ERM Proteins at the Crossroad of Leukocyte Polarization, Migration and Intercellular Adhesion
Abstract
1. Introduction
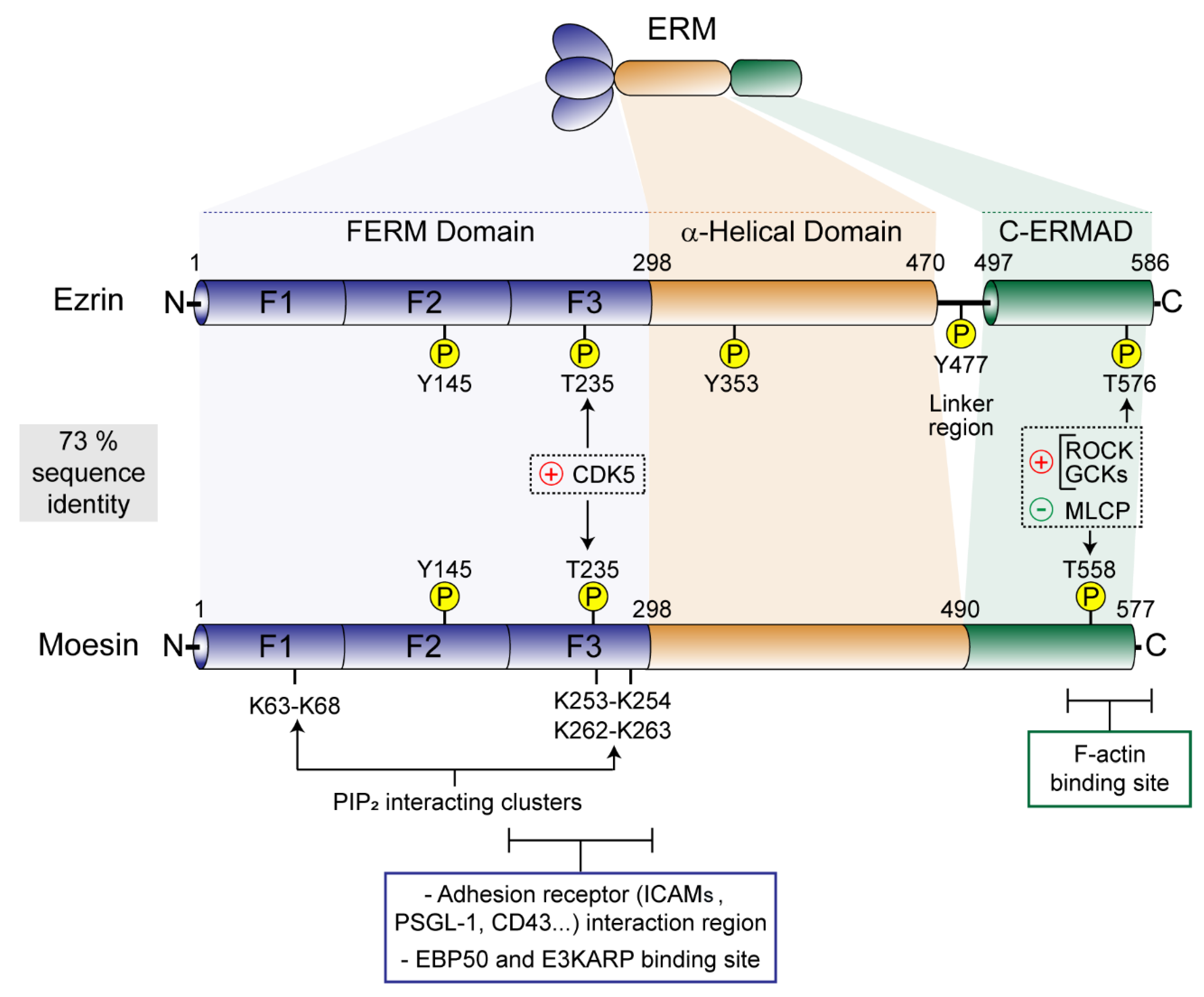
2. ERM Tools for Plasma Membrane-to-Cytoskeleton Bridging
3. ERMs in Leukocyte Polarization and Migration
3.1. Tethering and Rolling
3.2. Firm Adhesion
3.3. Transendothelial Migration
4. ERMs and Intercellular Adhesion: the Phagocytic Cup and the Immune Synapse as Paradigms of Ezrin- and Moesin-Mediated PM Organization in Leukocytes
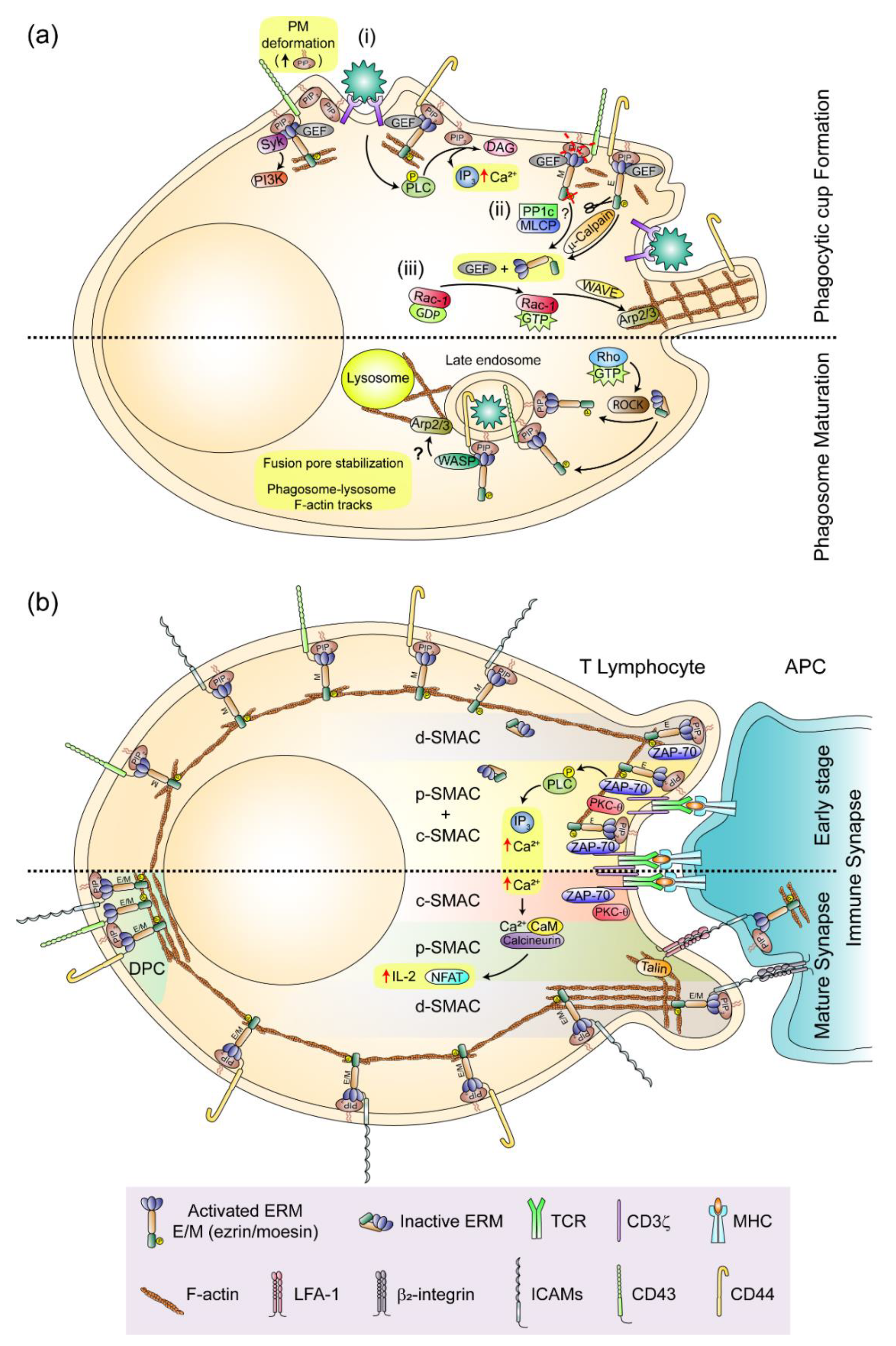
4.1. The phagocytic Cup and the Phagosome
4.2. The Immune Synapse
5. ERMs and Immune Regulation
6. Conclusions and Future Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chugh, P.; Clark, A.G.; Smith, M.B.; Cassani, D.A.; Dierkes, K.; Ragab, A.; Paluch, E.K. Actin cortex architecture regulates cell surface tension. Nat. Cell Biol. 2017, 19, 689–697. [Google Scholar] [CrossRef]
- Fehon, R.G.; McClatchey, A.I.; Bretscher, A. Organizing the cell cortex: The role of ERM proteins. Nat. Rev. Mol. Cell Biol. 2010, 11, 276–287. [Google Scholar] [CrossRef]
- Michie, K.A.; Bermeister, A.; Robertson, N.O.; Goodchild, S.C.; Curmi, P.M. Two Sides of the Coin: Ezrin/Radixin/Moesin and Merlin Control Membrane Structure and Contact Inhibition. Int. J. Mol. Sci. 2019, 20, 1996. [Google Scholar] [CrossRef] [PubMed]
- Lankes, W.; Griesmacher, A.; Grünwald, J.; Schwartz-Albiez, R.; Keller, R. A heparin-binding protein involved in inhibition of smooth-muscle cell proliferation. Biochem. J. 1988, 251, 831–842. [Google Scholar] [CrossRef] [PubMed]
- Bretscher, A. Purification of an 80,000-dalton protein that is a component of the isolated microvillus cytoskeleton, and its localization in nonmuscle cells. J. Cell Biol. 1983, 97, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Tsukita, S.; Hieda, Y.; Tsukita, S. A new 82-kD barbed end-capping protein (radixin) localized in the cell-to-cell adherens junction: Purification and characterization. J. Cell Biol. 1989, 108, 2369–2382. [Google Scholar] [CrossRef]
- Crawley, S.W.; Mooseker, M.S.; Tyska, M.J. Shaping the intestinal brush border. J. Cell Biol. 2014, 207, 441–451. [Google Scholar] [CrossRef]
- Chorna-Ornan, I.; Tzarfaty, V.; Ankri-Eliahoo, G.; Joel-Almagor, T.; Meyer, N.E.; Huber, A.; Minke, B. Light-regulated interaction of Dmoesin with TRP and TRPL channels is required for maintenance of photoreceptors. J. Cell Biol. 2005, 171, 143–152. [Google Scholar] [CrossRef]
- Bonilha, V.L.; Finnemann, S.C.; Rodriguez-Boulan, E. Ezrin promotes morphogenesis of apical microvilli and basal infoldings in retinal pigment epithelium. J. Cell Biol. 1999, 147, 1533–1548. [Google Scholar] [CrossRef]
- Wang, Y.; Kaiser, M.S.; Larson, J.D.; Nasevicius, A.; Clark, K.J.; Wadman, S.A.; Essner, J.J. Moesin1 and Ve-cadherin are required in endothelial cells during in vivo tubulogenesis. Development 2010, 137, 3119–3128. [Google Scholar] [CrossRef]
- JayaNandanan, N.; Mathew, R.; Leptin, M. Guidance of subcellular tubulogenesis by actin under the control of a synaptotagmin-like protein and Moesin. Nat. Commun. 2014, 5, 3036. [Google Scholar] [CrossRef] [PubMed]
- Khan, L.A.; Jafari, G.; Zhang, N.; Membreno, E.; Yan, S.; Zhang, H.; Gobel, V. A tensile trilayered cytoskeletal endotube drives capillary-like lumenogenesis. J. Cell Biol. 2019, 218, 2403–2424. [Google Scholar] [CrossRef] [PubMed]
- Serrador, J.M.; Nieto, M.; Alonso-Lebrero, J.L.; del Pozo, M.A.; Calvo, J.; Furthmayr, H.; Sánchez-Madrid, F. CD43 interacts with moesin and ezrin and regulates its redistribution to the uropods of T lymphocytes at the cell-cell contacts. Blood 1998, 91, 4632–4644. [Google Scholar] [CrossRef] [PubMed]
- Parameswaran, N.; Matsui, K.; Gupta, N. Conformational switching in ezrin regulates morphological and cytoskeletal changes required for B cell chemotaxis. J. Immunol. 2011, 186, 4088–4097. [Google Scholar] [CrossRef] [PubMed]
- Ramoni, C.; Luciani, F.; Spadaro, F.; Lugini, L.; Lozupone, F.; Fais, S. Differential expression and distribution of ezrin, radixin and moesin in human natural killer cells. Eur. J. Immunol. 2002, 32, 3059–3065. [Google Scholar] [CrossRef]
- Yoshinaga-Ohara, N.; Takahashi, A.; Uchiyama, T.; Sasada, M. Spatiotemporal regulation of moesin phosphorylation and rear release by Rho and serine/threonine phosphatase during neutrophil migration. Exp. Cell Res. 2002, 278, 112–122. [Google Scholar] [CrossRef]
- Mori, T.; Kitano, K.; Terawaki, S.I.; Maesaki, R.; Fukami, Y.; Hakoshima, T. Structural basis for CD44 recognition by ERM proteins. J. Biol. Chem. 2008, 283, 29602–29612. [Google Scholar] [CrossRef]
- Takai, Y.; Kitano, K.; Terawaki, S.I.; Maesaki, R.; Hakoshima, T. Structural basis of the cytoplasmic tail of adhesion molecule CD43 and its binding to ERM proteins. J. Mol. Biol. 2008, 381, 634–644. [Google Scholar] [CrossRef]
