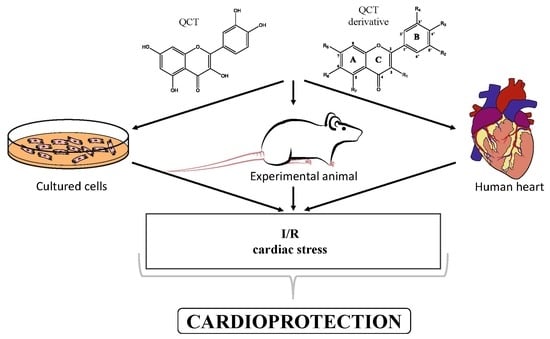
Potential Implications of Quercetin and its Derivatives in Cardioprotection
Abstract
1. Introduction
2. QCT and its Derivatives: Structure, Sources, Metabolism, Bioavailability
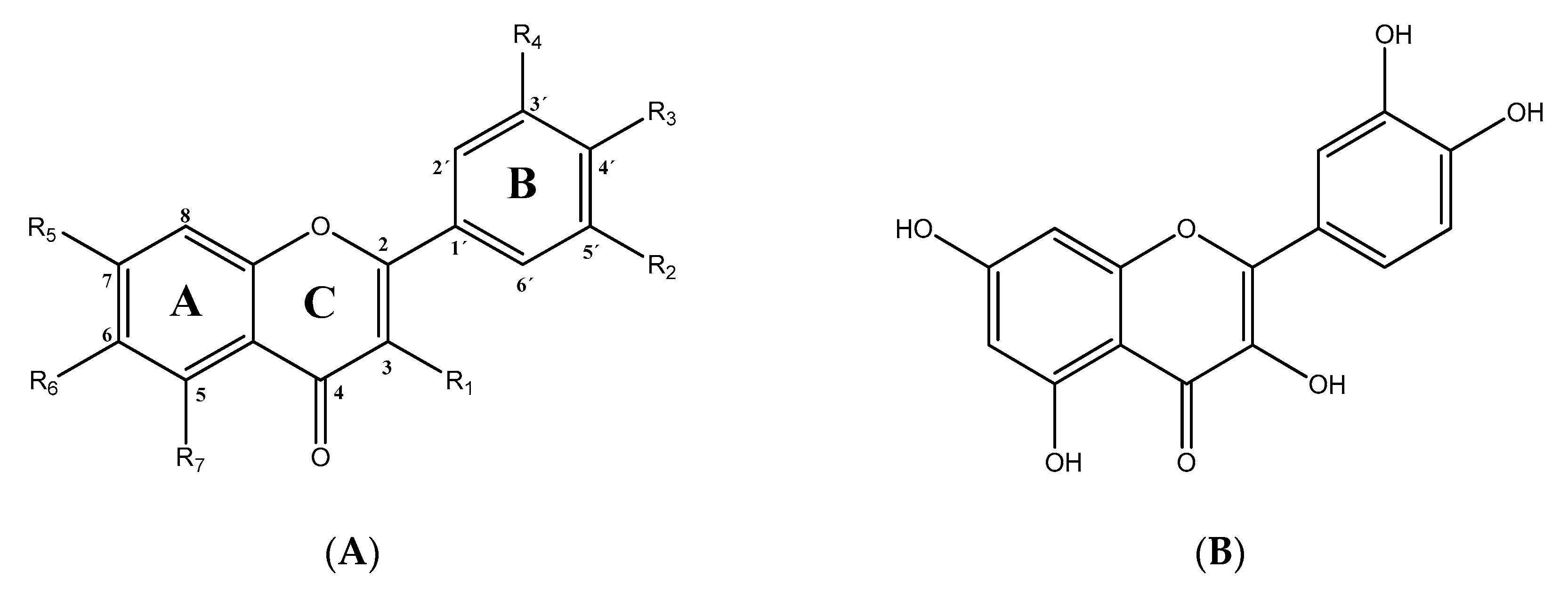
2.1. Chemistry of QCT and its Derivatives
2.1.1. QCT-O-Glycosides
2.1.2. QCT-C-Glycosides
2.1.3. QCT Ethers
2.1.4. Alkyl-Containing QCT Derivatives (Prenyls)
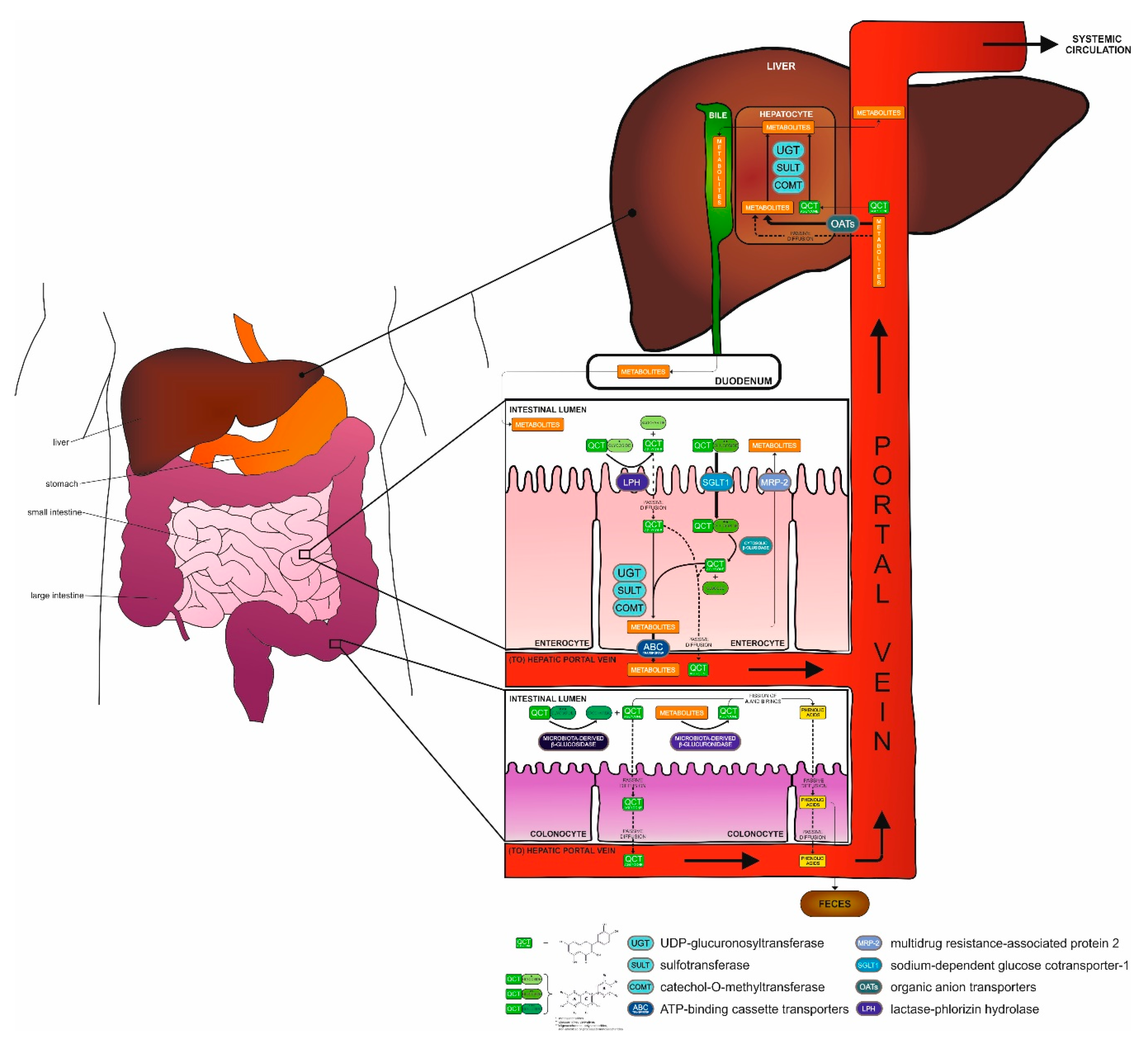
2.2. Metabolization of QCT in the Body
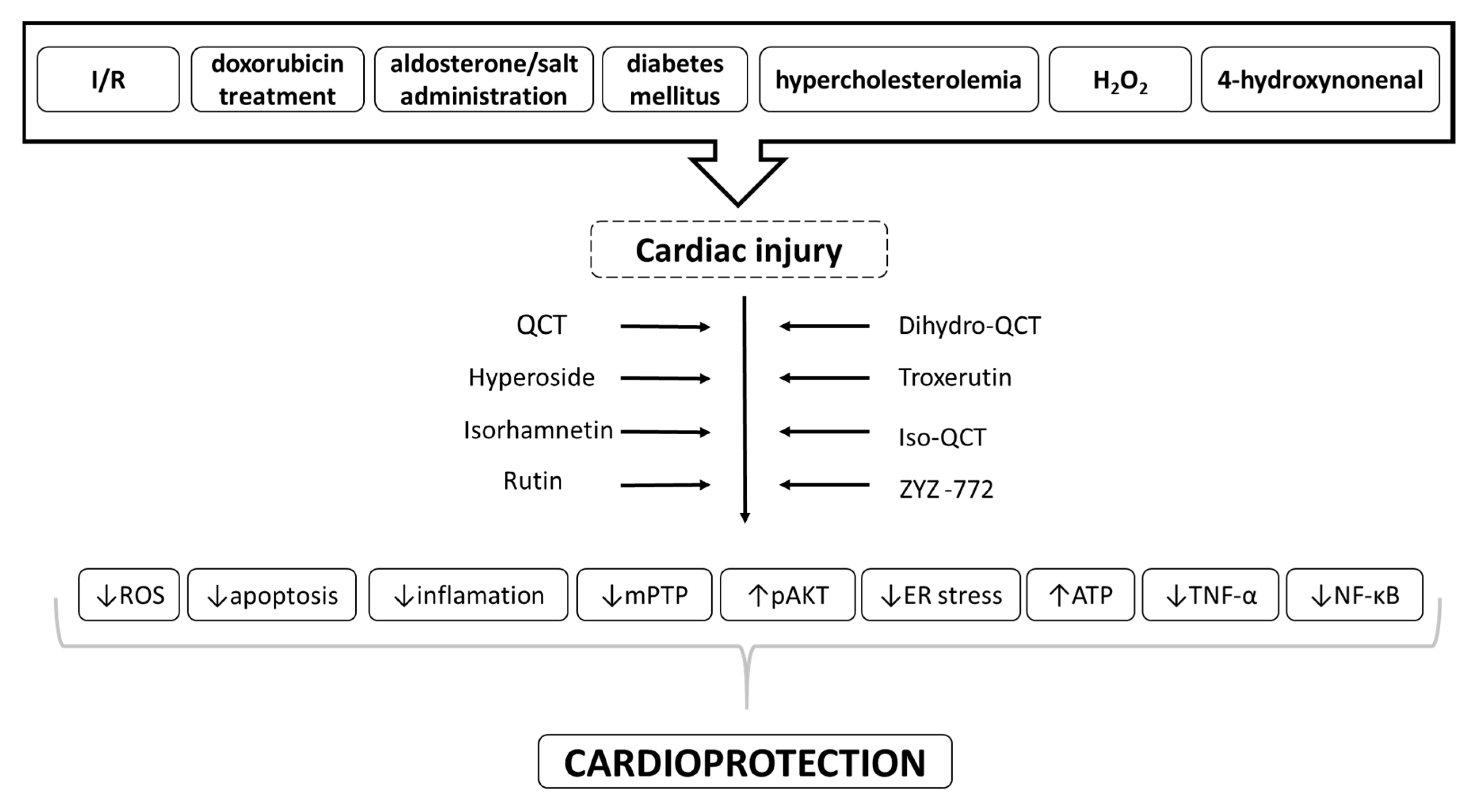
3. QCT and its Derivatives as Cardioprotective Agents
3.1. In Vitro and Ex Vivo Cardioprotection Afforded by QCT and QCT-Rich Plants
3.2. In Vitro Cardioprotection Afforded by QCT Derivatives
3.3. In Vivo Cardioprotection Afforded by QCT and QCT-Rich Plants
3.4. In Vivo Cardioprotection Afforded by QCT Derivatives
4. Role of Comorbidities in Cardioprotection by QCT and its Derivatives
5. Cardiovascular Effects of QCT in Human Studies and Clinical Trials
6. Controversial Findings and Potential Cardiotoxic Effects of QCT and its Derivatives
7. Conclusions and Future Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lovegrove, J.A.; Stainer, A.; Hobbs, D.A. Role of flavonoids and nitrates in cardiovascular health. Proc. Nutr. Soc. 2017, 76, 83–95. [Google Scholar] [CrossRef]
- Hussain, T.; Tan, B.; Murtaza, G.; Liu, G.; Rahu, N.; Saleem Kalhoro, M.; Hussain Kalhoro, D.; Adebowale, T.O.; Usman Mazhar, M.; ur Rehman, Z.; et al. Flavonoids and type 2 diabetes: Evidence of efficacy in clinical and animal studies and delivery strategies to enhance their therapeutic efficacy. Pharmacol. Res. 2020, 152, 104629. [Google Scholar] [CrossRef] [PubMed]
- Maaliki, D.; Shaito, A.A.; Pintus, G.; El-Yazbi, A.; Eid, A.H. Flavonoids in hypertension: A brief review of the underlying mechanisms. Curr. Opin. Pharmacol. 2019, 45, 57–65. [Google Scholar] [CrossRef]
- Khan, H.; Ullah, H.; Martorell, M.; Valdes, S.E.; Belwal, T.; Tejada, S.; Sureda, A.; Kamal, M.A. Flavonoids nanoparticles in cancer: Treatment, prevention and clinical prospects. Semin. Cancer Biol. 2019, 0–1. [Google Scholar] [CrossRef] [PubMed]
- D’Andrea, G. Quercetin: A flavonol with multifaceted therapeutic applications? Fitoterapia 2015, 106, 256–271. [Google Scholar] [CrossRef] [PubMed]
- Miles, S.L.; McFarland, M.; Niles, R.M. Molecular and physiological actions of quercetin: Need for clinical trials to assess its benefits in human disease. Nutr. Rev. 2014, 72, 720–734. [Google Scholar] [CrossRef] [PubMed]
- Hashemzaei, M.; Far, A.D.; Yari, A.; Heravi, R.E.; Tabrizian, K.; Taghdisi, S.M.; Sadegh, S.E.; Tsarouhas, K.; Kouretas, D.; Tzanakakis, G.; et al. Anticancer and apoptosis-inducing effects of quercetin in vitro and in vivo. Oncol. Rep. 2017, 38, 819–828. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Song, Y.; Liang, Y.; Li, R. Quercetin Treatment Improves Renal Function and Protects the Kidney in a Rat Model of Adenine-Induced Chronic Kidney Disease. Med. Sci. Monit. 2018, 24, 4760–4766. [Google Scholar] [CrossRef]
- Brüll, V.; Burak, C.; Stoffel-Wagner, B.; Wolffram, S.; Nickenig, G.; Müller, C.; Langguth, P.; Alteheld, B.; Fimmers, R.; Naaf, S.; et al. Effects of a quercetin-rich onion skin extract on 24 h ambulatory blood pressure and endothelial function in overweight-to-obese patients with (pre-)hypertension: A randomised double-blinded placebo-controlled cross-over trial. Br. J. Nutr. 2015, 114, 1263–1277. [Google Scholar] [CrossRef]
- Calabró, V.; Litterio, M.C.; Fraga, C.G.; Galleano, M.; Piotrkowski, B. Effects of quercetin on heart nitric oxide metabolism in l-NAME treated rats. Arch. Biochem. Biophys. 2018, 647, 47–53. [Google Scholar] [CrossRef]
- Kim, S.G.; Kim, J.R.; Choi, H.C. Quercetin-induced AMP-activated protein kinase activation attenuates vasoconstriction through LKB1-AMPK signaling pathway. J. Med. Food 2018, 21, 146–153. [Google Scholar] [CrossRef] [PubMed]
- Pereira, S.C.; Parente, J.M.; Belo, V.A.; Mendes, A.S.; Gonzaga, N.A.; do Vale, G.T.; Ceron, C.S.; Tanus-Santos, J.E.; Tirapelli, C.R.; Castro, M.M. Quercetin decreases the activity of matrix metalloproteinase-2 and ameliorates vascular remodeling in renovascular hypertension. Atherosclerosis 2018, 270, 146–153. [Google Scholar] [CrossRef] [PubMed]
- Barteková, M.; Čarnická, S.; Pancza, D.; Ondrejčáková, M.; Breier, A.; Ravingerová, T. Acute treatment with polyphenol quercetin improves postischemic recovery of isolated perfused rat hearts after global ischemia. Can. J. Physiol. Pharmacol. 2010, 88, 465–471. [Google Scholar] [CrossRef] [PubMed]
