Abstract
The tightly structured neural retina has a unique vascular network comprised of three interconnected plexuses in the inner retina (and choroid for outer retina), which provide oxygen and nutrients to neurons to maintain normal function. Clinical and experimental evidence suggests that neuronal metabolic needs control both normal retinal vascular development and pathological aberrant vascular growth. Particularly, photoreceptors, with the highest density of mitochondria in the body, regulate retinal vascular development by modulating angiogenic and inflammatory factors. Photoreceptor metabolic dysfunction, oxidative stress, and inflammation may cause adaptive but ultimately pathological retinal vascular responses, leading to blindness. Here we focus on the factors involved in neurovascular interactions, which are potential therapeutic targets to decrease energy demand and/or to increase energy production for neovascular retinal disorders.
1. Retinal Neurovascular Development
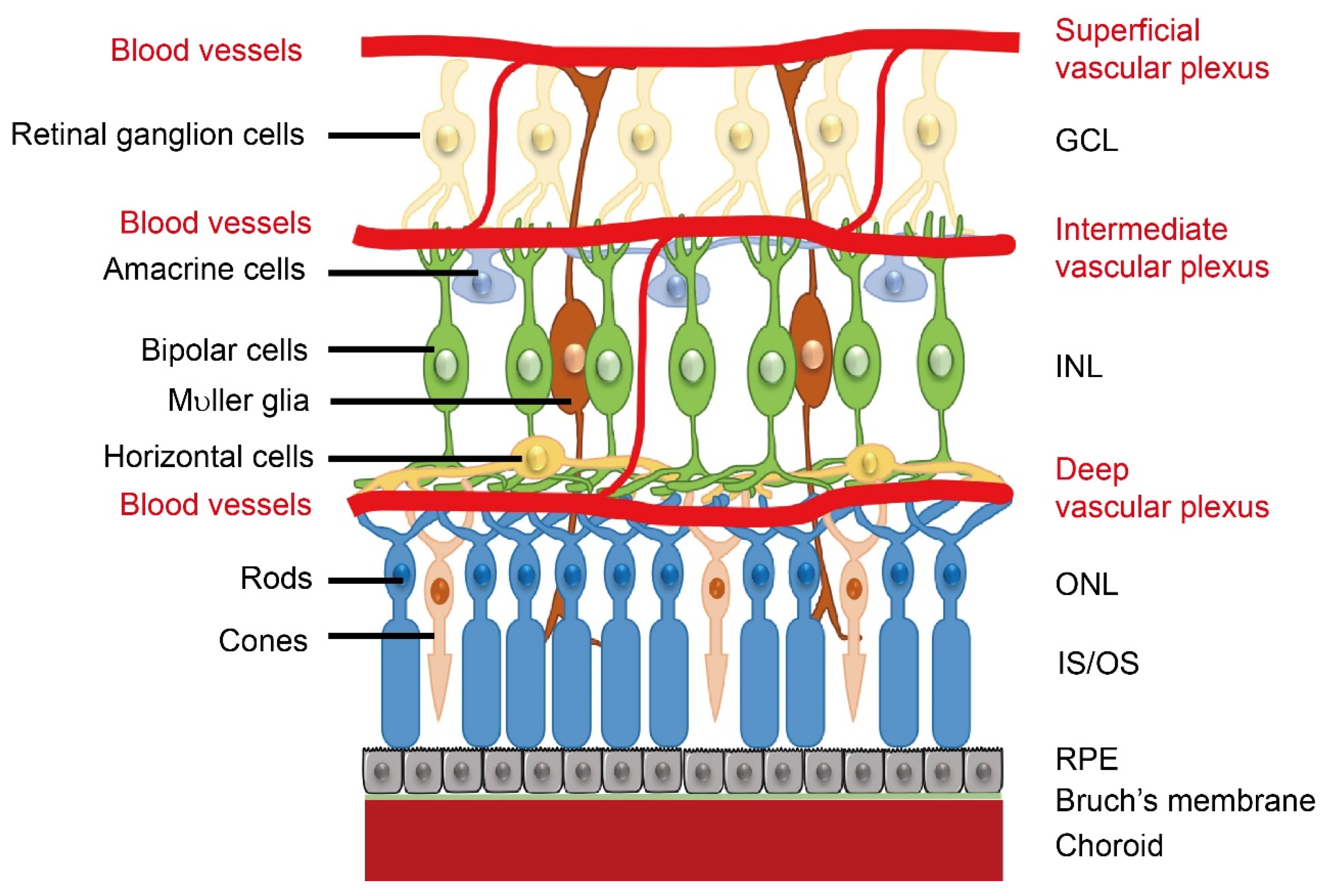
Retinal neurovascular crosstalk increases with the development of photoreceptors. As birth date studies of retinal neurogenesis in monkeys are relevant to human retinal development, human retinal development is referenced to that in monkeys. Neural development starts from the central retina and extends to the periphery [1]. In both human and monkey, the fovea, a region with high concentrations of cone photoreceptors, develops at 26–30% of full gestation while cone outer segments develop by 63–65% of full gestation [2]. Around the end of the first trimester (~30% gestation), the hyaloid artery (branched from the primitive dorsal ophthalmic artery), which extends through the primitive vitreous and provides nutrients to the developing eye [3], begins to regress, and in the following four months (~30 to 70% gestation), as the retinal vascular network extends from the central to the peripheral retina, the development of photoreceptors takes place [1]. The formation of the retinal vasculature is completed shortly after birth. In general, retinal angiogenesis begins in the superficial layers at the nerve fiber layer/ganglion cell layer interface. The first superficial primary capillary bed next sends branches into the retina to form the deep capillary bed, a new layer of vessels at the border of the INL/outer plexiform layer. The intermediate layer forms last in the inner plexiform layer and inner nuclear layer (INL). (Figure 1). The timing and spatial arrangement of the retinal vasculature and retinal neurons suggest a neurovascular link. However, it is important to note that the signals involved in the neurovascular interaction and their impact might be different between developmental and pathological conditions. For example, retinal glia cell-derived vascular endothelial growth factor (VEGF) is important for physiological retinal angiogenesis, but high VEGF expression in ischemic retinopathy induces pathological angiogenesis [4]. In addition, loss of WNT signaling leads to delayed hyaloid vessel regression and delayed retinal vascular growth, but over activation of WNT signaling leads to retinal neovascularization [5]. During the developmental stage, signals are tightly controlled for the proper formation of retinal vasculature. During disease conditions, the signals are uncontrolled and result in retinal vessel overgrowth.
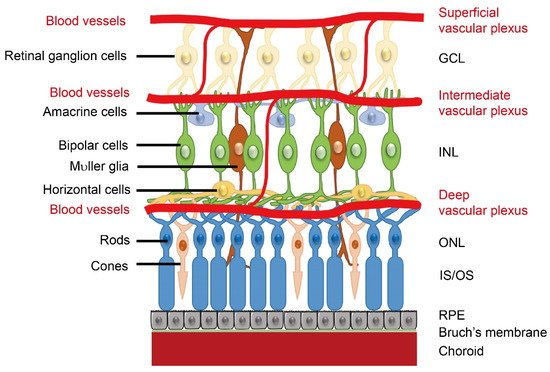
Figure 1.
Schematics of retinal neuronal and vascular structure. There are three layers of retinal vascular plexuses tightly coordinated with retinal neurons. GCL, ganglion cell layer; INL, inner nuclear layer; ONL, outer nuclear layer; OS/IS, outer segments/inner segments; RPE, retinal pigment epithelium. Schematics were drawn by Dr. Shuo Huang, Ophthalmology, BCH.
The endothelial cell Norrin/Frizzled-4 pathway plays a key role in regulating retinal angiogenesis [6,7]. The formation of retinal vascular patterns is also tightly regulated by factors from the surrounding environment. For example, endothelial stalk cells proliferate in response to a gradient of tissue-hypoxia-induced VEGF [8]. Retinal ganglion cell GPR91 responds to increased succinate levels and induces retinal vascular sprouting in hypoxic rodent retinas [9]. We will discuss the inductive signals and guidance cues involved in the neurovascular interaction and their contribution to the development of retinal disorders.
2. Factors Influence Retinal Neurovascular Interaction
Retinal vasculature is critical for the preservation of normal retinal function. This is because most nutrients (oxygen, glucose, lipids, ions) delivered to neurons of the inner retina come from the retinal vasculature, with the majority of photoreceptor cell nutrients provided by the choriocapillaris, which lies just below the retinal pigmentary epithelium (RPE).
Retinal photoreceptor cells are critical to the normal function of the retina, and play a major role in the neurovascular unit. Photoreceptor cell signaling is implicated in the degeneration of retinal capillaries in a variety of diseases, including DR, genetic diseases that lead to photoreceptor degeneration, and also in retinopathy of prematurity (ROP). It has been postulated that oxygen demands of the photoreceptors cause the development of the vascular abnormalities that are characteristic of ROP [10,11]. In photoreceptor-degenerating mice exposed to oxygen in oxygen-induced retinopathy (OIR, modeling ROP), there are fewer preretinal vascular endothelial cell nuclei and reduced retinal VEGF expression level versus wild type (WT) OIR controls [12]. Therefore, understanding the roles of photoreceptor metabolic needs and factors involved in regulating retinal vascular responses is critical for disease prevention and treatment. In addition, retinal ganglion cells (RGCs) play an important role in maintaining the normal structure and function of retinal blood vessels. Previous studies show that retinal vascular networks fail to develop in mice lacking RGCs [9]. RGCs also contribute to retinal blood vessels in experimental models of retinal regeneration induced by ischemia–reperfusion [13,14]. These findings strongly suggest that RGCs play an important functional role in both physiological and pathological retinal angiogenesis [15,16].
2.1. Oxygen Shortage and Retinal Neovascularization
The retinal vasculature provides oxygen and nutrients to support neurons. Oxygen is required for oxidative phosphorylation for mitochondrial energy production, in addition to lipid β-oxidation. In the light, retinal oxygen tension is highest in the choroid and decreases steeply between the choriocapillaris and the outer plexiform layer [17]. Photoreceptor inner segments have abundant mitochondria and consume the most oxygen in the retina [18,19]. It has also been reported that purified bovine rod outer segment, although devoid of mitochondria, have ectopically located mitochondrial proteins (respiratory chain complexes I-V) present, consume oxygen, and synthesize ATP [20,21,22]. In isolated bovine rod outer segment following photoexcitation, glycolysis alone is not sufficient to provide enough energy for phototransduction [23]. Phosphocreatine shuttle transports high energy phosphate groups in the form of creatine phosphate from rod inner segment to outer segment for conversion to ATP [23]. The pentose phosphate pathway also contributes to NADPH production [23]. However, in retinal metabolic disorders, oxygen saturation is altered [24]. In retinitis pigmentosa photoreceptors are lost and retinal oxygen consumption is reduced. This is correlated with visual field damage and retinal atrophy. In diabetic retinopathy, venous oxygen saturation increases and arteriovenous differences decrease. The magnitude of this difference correlates with disease severity.
Most cells respond to hypoxia by increasing the stability of hypoxia-inducible factor 1 protein (HIF-1) [25], which in turn induces the transcription of HIF-1-dependent VEGF-A [26]. VEGF-A is critical for blood vessel and neural growth and stability [27,28]. In rodent models of proliferative retinopathy, relative hypoxia induces high levels of VEGF-A production, causing uncontrolled vessel growth [29,30]. Photoreceptor metabolic needs regulate this process [10,31]. In addition, lactate, produced with anaerobic glycolysis, stabilizes N-myc downstream-regulated genes, to induce the Raf-ERK pathway to promote angiogenesis in tumors via an HIF-independent pathway [32]. Overall, oxygen is essential for the maintenance of retinal cell function and cells respond to hypoxia by inducing vessel growth to compensate for oxygen shortage.
2.2. Energy Shortage Drives Retinal Neovascularization
To produce energy for cells, fuel is needed. Previously, it was common knowledge that glucose was the only energy source for the retina [33]. However, we found that lipids are also used as mitochondrial fuel (oxidative phosphorylation) [34] accounting for the energy gap noted in 1960 that most glucose (~65%) is used for aerobic glycolysis rather than oxidative phosphorylation [35]. Thus, aberrant mitochondrial and peroxisomal fatty acid β-oxidation may lead to an energy shortage.
It is known that mutations in fatty acid transport proteins lead to retinal degeneration [36]. Studies have shown that very-low-density-lipoprotein receptor (VLDLR) loss-of-function in photoreceptors decreases retinal lipid uptake and results in accumulation of extracellular lipids. This in turn inhibits retinal glucose transporter 1 activity [34]. Ultimately, the resulting mitochondrial fuel shortage drives retinal neovascularization [34]. In addition, gene mutations in peroxisomal lipid transporters and metabolic enzymes may lead to retinal abnormalities, including pigmentary retinopathy and optic atrophy [37]. Mutations in Acyl-CoA binding domains containing protein 5 (which preferentially binds very-long-chain fatty acyl-CoAs) is associated with retinal dystrophy [38]. Deficiency of acyl-CoA binding domain containing protein 5 in HeLa cells leads to impaired peroxisomal β-oxidation and accumulation of very long-chain fatty acids [39]. However, knowledge of peroxisome function in retinal lipid metabolism is limited.
Energy shortage promotes pathological vascular growth. A fuel shortage of both lipid and glucose in photoreceptors of VLDLR knockout mice leads to a reduction in the levels of the Krebs cycle intermediate α-ketoglutarate. Low α-ketoglutarate levels (as well as low oxygen levels) stabilize HIF-1α protein and increase the production of VEGF-A, which in turn induces pathological vessel formation [34]. In the neonatal mouse model of hyperglycemia-associated retinopathy, decreased photoreceptor glucose metabolism causes a reduction in the photoreceptor platelet-derived growth factor beta expression, thereby delaying normal retinal vascular development [40]. Ketone bodies, mainly beta hydroxybutyrate and acetoacetate, are alternative energy sources in the fasting state [41]. Beta hydroxybutyrate activates G protein-coupled receptors GPR109A, which exhibits anti-inflammatory and neuroprotective effects [42]. Acetoacetate, as an endogenous agonist for GPR43, regulates energy expenditure and lipid metabolism in mice under fasting conditions [43]. Ketogenic diets are neuroprotective in some neuronal disorders, including ischemic stroke [42], Parkinson’s disease [44], and Alzheimer’s disease [45]. Exogenous ketone bodies also show neuroprotective effects on retinas with enhanced antioxidative defenses [46]. RPE cells in vitro have high expression of key enzymes involved in ketogenesis and phagocytose photoreceptor outer segment to produce ketone bodies [47,48]. RPE cells also metabolize ketone bodies for energy production [47]. High levels of ketone bodies are observed in patients with type 2 diabetes [49,50]. Insulin treatment elevates serum ketone bodies in type 1 diabetes [51]. A recent conference report shows that beta hydroxybutyrate via GPR109A reduces NLRP3-derived inflammation and loss of GPR109A worsens retinal vascular pathology in streptozotocin-induced type 1 diabetic retinas [52]. In general, there might be a protective role of ketone bodies in diabetic retinopathy but the impacts of ketone bodies on diabetic retina need to be further investigated. Taken together, further exploration in the interaction between photoreceptor energy deficiency and vascular development is needed for disease prevention and treatment at the early stages.
2.3. Oxidative Stress and Retinal Neovascularization
Oxidative stress results from an imbalance between the antioxidant defense system and the production of reactive oxygen species (ROS). Phototransduction, oxidization of polyunsaturated acids, and phagocytosis of photoreceptor outer segment leads to chronic production of ROS, resulting in potential oxidative stress [53]. Oxygen-consuming mitochondria in the inner segments play a primary role in oxidative stress in the outer retina [54]. Recent studies also report that oxidative stress may occur directly in the photoreceptor OS after blue light irradiation [55]. In the purified bovine rod outer segment, a dose response is observed to varying light intensity and duration in terms of both reactive oxygen intermediates and ATP synthesis [20]. In normal conditions, retinal cells maintain homeostasis between pro- and anti-oxidative signaling [56]. For example, in the mitochondria, superoxide dismutase 2 converts the major form of ROS in living cells, superoxide radicals (O2–), into H2O2 and O2 [57]. In the peroxisome, oxidases reduce O2 to H2O2 during lipid breakdown, while catalase removes H2O2 [56,58]. However, when the antioxidant defense system is compromised, excessive ROS lead to damaged proteins, nucleic acids, and lipids, contributing to neuronal loss in many retinal diseases [59,60]. Increasing anti-oxidative signaling prevents neuronal loss in photoreceptor-degenerating mouse mutants [56], as well as in the mouse model of ischemic retina [61]. Oxidative stress is a significant risk factor for age-related macular degeneration (AMD). Diet poor in antioxidant micronutrients (vitamin C, E, carotenoids, zinc) and low plasma levels of antioxidants may favor the development of AMD [62]. Inhibition of oxidative stress with N-acetylcysteine attenuates spermidine-induced hyperpermeability of the blood-retinal barrier, decreased rod function, as well as RPE degeneration in rats [63]. In hyperglycemic states, metabolic pathways producing ROS are activated which enhance inflammatory, apoptotic, and degeneration pathways, ultimately leading to the development of diabetic retinopathy [64,65]. Therefore, increasing anti-oxidant defenses under conditions of oxidative stress may help treat retinal metabolic disorders.