- Takai, Y.; Kitano, K.; Terawaki, S.I.; Maesaki, R.; Hakoshima, T. Structural basis of PSGL-1 binding to ERM proteins. Genes Cells 2007, 12, 1329–1338. [Google Scholar] [CrossRef]
- Yonemura, S.; Hirao, M.; Doi, Y.; Takahashi, N.; Kondo, T.; Tsukita, S.; Tsukita, S. Ezrin/radixin/moesin (ERM) proteins bind to a positively charged amino acid cluster in the juxta-membrane cytoplasmic domain of CD44, CD43, and ICAM-2. J. Cell Biol. 1998, 140, 885–895. [Google Scholar] [CrossRef]
- Alonso-Lebrero, J.L.; Serrador, J.M.; Domınguez-Jiménez, C.; Barreiro, O.; Luque, A.; Del Pozo, M.A.; Lozano, F. Polarization and interaction of adhesion molecules P-selectin glycoprotein ligand 1 and intercellular adhesion molecule 3 with moesin and ezrin in myeloid cells. Blood 2000, 95, 2413–2419. [Google Scholar] [CrossRef] [PubMed]
- Heiska, L.; Alfthan, K.; Grönholm, M.; Vilja, P.; Vaheri, A.; Carpén, O. Association of ezrin with intercellular adhesion molecule-1 and -2 (ICAM-1 and ICAM-2) Regulation by phosphatidylinositol 4, 5-bisphosphate. J. Biol. Chem. 1998, 273, 21893–21900. [Google Scholar] [CrossRef] [PubMed]
- Serrador, J.M.; Alonso-Lebrero, J.L.; Pozo, M.A.D.; Furthmayr, H.; Schwartz-Albiez, R.; Calvo, J.; Sánchez-Madrid, F. Moesin interacts with the cytoplasmic region of intercellular adhesion molecule-3 and is redistributed to the uropod of T lymphocytes during cell polarization. J. Cell Biol. 1997, 138, 1409–1423. [Google Scholar] [CrossRef] [PubMed]
- Barreiro, O.; Yáñez-Mó, M.; Serrador, J.M.; Montoya, M.C.; Vicente-Manzanares, M.; Tejedor, R.; Sánchez-Madrid, F. Dynamic interaction of VCAM-1 and ICAM-1 with moesin and ezrin in a novel endothelial docking structure for adherent leukocytes. J. Cell Biol. 2002, 157, 1233–1245. [Google Scholar] [CrossRef] [PubMed]
- Dickson, T.C.; Mintz, C.D.; Benson, D.L.; Salton, S.R. Functional binding interaction identified between the axonal CAM L1 and members of the ERM family. J. Cell Biol. 2002, 157, 1105–1112. [Google Scholar] [CrossRef] [PubMed]
- Serrador, J.M.; Vicente-Manzanares, M.; Calvo, J.; Barreiro, O.; Montoya, M.C.; Schwartz-Albiez, R.; Sánchez-Madrid, F. A novel serine-rich motif in the intercellular adhesion molecule 3 is critical for its ezrin/radixin/moesin-directed subcellular targeting. J. Biol. Chem. 2002, 277, 10400–10409. [Google Scholar] [CrossRef]
- Serrador, J.M.; Urzainqui, A.; Alonso-Lebrero, J.L.; Cabrero, J.R.; Montoya, M.C.; Vicente-Manzanares, M.; Sánchez-Madrid, F. A juxta-membrane amino acid sequence of P-selectin glycoprotein ligand-1 is involved in moesin binding and ezrin/radixin/moesin-directed targeting at the trailing edge of migrating lymphocytes. Eur. J. Immunol. 2002, 32, 1560–1566. [Google Scholar] [CrossRef]
- Cheng, L.; Itoh, K.; Lemmon, V. L1-mediated branching is regulated by two ezrin-radixin-moesin (ERM)-binding sites, the RSLE region and a novel juxtamembrane ERM-binding region. J. Neurosci. 2005, 25, 395–403. [Google Scholar] [CrossRef]
- Cannon, J.L.; Mody, P.D.; Blaine, K.M.; Chen, E.J.; Nelson, A.D.; Sayles, L.J.; Burkhardt, J.K. CD43 interaction with ezrin-radixin-moesin (ERM) proteins regulates T-cell trafficking and CD43 phosphorylation. Mol. Biol. Cell 2011, 22, 954–963. [Google Scholar] [CrossRef]
- Newe, A.; Rzeniewicz, K.; König, M.; Schroer, C.F.; Joachim, J.; Rey-Gallardo, A.; Ivetic, A. Serine Phosphorylation of L-Selectin Regulates ERM Binding, Clustering, and Monocyte Protrusion in Transendothelial Migration. Front. Immunol. 2019, 10, 2227. [Google Scholar] [CrossRef]
- Bretscher, A.; Chambers, D.; Nguyen, R.; Reczek, D. ERM-Merlin and EBP50 protein families in plasma membrane organization and function. Annu. Rev. Cell Dev. Biol. 2000, 16, 113–143. [Google Scholar] [CrossRef] [PubMed]
- Terawaki, S.; Maesaki, R.; Hakoshima, T. Structural basis for NHERF recognition by ERM proteins. Structure 2006, 14, 777–789. [Google Scholar] [CrossRef] [PubMed]
- Gary, R.; Bretscher, A. Ezrin self-association involves binding of an N-terminal domain to a normally masked C-terminal domain that includes the F-actin binding site. Mol. Biol. Cell 1995, 6, 1061–1075. [Google Scholar] [CrossRef] [PubMed]
- Magendantz, M.; Henry, M.D.; Lander, A.; Solomon, F. Interdomain interactions of radixin in vitro. J. Biol. Chem. 1995, 270, 25324–25327. [Google Scholar] [CrossRef]
- Nakamura, F.; Amieva, M.R.; Furthmayr, H. Phosphorylation of threonine 558 in the carboxyl-terminal actin-binding domain of moesin by thrombin activation of human platelets. J. Biol. Chem. 1995, 270, 31377–31385. [Google Scholar] [CrossRef] [PubMed]
- Matsui, T.; Maeda, M.; Doi, Y.; Yonemura, S.; Amano, M.; Kaibuchi, K.; Tsukita, S. Rho-kinase phosphorylates COOH-terminal threonines of ezrin/radixin/moesin (ERM) proteins and regulates their head-to-tail association. J. Cell Biol. 1998, 140, 647–657. [Google Scholar] [CrossRef] [PubMed]