- Barteková, M.; Šimončíková, P.; Fogarassyová, M.; Ivanová, M.; Okruhlicová, Ľ.; Tribulová, N.; Dovinová, I.; Barančík, M. Quercetin Improves Postischemic Recovery of Heart Function in Doxorubicin-Treated Rats and Prevents Doxorubicin-Induced Matrix Metalloproteinase-2 Activation and Apoptosis Induction. Int. J. Mol. Sci. 2015, 16, 8168–8185. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, Z.Z.; Wu, Y.; Ke, J.J.; He, X.H.; Wang, Y.L. Quercetin postconditioning attenuates myocardial ischemia/reperfusion injury in rats through the PI3K/Akt pathway. Braz. J. Med. Biol. Res. 2013, 46, 861–867. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Guo, X.; Chu, Y.; Lu, S. Heart protective effects and mechanism of quercetin preconditioning on anti-myocardial ischemia reperfusion (IR) injuries in rats. Gene 2014, 545, 149–155. [Google Scholar] [CrossRef]
- Castillo, R.L.; Herrera, E.A.; Gonzalez-Candia, A.; Reyes-Farias, M.; de la Jara, N.; Peña, J.P.; Carrasco-Pozo, C. Quercetin Prevents Diastolic Dysfunction Induced by a High-Cholesterol Diet: Role of Oxidative Stress and Bioenergetics in Hyperglycemic Rats. Oxid. Med. Cell. Longev. 2018, 2018, 1–14. [Google Scholar] [CrossRef]
- Li, Y.; Yao, J.; Han, C.; Yang, J.; Chaudhry, M.T.; Wang, S.; Liu, H.; Yin, Y. Quercetin, Inflammation and Immunity. Nutrients 2016, 8, 167. [Google Scholar] [CrossRef]
- Anand David, A.; Arulmoli, R.; Parasuraman, S. Overviews of biological importance of quercetin: A bioactive flavonoid. Pharmacogn. Rev. 2016, 10, 84. [Google Scholar]
- Kaşıkcı, M.; Bağdatlıoğlu, N. Bioavailability of Quercetin. Curr. Res. Nutr. Food Sci. J. 2016, 4, 146–151. [Google Scholar] [CrossRef]
- Gao, L.; Liu, G.; Wang, X.; Liu, F.; Xu, Y.; Ma, J. Preparation of a chemically stable quercetin formulation using nanosuspension technology. Int. J. Pharm. 2011, 404, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Konrad, M.; Nieman, D.C. Evaluation of Quercetin as a Countermeasure to Exercise-Induced Physiological Stress; Lamprecht, M., Ed.; CRC Press/Taylor & Francis: Abingdon, UK, 2015; ISBN 9781466567573. [Google Scholar]
- Cai, X.; Fang, Z.; Dou, J.; Yu, A.; Zhai, G. Bioavailability of Quercetin: Problems and Promises. Curr. Med. Chem. 2013, 20, 2572–2582. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Pandey, A.K. Chemistry and Biological Activities of Flavonoids: An Overview. Sci. World J. 2013, 2013, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Materska, M. Quercetin and its derivatives: Chemical structure and bioactivity—A review. Pol. J. FOOD Nutr. Sci. 2008, 58, 407–413. [Google Scholar]
- Makino, T.; Shimizu, R.; Kanemaru, M.; Suzuki, Y.; Moriwaki, M.; Mizukami, H. Enzymatically Modified Isoquercitrin, α-Oligoglucosyl Quercetin 3-O-Glucoside, Is Absorbed More Easily than Other Quercetin Glycosides or Aglycone after Oral Administration in Rats. Biol. Pharm. Bull. 2009, 32, 2034–2040. [Google Scholar] [CrossRef]
- Yang, B.; Liu, H.; Yang, J.; Gupta, V.K.; Jiang, Y. New insights on bioactivities and biosynthesis of flavonoid glycosides. Trends Food Sci. Technol. 2018, 79, 116–124. [Google Scholar] [CrossRef]
- Ferreres, F.; Figueiredo, R.; Bettencourt, S.; Carqueijeiro, I.; Oliveira, J.; Gil-Izquierdo, A.; Pereira, D.M.; Valentão, P.; Andrade, P.B.; Duarte, P.; et al. Identification of phenolic compounds in isolated vacuoles of the medicinal plant Catharanthus roseus and their interaction with vacuolar class III peroxidase: An H2O2 affair? J. Exp. Bot. 2011, 62, 2841–2854. [Google Scholar] [CrossRef]
- Wang, W.; Sun, C.; Mao, L.; Ma, P.; Liu, F.; Yang, J.; Gao, Y. The biological activities, chemical stability, metabolism and delivery systems of quercetin: A review. Trends Food Sci. Technol. 2016, 56, 21–38. [Google Scholar] [CrossRef]
- Lesjak, M.; Beara, I.; Simin, N.; Pintać, D.; Majkić, T.; Bekvalac, K.; Orčić, D.; Mimica-Dukić, N. Antioxidant and anti-inflammatory activities of quercetin and its derivatives. J. Funct. Foods 2018, 40, 68–75. [Google Scholar] [CrossRef]
- Lakhanpal, P.; Rai, D.K. Quercetin: A Versatile Flavonoid. Internet J. Med. Updat. EJOURNAL 2007, 2. [Google Scholar] [CrossRef]
- Berardini, N.; Fezer, R.; Conrad, J.; Beifuss, U.; Carle, R.; Schieber, A. Screening of Mango (Mangifera indica L.) Cultivars for Their Contents of Flavonol O - and Xanthone C -Glycosides, Anthocyanins, and Pectin. J. Agric. Food Chem. 2005, 53, 1563–1570. [Google Scholar] [CrossRef]