2.4. Retinal Circuitry and Retinal Remodeling
Progressive photoreceptor (especially cone) loss in retinal degenerative diseases, such as retinal detachment, AMD, and retinitis pigmentosa, leads to extensive, phased changes in the remnant retinal circuitry—termed “retinal remodeling,” which is unavoidable at the cellular and molecular level in the inner retinal neurons and glial cells [66,67]. First, photoreceptor stress and metabolic alterations in glia are initiated; second, glial remodeling occurs with the loss of photoreceptors; third, there is a neural, glial, and vascular remodeling of the surviving retina. In retinitis pigmentosa patients, the severity of visual field loss is correlated with retinal vessel attenuation [68,69]. In retinitis pigmentosa animal models, progressive loss of retinal blood vessels is also observed and a significant decrease in capillary density and capillary loop is found particularly in the deep (close to photoreceptors), but not in the superficial and intermediate capillary plexus [70,71]. In ROP mouse models, hyperoxia causes reduced density of retinal astrocytes in the avascular zone and maintaining retinal astrocytes normalizes revascularization [72,73]. Migrating astrocytes associate closely with the axons of retinal ganglion cells and subsequently direct vessel development in mice [74]. However, the thickness changes are only observed in the inner nuclear and plexiform layer but not photoreceptors in the mouse OIR model at the time when maximum neovascularization occurs [75]. Therefore, photoreceptor stress-induced retinal remodeling may also control retinal vascular patterning possibly through modulating glial responses.
2.5. Inflammation and Retinal Neovascularization
Immune response and inflammation are associated with pathological angiogenesis as seen in ROP, AMD, and diabetic retinopathy [76,77,78,79,80]. An immature immune system may increase the risk for ROP in premature neonates [81]. Recent work suggests a strong link between the suppressor of cytokine signaling-3 (SOCS3) signaling pathway and ROP [82,83,84]. SOCS proteins are key regulators of both innate and adaptive immunity and inflammatory responses, and can positively and negatively regulate macrophage and dendritic-cell activation, respectively, and is essential for T-cell development and differentiation. SOCS proteins may be involved in diseases of the immune system in ocular neovascularization [85].
Toll-like receptors [86] and the transcriptional activator nuclear factor NF-ĸB [87] can potentiate inflammatory responses and promote further endothelial injury, resulting in generalized systemic inflammation [88]. Prolonged supplemental oxygen exposure as well as systemic inflammation markers, such as plasma interleukin 6 (IL6) and tumor necrosis factor-alpha (TNFα) are strongly associated with severe ROP [89]. Markers of inflammation appear to be associated with ROP in human studies [90]. Inflammatory processes might interfere with normal retinal vascularization in preterm retinas [91]. Suppression of TNFα in the mouse OIR model appears to be protective [92]. Omega-3-polyunsaturated fatty acids in OIR decrease the size of the avascular area and this protective effect may be mediated, in part, via suppression of TNFα [93]. Besides the OIR model, the expression levels of inflammatory cytokine (IL6 and TNFα) modulated by key inflammatory factor c-Fos in photoreceptors are increased in VLDLR knockout mice [94].
Immune dysfunction and inflammation changes has been clinically and experimentally linked to development of choroidal neovascularization (CNV) [95,96,97,98,99,100,101,102], which affects ~10% of AMD patients, but it accounts for up to 90% of vision loss associated with AMD [103]. Immune cells play a key role in linking innate and adaptive immunity, primarily through antigen presentation and recruitment of adaptive immune cells. Choroidal circulation brings to the eye a large number of immune cells, specifically those of myeloid origin [104,105,106], which play important roles in retinal and choroidal vascular pathology [100,101,107,108,109,110]. In response to chronic insults, such as those occurring in AMD, myeloid cells become activated and release inflammatory mediators (such as VEGF, matrix metalloproteinases, interleukins, and chemokines) that stimulate ocular NV [109,111,112]. A number of chemokines attract immune cells to invade and infiltrate ocular tissues, and furthermore, stimulate the secretion of more trafficking molecules that influence immune cell migration [113]. Therefore, inflammatory mediators and the degree of myeloid cell activation and infiltration around CNV is critical to the innate inflammatory response that contributes to CNV onset and progression.
Development of pathological neovascularization in vascular eye diseases is linked with altered inflammation and macrophage function/polarization [114]. Lipid-induced alteration in inflammatory response is mediated in part by macrophages, which are phenotypically plastic with different polarization states (M1 and M2-like), and play critical roles in regulating ocular angiogenesis during development and in disease [115]. Retinoic-acid-receptor-related orphan receptor alpha (RORα), a lipid sensing nuclear receptor and transcription factor [116], which regulates lipid homeostasis and inflammatory cell differentiation and cytokine production [117], controls pathological angiogenesis through direct transcriptional control of SOCS3. This suggests the importance of lipid metabolism driven inflammation in ocular angiogenesis [84]. In addition, retinal gliosis had been reported to associate with ocular neovascularization, such as ROP [118] and diabetic retinopathy [119].
2.6. Neuron-Derived Factors and Neovascularization
Dysregulated crosstalk between the vasculature and retinal neurons, modulated by neuron-derived growth factors and guidance cues, contributes to the pathogenesis of ocular vascular diseases [15,120,121], stroke [122], Alzheimer’s disease [123], and epilepsy [124]. Among those neuron-derived factors, semaphorins (SEMAs), also known as collapsins, are critical regulators of angiogenesis during development and in diseases with neovascularization such as cancer and retinopathy. Semaphorins were first identified as a family of genes encoding guidance molecules for the embryologic development of the nervous system [125,126]. The action mechanisms underlying semaphorins through their receptors from different cell types in different disease conditions had been summarized previously [127,128,129]. Cerani et al. reported that neuron-derived Semaphorin3A is an early inducer of vascular permeability in diabetic retinopathy via neuropilin-1 [130]. Yang et al. reported that Semaphorin-3C signals through Neuropilin-1 and PlexinD1 receptors to inhibit pathological angiogenesis [131]. Wu et al. reported that inhibition of Sema4D and its receptor Plexin B1 signaling alleviates vascular dysfunction in diabetic retinopathy [132]. Fukushima et al. reported that Sema3E-PlexinD1 signaling selectively suppresses disoriented angiogenesis in ischemic retinopathy in mice [133]. These findings demonstrate that semaphorin family proteins are involved in neovascularization.
3. Therapeutic Potentials of Manipulating Pathways Controlling Neurovascular Crosstalk
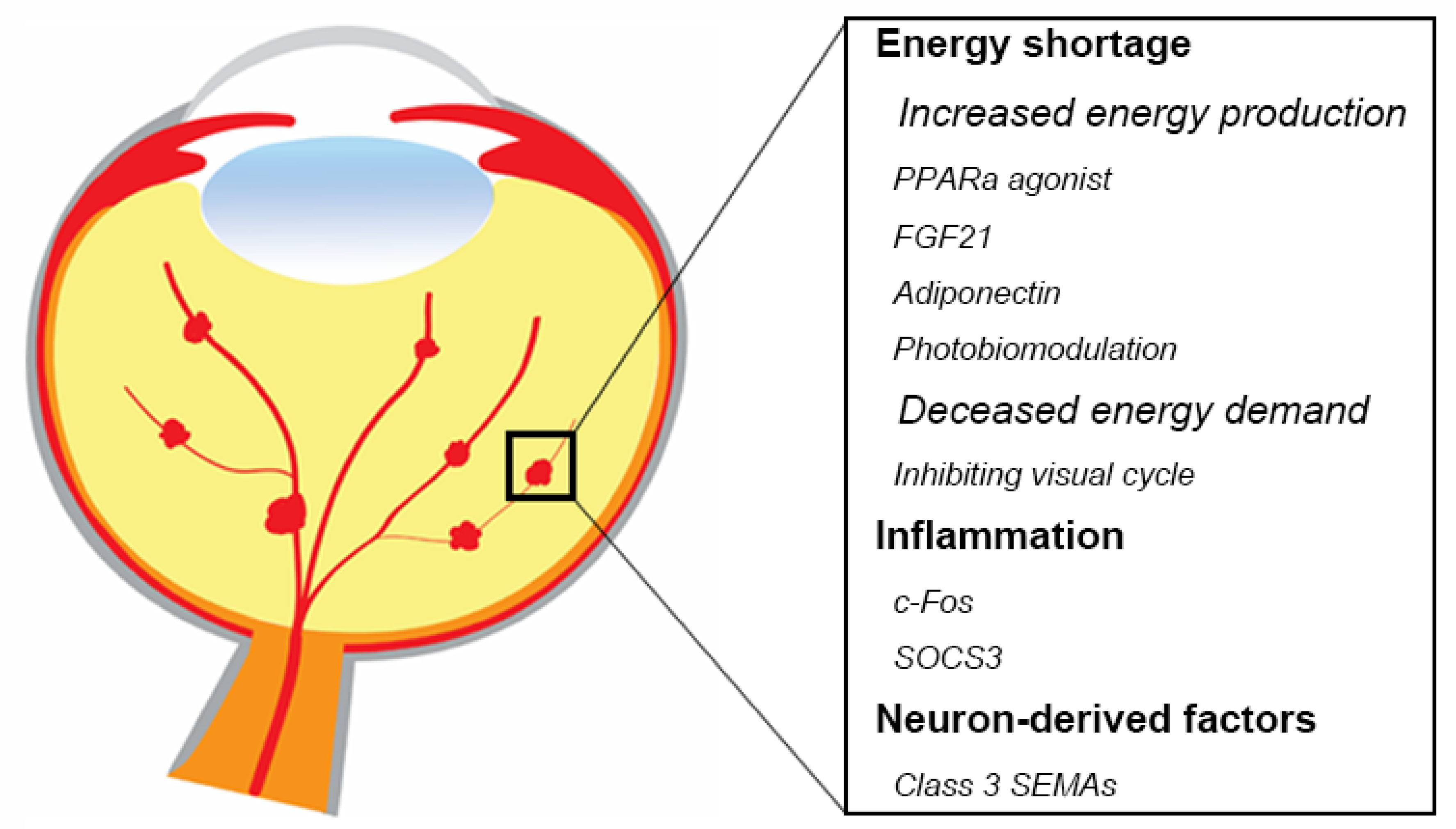
Anti-VEGF agents are the standard of care to treat most pathological retinal vessel growth in eye diseases [134,135]. However, anti-VEGF treatment is not completely effective and is associated with some adverse effects [136,137,138]. Systemic inhibition of VEGF levels following intravitreal anti-VEGF administration in patients with proliferative retinopathies is particularly concerning in preterm infants with ROP and incomplete vascular development of many organs [139,140,141,142,143,144], as VEGF is an important factor to maintain normal neuronal and vascular function. Therefore, there is a need for new safe therapeutic approaches. Modulation of retinal metabolism and inflammatory factors might prevent or treat neurovascular retinal diseases (Figure 2). One approach might be to increase fatty acid β oxidation with hormonal modulation (peroxisome proliferator-activated receptor alpha (PPARα) agonists such as fenofibrate, long acting fibroblast growth factor 21 (FGF21), or adiponectin). Inhibition of the visual cycle (to decrease the energy demand), near infrared (IR) light photobiomodulation (to increase ATP production), as well as inflammatory regulation (c-Fos, SOCS3) may potentially improve photoreceptor function as a therapeutic approach. There are also other potential factors involved in neurovascular interaction. For example, abnormal expression of numerous retinal miRNAs is related to retinal disorders such as AMD, diabetic retinopathy, retinitis pigmentosa, and retinoblastoma in both human and animal models [145]. In addition, miRNAs also regulates angiogenic factors and controls angiogenesis in vitro and in vivo [146,147,148,149]. In the current review, we focus on the major signals regulating oxygen and nutrients that mostly related to retinal metabolism, as well as some of the potential therapeutic targets that have been investigated in our group.
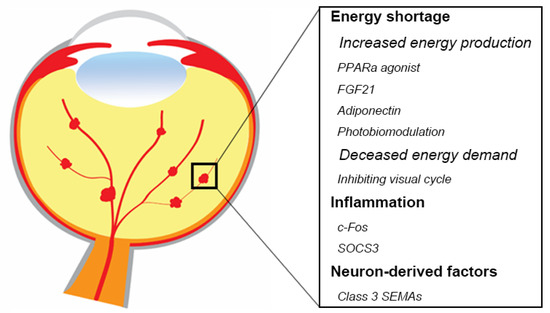
Figure 2.
Summary of potential therapeutic targets in retinal neurovascular disorders. Modulating energy shortage, inflammation, as well as neuron-derived factors would be beneficial to protect against retinal neurons and inhibits pathological angiogenesis. Schematics were drawn by Dr. Shuo Huang, Ophthalmology, BCH.
3.1. Hormonal Modulation
Fenofibrate, which is a PPARα agonist essential for lipid use and cytochrome P450 2C antagonist [150], prevents the progression of DR. In type 2 diabetic patients, fenofibrate treatment reduces the risk of proliferative DR by 35–40% [151,152]. In a rat model, fenofibrate reduces retinal neovascularization and ameliorates retinal vascular leakage in murine diabetes [153]. Inhibition of cytochrome P450 2C with fenofibrate or other inhibitors such as Montelukast decreases pathologic retinal angiogenesis in mice [154,155]. PPARα may also function through inhibition of the Wnt/Norrin pathway [156]. Wnt proteins and Norrin are ligands of receptors such as low-density lipoprotein receptor-related protein 5 or 6 (LRP5/6) and frizzled 4, which leads to the stabilization of β-catenin, which then translocates into the nucleus where it binds to transcription factors of lymphoid enhancer factor/T-cell factor to activate transcription of Wnt target genes [157,158]. The Wnt/Norrin pathway plays a crucial role in vascular development and retinal neuronal survival [5,159]. However, over-activated Wnt signaling is associated with pathologic retinal angiogenesis [160]. Therefore, inhibitors targeting aberrant Wnt/Norrin signaling may be benefit ocular neurovascular disorders.
Fasting or administration of PPARα agonist (such as fenofibrate) produces fibroblast growth factor (FGF21) in the liver [161,162,163,164]. FGF21, a secreted protein of 209 amino acids, was first reported in 2000 [165]. FGF21 administration lowers blood glucose levels and improves lipid metabolism in diabetic mice [166,167,168,169,170,171,172,173,174]. Clinically, FGF21 decreases body weight, decreases triglyceride levels and increases high-density lipoprotein cholesterol levels in type 2 diabetic patients [175,176]. In animal models of neurovascular eye disorders, including OIR, VLDLR knockout mice, and laser-induced choroidal neovascularization, FGF21 suppresses neovascularization in the retina and choroid [177], and protects against retinal dysfunction in the neural retina in diabetic mice [178], as well as decreasing blood-brain barrier leakage in type 2 diabetes mice and ameliorates transendothelial permeability in vitro [179].
Adiponectin is a circulating adipokine that is upregulated by FGF21 in adipose tissue [169,180,181]. Adiponectin is a key modulator of glucose and lipid metabolism, and is involved in many retinal metabolic disorders [182]. Circulating adiponectin levels are associated with the progression of retinal diseases such as ROP, DR, and AMD [183,184,185,186]. The depletion of AdipoR1, which is a major adiponectin receptor gene, causes a shortage of essential ω-3 very-long-chain polyunsaturated fatty acids in the retina and in turn photoreceptor degeneration in mice [186,187]. Modulating photoreceptor metabolism with adiponectin administration protects against retinal dysfunction and improves retinal vascular development in mice modeling early ROP [40]. The adiponectin pathway inhibits neovascularization in a number of animal models of proliferative retinopathy [183,188,189,190] and endothelial cell tube formation in vitro [191]. Adiponectin also dilates retinal arterioles via production of nitric oxide from endothelial cells [192]. The concentration of serum adiponectin is associated with increased retinal blood flow in type 2 diabetes patients [185]. Therefore, adiponectin is a potential therapeutic target in retinal metabolic diseases.