- Tsukita, S.; Yonemura, S. Cortical actin organization: Lessons from ERM (ezrin/radixin/moesin) proteins. J. Biol. Chem. 1999, 274, 34507–34510. [Google Scholar] [CrossRef]
- Fievet, B.T.; Gautreau, A.; Roy, C.; Del Maestro, L.; Mangeat, P.; Louvard, D.; Arpin, M. Phosphoinositide binding and phosphorylation act sequentially in the activation mechanism of ezrin. J. Cell Biol. 2004, 164, 653–659. [Google Scholar] [CrossRef]
- Nakamura, F.; Huang, L.; Pestonjamasp, K.; Luna, E.J.; Furthmayr, H. Regulation of F-actin binding to platelet moesin in vitro by both phosphorylation of threonine 558 and polyphosphatidylinositides. Mol. Biol. Cell 1999, 10, 2669–2685. [Google Scholar] [CrossRef]
- Ben-Aissa, K.; Patino-Lopez, G.; Belkina, N.V.; Maniti, O.; Rosales, T.; Hao, J.J.; Shaw, S. Activation of moesin, a protein that links actin cytoskeleton to the plasma membrane, occurs by phosphatidylinositol 4,5-bisphosphate (PIP2) binding sequentially to two sites and releasing an autoinhibitory linker. J. Biol. Chem. 2012, 287, 16311–16323. [Google Scholar] [CrossRef]
- Chen, X.; Khajeh, J.A.; Ju, J.H.; Gupta, Y.K.; Stanley, C.B.; Do, C.; Bu, Z. Phosphatidylinositol 4,5-bisphosphate clusters the cell adhesion molecule CD44 and assembles a specific CD44-Ezrin heterocomplex, as revealed by small angle neutron scattering. J. Biol. Chem. 2015, 290, 6639–6652. [Google Scholar] [CrossRef] [PubMed]
- Yonemura, S.; Matsui, T.; Tsukita, S.; Tsukita, S. Rho-dependent and -independent activation mechanisms of ezrin/radixin/moesin proteins: An essential role for polyphosphoinositides in vivo. J. Cell Sci. 2002, 115, 2569–2580. [Google Scholar] [PubMed]
- Lee, J.H.; Katakai, T.; Hara, T.; Gonda, H.; Sugai, M.; Shimizu, A. Roles of p-ERM and Rho-ROCK signaling in lymphocyte polarity and uropod formation. J. Cell Biol. 2004, 167, 327–337. [Google Scholar] [CrossRef] [PubMed]
- Pietromonaco, S.F.; Simons, P.C.; Altman, A.; Elias, L. Protein kinase C-theta phosphorylation of moesin in the actin-binding sequence. J. Biol. Chem. 1998, 273, 7594–7603. [Google Scholar] [CrossRef] [PubMed]
- Ng, T.; Parsons, M.; Hughes, W.E.; Monypenny, J.; Zicha, D.; Gautreau, A.; Parker, P.J. Ezrin is a downstream effector of trafficking PKC-integrin complexes involved in the control of cell motility. EMBO J. 2001, 20, 2723–2741. [Google Scholar] [CrossRef]
- Yin, H.; Shi, Z.; Jiao, S.; Chen, C.; Wang, W.; Greene, M.I.; Zhou, Z. Germinal center kinases in immune regulation. Cell Mol. Immunol. 2012, 9, 439–445. [Google Scholar] [CrossRef]
- Belkina, N.V.; Liu, Y.; Hao, J.J.; Karasuyama, H.; Shaw, S. LOK is a major ERM kinase in resting lymphocytes and regulates cytoskeletal rearrangement through ERM phosphorylation. Proc. Natl. Acad. Sci. USA 2009, 106, 4707–4712. [Google Scholar] [CrossRef]
- Kschonsak, Y.T.; Hoffmann, I. Activated ezrin controls MISP levels to ensure correct NuMA polarization and spindle orientation. J. Cell Sci. 2018, 131. [Google Scholar] [CrossRef]
- Ten Klooster, J.P.; Jansen, M.; Yuan, J.; Oorschot, V.; Begthel, H.; Di Giacomo, V.; Clevers, H. Mst4 and Ezrin induce brush borders downstream of the Lkb1/Strad/Mo25 polarization complex. Dev. Cell 2009, 16, 551–562. [Google Scholar] [CrossRef]
- Vitorino, P.; Yeung, S.; Crow, A.; Bakke, J.; Smyczek, T.; West, K.; Ndubaku, C. MAP4K4 regulates integrin-FERM binding to control endothelial cell motility. Nature 2015, 519, 425–430. [Google Scholar] [CrossRef]
- Baumgartner, M.; Sillman, A.L.; Blackwood, E.M.; Srivastava, J.; Madson, N.; Schilling, J.W.; Barber, D.L. The Nck-interacting kinase phosphorylates ERM proteins for formation of lamellipodium by growth factors. Proc. Natl. Acad. Sci. USA 2006, 103, 13391–13396. [Google Scholar] [CrossRef] [PubMed]
- Plutoni, C.; Keil, S.; Zeledon, C.; Delsin, L.E.A.; Decelle, B.; Roux, P.P.; Emery, G. Misshapen coordinates protrusion restriction and actomyosin contractility during collective cell migration. Nat. Commun. 2019, 10, 3940. [Google Scholar] [CrossRef] [PubMed]
- Machicoane, M.; de Frutos, C.A.; Fink, J.; Rocancourt, M.; Lombardi, Y.; Garel, S.; Echard, A. SLK-dependent activation of ERMs controls LGN-NuMA localization and spindle orientation. J. Cell Biol. 2014, 205, 791–799. [Google Scholar] [CrossRef] [PubMed]
- Carreno, S.; Kouranti, I.; Glusman, E.S.; Fuller, M.T.; Echard, A.; Payre, F. Moesin and its activating kinase Slik are required for cortical stability and microtubule organization in mitotic cells. J. Cell Biol. 2008, 180, 739–746. [Google Scholar] [CrossRef]
- Fukata, Y.; Kimura, K.; Oshiro, N.; Saya, H.; Matsuura, Y.; Kaibuchi, K. Association of the myosin-binding subunit of myosin phosphatase and moesin: Dual regulation of moesin phosphorylation by Rho-associated kinase and myosin phosphatase. J. Cell Biol. 1998, 141, 409–418. [Google Scholar] [CrossRef]
