The MICELI (MICrofluidic, ELectrical, Impedance): Prototyping a Point-of-Care Impedance Platelet Aggregometer
Abstract
1. Introduction
2. Results
2.1. Optimization of the MICELI aggregometer and assay protocol
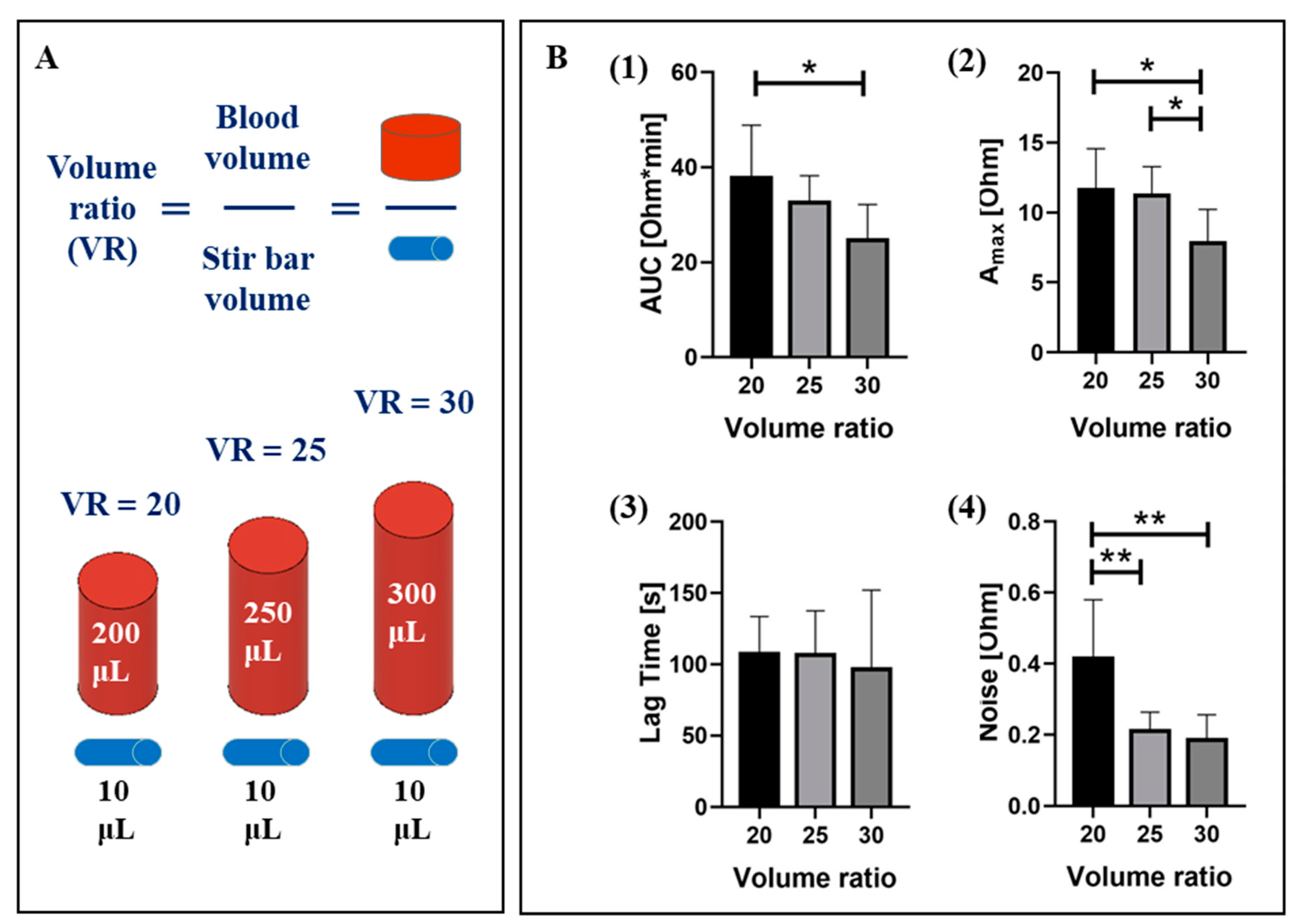
2.1.1. Sample Volume
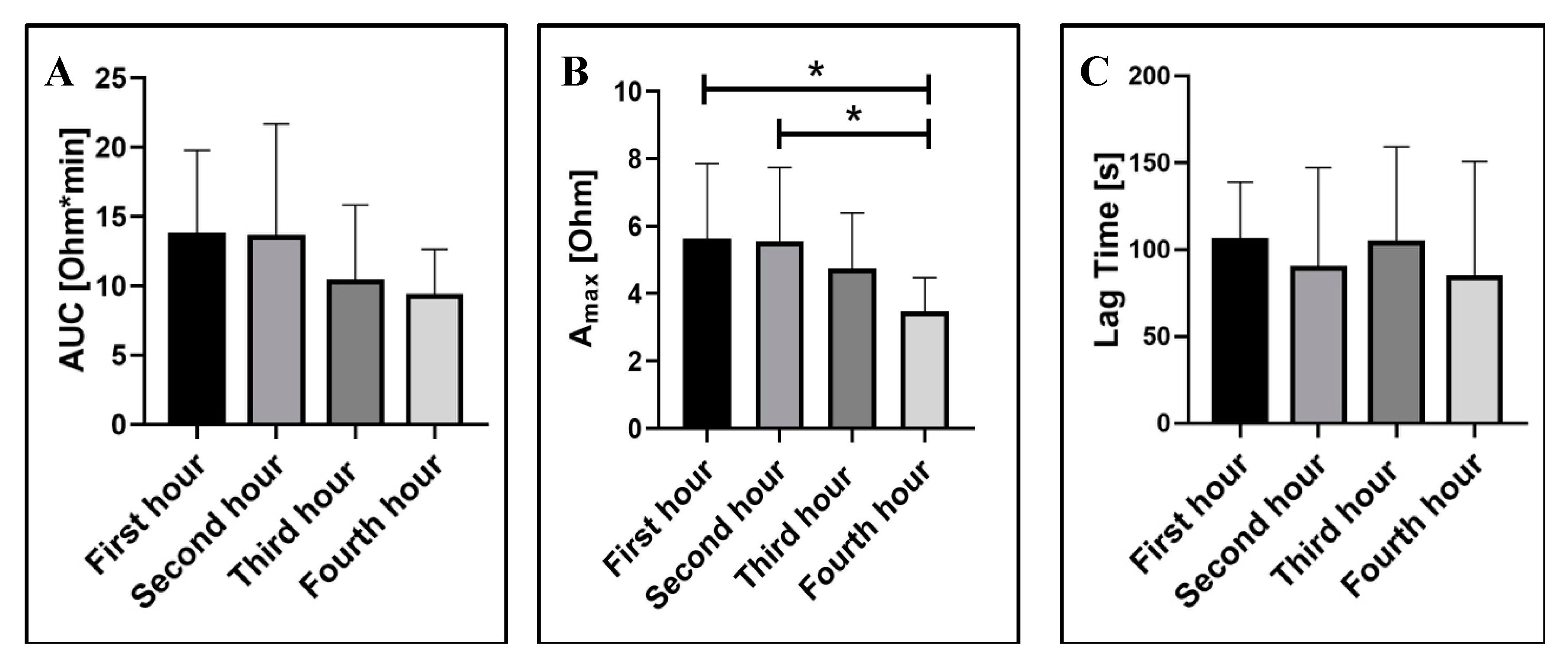
2.1.2. Blood Storage Time
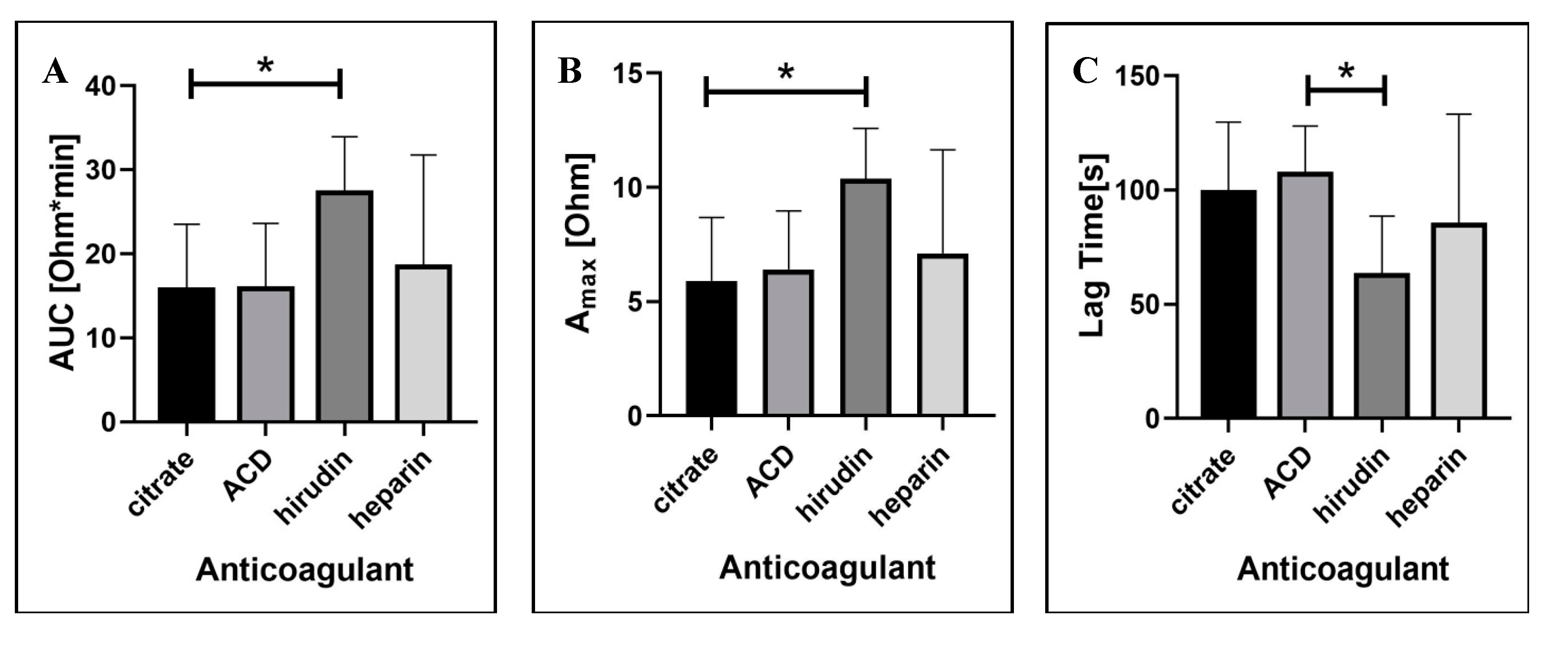
2.1.3. Blood Anticoagulation Regimen
2.2. Validation of the MICELI aggregometer
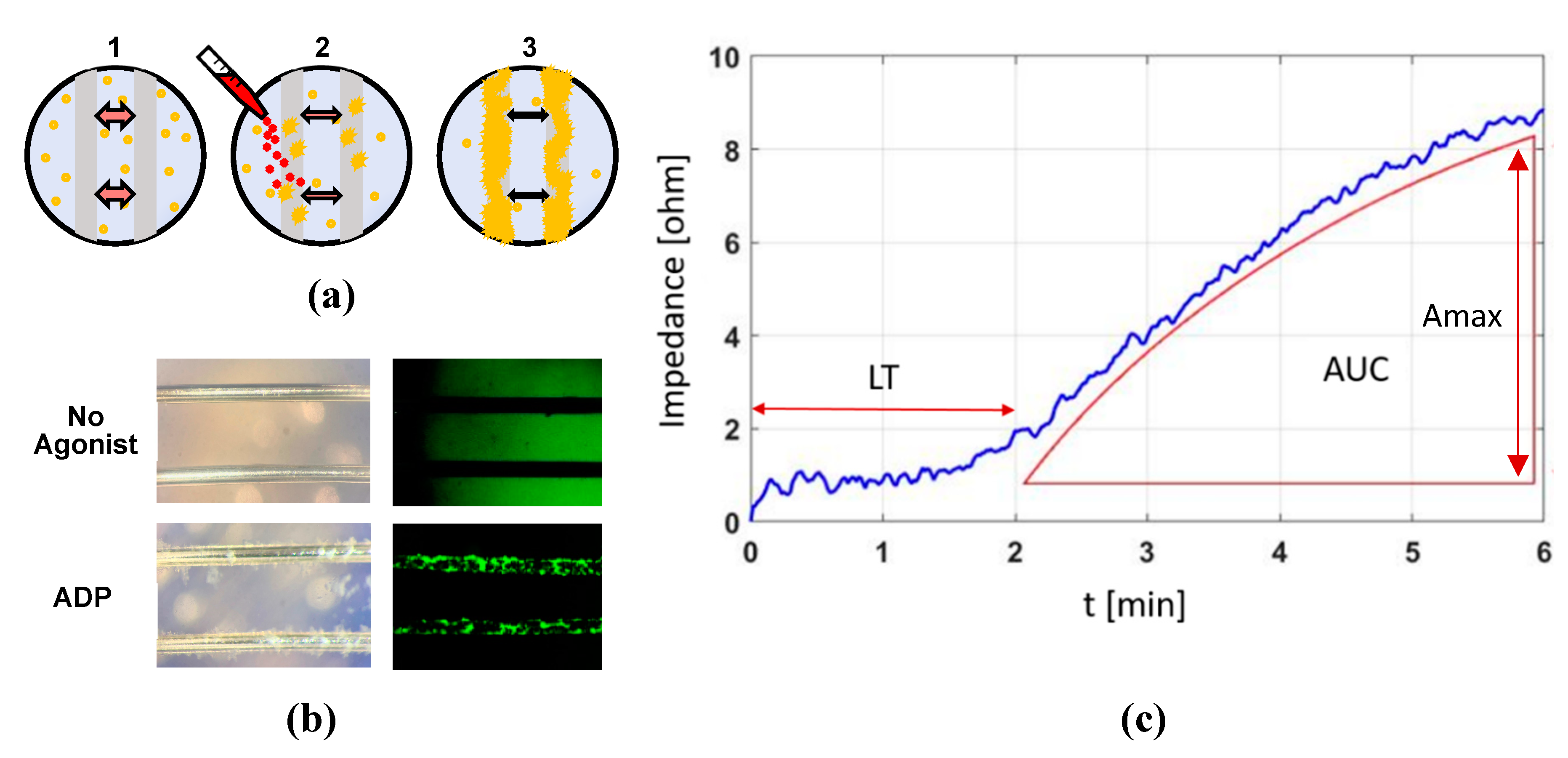
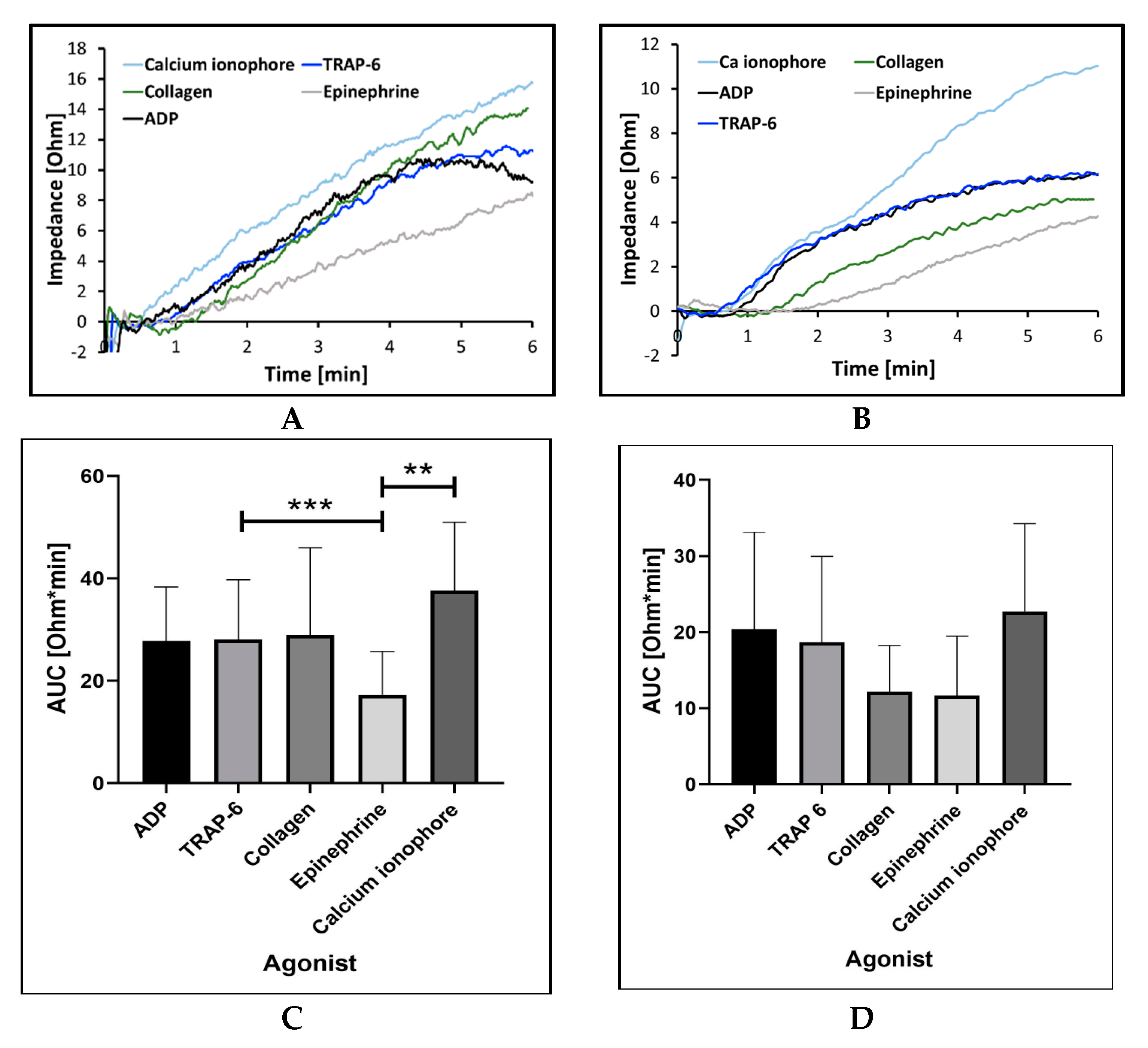
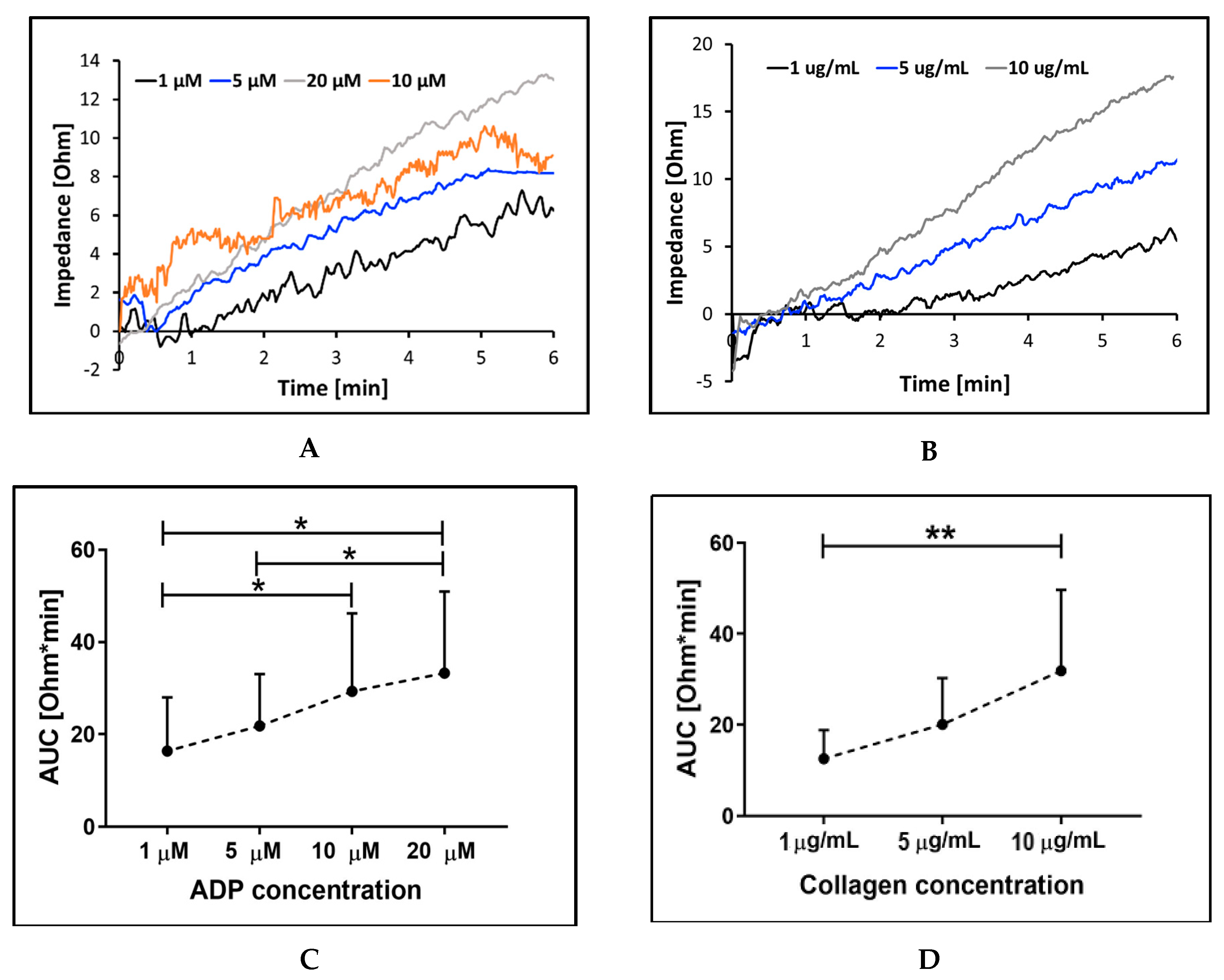
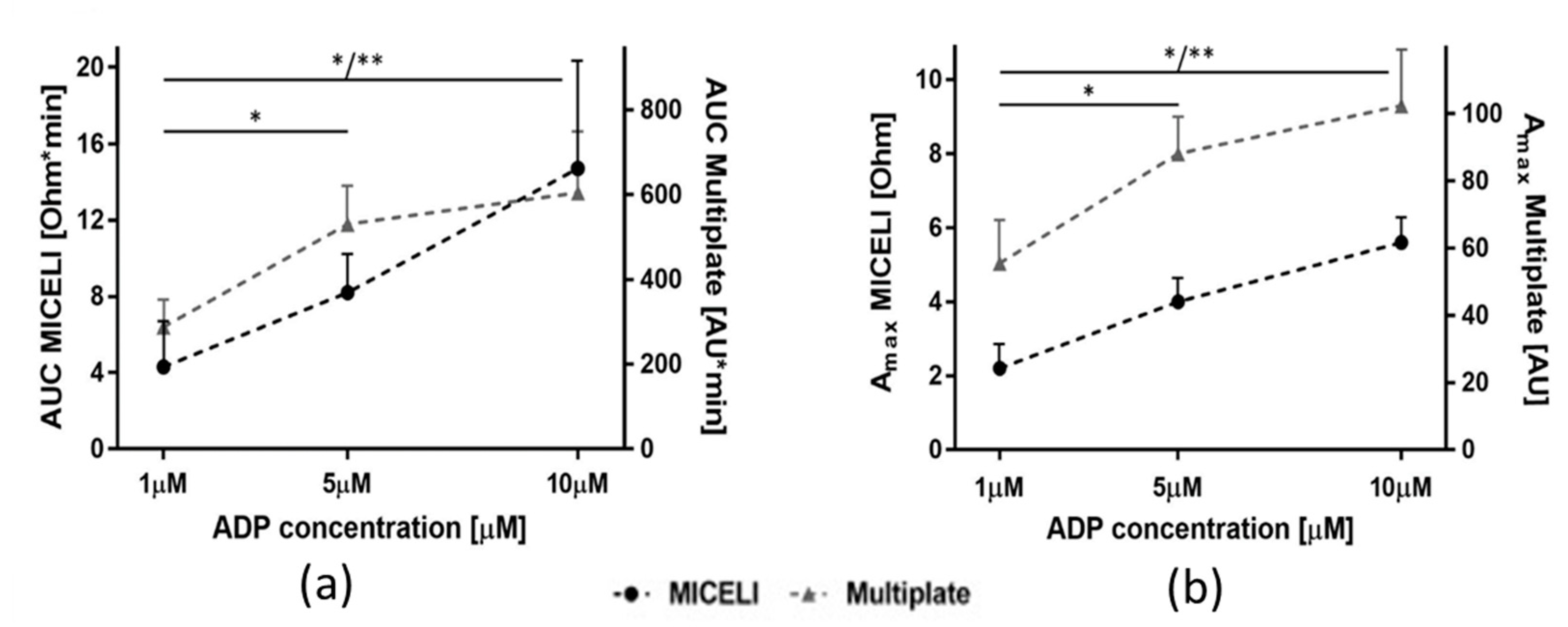
2.2.1. Sensitivity and Precision with Different Aggregation Agonists and Their Concentration
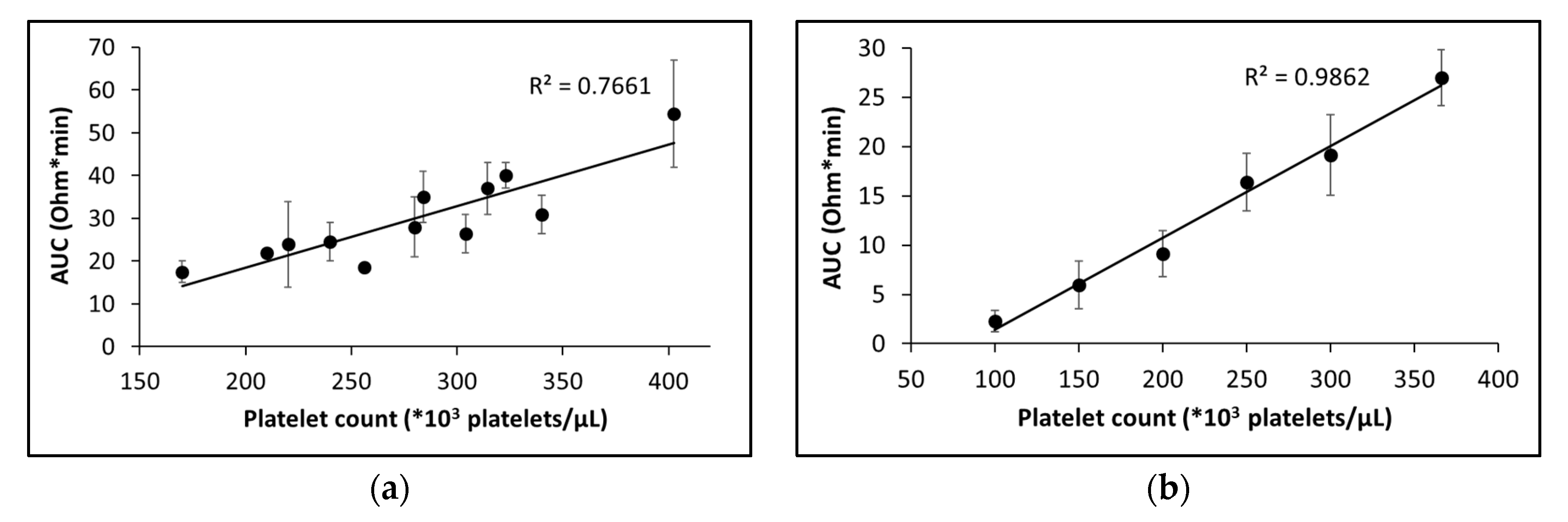
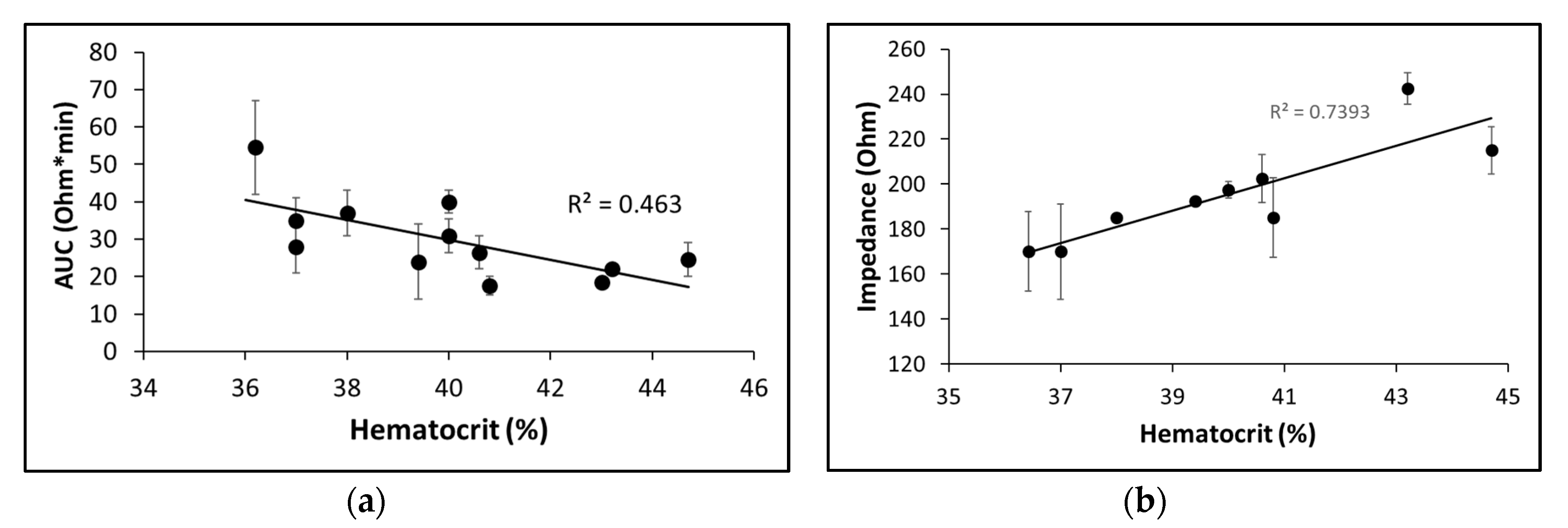
2.2.2. Sensitivity to platelet count and hematocrit
2.2.3. Operational Performance of the MICELI Aggregometer as Compared with Multiplate® Analyzer
3. Discussion
4. Materials and Methods
4.1. Blood Collection and Anticoagulation
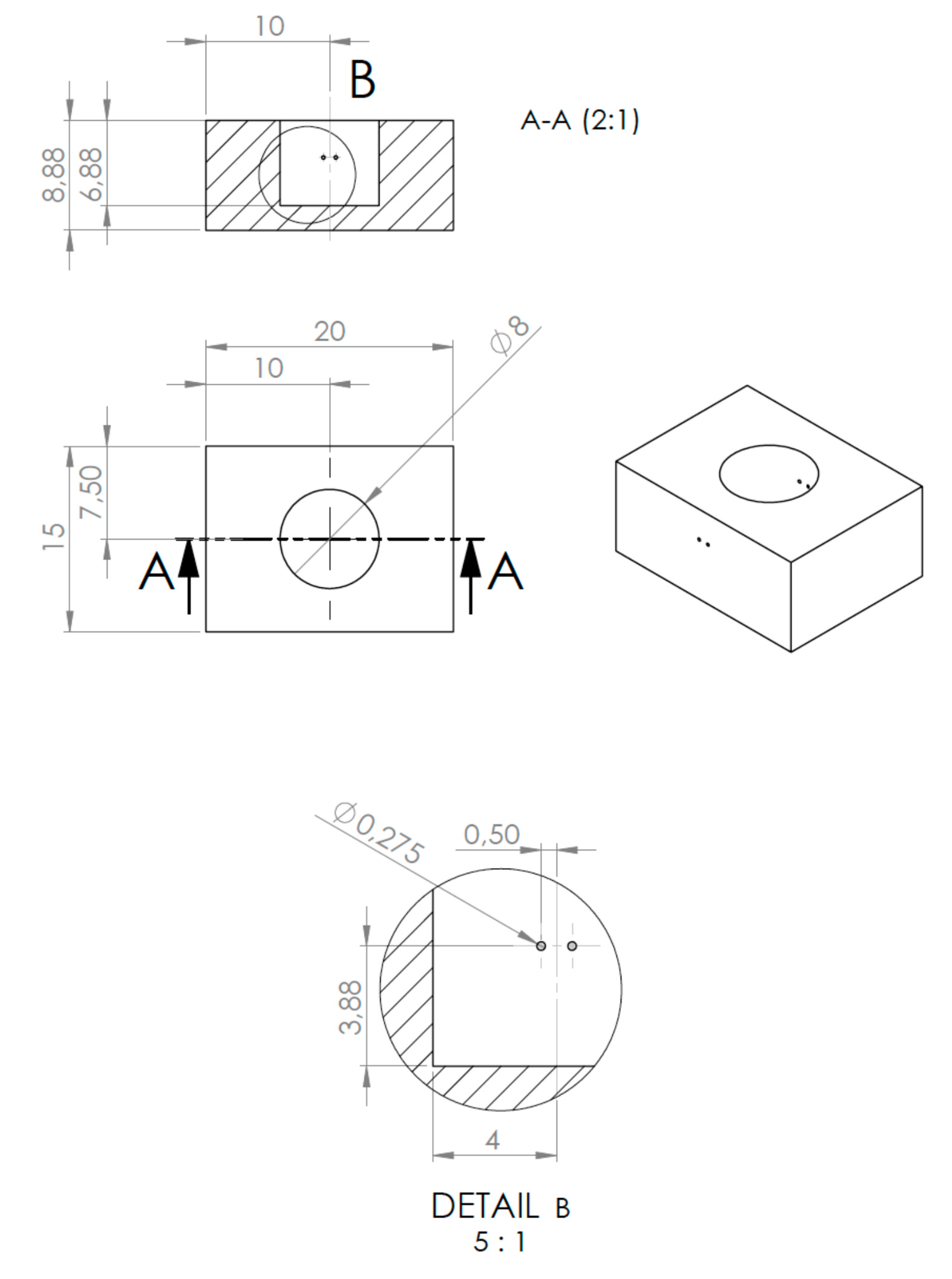
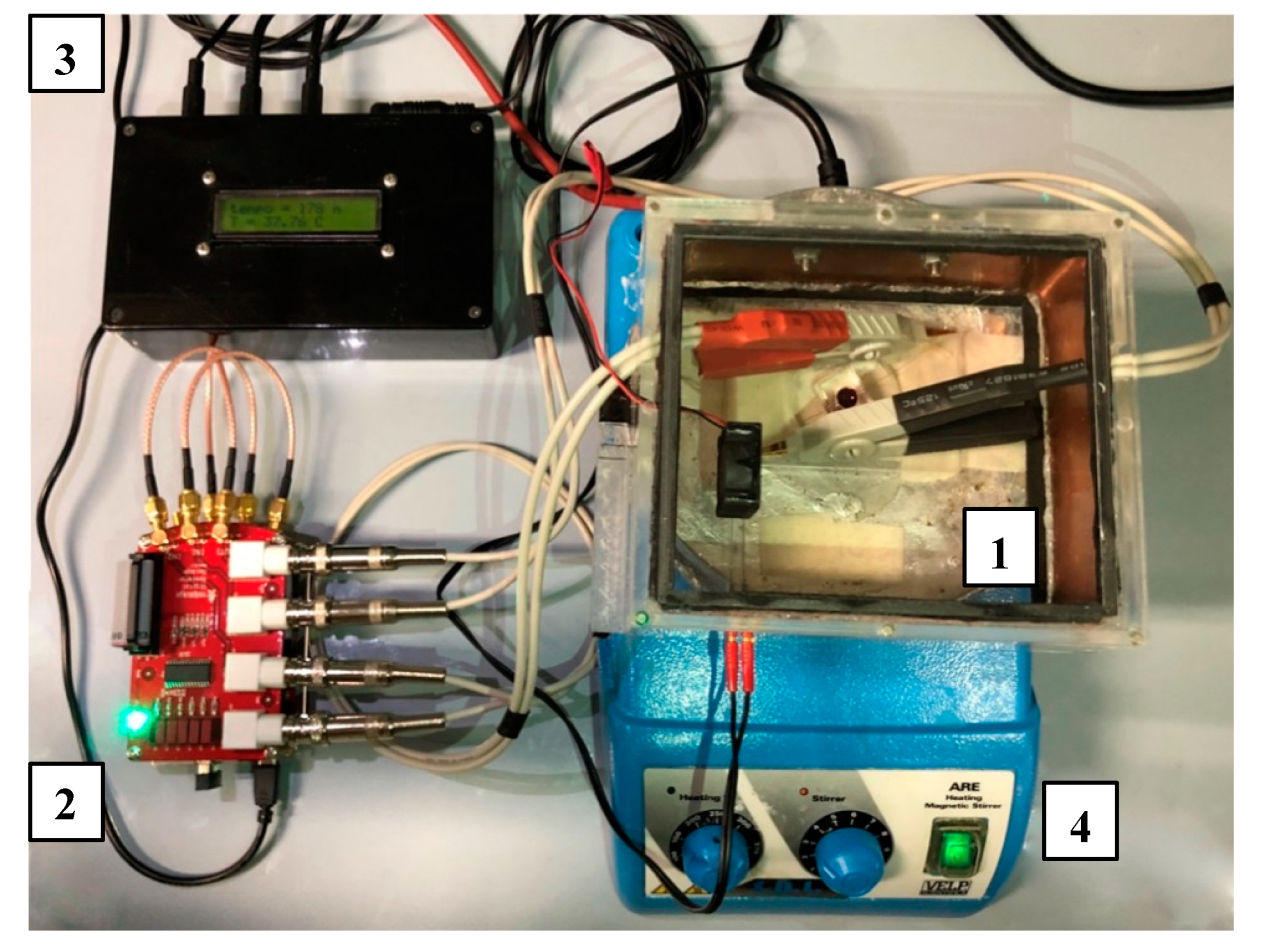
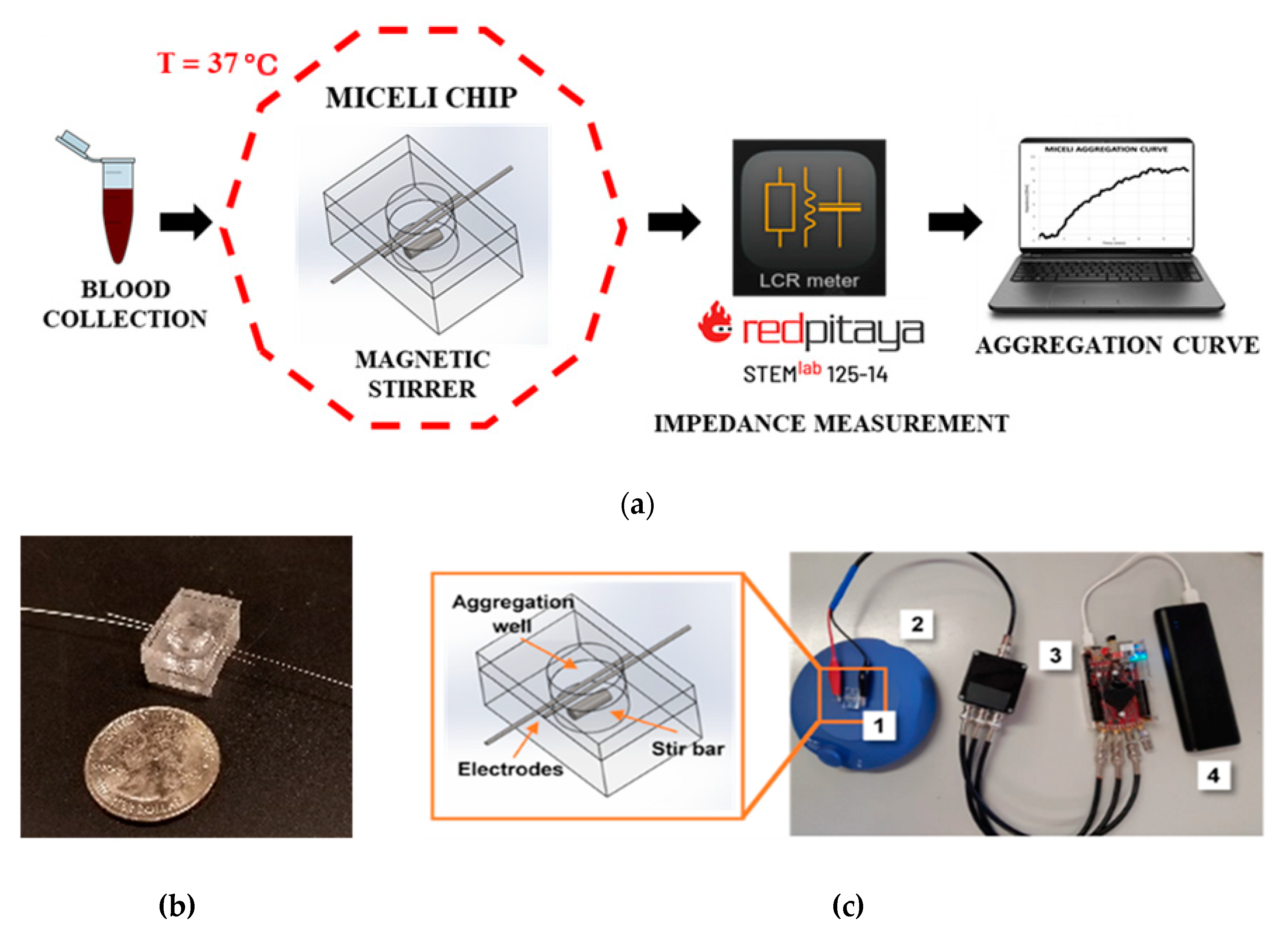
4.2. The MICELI Impedance Aggregometry System Setup