Adenosine monophosphate-activated protein kinase (AMPK), a crucial cellular energy sensor, is activated by falling energy status [193]. Activation of AMPK via attenuating NF-κB activation protects retinal neurons against lipopolysaccharide-induced inflammation [194]. Activation of AMPK with metformin protects light-induced retinal degeneration with decreased oxidative stress and DNA damage, as well as increased mitochondrial energy production [195]. Peroxisome proliferator-activated receptor coactivator-1alpha (PGC-1α), which is stimulated by metabolic sensor AMPK and sirtuin 1 (SIRT1), is a transcriptional coactivator of many genes involved in energy modulation and mitochondrial biogenesis [196,197]. PGC-1α repression and mitochondrial dysfunction are observed in RPE derived from AMD donor eyes versus age-matched normal controls [198]. A high-fat-diet induces AMD-like abnormalities in RPE and retinal morphology in mice with low levels of PGC-1α [199]. PGC-1α increases oxidative phosphorylation and fatty acid oxidation in RPE in vitro [200], as well as protecting RPE cells of the aging retina against oxidative stress-induced degeneration in vivo [196]. Loss of PGC-1α in mice leads to rapid RPE dysfunction and promotes photoreceptor degeneration [201], as well as severely protecting against a deterioration in retinal morphology and function with toxic light exposure [202]. PGC-1α also induces the expression of VEGFA in retinal cells and PGC-1α deficiency reduces early retinal vascular outgrowth, capillary density, and number of arteries and veins as adults [203]. In a mouse model of ROP, PGC-1α expression is dramatically induced in the inner nuclear layer, and loss of PGC-1α inhibits retinal neovascularization by decreasing VEGFA levels [203]. Adiponectin induces AMPK activation by promoting the cytosolic localization of LKB1 and stimulating Ca2+ release from intracellular stores in muscle cells [204]. In hyperglycemia-associated ROP, adiponectin via AMPK increases photoreceptor metabolism and pro-angiogenic growth factor Pdgfb production, and in turn improves retinal vascular growth [40]. Endocrine FGF21 is also an AMPK activator either directly through FGFR1/β-klotho signaling or indirectly by stimulating the secretion of adiponectin and corticosteroids [205]. FGF21 controls mitochondrial function by activating the AMPK-SIRT1-PGC-1α pathway in adipocytes [206].
3.2. Photobiomodulation
Photobiomodulation, also known as near infrared therapy (NIR) or far-infrared therapy (FIR), is a light-based treatment with a wave length between 600–1000 nm from a laser or a light emitting diode (LED). Over the last decades there has been an increased interest in the use of NIR and FIR in clinical and pre-clinical research. Clinically, it has shown promise in aiding wound healing [207], neurological neck pain [208], chronic joint disorders [208], peripheral nerve recovery [209], stroke recovery [210], and traumatic brain injury [211]. Photobiomodulation has also gained interest for the treatment of retinal disorders, having had positive effects in several mouse retinopathy models such as in light induced injury [212,213], OIR [214,215], streptozotocin-induced diabetes [216], methanol induced degeneration [217], and a model of complement factor H related degeneration [218]. These promising results have led to clinical trials in humans with AMD. In 2008, 203 patients with beginning “dry” and advanced AMD “wet” were treated with NIR light (0,3 J/cm2, 760 nm) transconjunctivally to the macula twice per week for 2 weeks [219]. Vision improved significantly in wet and dry AMD and was maintained for 3–36 months after treatment. In another study 2016 patients with dry AMD (AREDS. 2–4) were treated with multiwavelength light (590, 670, 790 nm) through the pupil three times per week for 6 weeks [220], which led to significant improvement of visual acuity and contrast sensitivity as well as a decrease in drusen volume and thickness. No adverse events were seen in these two studies.
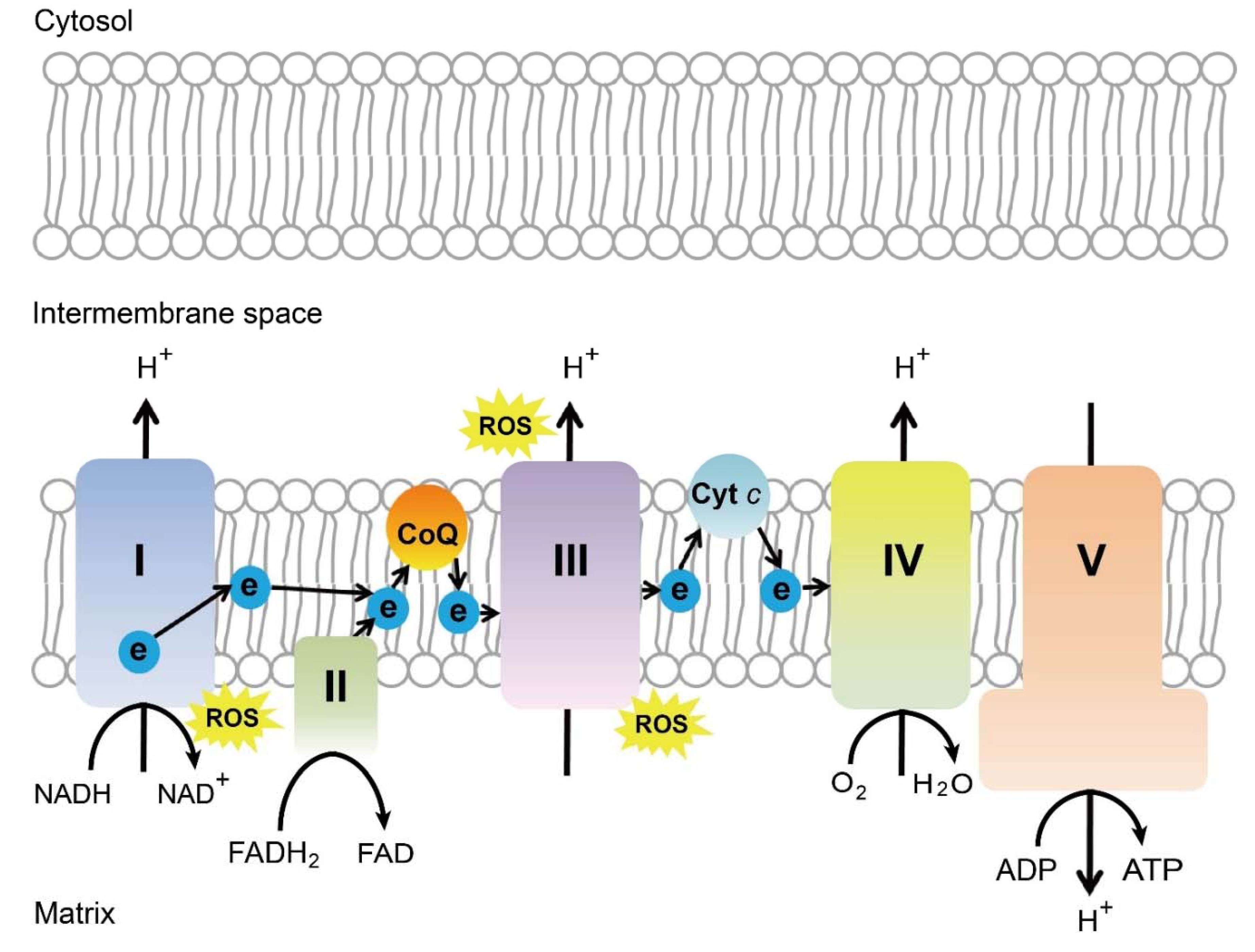
Many different molecular and cellular mechanisms for the neuroprotective effect of near infra-red light have been proposed. The most commonly accepted mechanism is that cytochrome c oxidase (or complex IV) of the mitochondrial respiratory chain absorbs wavelength in the red/near-infrared spectrum. The capability of cytochrome c oxidase to undergo oxidation and reduction is essential for the generation and maintenance of the mitochondrial transmembrane potential, which is critical for ATP production via oxidative phosphorylation [221] (Figure 3). A brief 670 nm exposure increases mitochondrial membrane potential [222], ATP production [221,223], and complex IV expression levels [221]. In vivo measurements in the retina with broadband near-infrared spectroscopy show a consistent progressive increase in cytochrome c oxidase 5 oxidation after 670 nm exposure for 5 minutes [224]. Photobiomodulation increases blood flow to neural tissue, increases nitric oxide levels, slows reactivation of the mitochondrial electron transport chain during reperfusion reduction in ROS levels, reduces nitric oxide levels, increases expression of cell survival mediators, and improves microglial function [225].
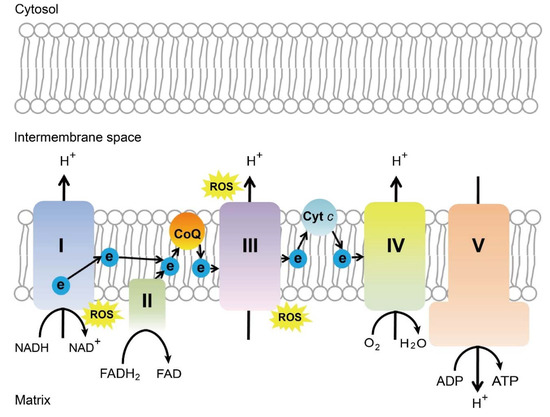
Figure 3.
Schematics of mitochondrial electron transport chain. ROS, reactive oxygen species, ATP, adenosine triphosphate, CoQ, coenzyme Q, Cyt c, cytochrome c, Complex I, NADH coenzyme Q reductase, complex II, succinate dehydrogenase, complex III, cytochrome bc1 complex, complex IV, cytochrome c oxidase, complex V, ATP synthase. Schematics were drawn by Dr. Zhongjie Fu, Ophthalmology, BCH. Adapted from EMBO Mol Med (2019)11: e10473. [226]
3.3. Suppression of the Visual Cycle to Decrease Energy Demands
Metabolism and function of photoreceptor cells and the retinal pigmentary epithelium show strong differences between day and night, and thus, diurnal differences on the outer retina can have important effects on the function of the retinal vasculature. The vertebrate visual system relies on two key pathways to detect and transform light signals into images; phototransduction and the visual (or retinoid) cycle. The high-energy-demanding phototransduction is vital for the conversion of external light into stimuli that can be processed by the brain to form an image, and is dependent on a constant supply of regenerated light-sensitive pigments. Evidence that neurovascular interactions, and specifically phototransduction, contribute to the vascular pathology at least in diabetic retinopathy can be derived from evidence that deletion of transducin, a key component of the phototransduction pathway, significantly inhibited the diabetes-induced degeneration of retinal capillaries and some molecular processes believed to participate in the pathogenesis of the retinopathy [227]. Likewise for the visual cycle, inhibition of a key enzyme of the visual cycle, RPE65, significantly inhibited both the diabetes-induced increase in retinal vascular permeability and retinal capillary degeneration over an 8 month period [228]. Administration of a visual cycle inhibitor (Emixustat) during the period of ischemia and reperfusion injury produced a ~30% reduction in retinal neovascularization in a mouse OIR model [229]. These observations suggest that signaling processes that are affected by the presence or absence of light can affect the integrity of the retinal neurovascular unit, and specifically the vasculature. Many more studies will be required to better understand the mechanisms and significance of these findings.
3.4. Inflammatory Regulation
Recently, there have been attempts to suppress neovascularization through controlling key regulators of inflammatory signals in the eye. Inflammatory changes in photoreceptors influence pathological angiogenesis in DR [230,231,232,233] and photoreceptor c-Fos controls proliferative angiogenesis by modulating photoreceptor inflammatory signals [94].
The expression levels of c-Fos and inflammatory cytokine (IL6 and TNFα) in photoreceptors are increased in VLDLR knockout mouse retinas, a retinal angiogenesis mouse model [94]. The immediate early gene and pro-oncogene c-Fos encodes a nuclear phosphoprotein that forms heterodimeric complexes with the Jun-family proteins to constitute activator protein 1 complex [234,235], which regulates inflammatory signals [236] during inflammatory disease development [237,238]. c-Fos is present in human photoreceptor cells throughout development [239] and regulates rod-specific gene expression [240] and photoreceptor apoptosis [235,241,242,243], and strongly associates with metabolic demands [94,244]. c-Fos is induced throughout the process of neovascularization formation in photoreceptors and controls retinal angiogenesis by modulating inflammatory signals in a retinal angiogenesis model [94]. Therefore, the c-Fos pathway may be a novel target to control inflammatory signals in stressed photoreceptors to mediate the development of neovascularization in ocular vascular diseases.
In addition, SOCS proteins are key physiological regulators of both innate and adaptive immunity and inflammatory responses. SOCS3 positively and negatively regulates macrophage and dendritic-cell activation, respectively, and it is essential for T-cell development and differentiation. SOCS proteins are involved in diseases of the immune system [85]. SOCS3 is also required to temporally fine-tune photoreceptor cell differentiation [245]. Therefore, the SOCS3 pathway might also play a role in regulating photoreceptor inflammatory signals in ocular neovascularization.
3.5. Class 3 SEMAs
Class 3 SEMAs consist of seven secreted soluble proteins of ∼100 kDa in size (designated by the letters a-g), which are secreted by cells of multiple lineages, including epithelial cells, neurons, and specific tumor cells [126]. Class 3 SEMAs regulate cell–cell interactions, cell differentiation, morphology, and function [246]. SEMA3A, 3E, and 3F are vaso-repulsive cues in developmental retinal angiogenesis [247,248,249]. SEMA3A, 3C, and 3E are all involved in pathological retinal neovascularization in eye disease models [131,133,250]. Both SEMA3A and 3E are expressed in retinal ganglion cells [16,133]. SEMA3F is expressed in the outer retina while Sema3a is predominantly induced in the inner retina under hypoxic conditions [248]. SEMAs and their neuropilin and plexin receptors can modulate angiogenesis [133,250,251]. Inhibition of SEMA3E–plexin-D1 signaling results in an increase of pathological neovessels, whereas activation of SEMA3E–plexin-D1 signaling suppresses the outgrowth of neovessels in the mouse model of OIR [133]. However, the upstream factors that mediate the expression of SEMA3s in the retinas to influence neurovascular interaction and pathological retinal angiogenesis are largely unknown. RORα is a critical upstream regulator of SEMA3E expression, and controls SEM3E-dependent neurovascular coupling in retinopathy [16]. RORα directly binds to a specific ROR response element on the promoter of Sema3e and negatively regulates Sema3e transcription, which can impact retinopathy development via signaling through its receptor complexes located on pathological neovessels. Transcriptional regulation of the RORα-SEMA3E axis in the retinal ganglion cell layer mediates neurovascular crosstalk in pathological retinal angiogenesis. Taken together, therapies targeting SEMA3E are potentially promising approaches for the treatment of microvascular diseases, particularly microvascular retinopathies [246].
SEMA3F acts as an anti-angiogenic modulator suppressing the formation of pathological neovascularization in the outer retina [252]. Anti-angiogenic activity of SEMA3F in ocular neovascularization was found in VLDLR knockout mice and the mouse model of laser-induced choroidal neovascularization. SEMA3F is physiologically expressed in the retina of both mice and humans [248], suggesting that SEMA3F a promising target for treating pathological neovascularization. Intravitreal injection of anti-VEGF compounds is clinically well established [253,254,255] and combinatory treatments are currently under investigation in clinical studies [256]. Intravitreal treatment with SEMA3F alone or in combination with other compounds may be feasible for future treatment strategies in neovascular eye diseases.
4. Conclusions and Perspectives
Neuronal metabolism and inflammatory responses control retinal vascular function. Hormonal modulation and photobiomodulation to increase ATP production, or inhibition of the visual cycle may improve retinal function. Furthermore, controlling photoreceptor inflammatory responses by modulating c-Fos and SOCS3 pathways, as well as regulating neuron-derived Class 3 SEMAs to inhibit the pathologic vessel formation is also a promising field of study. To date, fenofibrate treatment in type 2 diabetic patients reduces proliferative diabetic retinopathy. Photobiomodulation in AMD patients also improves visual function. Long-acting FGF21 improves circulating lipid profiles and decreases body weight in type 2 diabetic patients. The investigation of FGF21 on retinal metabolic disorders are in the pre-clinical phase but clinical trials of long-acting FGF21 in patients with non-alcoholic steatohepatitis is ongoing. The development of safe and effective visual cycle inhibitors for retinal degeneration and proliferative retinopathies, and further exploration of modulating retinal inflammatory responses by c-Fos and SOCS3 in proliferative retinopathies are currently in process.