- Rodrigues, N.T.; Lekomtsev, S.; Jananji, S.; Kriston-Vizi, J.; Hickson, G.R.; Baum, B. Kinetochore-localized PP1-Sds22 couples chromosome segregation to polar relaxation. Nature 2015, 524, 489–492. [Google Scholar] [CrossRef]
- Hishiya, A.; Ohnishi, M.; Tamura, S.; Nakamura, F. Protein phosphatase 2C inactivates F-actin binding of human platelet moesin. J. Biol. Chem. 1999, 274, 26705–26712. [Google Scholar] [CrossRef][Green Version]
- Li, Y.; Jin, Y.; Liu, B.; Lu, D.; Zhu, M.; Jin, Y.; Yin, Y. PTENalpha promotes neutrophil chemotaxis through regulation of cell deformability. Blood 2019, 133, 2079–2089. [Google Scholar] [CrossRef]
- Yang, H.S.; Hinds, P.W. Increased ezrin expression and activation by CDK5 coincident with acquisition of the senescent phenotype. Mol. Cell. 2003, 11, 1163–1176. [Google Scholar] [CrossRef]
- Krieg, J.; Hunter, T. Identification of the two major epidermal growth factor-induced tyrosine phosphorylation sites in the microvillar core protein ezrin. J. Biol. Chem. 1992, 267, 19258–19265. [Google Scholar]
- Heiska, L.; Carpen, O. Src phosphorylates ezrin at tyrosine 477 and induces a phosphospecific association between ezrin and a kelch-repeat protein family member. J. Biol. Chem. 2005, 280, 10244–10252. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.S.; Hinds, P.W. Phosphorylation of ezrin by cyclin-dependent kinase 5 induces the release of Rho GDP dissociation inhibitor to inhibit Rac1 activity in senescent cells. Cancer Res. 2006, 66, 2708–2715. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Srivastava, J.; Elliott, B.E.; Louvard, D.; Arpin, M. Src-dependent ezrin phosphorylation in adhesion-mediated signaling. Mol. Biol. Cell 2005, 16, 1481–1490. [Google Scholar] [CrossRef] [PubMed]
- Elliott, B.E.; Meens, J.A.; SenGupta, S.K.; Louvard, D.; Arpin, M. The membrane cytoskeletal crosslinker ezrin is required for metastasis of breast carcinoma cells. Breast Cancer Res. 2005, 7, R365–R373. [Google Scholar] [CrossRef]
- Parameswaran, N.; Enyindah-Asonye, G.; Bagheri, N.; Shah, N.B.; Gupta, N. Spatial coupling of JNK activation to the B cell antigen receptor by tyrosine-phosphorylated ezrin. J. Immunol. 2013, 190, 2017–2026. [Google Scholar] [CrossRef]
- Coffey, G.P.; Rajapaksa, R.; Liu, R.; Sharpe, O.; Kuo, C.C.; Krauss, S.W.; Robinson, W.H. Engagement of CD81 induces ezrin tyrosine phosphorylation and its cellular redistribution with filamentous actin. J. Cell Sci. 2009, 122, 3137–3144. [Google Scholar] [CrossRef]
- Sanchez-Madrid, F.; del Pozo, M.A. Leukocyte polarization in cell migration and immune interactions. EMBO J. 1999, 18, 501–511. [Google Scholar] [CrossRef]
- Del Pozo, M.A.; Vicente-Manzanares, M.; Tejedor, R.; Serrador, J.M.; Sánchez-Madrid, F. Rho GTPases control migration and polarization of adhesion molecules and cytoskeletal ERM components in T lymphocytes. Eur. J. Immunol. 1999, 29, 3609–3620. [Google Scholar]
- McFarland, W. Microspikes on the lymphocyte uropod. Science 1969, 163, 818–820. [Google Scholar] [CrossRef]
- Bhalla, D.K.; Braun, J.; Karnovsky, M.J. Lymphocyte surface and cytoplasmic changes associated with translational motility and spontaneous capping of Ig. J. Cell Sci. 1979, 39, 137–147. [Google Scholar]
- Zabel, B.A.; Rott, A.; Butcher, E.C. Leukocyte chemoattractant receptors in human disease pathogenesis. Annu. Rev. Pathol. 2015, 10, 51–81. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Wang, F.; Van Keymeulen, A.; Herzmark, P.; Straight, A.; Kelly, K.; Bourne, H.R. Divergent signals and cytoskeletal assemblies regulate self-organizing polarity in neutrophils. Cell 2003, 114, 201–214. [Google Scholar] [CrossRef]
- Sanchez-Madrid, F.; Serrador, J.M. Bringing up the rear: Defining the roles of the uropod. Nat. Rev. Mol. Cell Biol. 2009, 10, 353–359. [Google Scholar] [CrossRef] [PubMed]
- Hao, J.J.; Liu, Y.; Kruhlak, M.; Debell, K.E.; Rellahan, B.L.; Shaw, S. Phospholipase C-mediated hydrolysis of PIP2 releases ERM proteins from lymphocyte membrane. J. Cell Biol. 2009, 184, 451–462. [Google Scholar] [CrossRef]
- Treanor, B.; Depoil, D.; Bruckbauer, A.; Batista, F.D. Dynamic cortical actin remodeling by ERM proteins controls BCR microcluster organization and integrity. J. Exp. Med. 2011, 208, 1055–1068. [Google Scholar] [CrossRef]
- Liu, X.; Yang, T.; Suzuki, K.; Tsukita, S.; Ishii, M.; Zhou, S.; Oh, M.J. Moesin and myosin phosphatase confine neutrophil orientation in a chemotactic gradient. J. Exp. Med. 2015, 212, 267–280. [Google Scholar] [CrossRef]
- Ren, C.; Yuan, Q.; Braun, M.; Zhang, X.; Petri, B.; Zhang, J.; Fan, R. Leukocyte Cytoskeleton Polarization Is Initiated by Plasma Membrane Curvature from Cell Attachment. Dev. Cell 2019, 49, 206–219. [Google Scholar] [CrossRef]