4.3. Impedance Aggregometry Using the MICELI System
4.4. Impedance Aggregometry Using Multiplate® Analyzer
4.5. Statistical Analysis
5. Conclusions
6. Patents
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| POC | Point-of-care |
| LTA | Electrical impedance aggregometry |
| EIA | Light transmission aggregometry |
| PRP | Platelet-rich plasma |
| PPP | Platelet-poor plasma |
| ADP | Adenosine diphosphate |
| TRAP-6 | Thrombin receptor-activating peptide 6 |
| AUC | Area under the curve |
| AU | Arbitrary unit |
| ACD | Acid citrate dextrose |
| Amax | Maximal aggregation |
| LT | Lag time |
| VR | Volume ratio |
Appendix A
| Statistical Value | MICELI (5 μM ADP) | Multiplate® (5 μM ADP) | Multiplate® User Manual (6.4 μM ADP) |
|---|---|---|---|
| n | 6 | 6 | 64 |
| Median | 8.65 | 500 | 775 |
| 5° Percentile | 5.83 | 410 | 474 |
| 95° Percentile | 10.77 | 630 | 1076 |
| Reference Range AUC | 8.65–10.67 | 410–630 | 474–1076 |
| 5° and 50° Percentile Difference | −33% | −18% | −39% |
| 50° and 95° Percentile Difference | 26% | 24% | 39% |
References
- Holinstat, M. Normal platelet function. Cancer Metastasis Rev. 2017, 36, 195–198. [Google Scholar] [PubMed]