- Zheng, W.; Wang, S.Y. Oxygen Radical Absorbing Capacity of Phenolics in Blueberries, Cranberries, Chokeberries, and Lingonberries. J. Agric. Food Chem. 2003, 51, 502–509. [Google Scholar] [CrossRef]
- Kuti, J.O.; Konuru, H.B. Antioxidant Capacity and Phenolic Content in Leaf Extracts of Tree Spinach (Cnidoscolus spp.). J. Agric. Food Chem. 2004, 52, 117–121. [Google Scholar] [CrossRef] [PubMed]
- Chang, Q.; Wong, Y.-S. Identification of Flavonoids in Hakmeitau Beans (Vigna sinensis) by High-Performance Liquid Chromatography−Electrospray Mass Spectrometry (LC-ESI/MS). J. Agric. Food Chem. 2004, 52, 6694–6699. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.-O.; Chun, O.K.; Kim, Y.J.; Moon, H.-Y.; Lee, C.Y. Quantification of Polyphenolics and Their Antioxidant Capacity in Fresh Plums. J. Agric. Food Chem. 2003, 51, 6509–6515. [Google Scholar] [CrossRef] [PubMed]
- Nemeth, K.; Piskula, M.K. Food Content, Processing, Absorption and Metabolism of Onion Flavonoids. Crit. Rev. Food Sci. Nutr. 2007, 47, 397–409. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, B.; Landbo, A.-K.; Knudsen, D.; Silva, A.P.; Moutinho-Pereira, J.; Rosa, E.; Meyer, A.S. Effect of Ripeness and Postharvest Storage on the Phenolic Profiles of Cherries (Prunus avium L.). J. Agric. Food Chem. 2004, 52, 523–530. [Google Scholar] [CrossRef]
- Slimestad, R.; Verheul, M.J. Seasonal Variations in the Level of Plant Constituents in Greenhouse Production of Cherry Tomatoes. J. Agric. Food Chem. 2005, 53, 3114–3119. [Google Scholar] [CrossRef]
- Oomah, B.D.; Mazza, G. Flavonoids and Antioxidative Activities in Buckwheat. J. Agric. Food Chem. 1996, 44, 1746–1750. [Google Scholar] [CrossRef]
- Price, K.R.; Casuscelli, F.; Colquhoun, I.J.; Rhodes, M.J.C. Composition and Content of Flavonol Gl y cosides in Broccoli Florets (Brassica olearacea) and their Fate during Cooking. J. Sci. Food Agric. 1998, 468, 468–472. [Google Scholar] [CrossRef]
- Materska, M.; Perucka, I. Antioxidant Activity of the Main Phenolic Compounds Isolated from Hot Pepper Fruit (Capsicum annuum L.). J. Agric. Food Chem. 2005, 53, 1750–1756. [Google Scholar] [CrossRef]
- Jürgenliemk, G.; Nahrstedt, A. Phenolic Compounds from Hypericum perforatum. Planta Med. 2002, 68, 88–91. [Google Scholar] [CrossRef] [PubMed]
- Flamini, G.; Antognoli, E.; Morelli, I. Two flavonoids and other compounds from the aerial parts of Centaurea bracteata from Italy. Phytochemistry 2001, 57, 559–564. [Google Scholar] [CrossRef]
- Özïpek, M.; Çaliş, İ.; Ertan, M.; Rüedi, P. Rhamnetin 3-p-coumaroylrhamninoside from Rhamnus petiolaris. Phytochemistry 1994, 37, 249–253. [Google Scholar] [CrossRef]
- Olsson, M.E.; Gustavsson, K.-E.; Vågen, I.M. Quercetin and Isorhamnetin in Sweet and Red Cultivars of Onion (Allium cepa L.) at Harvest, after Field Curing, Heat Treatment, and Storage. J. Agric. Food Chem. 2010, 58, 2323–2330. [Google Scholar] [CrossRef] [PubMed]
- Yao, L.; Datta, N.; Tomás-Barberán, F.A.; Ferreres, F.; Martos, I.; Singanusong, R. Flavonoids, phenolic acids and abscisic acid in Australian and New Zealand Leptospermum honeys. Food Chem. 2003, 81, 159–168. [Google Scholar] [CrossRef]
- Hisanaga, A.; Mukai, R.; Sakao, K.; Terao, J.; Hou, D. Anti-inflammatory effects and molecular mechanisms of 8-prenyl quercetin. Mol. Nutr. Food Res. 2016, 60, 1020–1032. [Google Scholar] [CrossRef]
- Crespy, V.; Morand, C.; Besson, C.; Manach, C.; Demigne, C.; Remesy, C. Quercetin, but not Its Glycosides, Is Absorbed from the Rat Stomach. J. Agric. Food Chem. 2002, 50, 618–621. [Google Scholar] [CrossRef]
- Murota, K.; Shimizu, S.; Chujo, H.; Moon, J.; Terao, J. Efficiency of Absorption and Metabolic Conversion of Quercetin and Its Glucosides in Human Intestinal Cell Line Caco-2. Arch. Biochem. Biophys. 2000, 384, 391–397. [Google Scholar] [CrossRef]
- Murota, K.; Shimizu, S.; Miyamoto, S.; Izumi, T.; Obata, A.; Kikuchi, M.; Terao, J. Unique Uptake and Transport of Isoflavone Aglycones by Human Intestinal Caco-2 Cells: Comparison of Isoflavonoids and Flavonoids. J. Nutr. 2002, 132, 1956–1961. [Google Scholar] [CrossRef]
- Day, A.J.; Gee, J.M.; DuPont, M.S.; Johnson, I.T.; Williamson, G. Absorption of quercetin-3-glucoside and quercetin-4′-glucoside in the rat small intestine: The role of lactase phlorizin hydrolase and the sodium-dependent glucose transporter. Biochem. Pharmacol. 2003, 65, 1199–1206. [Google Scholar] [CrossRef]