- Lacalle, R.A.; Peregil, R.M.; Albar, J.P.; Merino, E.; Martínez-A, C.; Mérida, I.; Mañes, S. Type I phosphatidylinositol 4-phosphate 5-kinase controls neutrophil polarity and directional movement. J. Cell Biol. 2007, 179, 1539–1553. [Google Scholar] [CrossRef]
- Chen, E.J.; Shaffer, M.H.; Williamson, E.K.; Huang, Y.; Burkhardt, J.K. Ezrin and moesin are required for efficient T cell adhesion and homing to lymphoid organs. PLoS ONE 2013, 8, e52368. [Google Scholar] [CrossRef]
- Martinelli, S.; Chen, E.J.; Clarke, F.; Lyck, R.; Affentranger, S.; Burkhardt, J.K.; Niggli, V. Ezrin/Radixin/Moesin proteins and flotillins cooperate to promote uropod formation in T cells. Front. Immunol. 2013, 4, 84. [Google Scholar] [CrossRef]
- Doi, Y.; Itoh, M.; Yonemura, S.; Ishihara, S.; Takano, H.; Noda, T.; Tsukita, S. Normal development of mice and unimpaired cell adhesion/cell motility/actin-based cytoskeleton without compensatory up-regulation of ezrin or radixin in moesin gene knockout. J. Biol. Chem. 1999, 274, 2315–2321. [Google Scholar] [CrossRef] [PubMed]
- Hirata, T.; Nomachi, A.; Tohya, K.; Miyasaka, M.; Tsukita, S.; Watanabe, T.; Narumiya, S. Moesin-deficient mice reveal a non-redundant role for moesin in lymphocyte homeostasis. Int. Immunol. 2012, 24, 705–717. [Google Scholar] [CrossRef] [PubMed]
- Sanz, M.J.; Kubes, P. Neutrophil-active chemokines in in vivo imaging of neutrophil trafficking. Eur. J. Immunol. 2012, 42, 278–283. [Google Scholar] [CrossRef] [PubMed]
- Yonemura, S.; Tsukita, S.; Tsukita, S. Direct involvement of ezrin/radixin/moesin (ERM)-binding membrane proteins in the organization of microvilli in collaboration with activated ERM proteins. J. Cell Biol. 1999, 145, 1497–1509. [Google Scholar] [CrossRef]
- Ivetič, A.; Florey, O.; Deka, J.; Haskard, D.O.; Ager, A.; Ridley, A.J. Mutagenesis of the ezrin-radixin-moesin binding domain of L-selectin tail affects shedding, microvillar positioning, and leukocyte tethering. J. Biol. Chem. 2004, 279, 33263–33272. [Google Scholar] [CrossRef]
- Ishihara, S.; Nishikimi, A.; Umemoto, E.; Miyasaka, M.; Saegusa, M.; Katagiri, K. Dual functions of Rap1 are crucial for T-cell homeostasis and prevention of spontaneous colitis. Nat. Commun. 2015, 6, 8982. [Google Scholar] [CrossRef]
- Matsumoto, M.; Hirata, T. Moesin regulates neutrophil rolling velocity in vivo. Cell Immunol. 2016, 304–305, 59–62. [Google Scholar] [CrossRef]
- Spertini, C.; Baisse, B.; Spertini, O. Ezrin-radixin-moesin-binding sequence of PSGL-1 glycoprotein regulates leukocyte rolling on selectins and activation of extracellular signal-regulated kinases. J. Biol. Chem. 2012, 287, 10693–10702. [Google Scholar] [CrossRef]
- Nijhara, R.; van Hennik, P.B.; Gignac, M.L.; Kruhlak, M.J.; Hordijk, P.L.; Delon, J.; Shaw, S. Rac1 mediates collapse of microvilli on chemokine-activated T lymphocytes. J. Immunol. 2004, 173, 4985–4993. [Google Scholar] [CrossRef]
- Barreiro, O.; Yáñez-Mó, M.; Sala-Valdés, M.; Gutiérrez-López, M.D.; Ovalle, S.; Higginbottom, A.; Sánchez-Madrid, F. Endothelial tetraspanin microdomains regulate leukocyte firm adhesion during extravasation. Blood 2005, 105, 2852–2861. [Google Scholar] [CrossRef]
- Sala-Valdés, M.; Ursa, Á.; Charrin, S.; Rubinstein, E.; Hemler, M.E.; Sánchez-Madrid, F.; Yáñez-Mó, M. EWI-2 and EWI-F link the tetraspanin web to the actin cytoskeleton through their direct association with ezrin-radixin-moesin proteins. J. Biol. Chem. 2006, 281, 19665–19675. [Google Scholar] [CrossRef] [PubMed]
- Carman, C.V.; Jun, C.D.; Salas, A.; Springer, T.A. Endothelial cells proactively form microvilli-like membrane projections upon intercellular adhesion molecule 1 engagement of leukocyte LFA-1. J. Immunol. 2003, 171, 6135–6144. [Google Scholar] [CrossRef] [PubMed]
- Doulet, N.; Donnadieu, E.; Laran-Chich, M.P.; Niedergang, F.; Nassif, X.; Couraud, P.O.; Bourdoulous, S. Neisseria meningitidis infection of human endothelial cells interferes with leukocyte transmigration by preventing the formation of endothelial docking structures. J. Cell Biol. 2006, 173, 627–637. [Google Scholar] [CrossRef] [PubMed]
- Oh, H.M.; Lee, S.; Na, B.R.; Wee, H.; Kim, S.H.; Choi, S.C.; Jun, C.D. RKIKK motif in the intracellular domain is critical for spatial and dynamic organization of ICAM-1: Functional implication for the leukocyte adhesion and transmigration. Mol. Biol. Cell 2007, 18, 2322–2335. [Google Scholar] [CrossRef][Green Version]
- Filippi, M.D. Mechanism of Diapedesis: Importance of the Transcellular Route. Adv. Immunol. 2016, 129, 25–53. [Google Scholar]
- Liu, Y.; Belkina, N.V.; Park, C.; Nambiar, R.; Loughhead, S.M.; Patino-Lopez, G.; von Andrian, U.H. Constitutively active ezrin increases membrane tension, slows migration, and impedes endothelial transmigration of lymphocytes in vivo in mice. Blood 2012, 119, 445–453. [Google Scholar] [CrossRef]