Author Contributions
Z.F., Y.S., B.C., Y.T., S.H., Z.W., C.-H.L., S.S.C., W.B., T.S.K., D.A.A., A.H., and L.E.H.S. contributed to the draft and edits of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
L.E.H.S. is supported by NIH R24EY024868, R01EY017017, R01EY01717-13S1, R01EY030904-01, BCH IDDRC (1U54HD090255), Mass Lions Eye Foundation; A.H. is supported by The Swedish Research Council (DNR# #2016-01131), Government grants under the ALF agreement ALFGBG-717971, The Wallenberg Foundations and long-term support by De Blindas Vänner; T.S.K. was supported by NIH grants EY022938 and R24 EY024864, and BX003604 from the Department of Veterans Affairs, T.S.K. is the recipient of a Research Career Scientist award from the Department of Veterans Affairs; Z.F. is supported by the Boston Children’s Hospital OFD/BTREC/CTREC Faculty Career Development Grant, Boston Children’s Hospital Ophthalmology Foundation, Little Giraffe Foundation, Manton Center for Orphan Disease Research; Y.S. is supported by the BrightFocus Foundation, NIH R01EY030140, NIH/R01EY029238, Boston Children’s Hospital Ophthalmology Foundation; B.C. is supported by the Deutsche Forschungsgemeinschaft (CA 1940/1-1); Y.T. is supported by the Manpei Suzuki Diabetes Foundation.
Conflicts of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Oyster, C.W. The Human Eye: Structure and Function; Sinauer Associates: Sunderland, MA, USA, 1999. [Google Scholar]
- Hendrickson, A. A morphological comparison of foveal development in man and monkey. Eye (Lond.) 1992, 6, 136–144. [Google Scholar] [CrossRef] [PubMed]
- Saint-Geniez, M.; D’Amore, P.A. Development and pathology of the hyaloid, choroidal and retinal vasculature. Int. J. Dev. Biol. 2004, 48, 1045–1058. [Google Scholar] [CrossRef] [PubMed]
- Penn, J.S.; Madan, A.; Caldwell, R.B.; Bartoli, M.; Caldwell, R.W.; Hartnett, M.E. Vascular endothelial growth factor in eye disease. Prog. Retin. Eye Res. 2008, 27, 331–371. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Liu, C.H.; Huang, S.; Chen, J. Wnt Signaling in vascular eye diseases. Prog. Retin. Eye Res. 2019, 70, 110–133. [Google Scholar] [CrossRef]
- Ye, X.; Wang, Y.; Cahill, H.; Yu, M.; Badea, T.C.; Smallwood, P.M.; Peachey, N.S.; Nathans, J. Norrin, frizzled-4, and Lrp5 signaling in endothelial cells controls a genetic program for retinal vascularization. Cell 2009, 139, 285–298. [Google Scholar] [CrossRef]
- Wang, Y.; Cho, C.; Williams, J.; Smallwood, P.M.; Zhang, C.; Junge, H.J.; Nathans, J. Interplay of the Norrin and Wnt7a/Wnt7b signaling systems in blood-brain barrier and blood-retina barrier development and maintenance. Proc. Natl. Acad. Sci. USA 2018, 115, E11827–E11836. [Google Scholar] [CrossRef]
- Gerhardt, H.; Golding, M.; Fruttiger, M.; Ruhrberg, C.; Lundkvist, A.; Abramsson, A.; Jeltsch, M.; Mitchell, C.; Alitalo, K.; Shima, D.; et al. VEGF guides angiogenic sprouting utilizing endothelial tip cell filopodia. J. Cell Biol. 2003, 161, 1163–1177. [Google Scholar] [CrossRef]
- Sapieha, P.; Sirinyan, M.; Hamel, D.; Zaniolo, K.; Joyal, J.S.; Cho, J.H.; Honore, J.C.; Kermorvant-Duchemin, E.; Varma, D.R.; Tremblay, S.; et al. The succinate receptor GPR91 in neurons has a major role in retinal angiogenesis. Nat. Med. 2008, 14, 1067–1076. [Google Scholar] [CrossRef]
- Fulton, A.B.; Akula, J.D.; Mocko, J.A.; Hansen, R.M.; Benador, I.Y.; Beck, S.C.; Fahl, E.; Seeliger, M.W.; Moskowitz, A.; Harris, M.E. Retinal degenerative and hypoxic ischemic disease. Doc. Ophthalmol. Adv. Ophthalmol. 2009, 118, 55–61. [Google Scholar] [CrossRef]
- Akula, J.D.; Hansen, R.M.; Martinez-Perez, M.E.; Fulton, A.B. Rod photoreceptor function predicts blood vessel abnormality in retinopathy of prematurity. Investig. Ophthalmol. Vis. Sci. 2007, 48, 4351–4359. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, Z.M. Oxygen-induced retinopathy in mice with retinal photoreceptor cell degeneration. Life Sci. 2014, 102, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Gong, B.; Hatala, D.A.; Kern, T.S. Retinal ischemia and reperfusion causes capillary degeneration: Similarities to diabetes. Investig. Ophthalmol. Vis. Sci. 2007, 48, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Ueda, K.; Nakahara, T.; Hoshino, M.; Mori, A.; Sakamoto, K.; Ishii, K. Retinal blood vessels are damaged in a rat model of NMDA-induced retinal degeneration. Neurosci. Lett. 2010, 485, 55–59. [Google Scholar] [CrossRef] [PubMed]
- Nakahara, T.; Mori, A.; Kurauchi, Y.; Sakamoto, K.; Ishii, K. Neurovascular interactions in the retina: Physiological and pathological roles. J. Pharmacol. Sci. 2013, 123, 79–84. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, C.H.; Wang, Z.; Meng, S.S.; Burnim, S.B.; SanGiovanni, J.P.; Kamenecka, T.M.; Solt, L.A.; Chen, J. RORalpha modulates semaphorin 3E transcription and neurovascular interaction in pathological retinal angiogenesis. FASEB J. 2017, 31, 4492–4502. [Google Scholar] [CrossRef]
- Lange, C.A.; Bainbridge, J.W. Oxygen sensing in retinal health and disease. Ophthalmologica 2012, 227, 115–131. [Google Scholar] [CrossRef]
- Hoang, Q.V.; Linsenmeier, R.A.; Chung, C.K.; Curcio, C.A. Photoreceptor inner segments in monkey and human retina: Mitochondrial density, optics, and regional variation. Vis. Neurosci. 2002, 19, 395–407. [Google Scholar] [CrossRef]
- Yu, D.Y.; Cringle, S.J. Oxygen distribution and consumption within the retina in vascularised and avascular retinas and in animal models of retinal disease. Prog. Retin. Eye Res. 2001, 20, 175–208. [Google Scholar] [CrossRef]
- Calzia, D.; Degan, P.; Caicci, F.; Bruschi, M.; Manni, L.; Ramenghi, L.A.; Candiano, G.; Traverso, C.E.; Panfoli, I. Modulation of the rod outer segment aerobic metabolism diminishes the production of radicals due to light absorption. Free Radic. Biol. Med. 2018, 117, 110–118. [Google Scholar] [CrossRef]
- Panfoli, I.; Calzia, D.; Bruschi, M.; Oneto, M.; Bianchini, P.; Ravera, S.; Petretto, A.; Diaspro, A.; Candiano, G. Functional expression of oxidative phosphorylation proteins in the rod outer segment disc. Cell Biochem. Funct. 2013, 31, 532–538. [Google Scholar] [CrossRef]
- Panfoli, I.; Calzia, D.; Bianchini, P.; Ravera, S.; Diaspro, A.; Candiano, G.; Bachi, A.; Monticone, M.; Aluigi, M.G.; Barabino, S.; et al. Evidence for aerobic metabolism in retinal rod outer segment disks. Int. J. Biochem. Cell Biol. 2009, 41, 2555–2565. [Google Scholar] [CrossRef] [PubMed]
- Hsu, S.C.; Molday, R.S. Glucose metabolism in photoreceptor outer segments. Its role in phototransduction and in NADPH-requiring reactions. J. Biol. Chem. 1994, 269, 17954–17959. [Google Scholar] [PubMed]
- Stefansson, E.; Olafsdottir, O.B.; Einarsdottir, A.B.; Eliasdottir, T.S.; Eysteinsson, T.; Vehmeijer, W.; Vandewalle, E.; Bek, T.; Hardarson, S.H. Retinal Oximetry Discovers Novel Biomarkers in Retinal and Brain Diseases. Investig. Ophthalmol. Vis. Sci. 2017, 58, BIO227–BIO233. [Google Scholar] [CrossRef] [PubMed]
- Semenza, G.L. Vascular responses to hypoxia and ischemia. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 648–652. [Google Scholar] [CrossRef] [PubMed]
- Forsythe, J.A.; Jiang, B.H.; Iyer, N.V.; Agani, F.; Leung, S.W.; Koos, R.D.; Semenza, G.L. Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor 1. Mol. Cell. Biol. 1996, 16, 4604–4613. [Google Scholar] [CrossRef]
- Rosenstein, J.M.; Krum, J.M.; Ruhrberg, C. VEGF in the nervous system. Organogenesis 2010, 6, 107–114. [Google Scholar] [CrossRef]
- Mackenzie, F.; Ruhrberg, C. Diverse roles for VEGF-A in the nervous system. Development 2012, 139, 1371–1380. [Google Scholar] [CrossRef]
- Smith, L.E.; Wesolowski, E.; McLellan, A.; Kostyk, S.K.; D’Amato, R.; Sullivan, R.; D’Amore, P.A. Oxygen-induced retinopathy in the mouse. Investig. Ophthalmol. Vis. Sci. 1994, 35, 101–111. [Google Scholar]
- Pierce, E.A.; Avery, R.L.; Foley, E.D.; Aiello, L.P.; Smith, L.E. Vascular endothelial growth factor/vascular permeability factor expression in a mouse model of retinal neovascularization. Proc. Natl. Acad. Sci. USA 1995, 92, 905–909. [Google Scholar] [CrossRef]
- Akula, J.D.; Hansen, R.M.; Tzekov, R.; Favazza, T.L.; Vyhovsky, T.C.; Benador, I.Y.; Mocko, J.A.; McGee, D.; Kubota, R.; Fulton, A.B. Visual cycle modulation in neurovascular retinopathy. Exp. Eye Res. 2010, 91, 153–161. [Google Scholar] [CrossRef]
- Lee, D.C.; Sohn, H.A.; Park, Z.Y.; Oh, S.; Kang, Y.K.; Lee, K.M.; Kang, M.; Jang, Y.J.; Yang, S.J.; Hong, Y.K.; et al. A lactate-induced response to hypoxia. Cell 2015, 161, 595–609. [Google Scholar] [CrossRef] [PubMed]
- Mergenthaler, P.; Lindauer, U.; Dienel, G.A.; Meisel, A. Sugar for the brain: The role of glucose in physiological and pathological brain function. Trends Neurosci. 2013, 36, 587–597. [Google Scholar] [CrossRef] [PubMed]
- Joyal, J.S.; Sun, Y.; Gantner, M.L.; Shao, Z.; Evans, L.P.; Saba, N.; Fredrick, T.; Burnim, S.; Kim, J.S.; Patel, G.; et al. Retinal lipid and glucose metabolism dictates angiogenesis through the lipid sensor Ffar1. Nat. Med. 2016, 22, 439–445. [Google Scholar] [CrossRef] [PubMed]
- Cohen, L.H.; Noell, W.K. Glucose catabolism of rabbit retina before and after development of visual function. J. Neurochem. 1960, 5, 253–276. [Google Scholar] [CrossRef]
- Dourlen, P.; Sujkowski, A.; Wessells, R.; Mollereau, B. Fatty acid transport proteins in disease: New insights from invertebrate models. Prog. Lipid Res. 2015, 60, 30–40. [Google Scholar] [CrossRef]
- Folz, S.J.; Trobe, J.D. The peroxisome and the eye. Surv. Ophthalmol. 1991, 35, 353–368. [Google Scholar] [CrossRef]
- Yagita, Y.; Shinohara, K.; Abe, Y.; Nakagawa, K.; Al-Owain, M.; Alkuraya, F.S.; Fujiki, Y. Deficiency of a Retinal Dystrophy Protein, Acyl-CoA Binding Domain-containing 5 (ACBD5), Impairs Peroxisomal beta-Oxidation of Very-long-chain Fatty Acids. J. Biol. Chem. 2017, 292, 691–705. [Google Scholar] [CrossRef]
- Ferdinandusse, S.; Falkenberg, K.D.; Koster, J.; Mooyer, P.A.; Jones, R.; van Roermund, C.W.T.; Pizzino, A.; Schrader, M.; Wanders, R.J.A.; Vanderver, A.; et al. ACBD5 deficiency causes a defect in peroxisomal very long-chain fatty acid metabolism. J. Med. Genet. 2017, 54, 330–337. [Google Scholar] [CrossRef]
- Fu, Z.; Lofqvist, C.A.; Liegl, R.; Wang, Z.; Sun, Y.; Gong, Y.; Liu, C.H.; Meng, S.S.; Burnim, S.B.; Arellano, I.; et al. Photoreceptor glucose metabolism determines normal retinal vascular growth. EMBO Mol. Med. 2018, 10, 79–90. [Google Scholar] [CrossRef]
- Puchalska, P.; Crawford, P.A. Multi-dimensional Roles of Ketone Bodies in Fuel Metabolism, Signaling, and Therapeutics. Cell Metab. 2017, 25, 262–284. [Google Scholar] [CrossRef]
- Rahman, M.; Muhammad, S.; Khan, M.A.; Chen, H.; Ridder, D.A.; Muller-Fielitz, H.; Pokorna, B.; Vollbrandt, T.; Stolting, I.; Nadrowitz, R.; et al. The beta-hydroxybutyrate receptor HCA2 activates a neuroprotective subset of macrophages. Nat. Commun. 2014, 5, 3944. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, J.; Ohue-Kitano, R.; Mukouyama, H.; Nishida, A.; Watanabe, K.; Igarashi, M.; Irie, J.; Tsujimoto, G.; Satoh-Asahara, N.; Itoh, H.; et al. Ketone body receptor GPR43 regulates lipid metabolism under ketogenic conditions. Proc. Natl. Acad. Sci. USA 2019, 116, 23813–23821. [Google Scholar] [CrossRef] [PubMed]
- Tieu, K.; Perier, C.; Caspersen, C.; Teismann, P.; Wu, D.C.; Yan, S.D.; Naini, A.; Vila, M.; Jackson-Lewis, V.; Ramasamy, R.; et al. D-beta-hydroxybutyrate rescues mitochondrial respiration and mitigates features of Parkinson disease. J. Clin. Investig. 2003, 112, 892–901. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.X.; Maalouf, M.; Han, P.; Zhao, M.; Gao, M.; Dharshaun, T.; Ryan, C.; Whitelegge, J.; Wu, J.; Eisenberg, D.; et al. Ketones block amyloid entry and improve cognition in an Alzheimer’s model. Neurobiol. Aging 2016, 39, 25–37. [Google Scholar] [CrossRef] [PubMed]
- Izuta, Y.; Imada, T.; Hisamura, R.; Oonishi, E.; Nakamura, S.; Inagaki, E.; Ito, M.; Soga, T.; Tsubota, K. Ketone body 3-hydroxybutyrate mimics calorie restriction via the Nrf2 activator, fumarate, in the retina. Aging Cell 2018, 17, e12699. [Google Scholar] [CrossRef] [PubMed]
- Adijanto, J.; Du, J.; Moffat, C.; Seifert, E.L.; Hurle, J.B.; Philp, N.J. The retinal pigment epithelium utilizes fatty acids for ketogenesis. J. Biol. Chem. 2014, 289, 20570–20582. [Google Scholar] [CrossRef]
- Reyes-Reveles, J.; Dhingra, A.; Alexander, D.; Bragin, A.; Philp, N.J.; Boesze-Battaglia, K. Phagocytosis-dependent ketogenesis in retinal pigment epithelium. J. Biol. Chem. 2017, 292, 8038–8047. [Google Scholar] [CrossRef]