- Wolffram, S.; Blöck, M.; Ader, P. Quercetin-3-Glucoside Is Transported by the Glucose Carrier SGLT1 across the Brush Border Membrane of Rat Small Intestine. J. Nutr. 2002, 132, 630–635. [Google Scholar] [CrossRef] [PubMed]
- Németh, K.; Plumb, G.W.; Berrin, J.-G.; Juge, N.; Jacob, R.; Naim, H.Y.; Williamson, G.; Swallow, D.M.; Kroon, P.A. Deglycosylation by small intestinal epithelial cell?-glucosidases is a critical step in the absorption and metabolism of dietary flavonoid glycosides in humans. Eur. J. Nutr. 2003, 42, 29–42. [Google Scholar] [CrossRef] [PubMed]
- Aura, A.-M.; O’Leary, K.A.; Williamson, G.; Ojala, M.; Bailey, M.; Puupponen-Pimiä, R.; Nuutila, A.M.; Oksman-Caldentey, K.-M.; Poutanen, K. Quercetin Derivatives Are Deconjugated and Converted to Hydroxyphenylacetic Acids but Not Methylated by Human Fecal Flora in Vitro. J. Agric. Food Chem. 2002, 50, 1725–1730. [Google Scholar] [CrossRef]
- Schoefer, L.; Mohan, R.; Schwiertz, A.; Braune, A.; Blaut, M. Anaerobic degradation of flavonoids by Clostridium orbiscindens. Appl. Environ. Microbiol. 2003, 69, 5849–5854. [Google Scholar] [CrossRef]
- Galijatovic, A.; Walle, U.K.; Walle, T. Induction of UDP-glucuronosyltransferase by the flavonoids chrysin and quercetin in Caco-2 cells. Pharm. Res. 2000, 17, 21–26. [Google Scholar] [CrossRef]
- Graf, B.A.; Ameho, C.; Dolnikowski, G.G.; Milbury, P.E.; Chen, C.; Blumberg, J.B. Rat Gastrointestinal Tissues Metabolize Quercetin. J. Nutr. 2006, 136, 39–44. [Google Scholar] [CrossRef]
- Cao, Y.; Chen, Z.-J.; Jiang, H.; Chen, J. Computational Studies of the Regioselectivities of COMT-Catalyzed Meta -/ Para -O Methylations of Luteolin and Quercetin. J. Phys. Chem. B 2014, 118, 470–481. [Google Scholar] [CrossRef]
- Williamson, G.; Kay, C.D.; Crozier, A. The Bioavailability, Transport, and Bioactivity of Dietary Flavonoids: A Review from a Historical Perspective. Compr. Rev. Food Sci. Food Saf. 2018, 17, 1054–1112. [Google Scholar] [CrossRef]
- Murota, K.; Hotta, A.; Ido, H.; Kawai, Y.; Moon, J.; Sekido, K.; Hayashi, H.; Inakuma, T.; Terao, J. Antioxidant capacity of albumin-bound quercetin metabolites after onion consumption in humans. J. Med. Investig. 2007, 54, 370–374. [Google Scholar] [CrossRef]
- Murota, K.; Cermak, R.; Terao, J.; Wolffram, S. Influence of fatty acid patterns on the intestinal absorption pathway of quercetin in thoracic lymph duct-cannulated rats. Br. J. Nutr. 2013, 109, 2147–2153. [Google Scholar] [CrossRef] [PubMed]
- Arts, I.C.W.; Sesink, A.L.A.; Faassen-Peters, M.; Hollman, P.C.H. The type of sugar moiety is a major determinant of the small intestinal uptake and subsequent biliary excretion of dietary quercetin glycosides. Br. J. Nutr. 2004, 91, 841–847. [Google Scholar] [CrossRef]
- Guo, Y.; Bruno, R.S. Endogenous and exogenous mediators of quercetin bioavailability. J. Nutr. Biochem. 2015, 26, 201–210. [Google Scholar] [CrossRef]
- Kawabata, K.; Mukai, R.; Ishisaka, A. Quercetin and related polyphenols: New insights and implications for their bioactivity and bioavailability. Food Funct. 2015, 6, 1399–1417. [Google Scholar] [CrossRef] [PubMed]
- Williamson, G.; Aeberli, I.; Miguet, L.; Zhang, Z.; Sanchez, M.-B.; Crespy, V.; Barron, D.; Needs, P.; Kroon, P.A.; Glavinas, H.; et al. Interaction of Positional Isomers of Quercetin Glucuronides with the Transporter ABCC2 (cMOAT, MRP2). Drug Metab. Dispos. 2007, 35, 1262–1268. [Google Scholar] [CrossRef] [PubMed]
- Ulusoy, H.G.; Sanlier, N. A minireview of quercetin: From its metabolism to possible mechanisms of its biological activities. Crit. Rev. Food Sci. Nutr. 2019, 0, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Abrahamse, S.L.; Kloots, W.J.; van Amelsvoort, J.M.M. Absorption, distribution, and secretion of epicatechin and quercetin in the rat. Nutr. Res. 2005, 25, 305–317. [Google Scholar] [CrossRef]
- Bali, E.; Ergin, V.; Rackova, L.; Bayraktar, O.; Küçükboyacı, N.; Karasu, Ç. Olive Leaf Extracts Protect Cardiomyocytes against 4-Hydroxynonenal-Induced Toxicity In Vitro: Comparison with Oleuropein, Hydroxytyrosol, and Quercetin. Planta Med. 2014, 80, 984–992. [Google Scholar] [CrossRef]
- Chen, J.-Y.; Hu, R.-Y.; Chou, H.-C. Quercetin-induced cardioprotection against doxorubicin cytotoxicity. J. Biomed. Sci. 2013, 20, 95. [Google Scholar] [CrossRef]
- Zhang, X.; Li, P.; Guo, S.; Wang, S.; Liu, D. Quantitation of β-carboline and quercetin in alligator weed (Alternanthera philoxeroides (Mart.) Griseb.) by LC-MS/MS and evaluation of cardioprotective effects of the methanol extracts. Drug Discov. Ther. 2018, 12, 341–346. [Google Scholar] [CrossRef]