- Müller, N.; Fischer, H.J.; Tischner, D.; van den Brandt, J.; Reichardt, H.M. Glucocorticoids induce effector T cell depolarization via ERM proteins, thereby impeding migration and APC conjugation. J. Immunol. 2013, 190, 4360–4370. [Google Scholar] [CrossRef]
- Shulman, Z.; Shinder, V.; Klein, E.; Grabovsky, V.; Yeger, O.; Geron, E.; Laudanna, C. Lymphocyte crawling and transendothelial migration require chemokine triggering of high-affinity LFA-1 integrin. Immunity 2009, 30, 384–396. [Google Scholar] [CrossRef]
- Carman, C.V.; Sage, P.T.; Sciuto, T.E.; Miguel, A.; Geha, R.S.; Ochs, H.D.; Springer, T.A. Transcellular diapedesis is initiated by invasive podosomes. Immunity 2007, 26, 784–797. [Google Scholar] [CrossRef]
- Koduru, S.; Kumar, L.; Massaad, M.J.; Ramesh, N.; Le Bras, S.; Ozcan, E.; King, S. Cdc42 interacting protein 4 (CIP4) is essential for integrin-dependent T-cell trafficking. Proc. Natl. Acad. Sci. USA 2010, 107, 16252–16256. [Google Scholar] [CrossRef]
- Rey-Gallardo, A.; Tomlins, H.; Joachim, J.; Rahman, I.; Kitscha, P.; Frudd, K.; Ivetic, A. Sequential binding of ezrin and moesin to L-selectin regulates monocyte protrusive behaviour during transendothelial migration. J. Cell Sci. 2018, 131. [Google Scholar] [CrossRef]
- Ilina, O.; Friedl, P. Mechanisms of collective cell migration at a glance. J. Cell Sci. 2009, 122, 3203–3208. [Google Scholar] [CrossRef]
- Ramel, D.; Wang, X.; Laflamme, C.; Montell, D.J.; Emery, G. Rab11 regulates cell-cell communication during collective cell movements. Nat. Cell boil. 2013, 15, 317–324. [Google Scholar] [CrossRef]
- Malet-Engra, G.; Yu, W.; Oldani, A.; Rey-Barroso, J.; Gov, N.S.; Scita, G.; Dupré, L. Collective cell motility promotes chemotactic prowess and resistance to chemorepulsion. Curr. Biol. 2015, 25, 242–250. [Google Scholar] [CrossRef]
- Grakoui, A.; Bromley, S.K.; Sumen, C.; Davis, M.M.; Shaw, A.S.; Allen, P.M.; Dustin, M.L. The immunological synapse: A molecular machine controlling T cell activation. Science 1999, 285, 221–227. [Google Scholar] [CrossRef]
- Tolar, P. Cytoskeletal control of B cell responses to antigens. Nat. Rev. Immunol. 2017, 17, 621–634. [Google Scholar] [CrossRef]
- Lagrue, K.; Carisey, A.; Oszmiana, A.; Kennedy, P.R.; Williamson, D.J.; Cartwright, A.; Davis, D.M. The central role of the cytoskeleton in mechanisms and functions of the NK cell immune synapse. Immunol. Rev. 2013, 256, 203–221. [Google Scholar] [CrossRef]
- Niedergang, F.; Di Bartolo, V.; Alcover, A. Comparative Anatomy of Phagocytic and Immunological Synapses. Front. Immunol. 2016, 7, 18. [Google Scholar] [CrossRef]
- Freeman, S.A.; Vega, A.; Riedl, M.; Collins, R.F.; Ostrowski, P.P.; Woods, E.C.; Mayor, S. Transmembrane Pickets Connect Cyto- and Pericellular Skeletons Forming Barriers to Receptor Engagement. Cell 2018, 172, 305–317. [Google Scholar] [CrossRef]
- Urzainqui, A.; Serrador, J.M.; Viedma, F.; Yáñez-Mó, M.; Rodríguez, A.; Corbí, A.L.; Sánchez-Madrid, F. ITAM-based interaction of ERM proteins with Syk mediates signaling by the leukocyte adhesion receptor PSGL-1. Immunity 2002, 17, 401–412. [Google Scholar] [CrossRef]
- Mu, L.; Tu, Z.; Miao, L.; Ruan, H.; Kang, N.; Hei, Y.; Du, Y. A phosphatidylinositol 4,5-bisphosphate redistribution-based sensing mechanism initiates a phagocytosis programing. Nat. Commun. 2018, 9, 4259. [Google Scholar] [CrossRef]
- Defacque, H.; Egeberg, M.; Habermann, A.; Diakonova, M.; Roy, C.; Mangeat, P.; Griffiths, G. Involvement of ezrin/moesin in de novo actin assembly on phagosomal membranes. EMBO J. 2000, 19, 199–212. [Google Scholar] [CrossRef]
- Shcherbina, A.; Bretscher, A.; Kenney, D.M.; Remold-O’Donnell, E. Moesin, the major ERM protein of lymphocytes and platelets, differs from ezrin in its insensitivity to calpain. FEBS Lett. 1999, 443, 31–36. [Google Scholar] [CrossRef]
- Roberts, R.E.; Hallett, M.B. Neutrophil Cell Shape Change: Mechanism and Signalling during Cell Spreading and Phagocytosis. Int. J. Mol. Sci. 2019, 20, 1383. [Google Scholar] [CrossRef]
- Ferreira, É.R.; Bonfim-Melo, A.; Cordero, E.M.; Mortara, R.A. ERM Proteins Play Distinct Roles in Cell Invasion by Extracellular Amastigotes of Trypanosoma cruzi. Front. Microbiol. 2017, 8, 2230. [Google Scholar] [CrossRef]
- Nakaya, M.; Kitano, M.; Matsuda, M.; Nagata, S. Spatiotemporal activation of Rac1 for engulfment of apoptotic cells. Proc. Natl. Acad. Sci. USA 2008, 105, 9198–9203. [Google Scholar] [CrossRef]
- Kim, S.Y.; Kim, S.; Bae, D.J.; Park, S.Y.; Lee, G.Y.; Park, G.M.; Kim, I.S. Coordinated balance of Rac1 and RhoA plays key roles in determining phagocytic appetite. PLoS ONE 2017, 12, e0174603. [Google Scholar] [CrossRef]