- Avogaro, A.; Crepaldi, C.; Miola, M.; Maran, A.; Pengo, V.; Tiengo, A.; Del Prato, S. High blood ketone body concentration in type 2 non-insulin dependent diabetic patients. J. Endocrinol. Investig. 1996, 19, 99–105. [Google Scholar] [CrossRef]
- Mahendran, Y.; Vangipurapu, J.; Cederberg, H.; Stancakova, A.; Pihlajamaki, J.; Soininen, P.; Kangas, A.J.; Paananen, J.; Civelek, M.; Saleem, N.K.; et al. Association of ketone body levels with hyperglycemia and type 2 diabetes in 9398 Finnish men. Diabetes 2013, 62, 3618–3626. [Google Scholar] [CrossRef]
- Harano, Y.; Kosugi, K.; Hyosu, T.; Suzuki, M.; Hidaka, H.; Kashiwagi, A.; Uno, S.; Shigeta, Y. Ketone bodies as markers for type 1 (insulin-dependent) diabetes and their value in the monitoring of diabetic control. Diabetologia 1984, 26, 343–348. [Google Scholar] [CrossRef]
- Fromal, O.; Lamoke, F.; Kaufman, M.; Bartoli, M.; Martin, P.M. Blockade of NLRP3 inflammasome activation in diabetic retina by the ketone metabolite beta-hydroxybutyrate is mediated by GPR109A. In ARVO Annual Meeting; ARVO: Seattle, WA, USA, 2016; Volume 57, p. 5451. [Google Scholar]
- Nishimura, Y.; Hara, H.; Kondo, M.; Hong, S.; Matsugi, T. Oxidative Stress in Retinal Diseases. Oxid. Med. Cell. Longev. 2017, 2017, 4076518. [Google Scholar] [CrossRef] [PubMed]
- Jarrett, S.G.; Lin, H.; Godley, B.F.; Boulton, M.E. Mitochondrial DNA damage and its potential role in retinal degeneration. Prog. Retin. Eye Res. 2008, 27, 596–607. [Google Scholar] [CrossRef] [PubMed]
- Roehlecke, C.; Schumann, U.; Ader, M.; Brunssen, C.; Bramke, S.; Morawietz, H.; Funk, R.H. Stress reaction in outer segments of photoreceptors after blue light irradiation. PLoS ONE 2013, 8, e71570. [Google Scholar] [CrossRef] [PubMed]
- Xiong, W.; MacColl Garfinkel, A.E.; Li, Y.; Benowitz, L.I.; Cepko, C.L. NRF2 promotes neuronal survival in neurodegeneration and acute nerve damage. J. Clin. Investig. 2015, 125, 1433–1445. [Google Scholar] [CrossRef] [PubMed]
- Fukui, M.; Zhu, B.T. Mitochondrial superoxide dismutase SOD2, but not cytosolic SOD1, plays a critical role in protection against glutamate-induced oxidative stress and cell death in HT22 neuronal cells. Free Radic. Biol. Med. 2010, 48, 821–830. [Google Scholar] [CrossRef]
- Wanders, R.J.; Waterham, H.R. Biochemistry of mammalian peroxisomes revisited. Annu. Rev. Biochem. 2006, 75, 295–332. [Google Scholar] [CrossRef]
- Datta, S.; Cano, M.; Ebrahimi, K.; Wang, L.; Handa, J.T. The impact of oxidative stress and inflammation on RPE degeneration in non-neovascular AMD. Prog. Retin. Eye Res. 2017, 60, 201–218. [Google Scholar] [CrossRef]
- Calderon, G.D.; Juarez, O.H.; Hernandez, G.E.; Punzo, S.M.; De la Cruz, Z.D. Oxidative stress and diabetic retinopathy: Development and treatment. Eye (Lond.) 2017, 31, 1122–1130. [Google Scholar] [CrossRef]
- Li, S.Y.; Fu, Z.J.; Ma, H.; Jang, W.C.; So, K.F.; Wong, D.; Lo, A.C. Effect of lutein on retinal neurons and oxidative stress in a model of acute retinal ischemia/reperfusion. Investig. Ophthalmol. Vis. Sci. 2009, 50, 836–843. [Google Scholar] [CrossRef]
- Drobek-Slowik, M.; Karczewicz, D.; Safranow, K. The potential role of oxidative stress in the pathogenesis of the age-related macular degeneration (AMD). Postepy Hig. Med. Dosw. 2007, 61, 28–37. [Google Scholar]
- Ohashi, K.; Kageyama, M.; Shinomiya, K.; Fujita-Koyama, Y.; Hirai, S.I.; Katsuta, O.; Nakamura, M. Spermidine Oxidation-Mediated Degeneration of Retinal Pigment Epithelium in Rats. Oxid. Med. Cell. Longev. 2017, 2017, 4128061. [Google Scholar] [CrossRef] [PubMed]
- Cecilia, O.M.; Jose Alberto, C.G.; Jose, N.P.; Ernesto German, C.M.; Ana Karen, L.C.; Luis Miguel, R.P.; Ricardo Raul, R.R.; Adolfo Daniel, R.C. Oxidative Stress as the Main Target in Diabetic Retinopathy Pathophysiology. J. Diabetes Res. 2019, 2019, 8562408. [Google Scholar] [CrossRef] [PubMed]
- Kowluru, R.A.; Mishra, M. Oxidative stress, mitochondrial damage and diabetic retinopathy. Biochim. Biophys. Acta 2015, 1852, 2474–2483. [Google Scholar] [CrossRef] [PubMed]
- Krishnamoorthy, V.; Cherukuri, P.; Poria, D.; Goel, M.; Dagar, S.; Dhingra, N.K. Retinal Remodeling: Concerns, Emerging Remedies and Future Prospects. Front. Cell Neurosci. 2016, 10, 38. [Google Scholar] [CrossRef] [PubMed]
- Jones, B.W.; Pfeiffer, R.L.; Ferrell, W.D.; Watt, C.B.; Marmor, M.; Marc, R.E. Retinal remodeling in human retinitis pigmentosa. Exp. Eye Res. 2016, 150, 149–165. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Kawasaki, R.; Dobson, L.P.; Ruddle, J.B.; Kearns, L.S.; Wong, T.Y.; Mackey, D.A. Quantitative analysis of retinal vessel attenuation in eyes with retinitis pigmentosa. Investig. Ophthalmol. Vis. Sci. 2012, 53, 4306–4314. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, S.; Oishi, A.; Ogino, K.; Makiyama, Y.; Kurimoto, M.; Yoshimura, N. Association of retinal vessel attenuation with visual function in eyes with retinitis pigmentosa. Clin. Ophthalmol. 2014, 8, 1487–1493. [Google Scholar]
- Hanna, J.; Yucel, Y.H.; Zhou, X.; Mathieu, E.; Paczka-Giorgi, L.A.; Gupta, N. Progressive loss of retinal blood vessels in a live model of retinitis pigmentosa. Can. J. Ophthalmol. 2018, 53, 391–401. [Google Scholar] [CrossRef]
- Fernandez-Sanchez, L.; Esquiva, G.; Pinilla, I.; Lax, P.; Cuenca, N. Retinal Vascular Degeneration in the Transgenic P23H Rat Model of Retinitis Pigmentosa. Front. Neuroanat. 2018, 12, 55. [Google Scholar] [CrossRef]
- Dorrell, M.I.; Aguilar, E.; Jacobson, R.; Trauger, S.A.; Friedlander, J.; Siuzdak, G.; Friedlander, M. Maintaining retinal astrocytes normalizes revascularization and prevents vascular pathology associated with oxygen-induced retinopathy. Glia 2010, 58, 43–54. [Google Scholar] [CrossRef]
- Bucher, F.; Stahl, A.; Agostini, H.T.; Martin, G. Hyperoxia causes reduced density of retinal astrocytes in the central avascular zone in the mouse model of oxygen-induced retinopathy. Mol. Cell Neurosci. 2013, 56, 225–233. [Google Scholar] [CrossRef]
- O’Sullivan, M.L.; Punal, V.M.; Kerstein, P.C.; Brzezinski, J.A.T.; Glaser, T.; Wright, K.M.; Kay, J.N. Astrocytes follow ganglion cell axons to establish an angiogenic template during retinal development. Glia 2017, 65, 1697–1716. [Google Scholar] [CrossRef]
- Fu, Z.; Nian, S.; Li, S.Y.; Wong, D.; Chung, S.K.; Lo, A.C. Deficiency of aldose reductase attenuates inner retinal neuronal changes in a mouse model of retinopathy of prematurity. Graefe’s Arch. Clin. Exp. Ophthalmol. 2015, 253, 1503–1513. [Google Scholar] [CrossRef]
- Rubsam, A.; Parikh, S.; Fort, P.E. Role of Inflammation in Diabetic Retinopathy. Int. J. Mol. Sci. 2018, 19, 942. [Google Scholar] [CrossRef]
- Rathi, S.; Jalali, S.; Patnaik, S.; Shahulhameed, S.; Musada, G.R.; Balakrishnan, D.; Rani, P.K.; Kekunnaya, R.; Chhablani, P.P.; Swain, S.; et al. Abnormal Complement Activation and Inflammation in the Pathogenesis of Retinopathy of Prematurity. Front. Immunol. 2017, 8, 1868. [Google Scholar] [CrossRef]
- Rivera, J.C.; Holm, M.; Austeng, D.; Morken, T.S.; Zhou, T.E.; Beaudry-Richard, A.; Sierra, E.M.; Dammann, O.; Chemtob, S. Retinopathy of prematurity: Inflammation, choroidal degeneration, and novel promising therapeutic strategies. J. Neuroinflam. 2017, 14, 165. [Google Scholar] [CrossRef]
- Kauppinen, A.; Paterno, J.J.; Blasiak, J.; Salminen, A.; Kaarniranta, K. Inflammation and its role in age-related macular degeneration. Cell Mol. Life Sci. 2016, 73, 1765–1786. [Google Scholar] [CrossRef]
- Mansoor, N.; Wahid, F.; Azam, M.; Shah, K.; den Hollander, A.I.; Qamar, R.; Ayub, H. Molecular Mechanisms of Complement System Proteins and Matrix Metalloproteinases in the Pathogenesis of Age-Related Macular Degeneration. Curr. Mol. Med. 2019, 19, 705–718. [Google Scholar] [CrossRef]
- Chen, M.; Citil, A.; McCabe, F.; Leicht, K.M.; Fiascone, J.; Dammann, C.E.; Dammann, O. Infection, oxygen, and immaturity: Interacting risk factors for retinopathy of prematurity. Neonatology 2011, 99, 125–132. [Google Scholar] [CrossRef]
- Stahl, A.; Joyal, J.S.; Chen, J.; Sapieha, P.; Juan, A.M.; Hatton, C.J.; Pei, D.T.; Hurst, C.G.; Seaward, M.R.; Krah, N.M.; et al. SOCS3 is an endogenous inhibitor of pathologic angiogenesis. Blood 2012, 120, 2925–2929. [Google Scholar] [CrossRef]
- Sun, Y.; Ju, M.; Lin, Z.; Fredrick, T.W.; Evans, L.P.; Tian, K.T.; Saba, N.J.; Morss, P.C.; Pu, W.T.; Chen, J.; et al. SOCS3 in retinal neurons and glial cells suppresses VEGF signaling to prevent pathological neovascular growth. Sci. Signal. 2015, 8, ra94. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Liu, C.H.; SanGiovanni, J.P.; Evans, L.P.; Tian, K.T.; Zhang, B.; Stahl, A.; Pu, W.T.; Kamenecka, T.M.; Solt, L.A.; et al. Nuclear receptor RORalpha regulates pathologic retinal angiogenesis by modulating SOCS3-dependent inflammation. Proc. Natl. Acad. Sci. USA 2015, 112, 10401–10406. [Google Scholar] [CrossRef]
- Yoshimura, A.; Naka, T.; Kubo, M. SOCS proteins, cytokine signalling and immune regulation. Nat. Rev. Immunol. 2007, 7, 454–465. [Google Scholar] [CrossRef]
- Imai, Y.; Kuba, K.; Neely, G.G.; Yaghubian-Malhami, R.; Perkmann, T.; van Loo, G.; Ermolaeva, M.; Veldhuizen, R.; Leung, Y.H.; Wang, H.; et al. Identification of oxidative stress and Toll-like receptor 4 signaling as a key pathway of acute lung injury. Cell 2008, 133, 235–249. [Google Scholar] [CrossRef]
- de Oliveira-Marques, V.; Cyrne, L.; Marinho, H.S.; Antunes, F. A quantitative study of NF-kappaB activation by H2O2: Relevance in inflammation and synergy with TNF-alpha. J. Immunol. 2007, 178, 3893–3902. [Google Scholar] [CrossRef]
- Volpe, J.J. Postnatal sepsis, necrotizing entercolitis, and the critical role of systemic inflammation in white matter injury in premature infants. J. Pediatr. 2008, 153, 160–163. [Google Scholar] [CrossRef]
- Silveira, R.C.; Fortes Filho, J.B.; Procianoy, R.S. Assessment of the contribution of cytokine plasma levels to detect retinopathy of prematurity in very low birth weight infants. Investig. Ophthalmol. Vis. Sci. 2011, 52, 1297–1301. [Google Scholar] [CrossRef]
- Lavoie, P.M.; Lavoie, J.C.; Watson, C.; Rouleau, T.; Chang, B.A.; Chessex, P. Inflammatory response in preterm infants is induced early in life by oxygen and modulated by total parenteral nutrition. Pediatr. Res. 2010, 68, 248–251. [Google Scholar] [CrossRef]
- Dammann, O. Inflammation and retinopathy of prematurity. Acta Paediatr. 2010, 99, 975–977. [Google Scholar] [CrossRef]
- Gardiner, T.A.; Gibson, D.S.; de Gooyer, T.E.; de la Cruz, V.F.; McDonald, D.M.; Stitt, A.W. Inhibition of tumor necrosis factor-alpha improves physiological angiogenesis and reduces pathological neovascularization in ischemic retinopathy. Am. J. Pathol. 2005, 166, 637–644. [Google Scholar] [CrossRef]
- Connor, K.M.; SanGiovanni, J.P.; Lofqvist, C.; Aderman, C.M.; Chen, J.; Higuchi, A.; Hong, S.; Pravda, E.A.; Majchrzak, S.; Carper, D.; et al. Increased dietary intake of omega-3-polyunsaturated fatty acids reduces pathological retinal angiogenesis. Nat. Med. 2007, 13, 868–873. [Google Scholar] [CrossRef]
- Sun, Y.; Lin, Z.; Liu, C.H.; Gong, Y.; Liegl, R.; Fredrick, T.W.; Meng, S.S.; Burnim, S.B.; Wang, Z.; Akula, J.D.; et al. Inflammatory signals from photoreceptor modulate pathological retinal angiogenesis via c-Fos. J. Exp. Med. 2017, 214, 1753–1767. [Google Scholar] [CrossRef]
- Ambati, J.; Atkinson, J.P.; Gelfand, B.D. Immunology of age-related macular degeneration. Nat. Rev. Immunol. 2013, 13, 438–451. [Google Scholar] [CrossRef]
- Raychaudhuri, S.; Iartchouk, O.; Chin, K.; Tan, P.L.; Tai, A.K.; Ripke, S.; Gowrisankar, S.; Vemuri, S.; Montgomery, K.; Yu, Y.; et al. A rare penetrant mutation in CFH confers high risk of age-related macular degeneration. Nat. Genet. 2011, 43, 1232–1236. [Google Scholar] [CrossRef]
- Doyle, S.L.; Campbell, M.; Ozaki, E.; Salomon, R.G.; Mori, A.; Kenna, P.F.; Farrar, G.J.; Kiang, A.S.; Humphries, M.M.; Lavelle, E.C.; et al. NLRP3 has a protective role in age-related macular degeneration through the induction of IL-18 by drusen components. Nat. Med. 2012, 18, 791–798. [Google Scholar] [CrossRef]
- Miller, J.W. Age-related macular degeneration revisited--piecing the puzzle: The LXIX Edward Jackson memorial lecture. Am. J. Ophthalmol. 2013, 155, 1–35 e13. [Google Scholar] [CrossRef]
- Kumar, A.; Zhao, L.; Fariss, R.N.; McMenamin, P.G.; Wong, W.T. Vascular associations and dynamic process motility in perivascular myeloid cells of the mouse choroid: Implications for function and senescent change. Investig. Ophthalmol. Vis. Sci. 2014, 55, 1787–1796. [Google Scholar] [CrossRef]
- Skeie, J.M.; Mullins, R.F. Macrophages in neovascular age-related macular degeneration: Friends or foes? Eye 2009, 23, 747–755. [Google Scholar] [CrossRef]