- Syama, H.P.; Arya, A.D.; Dhanya, R.; Nisha, P.; Sundaresan, A.; Jacob, E.; Jayamurthy, P. Quantification of phenolics in Syzygium cumini seed and their modulatory role on tertiary butyl-hydrogen peroxide-induced oxidative stress in H9c2 cell lines and key enzymes in cardioprotection. J. Food Sci. Technol. 2017, 54, 2115–2125. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Wang, T.; Zhang, C.; Xuan, J.; Su, C.; Wang, Y. Quercetin attenuates cardiomyocyte apoptosis via inhibition of JNK and p38 mitogen-activated protein kinase signaling pathways. Gene 2016, 577, 275–280. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Peng, Y.; Xu, T.; Yi, X.; Liu, Y.; Luo, Y.; Yin, D.; He, M. The effects of quercetin protect cardiomyocytes from A/R injury is related to its capability to increasing expression and activity of PKCε protein. Mol. Cell. Biochem. 2013, 382, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Ai, X.; Lu, W.; Zeng, K.; Li, C.; Jiang, Y.; Tu, P. Microfluidic Coculture Device for Monitoring of Inflammation-Induced Myocardial Injury Dynamics. Anal. Chem. 2018, 90, 4485–4494. [Google Scholar] [CrossRef]
- Lozano, O.; Lázaro-Alfaro, A.; Silva-Platas, C.; Oropeza-Almazán, Y.; Torres-Quintanilla, A.; Bernal-Ramírez, J.; Alves-Figueiredo, H.; García-Rivas, G. Nanoencapsulated Quercetin Improves Cardioprotection during Hypoxia-Reoxygenation Injury through Preservation of Mitochondrial Function. Oxid. Med. Cell. Longev. 2019, 2019, 1–14. [Google Scholar] [CrossRef]
- Cote, B.; Carlson, L.J.; Rao, D.A.; Alani, A.W.G. Combinatorial resveratrol and quercetin polymeric micelles mitigate doxorubicin induced cardiotoxicity in vitro and in vivo. J. Control. Release 2015, 213, 128–133. [Google Scholar] [CrossRef]
- Dong, L.Y.; Chen, F.; Xu, M.; Yao, L.P.; Zhang, Y.J.; Zhuang, Y. Quercetin attenuates myocardial ischemia-reperfusion injury via downregulation of the HMGB1-TLR4-NF-кB signaling pathway. Am. J. Transl. Res. 2018, 10, 1273–1283. [Google Scholar]
- Huang, L.; He, H.; Liu, Z.; Liu, D.; Yin, D.; He, M. Protective Effects of Isorhamnetin on Cardiomyocytes Against Anoxia/Reoxygenation-induced Injury Is Mediated by SIRT1. J. Cardiovasc. Pharmacol. 2016, 67, 526–537. [Google Scholar] [CrossRef]
- Shu, Z.; Yang, Y.; Yang, L.; Jiang, H.; Yu, X.; Wang, Y. Cardioprotective effects of dihydroquercetin against ischemia reperfusion injury by inhibiting oxidative stress and endoplasmic reticulum stress-induced apoptosis via the PI3K/Akt pathway. Food Funct. 2019, 10, 203–215. [Google Scholar] [CrossRef]
- Wang, Y.; Zhong, L.; Liu, X.; Zhu, Y. ZYZ-772 Prevents Cardiomyocyte Injury by Suppressing Nox4-Derived ROS Production and Apoptosis. Molecules 2017, 22, 331. [Google Scholar] [CrossRef]
- Xiao, R.; Xiang, A.-L.; Pang, H.-B.; Liu, K.-Q. Hyperoside protects against hypoxia/reoxygenation induced injury in cardiomyocytes by suppressing the Bnip3 expression. Gene 2017, 629, 86–91. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Xu, H.; Zhu, G.; Liu, S. Isoquercetin ameliorated hypoxia/reoxygenation-induced H9C2 cardiomyocyte apoptosis via a mitochondrial-dependent pathway. Biomed. Pharmacother. 2017, 95, 938–943. [Google Scholar] [CrossRef] [PubMed]
- Daubney, J.; Bonner, P.L.; Hargreaves, A.J.; Dickenson, J.M. Cardioprotective and Cardiotoxic Effects of Quercetin and Two of Its In Vivo Metabolites on Differentiated H9c2 Cardiomyocytes. Basic Clin. Pharmacol. Toxicol. 2015, 116, 96–109. [Google Scholar] [CrossRef] [PubMed]
- Allawadhi, P.; Khurana, A.; Sayed, N.; Kumari, P.; Godugu, C. Isoproterenol-induced cardiac ischemia and fibrosis: Plant-based approaches for intervention. Phyther. Res. 2018, 32, 1908–1932. [Google Scholar] [CrossRef]
- Kumar, M.; Kasala, E.R.; Bodduluru, L.N.; Kumar, V.; Lahkar, M. Molecular and biochemical evidence on the protective effects of quercetin in isoproterenol-induced acute myocardial injury in rats. J. Biochem. Mol. Toxicol. 2017, 31, e21832. [Google Scholar] [CrossRef]
- Ballmann, C.; Denney, T.S.; Beyers, R.J.; Quindry, T.; Romero, M.; Amin, R.; Selsby, J.T.; Quindry, J.C. Lifelong quercetin enrichment and cardioprotection in Mdx/Utrn +/− mice. Am. J. Physiol. Circ. Physiol. 2017, 312, H128–H140. [Google Scholar] [CrossRef]