- Erwig, L.P.; McPhilips, K.A.; Wynes, M.W.; Ivetic, A.; Ridley, A.J.; Henson, P.M. Differential regulation of phagosome maturation in macrophages and dendritic cells mediated by Rho GTPases and ezrin-radixin-moesin (ERM) proteins. Proc. Natl. Acad. Sci. USA 2006, 103, 12825–12830. [Google Scholar] [CrossRef]
- Marion, S.; Hoffmann, E.; Holzer, D.; Le Clainche, C.; Martin, M.; Sachse, M.; Griffiths, G. Ezrin promotes actin assembly at the phagosome membrane and regulates phago-lysosomal fusion. Traffic 2011, 12, 421–437. [Google Scholar] [CrossRef]
- Dustin, M.L. Hunter to gatherer and back: Immunological synapses and kinapses as variations on the theme of amoeboid locomotion. Annu. Rev. Cell Dev. Biol. 2008, 24, 577–596. [Google Scholar] [CrossRef]
- Fooksman, D.R.; Vardhana, S.; Vasiliver-Shamis, G.; Liese, J.; Blair, D.A.; Waite, J.; Dustin, M.L. Functional anatomy of T cell activation and synapse formation. Annu. Rev. Immunol. 2009, 28, 79–105. [Google Scholar] [CrossRef]
- Vardhana, S.; Choudhuri, K.; Varma, R.; Dustin, M.L. Essential role of ubiquitin and TSG101 protein in formation and function of the central supramolecular activation cluster. Immunity 2010, 32, 531–540. [Google Scholar] [CrossRef]
- Roumier, A.; Olivo-Marin, J.C.; Arpin, M.; Michel, F.; Martin, M.; Mangeat, P.; Alcover, A. The membrane-microfilament linker ezrin is involved in the formation of the immunological synapse and in T cell activation. Immunity 2001, 15, 715–728. [Google Scholar] [CrossRef]
- Delon, J.; Kaibuchi, K.; Germain, R.N. Exclusion of CD43 from the immunological synapse is mediated by phosphorylation-regulated relocation of the cytoskeletal adaptor moesin. Immunity 2001, 15, 691–701. [Google Scholar] [CrossRef]
- Allenspach, E.J.; Cullinan, P.; Tong, J.; Tang, Q.; Tesciuba, A.G.; Cannon, J.L.; Sperling, A.I. ERM-dependent movement of CD43 defines a novel protein complex distal to the immunological synapse. Immunity 2001, 15, 739–750. [Google Scholar] [CrossRef]
- McCann, F.E.; Vanherberghen, B.; Eleme, K.; Carlin, L.M.; Newsam, R.J.; Goulding, D.; Davis, D.M. The size of the synaptic cleft and distinct distributions of filamentous actin, ezrin, CD43, and CD45 at activating and inhibitory human NK cell immune synapses. J. Immunol. 2003, 170, 2862–2870. [Google Scholar] [CrossRef]
- Masilamani, M.; Nguyen, C.; Kabat, J.; Borrego, F.; Coligan, J.E. CD94/NKG2A inhibits NK cell activation by disrupting the actin network at the immunological synapse. J. Immunol. 2006, 177, 3590–3596. [Google Scholar] [CrossRef]
- Tong, J.; Allenspach, E.J.; Takahashi, S.M.; Mody, P.D.; Park, C.; Burkhardt, J.K.; Sperling, A.I. CD43 regulation of T cell activation is not through steric inhibition of T cell-APC interactions but through an intracellular mechanism. J. Exp. Med. 2004, 199, 1277–1283. [Google Scholar] [CrossRef]
- Ilani, T.; Khanna, C.; Zhou, M.; Veenstra, T.D.; Bretscher, A. Immune synapse formation requires ZAP-70 recruitment by ezrin and CD43 removal by moesin. J. Cell Biol. 2007, 179, 733–746. [Google Scholar] [CrossRef]
- Shaffer, M.H.; Dupree, R.S.; Zhu, P.; Saotome, I.; Schmidt, R.F.; McClatchey, A.I.; Burkhardt, J.K. Ezrin and moesin function together to promote T cell activation. J. Immunol. 2009, 182, 1021–1032. [Google Scholar] [CrossRef]
- Blumenthal, D.; Burkhardt, J.K. Multiple actin networks coordinate mechanotransduction at the immunological synapse. J. Cell Biol. 2020, 219. [Google Scholar] [CrossRef] [PubMed]
- Faure, S.; Salazar-Fontana, L.I.; Semichon, M.; Tybulewicz, V.L.; Bismuth, G.; Trautmann, A.; Delon, J. ERM proteins regulate cytoskeleton relaxation promoting T cell-APC conjugation. Nat. Immunol. 2004, 5, 272–279. [Google Scholar] [CrossRef] [PubMed]
- Van Kooyk, Y.; van Vliet, S.J.; Figdor, C.G. The actin cytoskeleton regulates LFA-1 ligand binding through avidity rather than affinity changes. J. Biol. Chem. 1999, 274, 26869–26877. [Google Scholar] [CrossRef] [PubMed]
- Comrie, W.A.; Li, S.; Boyle, S.; Burkhardt, J.K. The dendritic cell cytoskeleton promotes T cell adhesion and activation by constraining ICAM-1 mobility. J. Cell Biol. 2015, 208, 457–473. [Google Scholar] [CrossRef]
- Gross, C.C.; Brzostowski, J.A.; Liu, D.; Long, E.O. Tethering of intercellular adhesion molecule on target cells is required for LFA-1-dependent NK cell adhesion and granule polarization. J. Immunol. 2010, 185, 2918–2926. [Google Scholar] [CrossRef]
- Treanor, B.; Depoil, D.; Gonzalez-Granja, A.; Barral, P.; Weber, M.; Dushek, O.; Batista, F.D. The membrane skeleton controls diffusion dynamics and signaling through the B cell receptor. Immunity 2010, 32, 187–199. [Google Scholar] [CrossRef]
- Parameswaran, N.; Gupta, N. Re-defining ERM function in lymphocyte activation and migration. Immunol. Rev. 2013, 256, 63–79. [Google Scholar] [CrossRef]