- Koupenova, M.; Kehrel, B.E.; Corkrey, H.A.; Freedman, J.E. Thrombosis and platelets: An update. Eur. Heart J. 2016, 38, ehw550. [Google Scholar]
- Cattaneo, M.; Hayward, C.P.M.; Moffat, K.A.; Pugliano, M.T.; Liu, Y.; Michelson, A.D. Results of a worldwide survey on the assessment of platelet function by light transmission aggregometry: A report from the platelet physiology subcommittee of the SSC of the ISTH. J. Thromb. Haemost. 2009, 7, 1029. [Google Scholar] [PubMed]
- Antithrombotic Trialists’ Collaboration. Collaborative meta-analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. BMJ 2002, 324, 71–86. [Google Scholar]
- Grove, E.L.; Hossain, R.; Storey, R.F. Platelet function testing and prediction of procedural bleeding risk. Thromb. Haemost. 2013, 109, 817–824. [Google Scholar]
- Cayla, G.; Cuisset, T.; Silvain, J.; Leclercq, F.; Manzo-Silberman, S.; Saint-Etienne, C.; Delarche, N.; Bellemain-Appaix, A.; Range, G.; El Mahmoud, R.; et al. Platelet function monitoring to adjust antiplatelet therapy in elderly patients stented for an acute coronary syndrome (ANTARCTIC): An open-label, blinded-endpoint, randomised controlled superiority trial. Lancet 2016, 388, 2015–2022. [Google Scholar]