- Arumugam, S.; Thandavarayan, R.A.; Arozal, W.; Sari, F.R.; Giridharan, V.V.; Soetikno, V.; Palaniyandi, S.S.; Harima, M.; Suzuki, K.; Nagata, M.; et al. Quercetin offers cardioprotection against progression of experimental autoimmune myocarditis by suppression of oxidative and endoplasmic reticulum stress via endothelin-1/MAPK signalling. Free Radic. Res. 2012, 46, 154–163. [Google Scholar] [CrossRef]
- Shahbaz, A.U.; Kamalov, G.; Zhao, W.; Zhao, T.; Johnson, P.L.; Sun, Y.; Bhattacharya, S.K.; Ahokas, R.A.; Gerling, I.C.; Weber, K.T. Mitochondria-targeted Cardioprotection in Aldosteronism. J. Cardiovasc. Pharmacol. 2011, 57, 37–43. [Google Scholar] [CrossRef]
- Putakala, M.; Gujjala, S.; Nukala, S.; Bongu, S.B.R.; Chintakunta, N.; Desireddy, S. Cardioprotective effect of Phyllanthus amarus against high fructose diet induced myocardial and aortic stress in rat model. Biomed. Pharmacother. 2017, 95, 1359–1368. [Google Scholar] [CrossRef]
- Fadda, L.M.; Attia, H.A.; Al-Rasheed, N.M.; Ali, H.M.; Al-Rasheed, N.M. Roles of some antioxidants in modulation of cardiac myopathy induced by sodium nitrite via down-regulation of mRNA expression of NF-κB, Bax, and flt-1 and suppressing DNA damage. Saudi Pharm. J. 2018, 26, 217–223. [Google Scholar] [CrossRef]
- Wiseman, R.L.; Zhang, Y.; Lee, K.P.K.; Harding, H.P.; Haynes, C.M.; Price, J.; Sicheri, F.; Ron, D. Flavonol Activation Defines an Unanticipated Ligand-Binding Site in the Kinase-RNase Domain of IRE1. Mol. Cell 2010, 38, 291–304. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Xiong, T.; Liu, P.; Guo, X.; Xiao, L.; Zhou, F.; Tang, Y.; Yao, P. Quercetin ameliorates HFD-induced NAFLD by promoting hepatic VLDL assembly and lipophagy via the IRE1a/XBP1s pathway. Food Chem. Toxicol. 2018, 114, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Binder, P.; Wang, S.; Radu, M.; Zin, M.; Collins, L.; Khan, S.; Li, Y.; Sekeres, K.; Humphreys, N.; Swanton, E.; et al. Pak2 as a Novel Therapeutic Target for Cardioprotective Endoplasmic Reticulum Stress Response. Circ. Res. 2019, 124, 696–711. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Yu, Z.; Huang, X.; Gao, Y.; Wang, X.; Gu, J.; Xue, S. Peroxisome proliferator-activated receptor γ (PPARγ) mediates the protective effect of quercetin against myocardial ischemia-reperfusion injury via suppressing the NF-κB pathway. Am. J. Transl. Res. 2016, 8, 5169–5186. [Google Scholar]
- Jin, H.-B.; Yang, Y.-B.; Song, Y.-L.; Zhang, Y.; Li, Y.-R. Protective roles of quercetin in acute myocardial ischemia and reperfusion injury in rats. Mol. Biol. Rep. 2012, 39, 11005–11009. [Google Scholar] [CrossRef]
- Tang, J.; Lu, L.; Liu, Y.; Ma, J.; Yang, L.; Li, L.; Guo, H.; Yu, S.; Ren, J.; Bai, H.; et al. Quercetin improve ischemia/reperfusion-induced cardiomyocyte apoptosis in vitro and in vivo study via SIRT1/PGC-1α signaling. J. Cell. Biochem. 2019, 120, 9747–9757. [Google Scholar] [CrossRef]
- Ma, C.; Jiang, Y.; Zhang, X.; Chen, X.; Liu, Z.; Tian, X. Isoquercetin ameliorates myocardial infarction through anti-inflammation and anti-apoptosis factor and regulating TLR4-NF-κB signal pathway. Mol. Med. Rep. 2018, 17, 6675–6680. [Google Scholar] [CrossRef]
- Shu, L.; Zhang, W.; Huang, C.; Huang, G.; Su, G. Troxerutin Protects Against Myocardial Ischemia/Reperfusion Injury Via Pi3k/Akt Pathway in Rats. Cell. Physiol. Biochem. 2017, 44, 1939–1948. [Google Scholar] [CrossRef]
- Badalzadeh, R.; Baradaran, B.; Alihemmati, A.; Yousefi, B.; Abbaszadeh, A. Troxerutin Preconditioning and Ischemic Postconditioning Modulate Inflammatory Response after Myocardial Ischemia/Reperfusion Injury in Rat Model. Inflammation 2017, 40, 136–143. [Google Scholar] [CrossRef]
- Saliu, J.A.; Oyeleye, S.I.; Olasehinde, T.A.; Oboh, G. Modulatory effects of stonebreaker (Phyllanthus amarus) and bitter gourd (Momordica charantia) on enzymes linked with cardiac function in heart tissue of doxorubicin-stressed rats. Drug Chem. Toxicol. 2019, 0545, 1–9. [Google Scholar] [CrossRef]
- Annapurna, A.; Reddy, C.S.; Akondi, R.B.; Rao, S.R.C. Cardioprotective actions of two bioflavonoids, quercetin and rutin, in experimental myocardial infarction in both normal and streptozotocin-induced type I diabetic rats. J. Pharm. Pharmacol. 2009, 61, 1365–1374. [Google Scholar] [CrossRef] [PubMed]
- Roslan, J.; Giribabu, N.; Karim, K.; Salleh, N. Quercetin ameliorates oxidative stress, inflammation and apoptosis in the heart of streptozotocin-nicotinamide-induced adult male diabetic rats. Biomed. Pharmacother. 2017, 86, 570–582. [Google Scholar] [CrossRef] [PubMed]