- Gupta, N.; Wollscheid, B.; Watts, J.D.; Scheer, B.; Aebersold, R.; DeFranco, A.L. Quantitative proteomic analysis of B cell lipid rafts reveals that ezrin regulates antigen receptor-mediated lipid raft dynamics. Nat. Immunol. 2006, 7, 625–633. [Google Scholar] [CrossRef]
- Kwak, K.; Quizon, N.; Sohn, H.; Saniee, A.; Manzella-Lapeira, J.; Holla, P.; Spillane, K.M. Intrinsic properties of human germinal center B cells set antigen affinity thresholds. Sci. Immunol. 2018, 3, eaau6598. [Google Scholar] [CrossRef]
- Delmonte, O.M.; Biggs, C.M.; Hayward, A.; Comeau, A.M.; Kuehn, H.S.; Rosenzweig, S.D.; Notarangelo, L.D. First Case of X-Linked Moesin Deficiency Identified After Newborn Screening for SCID. J. Clin. Immunol. 2017, 37, 336–338. [Google Scholar] [CrossRef]
- Lagresle-Peyrou, C.; Luce, S.; Ouchani, F.; Soheili, T.S.; Sadek, H.; Chouteau, M.; Lambert, N. X-linked primary immunodeficiency associated with hemizygous mutations in the moesin (MSN) gene. J. Allergy Clin. Immunol. 2016, 138, 1681–1689. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Harada, T.; Juang, Y.T.; Kyttaris, V.C.; Wang, Y.; Zidanic, M.; Tsokos, G.C. Phosphorylated ERM is responsible for increased T cell polarization, adhesion, and migration in patients with systemic lupus erythematosus. J. Immunol. 2007, 178, 1938–1947. [Google Scholar] [CrossRef] [PubMed]
- Satooka, H.; Nagakubo, D.; Sato, T.; Hirata, T. The ERM Protein Moesin Regulates CD8(+) Regulatory T Cell Homeostasis and Self-Tolerance. J. Immunol. 2017, 199, 3418–3426. [Google Scholar] [CrossRef] [PubMed]
- Ansa-Addo, E.A.; Zhang, Y.; Yang, Y.; Hussey, G.S.; Howley, B.V.; Salem, M.; Howe, P.H. Membrane-organizing protein moesin controls Treg differentiation and antitumor immunity via TGF-beta signaling. J. Clin. Investig. 2017, 127, 1321–1337. [Google Scholar] [CrossRef]
- Pore, D.; Huang, E.; Dejanovic, D.; Parameswaran, N.; Cheung, M.B.; Gupta, N. Cutting Edge: Deletion of Ezrin in B Cells of Lyn-Deficient Mice Downregulates Lupus Pathology. J. Immunol. 2018, 201, 1353–1358. [Google Scholar] [CrossRef]
- Pore, D.; Matsui, K.; Parameswaran, N.; Gupta, N. Cutting Edge: Ezrin Regulates Inflammation by Limiting B Cell IL-10 Production. J. Immunol. 2016, 196, 558–562. [Google Scholar] [CrossRef]
- Pore, D.; Parameswaran, N.; Matsui, K.; Stone, M.B.; Saotome, I.; McClatchey, A.I.; Gupta, N. Ezrin tunes the magnitude of humoral immunity. J. Immunol. 2013, 191, 4048–4058. [Google Scholar] [CrossRef]
- Birgbauer, E.; Solomon, F. A marginal band-associated protein has properties of both microtubule- and microfilament-associated proteins. J. Cell Biol. 1989, 109, 1609–1620. [Google Scholar] [CrossRef]
- Martin, M.; Roy, C.; Montcourrier, P.; Sahuquet, A.; Mangeat, P. Three determinants in ezrin are responsible for cell extension activity. Mol. Biol. Cell 1997, 8, 1543–1557. [Google Scholar] [CrossRef][Green Version]
- Solinet, S.; Mahmud, K.; Stewman, S.F.; Ben El Kadhi, K.; Decelle, B.; Talje, L.; Carreno, S. The actin-binding ERM protein Moesin binds to and stabilizes microtubules at the cell cortex. J. Cell Biol. 2013, 202, 251–260. [Google Scholar] [CrossRef]
- Takesono, A.; Heasman, S.J.; Wojciak-Stothard, B.; Garg, R.; Ridley, A.J. Microtubules regulate migratory polarity through Rho/ROCK signaling in T cells. PLoS ONE 2010, 5, e8774. [Google Scholar] [CrossRef] [PubMed]
- Prentice-Mott, H.V.; Meroz, Y.; Carlson, A.; Levine, M.A.; Davidson, M.W.; Irimia, D.; Shah, J.V. Directional memory arises from long-lived cytoskeletal asymmetries in polarized chemotactic cells. Proc. Natl. Acad. Sci. USA 2016, 113, 1267–1272. [Google Scholar] [CrossRef] [PubMed]
- Lasserre, R.; Charrin, S.; Cuche, C.; Danckaert, A.; Thoulouze, M.I.; De Chaumont, F.; Etienne-Manneville, S. Ezrin tunes T-cell activation by controlling Dlg1 and microtubule positioning at the immunological synapse. EMBO J. 2010, 29, 2301–2314. [Google Scholar] [CrossRef] [PubMed]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Ortiz, A.; Serrador, J.M. ERM Proteins at the Crossroad of Leukocyte Polarization, Migration and Intercellular Adhesion. Int. J. Mol. Sci. 2020, 21, 1502. https://doi.org/10.3390/ijms21041502
García-Ortiz A, Serrador JM. ERM Proteins at the Crossroad of Leukocyte Polarization, Migration and Intercellular Adhesion. International Journal of Molecular Sciences. 2020; 21(4):1502. https://doi.org/10.3390/ijms21041502
Chicago/Turabian StyleGarcía-Ortiz, Almudena, and Juan Manuel Serrador. 2020. "ERM Proteins at the Crossroad of Leukocyte Polarization, Migration and Intercellular Adhesion" International Journal of Molecular Sciences 21, no. 4: 1502. https://doi.org/10.3390/ijms21041502
APA StyleGarcía-Ortiz, A., & Serrador, J. M. (2020). ERM Proteins at the Crossroad of Leukocyte Polarization, Migration and Intercellular Adhesion. International Journal of Molecular Sciences, 21(4), 1502. https://doi.org/10.3390/ijms21041502