- Yeung, L.Y.Y.; Sarani, B.; Weinberg, J.A.; McBeth, P.B.; May, A.K. Surgeon’s guide to anticoagulant and antiplatelet medications part two: Antiplatelet agents and perioperative management of long-term anticoagulation. Trauma Surg. Acute Care Open 2016, 1, e000022. [Google Scholar]
- Vilahur, G.; Choi, B.G.; Zafar, M.U.; Viles-Gonzalez, J.F.; Vorchheimer, D.A.; Fuster, V.; Badimon, J.J. Normalization of platelet reactivity in clopidogrel-treated subjects. J. Thromb. Haemost. 2007, 5, 82–90. [Google Scholar]
- Triulzi, D.J.; Assmann, S.F.; Strauss, R.G.; Ness, P.M.; Hess, J.R.; Kaufman, R.M.; Granger, S.; Slichter, S.J. The impact of platelet transfusion characteristics on posttransfusion platelet increments and clinical bleeding in patients with hypoproliferative thrombocytopenia. Blood 2012, 119, 5553–5562. [Google Scholar]
- Kerkhoffs, J.-L.H.; Eikenboom, J.C.J.; van de Watering, L.M.G.; van Wordragen-Vlaswinkel, R.J.; Wijermans, P.W.; Brand, A. The clinical impact of platelet refractoriness: Correlation with bleeding and survival. Transfusion 2008, 48, 1959–1965. [Google Scholar]