- Najafi, M.; Noroozi, E.; Javadi, A.; Badalzadeh, R. Anti-arrhythmogenic and anti-inflammatory effects of troxerutin in ischemia/reperfusion injury of diabetic myocardium. Biomed. Pharmacother. 2018, 102, 385–391. [Google Scholar] [CrossRef] [PubMed]
- Seferović, P.M.; Paulus, W.J. Clinical diabetic cardiomyopathy: A two-faced disease with restrictive and dilated phenotypes. Eur. Heart J. 2015, 36, 1718–1727. [Google Scholar] [CrossRef]
- Soman, S.; Rajamanickam, C.; Rauf, A.A.; Madambath, I. Molecular mechanisms of the antiglycative and cardioprotective activities of Psidium guajava leaves in the rat diabetic myocardium. Pharm. Biol. 2016, 54, 3078–3085. [Google Scholar] [CrossRef]
- Ulasova, E.; Perez, J.; Hill, B.G.; Bradley, W.E.; Garber, D.W.; Landar, A.; Barnes, S.; Prasain, J.; Parks, D.A.; Dell’Italia, L.J.; et al. Quercetin prevents left ventricular hypertrophy in the Apo E knockout mouse. Redox Biol. 2013, 1, 381–386. [Google Scholar] [CrossRef]
- Zhang, J.; Liang, R.; Wang, L.; Yan, R.; Hou, R.; Gao, S.; Yang, B. Effects of an aqueous extract of Crataegus pinnatifida Bge. var. major N.E.Br. fruit on experimental atherosclerosis in rats. J. Ethnopharmacol. 2013, 148, 563–569. [Google Scholar] [CrossRef]
- Ferenczyova, K.; Kalocayova, B.; Kindernay, L.; Jelemensky, M.; Balis, P.; Berenyiova, A.; Zemancikova, A.; Farkasova, V.; Sykora, M.; Tothova, L.; et al. Quercetin Exerts Age-Dependent Beneficial Effects on Blood Pressure and Vascular Function, But Is Inefficient in Preventing Myocardial Ischemia-Reperfusion Injury in Zucker Diabetic Fatty Rats. Molecules 2020, 25, 187. [Google Scholar] [CrossRef]
- Knekt, P.; Jarvinen, R.; Reunanen, A.; Maatela, J. Flavonoid intake and coronary mortality in Finland: A cohort study. BMJ 1996, 312, 478–481. [Google Scholar] [CrossRef]
- Malishevskaia, I.V.; Ilashchuk, T.A.; Okipniak, I.V. [Therapeutic efficacy of quercetin in patients with is ischemic heart disease with underlying metabolic syndrome]. Georg. Med. News 2013, 225, 67–71. [Google Scholar]
- Chekalina, N.I.; Shut, S.V.; Trybrat, T.A.; Burmak, Y.H.; Petrov, Y.Y.; Manusha, Y.I.; Kazakov, Y.M. Effect of quercetin on parameters of central hemodynamics and myocardial ischemia in patients with stable coronary heart disease. Wiad. Lek. 2017, 70, 707–711. [Google Scholar] [PubMed]
- Zahedi, M.; Ghiasvand, R.; Feizi, A.; Asgari, G.; Darvish, L. Does quercetin improve cardiovascular risk factors and inflammatory biomarkers in women with type 2 diabetes: A double-blind randomized controlled clinical trial. Int. J. Prev. Med. 2013, 4, 777–785. [Google Scholar] [PubMed]
- Dower, J.I.; Geleijnse, J.M.; Gijsbers, L.; Zock, P.L.; Kromhout, D.; Hollman, P.C.H. Effects of the pure flavonoids epicatechin and quercetin on vascular function and cardiometabolic health: A randomized, double-blind, placebo-controlled, crossover trial. Am. J. Clin. Nutr. 2015, 101, 914–921. [Google Scholar] [CrossRef] [PubMed]
- Serban, M.C.; Sahebkar, A.; Zanchetti, A.; Mikhailidis, D.P.; Howard, G.; Antal, D.; Andrica, F.; Ahmed, A.; Aronow, W.S.; Muntner, P.; et al. Effects of Quercetin on Blood Pressure: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J. Am. Heart Assoc. 2016, 5, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Kondratiuk, V.E.; Synytsia, Y.P. Effect of quercetin on the echocardiographic parameters of left ventricular diastolic function in patients with gout and essential hypertension. Wiad. Lek. 2018, 71, 1554–1559. [Google Scholar]
- Yadav, H.N.; Singh, M.; Sharma, P.L. Pharmacological inhibition of GSK-3β produces late phase of cardioprotection in hyperlipidemic rat: Possible involvement of HSP 72. Mol. Cell. Biochem. 2012, 369, 227–233. [Google Scholar] [CrossRef]
- Yao, Y.; Zhang, G.; Zhang, Y.; Li, W.; Wang, C.; Yin, C.; Zhang, F. Lipopolysaccharide pretreatment protects against ischemia/reperfusion injury via increase of HSP70 and inhibition of NF-κB. Cell Stress Chaperones 2011, 16, 287–296. [Google Scholar]
- Eren-Guzelgun, B.; Ince, E.; Gurer-Orhan, H. In vitro antioxidant/prooxidant effects of combined use of flavonoids. Nat. Prod. Res. 2018, 32, 1446–1450. [Google Scholar] [CrossRef]
| Chemical Structure | Common Name/ Systematic Name | Food Sources | References |
|---|---|---|---|
| QCT-3-O-glycosides | |||
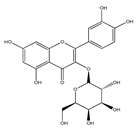 | Hyperoside/ QCT-3-O-galactoside | Mango Cranberries Blueberries Chokeberries | [32] [33] |
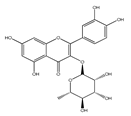 | Quercitrin/ QCT-3-O-rhamnoside | Mango Spinach | [32] [34] |