- Cherepanoff, S.; McMenamin, P.; Gillies, M.C.; Kettle, E.; Sarks, S.H. Bruch’s membrane and choroidal macrophages in early and advanced age-related macular degeneration. Br. J. Ophthalmol. 2010, 94, 918–925. [Google Scholar] [CrossRef]
- Chen, M.; Lechner, J.; Zhao, J.; Toth, L.; Hogg, R.; Silvestri, G.; Kissenpfennig, A.; Chakravarthy, U.; Xu, H. STAT3 Activation in Circulating Monocytes Contributes to Neovascular Age-Related Macular Degeneration. Curr. Mol. Med. 2016, 16, 412–423. [Google Scholar] [CrossRef]
- Apte, R.S.; Richter, J.; Herndon, J.; Ferguson, T.A. Macrophages inhibit neovascularization in a murine model of age-related macular degeneration. PLoS Med. 2006, 3, e310. [Google Scholar] [CrossRef]
- Yang, P.; de Vos, A.F.; Kijlstra, A. Macrophages and MHC class II positive cells in the choroid during endotoxin induced uveitis. Br. J. Ophthalmol. 1997, 81, 396–401. [Google Scholar] [CrossRef][Green Version]
- McMenamin, P.G. Dendritic cells and macrophages in the uveal tract of the normal mouse eye. Br. J. Ophthalmol. 1999, 83, 598–604. [Google Scholar] [CrossRef]
- Forrester, J.V.; Xu, H.; Kuffova, L.; Dick, A.D.; McMenamin, P.G. Dendritic cell physiology and function in the eye. Immunol. Rev. 2010, 234, 282–304. [Google Scholar] [CrossRef]
- Karlstetter, M.; Scholz, R.; Rutar, M.; Wong, W.T.; Provis, J.M.; Langmann, T. Retinal microglia: Just bystander or target for therapy? Prog. Retin. Eye Res. 2015, 45, 30–57. [Google Scholar] [CrossRef]
- Altmann, C.; Schmidt, M.H.H. The Role of Microglia in Diabetic Retinopathy: Inflammation, Microvasculature Defects and Neurodegeneration. Int. J. Mol. Sci. 2018, 19, 110. [Google Scholar] [CrossRef]
- Grigsby, J.G.; Cardona, S.M.; Pouw, C.E.; Muniz, A.; Mendiola, A.S.; Tsin, A.T.; Allen, D.M.; Cardona, A.E. The role of microglia in diabetic retinopathy. J. Ophthalmol. 2014, 2014, 705783. [Google Scholar] [CrossRef]
- Crespo-Garcia, S.; Reichhart, N.; Hernandez-Matas, C.; Zabulis, X.; Kociok, N.; Brockmann, C.; Joussen, A.M.; Strauss, O. In vivo analysis of the time and spatial activation pattern of microglia in the retina following laser-induced choroidal neovascularization. Exp. Eye Res. 2015, 139, 13–21. [Google Scholar] [CrossRef]
- Talia, D.M.; Deliyanti, D.; Agrotis, A.; Wilkinson-Berka, J.L. Inhibition of the Nuclear Receptor RORgamma and Interleukin-17A Suppresses Neovascular Retinopathy: Involvement of Immunocompetent Microglia. Arterioscler. Thromb. Vasc. Biol. 2016, 36, 1186–1196. [Google Scholar] [CrossRef]
- Vecino, E.; Rodriguez, F.D.; Ruzafa, N.; Pereiro, X.; Sharma, S.C. Glia-neuron interactions in the mammalian retina. Prog. Retin. Eye Res. 2016, 51, 1–40. [Google Scholar] [CrossRef]
- Schmid, M.C.; Varner, J.A. Myeloid cell trafficking and tumor angiogenesis. Cancer Lett. 2007, 250, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, T.A.; Apte, R.S. Angiogenesis in eye disease: Immunity gained or immunity lost? Semin. Immunopathol. 2008, 30, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Combadiere, C.; Feumi, C.; Raoul, W.; Keller, N.; Rodero, M.; Pezard, A.; Lavalette, S.; Houssier, M.; Jonet, L.; Picard, E.; et al. CX3CR1-dependent subretinal microglia cell accumulation is associated with cardinal features of age-related macular degeneration. J. Clin. Investig. 2007, 117, 2920–2928. [Google Scholar] [CrossRef] [PubMed]
- Solt, L.A.; Burris, T.P. Action of RORs and their ligands in (patho)physiology. Trends Endocrinol. Metab. 2012, 23, 619–627. [Google Scholar] [CrossRef] [PubMed]
- Jetten, A.M. Retinoid-related orphan receptors (RORs): Critical roles in development, immunity, circadian rhythm, and cellular metabolism. Nucl. Recept. Signal. 2009, 7, e003. [Google Scholar] [CrossRef]
- Houston, S.K.; Bourne, T.D.; Lopes, M.B.; Ghazi, N.G. Bilateral massive retinal gliosis associated with retinopathy of prematurity. Arch. Pathol. Lab. Med. 2009, 133, 1242–1245. [Google Scholar]
- Gu, L.; Xu, H.; Zhang, C.; Yang, Q.; Zhang, L.; Zhang, J. Time-dependent changes in hypoxia- and gliosis-related factors in experimental diabetic retinopathy. Eye 2019, 33, 600–609. [Google Scholar] [CrossRef]
- Kern, T.S. Interrelationships between the Retinal Neuroglia and Vasculature in Diabetes. Diabetes Metab. J. 2014, 38, 163–170. [Google Scholar] [CrossRef]
- Qian, H.; Ripps, H. Neurovascular interaction and the pathophysiology of diabetic retinopathy. Exp. Diabetes Res. 2011, 2011, 693426. [Google Scholar] [CrossRef]
- Guo, S.; Kim, W.J.; Lok, J.; Lee, S.R.; Besancon, E.; Luo, B.H.; Stins, M.F.; Wang, X.; Dedhar, S.; Lo, E.H. Neuroprotection via matrix-trophic coupling between cerebral endothelial cells and neurons. Proc. Natl. Acad. Sci. USA 2008, 105, 7582–7587. [Google Scholar] [CrossRef]
- Iadecola, C. Neurovascular regulation in the normal brain and in Alzheimer’s disease. Nat. Rev. Neurosci. 2004, 5, 347–360. [Google Scholar] [CrossRef]
- Nishijima, T.; Piriz, J.; Duflot, S.; Fernandez, A.M.; Gaitan, G.; Gomez-Pinedo, U.; Verdugo, J.M.; Leroy, F.; Soya, H.; Nunez, A.; et al. Neuronal activity drives localized blood-brain-barrier transport of serum insulin-like growth factor-I into the CNS. Neuron 2010, 67, 834–846. [Google Scholar] [CrossRef]
- Luo, Y.; Raible, D.; Raper, J.A. Collapsin: A protein in brain that induces the collapse and paralysis of neuronal growth cones. Cell 1993, 75, 217–227. [Google Scholar] [CrossRef]
- Gaur, P.; Bielenberg, D.R.; Samuel, S.; Bose, D.; Zhou, Y.; Gray, M.J.; Dallas, N.A.; Fan, F.; Xia, L.; Lu, J.; et al. Role of class 3 semaphorins and their receptors in tumor growth and angiogenesis. Clin. Cancer Res. 2009, 15, 6763–6770. [Google Scholar] [CrossRef]
- Alto, L.T.; Terman, J.R. Semaphorins and their Signaling Mechanisms. Methods Mol. Biol. 2017, 1493, 1–25. [Google Scholar]
- Toledano, S.; Nir-Zvi, I.; Engelman, R.; Kessler, O.; Neufeld, G. Class-3 Semaphorins and Their Receptors: Potent Multifunctional Modulators of Tumor Progression. Int. J. Mol. Sci. 2019, 20, 556. [Google Scholar] [CrossRef]
- Franzolin, G.; Tamagnone, L. Semaphorin Signaling in Cancer-Associated Inflammation. Int. J. Mol. Sci. 2019, 20, 377. [Google Scholar] [CrossRef]
- Cerani, A.; Tetreault, N.; Menard, C.; Lapalme, E.; Patel, C.; Sitaras, N.; Beaudoin, F.; Leboeuf, D.; De Guire, V.; Binet, F.; et al. Neuron-derived semaphorin 3A is an early inducer of vascular permeability in diabetic retinopathy via neuropilin-1. Cell Metab. 2013, 18, 505–518. [Google Scholar] [CrossRef]
- Yang, W.J.; Hu, J.; Uemura, A.; Tetzlaff, F.; Augustin, H.G.; Fischer, A. Semaphorin-3C signals through Neuropilin-1 and PlexinD1 receptors to inhibit pathological angiogenesis. EMBO Mol. Med. 2015, 7, 1267–1284. [Google Scholar] [CrossRef]
- Wu, J.H.; Li, Y.N.; Chen, A.Q.; Hong, C.D.; Zhang, C.L.; Wang, H.L.; Zhou, Y.F.; Li, P.C.; Wang, Y.; Mao, L.; et al. Inhibition of Sema4D/PlexinB1 signaling alleviates vascular dysfunction in diabetic retinopathy. EMBO Mol. Med. 2020, 12, e10154. [Google Scholar] [CrossRef]
- Fukushima, Y.; Okada, M.; Kataoka, H.; Hirashima, M.; Yoshida, Y.; Mann, F.; Gomi, F.; Nishida, K.; Nishikawa, S.; Uemura, A. Sema3E-PlexinD1 signaling selectively suppresses disoriented angiogenesis in ischemic retinopathy in mice. J. Clin. Investig. 2011, 121, 1974–1985. [Google Scholar] [CrossRef] [PubMed]
- Klufas, M.A.; Chan, R.V. Intravitreal anti-VEGF therapy as a treatment for retinopathy of prematurity: What we know after 7 years. J. Pediatr. Ophthalmol. Strabismus 2015, 52, 77–84. [Google Scholar] [CrossRef]
- Bakri, S.J.; Thorne, J.E.; Ho, A.C.; Ehlers, J.P.; Schoenberger, S.D.; Yeh, S.; Kim, S.J. Safety and Efficacy of Anti-Vascular Endothelial Growth Factor Therapies for Neovascular Age-Related Macular Degeneration: A Report by the American Academy of Ophthalmology. Ophthalmology 2019, 126, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Nigam, N.; Hedaya, J.; Freeman, W.R. Non-responders to bevacizumab (Avastin) therapy of choroidal neovascular lesions. Br. J. Ophthalmol. 2008, 92, 865–866. [Google Scholar]
- Lux, A.; Llacer, H.; Heussen, F.M.; Joussen, A.M. Non-responders to bevacizumab (Avastin) therapy of choroidal neovascular lesions. Br. J. Ophthalmol. 2007, 91, 1318–1322. [Google Scholar] [CrossRef]
- Ventrice, P.; Leporini, C.; Aloe, J.F.; Greco, E.; Leuzzi, G.; Marrazzo, G.; Scorcia, G.B.; Bruzzichesi, D.; Nicola, V.; Scorcia, V. Anti-vascular endothelial growth factor drugs safety and efficacy in ophthalmic diseases. J. Pharm. Pharm. 2013, 4, S38–S42. [Google Scholar]
- Avery, R.L.; Castellarin, A.A.; Steinle, N.C.; Dhoot, D.S.; Pieramici, D.J.; See, R.; Couvillion, S.; Nasir, M.A.; Rabena, M.D.; Le, K.; et al. Systemic pharmacokinetics following intravitreal injections of ranibizumab, bevacizumab or aflibercept in patients with neovascular AMD. Br. J. Ophthalmol. 2014, 98, 1636–1641. [Google Scholar] [CrossRef]
- Bressler, N.M.; Boyer, D.S.; Williams, D.F.; Butler, S.; Francom, S.F.; Brown, B.; Di Nucci, F.; Cramm, T.; Tuomi, L.L.; Ianchulev, T.; et al. Cerebrovascular accidents in patients treated for choroidal neovascularization with ranibizumab in randomized controlled trials. Retina 2012, 32, 1821–1828. [Google Scholar] [CrossRef]
- Moorthy, S.; Cheung, N. Cerebrovascular accidents and ranibizumab. Ophthalmology 2009, 116, 1835. [Google Scholar] [CrossRef]
- Ueta, T.; Yanagi, Y.; Tamaki, Y.; Yamaguchi, T. Cerebrovascular accidents in ranibizumab. Ophthalmology 2009, 116, 362. [Google Scholar] [CrossRef]
- Hapani, S.; Sher, A.; Chu, D.; Wu, S. Increased risk of serious hemorrhage with bevacizumab in cancer patients: A meta-analysis. Oncology 2010, 79, 27–38. [Google Scholar] [CrossRef] [PubMed]
- Jalali, S.; Balakrishnan, D.; Zeynalova, Z.; Padhi, T.R.; Rani, P.K. Serious adverse events and visual outcomes of rescue therapy using adjunct bevacizumab to laser and surgery for retinopathy of prematurity. The Indian Twin Cities Retinopathy of Prematurity Screening database Report number 5. Arch. Dis. Child. Fetal Neonatal Ed. 2013, 98, F327–F333. [Google Scholar] [CrossRef] [PubMed]
- Andreeva, K.; Cooper, N.G. MicroRNAs in the Neural Retina. Int. J. Genom. 2014, 2014, 165897. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, S.; Chaqour, B. MicroRNA signature and function in retinal neovascularization. World J. Biol. Chem. 2014, 5, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.H.; Wang, Z.; Huang, S.; Sun, Y.; Chen, J. MicroRNA-145 Regulates Pathological Retinal Angiogenesis by Suppression of TMOD3. Mol. Nucleic Acids 2019, 16, 335–347. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.H.; Wang, Z.; Sun, Y.; SanGiovanni, J.P.; Chen, J. Retinal expression of small non-coding RNAs in a murine model of proliferative retinopathy. Sci. Rep. 2016, 6, 33947. [Google Scholar] [CrossRef]
- Liu, C.H.; Sun, Y.; Li, J.; Gong, Y.; Tian, K.T.; Evans, L.P.; Morss, P.C.; Fredrick, T.W.; Saba, N.J.; Chen, J. Endothelial microRNA-150 is an intrinsic suppressor of pathologic ocular neovascularization. Proc. Natl. Acad. Sci. USA 2015, 112, 12163–12168. [Google Scholar] [CrossRef]
- Walsky, R.L.; Gaman, E.A.; Obach, R.S. Examination of 209 drugs for inhibition of cytochrome P450 2C8. J. Clin. Pharm. 2005, 45, 68–78. [Google Scholar] [CrossRef]
- Keech, A.C.; Mitchell, P.; Summanen, P.A.; O’Day, J.; Davis, T.M.; Moffitt, M.S.; Taskinen, M.R.; Simes, R.J.; Tse, D.; Williamson, E.; et al. Effect of fenofibrate on the need for laser treatment for diabetic retinopathy (FIELD study): A randomised controlled trial. Lancet 2007, 370, 1687–1697. [Google Scholar] [CrossRef]
- Group, A.S.; Group, A.E.S.; Chew, E.Y.; Ambrosius, W.T.; Davis, M.D.; Danis, R.P.; Gangaputra, S.; Greven, C.M.; Hubbard, L.; Esser, B.A.; et al. Effects of medical therapies on retinopathy progression in type 2 diabetes. N. Engl. J. Med. 2010, 363, 233–244. [Google Scholar]
- Chen, Y.; Hu, Y.; Lin, M.; Jenkins, A.J.; Keech, A.C.; Mott, R.; Lyons, T.J.; Ma, J.X. Therapeutic effects of PPARalpha agonists on diabetic retinopathy in type 1 diabetes models. Diabetes 2013, 62, 261–272. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Shao, Z.; Fu, Z.; Edin, M.L.; Sun, Y.; Liegl, R.G.; Wang, Z.; Liu, C.H.; Burnim, S.B.; Meng, S.S.; et al. Fenofibrate Inhibits Cytochrome P450 Epoxygenase 2C Activity to Suppress Pathological Ocular Angiogenesis. EBioMedicine 2016, 13, 201–211. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Fu, Z.; Edin, M.L.; Liu, C.H.; Wang, Z.; Shao, Z.; Fredrick, T.W.; Saba, N.J.; Morss, P.C.; Burnim, S.B.; et al. Cytochrome P450 Oxidase 2C Inhibition Adds to omega-3 Long-Chain Polyunsaturated Fatty Acids Protection Against Retinal and Choroidal Neovascularization. Arterioscler. Thromb. Vasc. Biol. 2016, 36, 1919–1927. [Google Scholar] [CrossRef] [PubMed]