- Soliman, M.; Hartmann, M. Impedance Aggregometry Reveals Increased Platelet Aggregation during Liver Transplantation. J. Clin. Med. 2019, 8, 1803. [Google Scholar] [CrossRef] [PubMed]
- Yorkgitis, B.K.; Ruggia-Check, C.; Dujon, J.E. Antiplatelet and anticoagulation medications and the surgical patient. Am. J. Surg. 2014, 207, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Velik-Salchner, C.; Maier, S.; Innerhofer, P.; Streif, W.; Klingler, A.; Kolbitsch, C.; Fries, D. Point-of-care whole blood impedance aggregometry versus classical light transmission aggregometry for detecting aspirin and clopidogrel: The results of a pilot study. Anesth. Analg. 2008, 107, 1798–1806. [Google Scholar] [CrossRef] [PubMed]
- Koltai, K.; Kesmarky, G.; Feher, G.; Tibold, A.; Toth, K. Platelet aggregometry testing: Molecular mechanisms, techniques and clinical implications. Int. J. Mol. Sci. 2017, 18, 1–21. [Google Scholar] [CrossRef]
- Born, G.V.R.; Cross, M.J. The aggregation of blood platelets. J. Physiol. 1963, 168, 178–195. [Google Scholar] [CrossRef]
- Frontroth, J.P. Haemostasis; Springer Science Business Media: New York, NY, USA, 2013; Volume 992, pp. 227–240. [Google Scholar]