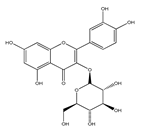 | Isoquercitrin/ QCT-3-O- glucoside | Beans Plums Onions Mango | [35] [36] [37] [32] |
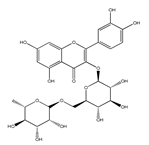 | Rutin/ QCT-3-O- rutinoside | Plums Cherries Tomatoes Buckwheat | [36] [38] [39] [40] |
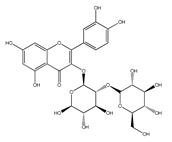 | QCT-3-O-sophoroside | Broccoli | [41] |
| QCT-7-O-glycosides | |||
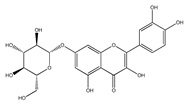 | QCT- 7-O-glucoside | Beans | [35] |
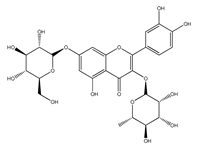 | QCT- 3-O-rhamnoside- 7-O-glucoside | Pepper | [42] |
| Acyl and sulfate QCT glycosides | |||
 | QCT- 3-O-(2″-acetylgalactoside) | Hypericum perforatum | [43] |
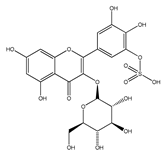 | QCT-3-O-glucoside-5′-sulfate | Cornflower | [44] |
| QCT-C-glycosides | |||
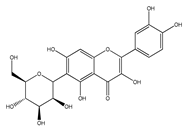 | QCT-6-C-glucoside | Ageratina calophylla | [25] |
| QCT ethers | |||
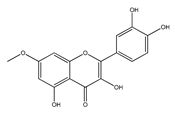 | Rhamnetin | Rhamnus petiolaris | [45] |
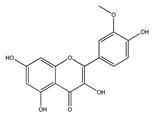 | Isorhamnetin | Onions Honey | [46] [47] |
| QCT prenyls | |||
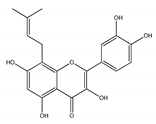 | 8-prenyl-QCT | Desmodium caudatum | [48] |
| Derivative | Dose | Exp. Model | Type of Injury | Effect | Mechanism | Reference |
|---|---|---|---|---|---|---|
| Quercetin (QCT) | 1–250 mg/kg | Rodents (mice/rats) | I/R | ↓oxidative stress ↓inflammation ↓infarct size ↑heart function | ↓ROS, ↓HMGB1, ↓NF-kB, ↓TNF- α, ↓apoptosis ↑PI3K/Akt, ↑SIRT1/PGC-1α | [14,16,78,91,95,96,97,102] |
| 20 mg/kg | Rats | Isoproterenol- induced MI | ↓oxidative stress ↓inflammation | ↓ROS, ↓calpain | [86] | |
| 0.2% in food | Mdx/Utrn+/− mice | Duchenne muscular dystrophy | ↑mitochondrial function | ↓NF-kB, ↓TGF-β1, ↓F4/80 | [87] | |
| 10 mg/kg | Rats | Autoimmune myocarditis | ↓oxidative stress | ↓ROS, ↓ER stress, ↑endothelin-1/MAPK | [88] | |
| 10–50 mg/kg | Rats | Diabetic cardiomyopathy | ↓oxidative stress ↓cardiac injury ↓inflammation ↓apoptosis | ↓troponin C, ↓CK-MB, ↓LDH, ↓ROS ↓ Bax, ↓caspases-3,-9 | [103] | |
| 10–80 µM | Cell cultures (H9c2, NRCM) | I/R | ↑cell viability ↓oxidative stress ↑mitochondrial function | ↓ROS, ↓JNK, ↓p38, ↓MAPK, ↑Bcl-2/Bax, ↑PKCε | [73,74] | |
| 0.1–10 µM | H9c2 | 4-hydroxynonenal – induced toxicity | ↓oxidative stress ↑cell viability | ↓ROS, ↓p-SAPK/JNK, ↓p-HSP27, ↓caspase 3 | [69] | |
| 500–200 µM | H9c2 | Doxorubicin – induced toxicity | ↑cell viability ↓ inflammation | ↓Src kinase activity, ↓ROS, ↓STAT3 | [70] | |
| 100µM | H9c2 | H2O2 – induced toxicity | ↓oxidative stress ↑cell viability | ↓ROS, ↓P38, ↓MAPK, ↓JNK | [84] | |
| Troxerutin | 150 mg/kg | Rats | I/R | ↓infarct size ↑cardiac function ↓arrhythmias ↓inflammation | ↑PI3K/Akt, ↓TNF-α, ↓IL-1b, ↓ICAM-1 | [90,100,104] |
| Hyperoside | 0.5–50 µM | NRCMs | I/R | ↑cell viability | ↓Bnip3 | [82] |
| IsoQCT | 20–80 µM/ml | H9c2 | I/R | ↑cell viability ↓cell apoptosis mitochondrial protection | ↓ROS generation ↓cytochrome c release | [83] |
| 80 mg/kg | Rats | AMI | ↓inflammation ↓apoptosis | ↓TLR4-NF-kB | [98] | |
| Isorhamnetin | 10–40µM | NRCMs | I/R | ↓oxidative stress mitochondrial protection | ↓ mPTP opening, ↓caspase-3 activity, ↓cytochrome c release, ↓ROS | [79] |
| DihydroQCT | 2,5–80 µM | H9c2 | I/R | ↓oxidative stress ↓apoptosis | ↓ROS, ↓ER stress, ↑PI3K/Akt | [80] |
| 5–20 µM in K-H | Rats | I/R | ↓oxidative stress ↓ apoptosis | ↓ROS, ↓ER stress, ↑PI3K/Akt | [80] | |
| ZYZ-772 | 1–50 µM | H9c2 | CoCl2 – induced H/R | ↑cell viability ↓oxidative stress ↓ apoptosis | ↓ROS, ↓Nox4/MAPK/p53 | [81] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferenczyova, K.; Kalocayova, B.; Bartekova, M. Potential Implications of Quercetin and its Derivatives in Cardioprotection. Int. J. Mol. Sci. 2020, 21, 1585. https://doi.org/10.3390/ijms21051585
Ferenczyova K, Kalocayova B, Bartekova M. Potential Implications of Quercetin and its Derivatives in Cardioprotection. International Journal of Molecular Sciences. 2020; 21(5):1585. https://doi.org/10.3390/ijms21051585
Chicago/Turabian StyleFerenczyova, Kristina, Barbora Kalocayova, and Monika Bartekova. 2020. "Potential Implications of Quercetin and its Derivatives in Cardioprotection" International Journal of Molecular Sciences 21, no. 5: 1585. https://doi.org/10.3390/ijms21051585
APA StyleFerenczyova, K., Kalocayova, B., & Bartekova, M. (2020). Potential Implications of Quercetin and its Derivatives in Cardioprotection. International Journal of Molecular Sciences, 21(5), 1585. https://doi.org/10.3390/ijms21051585