- Cheng, R.; Ding, L.; He, X.; Takahashi, Y.; Ma, J.X. Interaction of PPARalpha With the Canonic Wnt Pathway in the Regulation of Renal Fibrosis. Diabetes 2016, 65, 3730–3743. [Google Scholar] [CrossRef]
- Nusse, R.; Clevers, H. Wnt/beta-Catenin Signaling, Disease, and Emerging Therapeutic Modalities. Cell 2017, 169, 985–999. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Wang, Y.; Dabdoub, A.; Smallwood, P.M.; Williams, J.; Woods, C.; Kelley, M.W.; Jiang, L.; Tasman, W.; Zhang, K.; et al. Vascular development in the retina and inner ear: Control by Norrin and Frizzled-4, a high-affinity ligand-receptor pair. Cell 2004, 116, 883–895. [Google Scholar] [CrossRef]
- Moran, E.P.; Wang, Z.; Chen, J.; Sapieha, P.; Smith, L.E.; Ma, J.X. Neurovascular cross talk in diabetic retinopathy: Pathophysiological roles and therapeutic implications. Am. J. Physiol. 2016, 311, H738–H749. [Google Scholar] [CrossRef]
- Chen, J.; Stahl, A.; Krah, N.M.; Seaward, M.R.; Dennison, R.J.; Sapieha, P.; Hua, J.; Hatton, C.J.; Juan, A.M.; Aderman, C.M.; et al. Wnt signaling mediates pathological vascular growth in proliferative retinopathy. Circulation 2011, 124, 1871–1881. [Google Scholar] [CrossRef]
- Lundasen, T.; Hunt, M.C.; Nilsson, L.M.; Sanyal, S.; Angelin, B.; Alexson, S.E.; Rudling, M. PPARalpha is a key regulator of hepatic FGF21. Biochem. Biophys. Res. Commun. 2007, 360, 437–440. [Google Scholar] [CrossRef]
- Vernia, S.; Cavanagh-Kyros, J.; Garcia-Haro, L.; Sabio, G.; Barrett, T.; Jung, D.Y.; Kim, J.K.; Xu, J.; Shulha, H.P.; Garber, M.; et al. The PPARalpha-FGF21 hormone axis contributes to metabolic regulation by the hepatic JNK signaling pathway. Cell Metab. 2014, 20, 512–525. [Google Scholar] [CrossRef]
- Zhang, Y.; Pan, Y.; Xiong, R.; Zheng, J.; Li, Q.; Zhang, S.; Li, X.; Pan, X.; Yang, S. FGF21 mediates the protective effect of fenofibrate against acetaminophen -induced hepatotoxicity via activating autophagy in mice. Biochem. Biophys. Res. Commun. 2018, 503, 474–481. [Google Scholar] [CrossRef] [PubMed]
- Inagaki, T.; Lin, V.Y.; Goetz, R.; Mohammadi, M.; Mangelsdorf, D.J.; Kliewer, S.A. Inhibition of growth hormone signaling by the fasting-induced hormone FGF21. Cell Metab. 2008, 8, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, T.; Nakatake, Y.; Konishi, M.; Itoh, N. Identification of a novel FGF, FGF-21, preferentially expressed in the liver. Biochim. Biophys. Acta 2000, 1492, 203–206. [Google Scholar] [CrossRef]
- Dutchak, P.A.; Katafuchi, T.; Bookout, A.L.; Choi, J.H.; Yu, R.T.; Mangelsdorf, D.J.; Kliewer, S.A. Fibroblast growth factor-21 regulates PPARgamma activity and the antidiabetic actions of thiazolidinediones. Cell 2012, 148, 556–567. [Google Scholar] [CrossRef]
- Kim, S.H.; Kim, K.H.; Kim, H.K.; Kim, M.J.; Back, S.H.; Konishi, M.; Itoh, N.; Lee, M.S. Fibroblast growth factor 21 participates in adaptation to endoplasmic reticulum stress and attenuates obesity-induced hepatic metabolic stress. Diabetologia 2015, 58, 809–818. [Google Scholar] [CrossRef]
- Berglund, E.D.; Li, C.Y.; Bina, H.A.; Lynes, S.E.; Michael, M.D.; Shanafelt, A.B.; Kharitonenkov, A.; Wasserman, D.H. Fibroblast growth factor 21 controls glycemia via regulation of hepatic glucose flux and insulin sensitivity. Endocrinology 2009, 150, 4084–4093. [Google Scholar] [CrossRef]
- Lin, Z.; Tian, H.; Lam, K.S.; Lin, S.; Hoo, R.C.; Konishi, M.; Itoh, N.; Wang, Y.; Bornstein, S.R.; Xu, A.; et al. Adiponectin mediates the metabolic effects of FGF21 on glucose homeostasis and insulin sensitivity in mice. Cell Metab. 2013, 17, 779–789. [Google Scholar] [CrossRef]
- Kim, H.W.; Lee, J.E.; Cha, J.J.; Hyun, Y.Y.; Kim, J.E.; Lee, M.H.; Song, H.K.; Nam, D.H.; Han, J.Y.; Han, S.Y.; et al. Fibroblast growth factor 21 improves insulin resistance and ameliorates renal injury in db/db mice. Endocrinology 2013, 154, 3366–3376. [Google Scholar] [CrossRef]
- Zhang, C.; Shao, M.; Yang, H.; Chen, L.; Yu, L.; Cong, W.; Tian, H.; Zhang, F.; Cheng, P.; Jin, L.; et al. Attenuation of hyperlipidemia- and diabetes-induced early-stage apoptosis and late-stage renal dysfunction via administration of fibroblast growth factor-21 is associated with suppression of renal inflammation. PLoS ONE 2013, 8, e82275. [Google Scholar] [CrossRef]
- Liu, J.J.; Foo, J.P.; Liu, S.; Lim, S.C. The role of fibroblast growth factor 21 in diabetes and its complications: A review from clinical perspective. Diabetes Res. Clin. Pract. 2015, 108, 382–389. [Google Scholar] [CrossRef]
- Schlein, C.; Talukdar, S.; Heine, M.; Fischer, A.W.; Krott, L.M.; Nilsson, S.K.; Brenner, M.B.; Heeren, J.; Scheja, L. FGF21 Lowers Plasma Triglycerides by Accelerating Lipoprotein Catabolism in White and Brown Adipose Tissues. Cell Metab. 2016, 23, 441–453. [Google Scholar] [CrossRef] [PubMed]
- Ye, M.; Lu, W.; Wang, X.; Wang, C.; Abbruzzese, J.L.; Liang, G.; Li, X.; Luo, Y. FGF21-FGFR1 Coordinates Phospholipid Homeostasis, Lipid Droplet Function, and ER Stress in Obesity. Endocrinology 2016, 157, 4754–4769. [Google Scholar] [CrossRef] [PubMed]
- Talukdar, S.; Zhou, Y.; Li, D.; Rossulek, M.; Dong, J.; Somayaji, V.; Weng, Y.; Clark, R.; Lanba, A.; Owen, B.M.; et al. A Long-Acting FGF21 Molecule, PF-05231023, Decreases Body Weight and Improves Lipid Profile in Non-human Primates and Type 2 Diabetic Subjects. Cell Metab. 2016, 23, 427–440. [Google Scholar] [CrossRef] [PubMed]
- Gaich, G.; Chien, J.Y.; Fu, H.; Glass, L.C.; Deeg, M.A.; Holland, W.L.; Kharitonenkov, A.; Bumol, T.; Schilske, H.K.; Moller, D.E. The effects of LY2405319, an FGF21 analog, in obese human subjects with type 2 diabetes. Cell Metab. 2013, 18, 333–340. [Google Scholar] [CrossRef]
- Fu, Z.; Gong, Y.; Liegl, R.; Wang, Z.; Liu, C.H.; Meng, S.S.; Burnim, S.B.; Saba, N.J.; Fredrick, T.W.; Morss, P.C.; et al. FGF21 Administration Suppresses Retinal and Choroidal Neovascularization in Mice. Cell Rep. 2017, 18, 1606–1613. [Google Scholar] [CrossRef]
- Fu, Z.; Wang, Z.; Liu, C.H.; Gong, Y.; Cakir, B.; Liegl, R.; Sun, Y.; Meng, S.; Burnim, S.; Arellano, I.; et al. Fibroblast growth factor 21 protects photoreceptor function in type 1 diabetic mice. Diabetes 2018, 67, 974–985. [Google Scholar] [CrossRef]
- Yu, Z.; Lin, L.; Jiang, Y.; Chin, I.; Wang, X.; Li, X.; Lo, E.H.; Wang, X. Recombinant FGF21 Protects Against Blood-Brain Barrier Leakage Through Nrf2 Upregulation in Type 2 Diabetes Mice. Mol. Neurobiol. 2019, 56, 2314–2327. [Google Scholar] [CrossRef]
- Li, H.; Wu, G.; Fang, Q.; Zhang, M.; Hui, X.; Sheng, B.; Wu, L.; Bao, Y.; Li, P.; Xu, A.; et al. Fibroblast growth factor 21 increases insulin sensitivity through specific expansion of subcutaneous fat. Nat. Commun. 2018, 9, 272. [Google Scholar] [CrossRef]
- Holland, W.L.; Adams, A.C.; Brozinick, J.T.; Bui, H.H.; Miyauchi, Y.; Kusminski, C.M.; Bauer, S.M.; Wade, M.; Singhal, E.; Cheng, C.C.; et al. An FGF21-adiponectin-ceramide axis controls energy expenditure and insulin action in mice. Cell Metab. 2013, 17, 790–797. [Google Scholar] [CrossRef]
- Fu, Z.; Gong, Y.; Lofqvist, C.; Hellstrom, A.; Smith, L.E. Review: Adiponectin in retinopathy. Biochim. Biophys. Acta 2016, 1862, 1392–1400. [Google Scholar] [CrossRef]
- Fu, Z.; Lofqvist, C.A.; Shao, Z.; Sun, Y.; Joyal, J.S.; Hurst, C.G.; Cui, R.Z.; Evans, L.P.; Tian, K.; SanGiovanni, J.P.; et al. Dietary omega-3 polyunsaturated fatty acids decrease retinal neovascularization by adipose-endoplasmic reticulum stress reduction to increase adiponectin. Am. J. Clin. Nutr. 2015, 101, 879–888. [Google Scholar] [CrossRef]
- Mao, D.; Peng, H.; Li, Q.; Wang, J.; Li, P.; Hu, K.; Zhang, X.; Lei, B. Aqueous humor and plasma adiponectin levels in proliferative diabetic retinopathy patients. Curr. Eye Res. 2012, 37, 803–808. [Google Scholar] [CrossRef] [PubMed]
- Omae, T.; Nagaoka, T.; Yoshida, A. Relationship Between Retinal Blood Flow and Serum Adiponectin Concentrations in Patients With Type 2 Diabetes Mellitus. Investig. Ophthalmol. Vis. Sci. 2015, 56, 4143–4149. [Google Scholar] [CrossRef] [PubMed]
- Kaarniranta, K.; Paananen, J.; Nevalainen, T.; Sorri, I.; Seitsonen, S.; Immonen, I.; Salminen, A.; Pulkkinen, L.; Uusitupa, M. Adiponectin receptor 1 gene (ADIPOR1) variant is associated with advanced age-related macular degeneration in Finnish population. Neurosci. Lett. 2012, 513, 233–237. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, C.; Shen, Y.; Chen, N.; Wang, L.; Liang, L.; Guo, T.; Yin, X.; Ma, Z.; Zhang, B.; et al. A mutation in ADIPOR1 causes nonsyndromic autosomal dominant retinitis pigmentosa. Hum. Genet. 2016, 135, 1375–1387. [Google Scholar] [CrossRef]
- Higuchi, A.; Ohashi, K.; Kihara, S.; Walsh, K.; Ouchi, N. Adiponectin suppresses pathological microvessel formation in retina through modulation of tumor necrosis factor-alpha expression. Circ. Res. 2009, 104, 1058–1065. [Google Scholar] [CrossRef]
- Lyzogubov, V.V.; Tytarenko, R.G.; Bora, N.S.; Bora, P.S. Inhibitory role of adiponectin peptide I on rat choroidal neovascularization. Biochim. Biophys. Acta 2012, 1823, 1264–1272. [Google Scholar] [CrossRef]
- Fu, Z.; Liegl, R.; Wang, Z.; Gong, Y.; Liu, C.H.; Sun, Y.; Cakir, B.; Burnim, S.B.; Meng, S.S.; Lofqvist, C.; et al. Adiponectin Mediates Dietary Omega-3 Long-Chain Polyunsaturated Fatty Acid Protection Against Choroidal Neovascularization in Mice. Investig. Ophthalmol. Vis. Sci. 2017, 58, 3862–3870. [Google Scholar] [CrossRef]
- Palanisamy, K.; Nareshkumar, R.N.; Sivagurunathan, S.; Raman, R.; Sulochana, K.N.; Chidambaram, S. Anti-angiogenic effect of adiponectin in human primary microvascular and macrovascular endothelial cells. Microvasc. Res. 2019, 122, 136–145. [Google Scholar] [CrossRef]
- Omae, T.; Nagaoka, T.; Tanano, I.; Yoshida, A. Adiponectin-induced dilation of isolated porcine retinal arterioles via production of nitric oxide from endothelial cells. Investig. Ophthalmol. Vis. Sci. 2013, 54, 4586–4594. [Google Scholar] [CrossRef]
- Hardie, D.G.; Ross, F.A.; Hawley, S.A. AMPK: A nutrient and energy sensor that maintains energy homeostasis. Nat. Rev. Mol. Cell Biol. 2012, 13, 251–262. [Google Scholar] [CrossRef] [PubMed]
- Kamoshita, M.; Ozawa, Y.; Kubota, S.; Miyake, S.; Tsuda, C.; Nagai, N.; Yuki, K.; Shimmura, S.; Umezawa, K.; Tsubota, K. AMPK-NF-kappaB axis in the photoreceptor disorder during retinal inflammation. PLoS ONE 2014, 9, e103013. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Kong, L.; Wang, J.; Ash, J.D. Stimulation of AMPK prevents degeneration of photoreceptors and the retinal pigment epithelium. Proc. Natl. Acad. Sci. USA 2018, 115, 10475–10480. [Google Scholar] [CrossRef] [PubMed]
- Kaarniranta, K.; Kajdanek, J.; Morawiec, J.; Pawlowska, E.; Blasiak, J. PGC-1alpha Protects RPE Cells of the Aging Retina against Oxidative Stress-Induced Degeneration through the Regulation of Senescence and Mitochondrial Quality Control. The Significance for AMD Pathogenesis. Int. J. Mol. Sci. 2018, 19, 2317. [Google Scholar] [CrossRef]
- Canto, C.; Auwerx, J. PGC-1alpha, SIRT1 and AMPK, an energy sensing network that controls energy expenditure. Curr. Opin. Lipidol. 2009, 20, 98–105. [Google Scholar] [CrossRef]
- Golestaneh, N.; Chu, Y.; Cheng, S.K.; Cao, H.; Poliakov, E.; Berinstein, D.M. Repressed SIRT1/PGC-1alpha pathway and mitochondrial disintegration in iPSC-derived RPE disease model of age-related macular degeneration. J. Transl. Med. 2016, 14, 344. [Google Scholar] [CrossRef]
- Zhang, M.; Chu, Y.; Mowery, J.; Konkel, B.; Galli, S.; Theos, A.C.; Golestaneh, N. Pgc-1alpha repression and high-fat diet induce age-related macular degeneration-like phenotypes in mice. Dis. Model. Mech. 2018, 11, dmm032698. [Google Scholar] [CrossRef]
- Iacovelli, J.; Rowe, G.C.; Khadka, A.; Diaz-Aguilar, D.; Spencer, C.; Arany, Z.; Saint-Geniez, M. PGC-1alpha Induces Human RPE Oxidative Metabolism and Antioxidant Capacity. Investig. Ophthalmol. Vis. Sci. 2016, 57, 1038–1051. [Google Scholar] [CrossRef]
- Rosales, M.A.B.; Shu, D.Y.; Iacovelli, J.; Saint-Geniez, M. Loss of PGC-1alpha in RPE induces mesenchymal transition and promotes retinal degeneration. Life Sci. Alliance 2019, 2. [Google Scholar] [CrossRef]
- Egger, A.; Samardzija, M.; Sothilingam, V.; Tanimoto, N.; Lange, C.; Salatino, S.; Fang, L.; Garcia-Garrido, M.; Beck, S.; Okoniewski, M.J.; et al. PGC-1alpha determines light damage susceptibility of the murine retina. PLoS ONE 2012, 7, e31272. [Google Scholar] [CrossRef]