- Hvas, A.; Favaloro, E.J. Hemostasis and Thrombosis; Springer: New York, NY, USA, 2017; Volume 1646, pp. 321–331. [Google Scholar]
- Choi, J.-L.; Li, S.; Han, J.-Y. Platelet function tests: A review of progresses in clinical application. Biomed Res. Int. 2014, 2014, 456569. [Google Scholar] [CrossRef]
- Cardinal, D.C.; Flower, R.J. The electronic aggregometer: A novel device for assessing platelet behavior in blood. J. Pharmacol. Methods 1980, 3, 135–158. [Google Scholar] [CrossRef]
- Desconclois, C.; Valarche, V.; Boutekedjiret, T.; Raphael, M.; Dreyfus, M.; Proulle, V. Whole Blood Impedance Aggregometry: A New Tool for Severe Inherited Platelet Disorder Diagnosis? Blood 2011, 118, 5266. [Google Scholar] [CrossRef]
- Mullins, E.S.; Stroop, D.; Ingala, D.; Tarango, C.; Luchtman-Jones, L.; Lane, A.; Gruppo, R.A. Comparison of Multiple Electrode Impedance-Based Platelet Aggregometry to Light Transmission Aggregometry for Diagnosis of Qualitative Platelet Defects. Blood 2017, 130, 1048. [Google Scholar]
- 510(k) Number: K161329. Chrono-log Platelet Aggregometer, Model 490 4+4. Department of Health and Human Services Food and Drug Administration Indications for Use. Available online: https://www.accessdata.fda.gov/cdrh_docs/pdf16/K161329.pdf (accessed on 13 December 2019).
- 510(k) Substantial Equivalence Determination Decision Summary: k103555. Multiplate 5.0 aggregometer. Platelet aggregometer assays: ADPtest (10 µM ADP) and ASPItest (0.5 mM Arachidonic Acid). Available online: https://www.accessdata.fda.gov/cdrh_docs/reviews/k103555.pdf (accessed on 13 December 2019).
- Redaelli, A.; Slepian, M.J. Systems and Methods for Analyzing Platelet Function. Available online: http://hdl.handle.net/11311/1066190 (accessed on 22 June 2018).
- Santoleri, A.; Dimasi, A.; Consolo, F.; Rasponi, M.; Fiore, G.B.; Slepian, M.J.; Redaelli, A. The MICELI (MICrofluidic, ELectical, Impedance): A New Portable Device for Bedside Platelet Aggregation Measurement. In Proceedings of the ISMCS 2017 25th Anniversary Scientific Congress “Limitations, Controversies, and Gaps in MCS: Pathway to Solutions”, Controversies, Tucson, AZ, USA, 15–18 October 2017; p. 199. [Google Scholar]
- US8617468B2—Platelet Aggregation Test and Device—Google Patents. Available online: https://patents.google.com/patent/US8617468B2/en (accessed on 13 December 2019).