- Saint-Geniez, M.; Jiang, A.; Abend, S.; Liu, L.; Sweigard, H.; Connor, K.M.; Arany, Z. PGC-1alpha regulates normal and pathological angiogenesis in the retina. Am. J. Pathol. 2013, 182, 255–265. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Deepa, S.S.; Etzler, J.C.; Ryu, J.; Mao, X.; Fang, Q.; Liu, D.D.; Torres, J.M.; Jia, W.; Lechleiter, J.D.; et al. Adiponectin activates AMP-activated protein kinase in muscle cells via APPL1/LKB1-dependent and phospholipase C/Ca2+/Ca2+/calmodulin-dependent protein kinase kinase-dependent pathways. J. Biol. Chem. 2009, 284, 22426–22435. [Google Scholar] [CrossRef] [PubMed]
- Salminen, A.; Kauppinen, A.; Kaarniranta, K. FGF21 activates AMPK signaling: Impact on metabolic regulation and the aging process. J. Mol. Med. 2017, 95, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Chau, M.D.; Gao, J.; Yang, Q.; Wu, Z.; Gromada, J. Fibroblast growth factor 21 regulates energy metabolism by activating the AMPK-SIRT1-PGC-1alpha pathway. Proc. Natl. Acad. Sci. USA 2010, 107, 12553–12558. [Google Scholar] [CrossRef] [PubMed]
- Peplow, P.V.; Chung, T.Y.; Baxter, G.D. Laser photobiomodulation of wound healing: A review of experimental studies in mouse and rat animal models. Photomed. Laser Surg. 2010, 28, 291–325. [Google Scholar] [CrossRef] [PubMed]
- Chow, R.T.; Johnson, M.I.; Lopes-Martins, R.A.; Bjordal, J.M. Efficacy of low-level laser therapy in the management of neck pain: A systematic review and meta-analysis of randomised placebo or active-treatment controlled trials. Lancet 2009, 374, 1897–1908. [Google Scholar] [CrossRef]
- Anders, J.J.; Geuna, S.; Rochkind, S. Phototherapy promotes regeneration and functional recovery of injured peripheral nerve. Neurol. Res. 2004, 26, 233–239. [Google Scholar] [CrossRef]
- Lampl, Y.; Zivin, J.A.; Fisher, M.; Lew, R.; Welin, L.; Dahlof, B.; Borenstein, P.; Andersson, B.; Perez, J.; Caparo, C.; et al. Infrared laser therapy for ischemic stroke: A new treatment strategy: Results of the NeuroThera Effectiveness and Safety Trial-1 (NEST-1). Stroke 2007, 38, 1843–1849. [Google Scholar] [CrossRef]
- Fitzgerald, M.; Hodgetts, S.; Van Den Heuvel, C.; Natoli, R.; Hart, N.S.; Valter, K.; Harvey, A.R.; Vink, R.; Provis, J.; Dunlop, S.A. Red/near-infrared irradiation therapy for treatment of central nervous system injuries and disorders. Rev. Neurosci. 2013, 24, 205–226. [Google Scholar] [CrossRef]
- Rutar, M.; Natoli, R.; Albarracin, R.; Valter, K.; Provis, J. 670-nm light treatment reduces complement propagation following retinal degeneration. J. Neuroinflam. 2012, 9, 257. [Google Scholar] [CrossRef]
- Albarracin, R.; Eells, J.; Valter, K. Photobiomodulation protects the retina from light-induced photoreceptor degeneration. Investig. Ophthalmol. Vis. Sci. 2011, 52, 3582–3592. [Google Scholar] [CrossRef] [PubMed]
- Albarracin, R.; Natoli, R.; Rutar, M.; Valter, K.; Provis, J. 670 nm light mitigates oxygen-induced degeneration in C57BL/6J mouse retina. BMC Neurosci. 2013, 14, 125. [Google Scholar] [CrossRef] [PubMed]
- Natoli, R.; Valter, K.; Barbosa, M.; Dahlstrom, J.; Rutar, M.; Kent, A.; Provis, J. 670 nm photobiomodulation as a novel protection against retinopathy of prematurity: Evidence from oxygen induced retinopathy models. PLoS ONE 2013, 8, e72135. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Du, Y.; Lee, C.A.; Talahalli, R.; Eells, J.T.; Kern, T.S. Low-intensity far-red light inhibits early lesions that contribute to diabetic retinopathy: In vivo and in vitro. Investig. Ophthalmol. Vis. Sci. 2013, 54, 3681–3690. [Google Scholar] [CrossRef] [PubMed]
- Eells, J.T.; Henry, M.M.; Summerfelt, P.; Wong-Riley, M.T.; Buchmann, E.V.; Kane, M.; Whelan, N.T.; Whelan, H.T. Therapeutic photobiomodulation for methanol-induced retinal toxicity. Proc. Natl. Acad. Sci. USA 2003, 100, 3439–3444. [Google Scholar] [CrossRef]
- Begum, R.; Powner, M.B.; Hudson, N.; Hogg, C.; Jeffery, G. Treatment with 670 nm light up regulates cytochrome C oxidase expression and reduces inflammation in an age-related macular degeneration model. PLoS ONE 2013, 8, e57828. [Google Scholar] [CrossRef]
- Ivandic, B.T.; Ivandic, T. Low-level laser therapy improves vision in patients with age-related macular degeneration. Photomed. Laser Surg. 2008, 26, 241–245. [Google Scholar] [CrossRef]
- Merry, G.F.; Munk, M.R.; Dotson, R.S.; Walker, M.G.; Devenyi, R.G. Photobiomodulation reduces drusen volume and improves visual acuity and contrast sensitivity in dry age-related macular degeneration. Acta Ophthalmol. 2017, 95, e270–e277. [Google Scholar] [CrossRef]
- Gkotsi, D.; Begum, R.; Salt, T.; Lascaratos, G.; Hogg, C.; Chau, K.Y.; Schapira, A.H.; Jeffery, G. Recharging mitochondrial batteries in old eyes. Near infra-red increases ATP. Exp. Eye Res. 2014, 122, 50–53. [Google Scholar] [CrossRef]
- Kokkinopoulos, I.; Colman, A.; Hogg, C.; Heckenlively, J.; Jeffery, G. Age-related retinal inflammation is reduced by 670 nm light via increased mitochondrial membrane potential. Neurobiol. Aging 2013, 34, 602–609. [Google Scholar] [CrossRef]
- Karu, T.I. Mitochondrial signaling in mammalian cells activated by red and near-IR radiation. Photochem. Photobiol. 2008, 84, 1091–1099. [Google Scholar] [CrossRef] [PubMed]
- Kaynezhad, P.; Tachtsidis, I.; Jeffery, G. Optical monitoring of retinal respiration in real time: 670 nm light increases the redox state of mitochondria. Exp. Eye Res. 2016, 152, 88–93. [Google Scholar] [CrossRef] [PubMed]
- Beirne, K.; Rozanowska, M.; Votruba, M. Photostimulation of mitochondria as a treatment for retinal neurodegeneration. Mitochondrion 2017, 36, 85–95. [Google Scholar] [CrossRef]
- Fu, Z.; Chen, C.T.; Cagnone, G.; Heckel, E.; Sun, Y.; Cakir, B.; Tomita, Y.; Huang, S.; Li, Q.; Britton, W.; et al. Dyslipidemia in retinal metabolic disorders. EMBO Mol. Med. 2019, 11, e10473. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Tang, J.; Du, Y.; Saadane, A.; Samuels, I.; Veenstra, A.; Kiser, J.Z.; Palczewski, K.; Kern, T.S. Transducin1, Phototransduction and the Development of Early Diabetic Retinopathy. Investig. Ophthalmol. Vis. Sci. 2019, 60, 1538–1546. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Tang, J.; Du, Y.; Lee, C.A.; Golczak, M.; Muthusamy, A.; Antonetti, D.A.; Veenstra, A.A.; Amengual, J.; von Lintig, J.; et al. Retinylamine Benefits Early Diabetic Retinopathy in Mice. J. Biol. Chem. 2015, 290, 21568–21579. [Google Scholar] [CrossRef]
- Bavik, C.; Henry, S.H.; Zhang, Y.; Mitts, K.; McGinn, T.; Budzynski, E.; Pashko, A.; Lieu, K.L.; Zhong, S.; Blumberg, B.; et al. Visual Cycle Modulation as an Approach toward Preservation of Retinal Integrity. PLoS ONE 2015, 10, e0124940. [Google Scholar] [CrossRef]
- Tonade, D.; Liu, H.; Palczewski, K.; Kern, T.S. Photoreceptor cells produce inflammatory products that contribute to retinal vascular permeability in a mouse model of diabetes. Diabetologia 2017, 60, 2111–2120. [Google Scholar] [CrossRef]
- Du, Y.; Veenstra, A.; Palczewski, K.; Kern, T.S. Photoreceptor cells are major contributors to diabetes-induced oxidative stress and local inflammation in the retina. Proc. Natl. Acad. Sci. USA 2013, 110, 16586–16591. [Google Scholar] [CrossRef]
- Liu, H.; Tang, J.; Du, Y.; Saadane, A.; Tonade, D.; Samuels, I.; Veenstra, A.; Palczewski, K.; Kern, T.S. Photoreceptor Cells Influence Retinal Vascular Degeneration in Mouse Models of Retinal Degeneration and Diabetes. Investig. Ophthalmol. Vis. Sci. 2016, 57, 4272–4281. [Google Scholar] [CrossRef]
- Kern, T.S.; Berkowitz, B.A. Photoreceptors in diabetic retinopathy. J. Diabetes Investig. 2015, 6, 371–380. [Google Scholar] [CrossRef] [PubMed]
- Curran, T.; Franza, B.R., Jr. Fos and Jun: The AP-1 connection. Cell 1988, 55, 395–397. [Google Scholar] [CrossRef]
- Hafezi, F.; Steinbach, J.P.; Marti, A.; Munz, K.; Wang, Z.Q.; Wagner, E.F.; Aguzzi, A.; Reme, C.E. The absence of c-fos prevents light-induced apoptotic cell death of photoreceptors in retinal degeneration in vivo. Nat. Med. 1997, 3, 346–349. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, G.E.; Smith, M.S.; Verbalis, J.G. c-Fos and related immediate early gene products as markers of activity in neuroendocrine systems. Front. Neuroendocr. 1993, 14, 173–213. [Google Scholar] [CrossRef]
- Aikawa, Y.; Morimoto, K.; Yamamoto, T.; Chaki, H.; Hashiramoto, A.; Narita, H.; Hirono, S.; Shiozawa, S. Treatment of arthritis with a selective inhibitor of c-Fos/activator protein-1. Nat. Biotechnol. 2008, 26, 817–823. [Google Scholar] [CrossRef]
- Shiozawa, S.; Tsumiyama, K. Pathogenesis of rheumatoid arthritis and c-Fos/AP-1. Cell Cycle 2009, 8, 1539–1543. [Google Scholar] [CrossRef]
- Yu, M.C.; Li, W.W.; Liu, K.; Yew, D.T. An immunohistochemical study of the c-fos protooncogene in the developing human retina. Neuroscience 1994, 60, 983–987. [Google Scholar] [CrossRef]
- He, L.; Campbell, M.L.; Srivastava, D.; Blocker, Y.S.; Harris, J.R.; Swaroop, A.; Fox, D.A. Spatial and temporal expression of AP-1 responsive rod photoreceptor genes and bZIP transcription factors during development of the rat retina. Mol. Vis. 1998, 4, 32. [Google Scholar]
- Hobson, A.H.; Donovan, M.; Humphries, M.M.; Tuohy, G.; McNally, N.; Carmody, R.; Cotter, T.; Farrar, G.J.; Kenna, P.F.; Humphries, P. Apoptotic photoreceptor death in the rhodopsin knockout mouse in the presence and absence of c-fos. Exp. Eye Res. 2000, 71, 247–254. [Google Scholar] [CrossRef]
- Poon, H.K.; Tso, M.O.; Lam, T.T. c-Fos protein in photoreceptor cell death after photic injury in rats. Investig. Ophthalmol. Vis. Sci. 2000, 41, 2755–2758. [Google Scholar]
- Wenzel, A.; Grimm, C.; Marti, A.; Kueng-Hitz, N.; Hafezi, F.; Niemeyer, G.; Reme, C.E. c-fos controls the "private pathway" of light-induced apoptosis of retinal photoreceptors. J. Neurosci. 2000, 20, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Nathaniel, T.I.; Otukonyong, E.; Abdellatif, A.; Soyinka, J.O. Effect of hypoxia on metabolic rate, core body temperature, and c-fos expression in the naked mole rat. Int. J. Dev. Neurosci. 2012, 30, 539–544. [Google Scholar] [CrossRef] [PubMed]
- Ozawa, Y.; Nakao, K.; Shimazaki, T.; Shimmura, S.; Kurihara, T.; Ishida, S.; Yoshimura, A.; Tsubota, K.; Okano, H. SOCS3 is required to temporally fine-tune photoreceptor cell differentiation. Dev. Biol. 2007, 303, 591–600. [Google Scholar] [CrossRef] [PubMed]
- Worzfeld, T.; Offermanns, S. Semaphorins and plexins as therapeutic targets. Nat. Rev. Drug Discov. 2014, 13, 603–621. [Google Scholar] [CrossRef]
- Kim, J.; Oh, W.J.; Gaiano, N.; Yoshida, Y.; Gu, C. Semaphorin 3E-Plexin-D1 signaling regulates VEGF function in developmental angiogenesis via a feedback mechanism. Genes Dev. 2011, 25, 1399–1411. [Google Scholar] [CrossRef]
- Buehler, A.; Sitaras, N.; Favret, S.; Bucher, F.; Berger, S.; Pielen, A.; Joyal, J.S.; Juan, A.M.; Martin, G.; Schlunck, G.; et al. Semaphorin 3F forms an anti-angiogenic barrier in outer retina. FEBS Lett. 2013, 587, 1650–1655. [Google Scholar] [CrossRef]
- Ochsenbein, A.M.; Karaman, S.; Proulx, S.T.; Berchtold, M.; Jurisic, G.; Stoeckli, E.T.; Detmar, M. Endothelial cell-derived semaphorin 3A inhibits filopodia formation by blood vascular tip cells. Development 2016, 143, 589–594. [Google Scholar] [CrossRef]
- Joyal, J.S.; Sitaras, N.; Binet, F.; Rivera, J.C.; Stahl, A.; Zaniolo, K.; Shao, Z.; Polosa, A.; Zhu, T.; Hamel, D.; et al. Ischemic neurons prevent vascular regeneration of neural tissue by secreting semaphorin 3A. Blood 2011, 117, 6024–6035. [Google Scholar] [CrossRef]
- Soker, S.; Takashima, S.; Miao, H.Q.; Neufeld, G.; Klagsbrun, M. Neuropilin-1 is expressed by endothelial and tumor cells as an isoform-specific receptor for vascular endothelial growth factor. Cell 1998, 92, 735–745. [Google Scholar] [CrossRef]
- Sun, Y.; Liegl, R.; Gong, Y.; Buhler, A.; Cakir, B.; Meng, S.S.; Burnim, S.B.; Liu, C.H.; Reuer, T.; Zhang, P.; et al. Sema3f Protects Against Subretinal Neovascularization In Vivo. EBioMedicine 2017, 18, 281–287. [Google Scholar] [CrossRef]
- Ajlan, R.S.; Silva, P.S.; Sun, J.K. Vascular Endothelial Growth Factor and Diabetic Retinal Disease. Semin. Ophthalmol. 2016, 31, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Shao, J.; Choudhary, M.M.; Schachat, A.P. Neovascular Age-Related Macular Degeneration. Dev. Ophthalmol. 2016, 55, 125–136. [Google Scholar] [PubMed]
- Meyer, C.H.; Krohne, T.U.; Charbel Issa, P.; Liu, Z.; Holz, F.G. Routes for Drug Delivery to the Eye and Retina: Intravitreal Injections. Dev. Ophthalmol. 2016, 55, 63–70. [Google Scholar] [PubMed]
- Jaffe, G.J.; Eliott, D.; Wells, J.A.; Prenner, J.L.; Papp, A.; Patel, S. A Phase 1 Study of Intravitreous E10030 in Combination with Ranibizumab in Neovascular Age-Related Macular Degeneration. Ophthalmology 2016, 123, 78–85. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).