- Calatzis, A.; Kriger, B.; Wittwer, M. Cartridge Device For Blood Analysis. U.S. Patent No. 200,701,409,02, 21 June 2007. [Google Scholar]
- Silver, W.P.; Keller, M.P.; Teel, R.; Silver, D. Effects of donor characteristics and in vitro time and temperature on platelet aggregometry platelet. J. Vasc. Surg. 1993, 17, 726–733. [Google Scholar] [CrossRef]
- Zhou, L.; Schmaier, A.H. Platelet Aggregation Testing in Platelet-Rich Plasma Description of Procedures With the Aim to Develop Standards in the Field. Am. J. Clin. Pathol. 2005, 123, 172–183. [Google Scholar] [CrossRef] [PubMed]
- Peerschke, E.I.B.; Castellone, D.D.; Stroobants, A.K.; Francis, J. Reference Range Determination for Whole-Blood Platelet Aggregation Using the Multiplate Analyzer. Am. J. Clin. Pathol. 2014, 142, 647–656. [Google Scholar] [CrossRef]
- Kalb, M.L.; Potura, L.; Scharbert, G.; Kozek-Langenecker, S.A. The effect of ex vivo anticoagulants on whole blood platelet aggregation. Platelets 2009, 20, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Seyfert, U.T.; Haubelt, H.; Vogt, A.; Hellstern, P. Variables influencing Multiplate TM whole blood impedance platelet aggregometry and turbidimetric platelet aggregation in healthy individuals. Platelets 2007, 18, 199–206. [Google Scholar] [CrossRef]
- Michelson, A.D.; Frelinger, A.L.; Furman, M.I. Current Options in Platelet Function Testing. Am. J. Cardiol. 2006, 98, S4–S10. [Google Scholar] [CrossRef]
- Rasheed, H.; Tirmizi, A.H.; Salahuddin, F.; Rizvi, N.B.; Arshad, M.; Farooq, S.Z.; Saeed, S.A. Calcium Signaling in Human Platelet Aggregation Mediated by Platelet Activating Factor and Calcium Ionophore, A23187. J. Biol. Sci. 2004, 4, 117–121. [Google Scholar] [CrossRef]
- Sun, P.; McMillan-Ward, E.; Mian, R.; Israels, S.J. Comparison of light transmission aggregometry and multiple electrode aggregometry for the evaluation of patients with mucocutaneous bleeding. Int. J. Lab. Hematol. 2019, 41, 133–140. [Google Scholar] [CrossRef]
- Femia, E.A.; Scavone, M.; Lecchi, A.; Cattaneo, M. Effect of platelet count on platelet aggregation measured with impedance aggregometry (MultiplateTM analyzer) and with light transmission aggregometry. J. Thromb. Haemost. 2013, 11, 2193–2196. [Google Scholar] [CrossRef]
- Hanke, A.A.; Roberg, K.; Monaca, E.; Sellmann, T.; Weber, C.F.; Rahe-Meyer, N.; Görlinger, K. Impact of platelet count on results obtained from multiple electrode platelet aggregometry (multiplateTM). Eur. J. Med. Res. 2010, 15, 214–219. [Google Scholar] [CrossRef]
- Müller, M.R.; Salat, A.; Pulaki, S.; Stangl, P.; Ergun, E.; Schreiner, W.; Losert, U.; Wolner, E. Influence of hematocrit and platelet count on impedance and reactivity of whole blood for electrical aggregometry. J. Pharmacol. Toxicol. Methods 1995, 34, 17–22. [Google Scholar] [CrossRef]
- Dézsi, D.A.; Merkely, B.; Skopál, J.; Barabás, E.; Várnai, K.; Faluközy, J.; Veress, G.; Alotti, N.; Aradi, D. Impact of Test Conditions on ADP-Induced Platelet Function Results With the Multiplate Assay: Is Further Standardization Required? J. Cardiovasc. Pharmacol. Ther. 2018, 23, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Jilma-Stohlawetz, P.; Ratzinger, F.; Schörgenhofer, C.; Jilma, B.; Quehenberger, P. Effect of preanalytical time-delay on platelet function as measured by multiplate, PFA-100 and VerifyNow. Scand. J. Clin. Lab. Investig. 2016, 76, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Johnston, L.R.; Larsen, P.D.; La Flamme, A.C.; Harding, S.A. Methodological considerations for the assessment of ADP induced platelet aggregation using the Multiplate® analyser. Platelets 2013, 24, 303–307. [Google Scholar] [CrossRef] [PubMed]
- Wallén, N.H.; Ladjevardi, M.; Albert, J.; Bröijersén, A. Influence of different anticoagulants on platelet aggregation in whole blood; a comparison between citrate, low molecular mass heparin and hirudin. Thromb. Res. 1997, 87, 151–157. [Google Scholar] [CrossRef]
- Bonello, L.; Tantry, U.S.; Marcucci, R.; Blindt, R.; Angiolillo, D.J.; Becker, R.; Bhatt, D.L.; Cattaneo, M.; Collet, J.P.; Cuisset, T.; et al. Consensus and future directions on the definition of high on-treatment platelet reactivity to adenosine diphosphate. J. Am. Coll. Cardiol. 2010, 56, 919–933. [Google Scholar] [CrossRef]
- Mackie, I.J.; Jones, R.; Machin, S.J. Platelet impedance aggregation in whole blood and its inhibition by antiplatelet drugs. J. Clin. Pathol. 1984, 37, 874–878. [Google Scholar] [CrossRef]
- Swart, S.; Pearson, D.; Wood, J.; Barnett, D. Effects of adrenaline and alpha adrenergic antagonists on platelet aggregation in whole blood: Evaluation of electrical impedance aggregometry. Thromb. Res. 1984, 36, 411–418. [Google Scholar] [CrossRef]
- Beynon, C.; Scherer, M.; Jakobs, M.; Jung, C.; Sakowitz, O.W.; Unterberg, A.W. Initial experiences with Multiplate® for rapid assessment of antiplatelet agent activity in neurosurgical emergencies. Clin. Neurol. Neurosurg. 2013, 115, 2003–2008. [Google Scholar] [CrossRef]
- Briggs, A.; Gates, J.D.; Kaufman, R.M.; Calahan, C.; Gormley, W.B.; Havens, J.M. Platelet dysfunction and platelet transfusion in traumatic brain injury. J. Surg. Res. 2015, 193, 802–806. [Google Scholar] [CrossRef]
- Meißner, A.; Schlenke, P. Massive bleeding and massive transfusion. Transfus. Med. Hemotherapy 2012, 39, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Rahe-Meyer, N.; Winterhalter, M.; Boden, A.; Froemke, C.; Piepenbrock, S.; Calatzis, A.; Solomon, C. Platelet concentrates transfusion in cardiac surgery and platelet function assessment by multiple electrode aggregometry. Acta Anaesthesiol. Scand. 2009, 53, 168–175. [Google Scholar] [CrossRef] [PubMed]
- U.S Census Bureau QuickFacts: Pima County, Arizona. Available online: https://www.census.gov/quickfacts/fact/table/pimacountyarizona/PST045218 (accessed on 12 December 2019).
- CLSI. Procedure for Determining Packed Cell Volume by the Microhematocrit Method, 3rd ed.; CLSI document H07-A3; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2000. [Google Scholar]
| Intradonor Variability 1 | Interdonor Variability 2 | |||
|---|---|---|---|---|
| Agonist Type | PRP | Whole blood | PRP | Whole blood |
| ADP | 16 | 24 | 4 | 18 |
| TRAP-6 | 6 | 23 | 11 | 11 |
| Collagen | 4 | 26 | 14 | 22 |
| Ca ionophore | 23 | 21 | 8 | 6 |
| Agonist Type | Concentration | Volume, μL |
|---|---|---|
| ADP | 1, 5, 10, 20 μM | 5 1 |
| TRAP-6 | 32 μM | 6.4 |
| Collagen | 1, 5, 10 μg/mL | 25 1 |
| Epinephrine | 10 μM | 2.5 |
| Calcium ionophore | 5 μM | 2.5 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roka-Moiia, Y.; Bozzi, S.; Ferrari, C.; Mantica, G.; Dimasi, A.; Rasponi, M.; Santoleri, A.; Scavone, M.; Consolo, F.; Cattaneo, M.; et al. The MICELI (MICrofluidic, ELectrical, Impedance): Prototyping a Point-of-Care Impedance Platelet Aggregometer. Int. J. Mol. Sci. 2020, 21, 1174. https://doi.org/10.3390/ijms21041174
Roka-Moiia Y, Bozzi S, Ferrari C, Mantica G, Dimasi A, Rasponi M, Santoleri A, Scavone M, Consolo F, Cattaneo M, et al. The MICELI (MICrofluidic, ELectrical, Impedance): Prototyping a Point-of-Care Impedance Platelet Aggregometer. International Journal of Molecular Sciences. 2020; 21(4):1174. https://doi.org/10.3390/ijms21041174
Chicago/Turabian StyleRoka-Moiia, Yana, Silvia Bozzi, Chiara Ferrari, Gabriele Mantica, Annalisa Dimasi, Marco Rasponi, Andrea Santoleri, Mariangela Scavone, Filippo Consolo, Marco Cattaneo, and et al. 2020. "The MICELI (MICrofluidic, ELectrical, Impedance): Prototyping a Point-of-Care Impedance Platelet Aggregometer" International Journal of Molecular Sciences 21, no. 4: 1174. https://doi.org/10.3390/ijms21041174
APA StyleRoka-Moiia, Y., Bozzi, S., Ferrari, C., Mantica, G., Dimasi, A., Rasponi, M., Santoleri, A., Scavone, M., Consolo, F., Cattaneo, M., Slepian, M. J., & Redaelli, A. (2020). The MICELI (MICrofluidic, ELectrical, Impedance): Prototyping a Point-of-Care Impedance Platelet Aggregometer. International Journal of Molecular Sciences, 21(4), 1174. https://doi.org/10.3390/ijms21041174





