Epicardial Adipose Tissue, Adiponectin and Leptin: A Potential Source of Cardiovascular Risk in Chronic Kidney Disease
Abstract
1. Introduction
2. Cardiovascular Risk in Chronic Kidney Disease
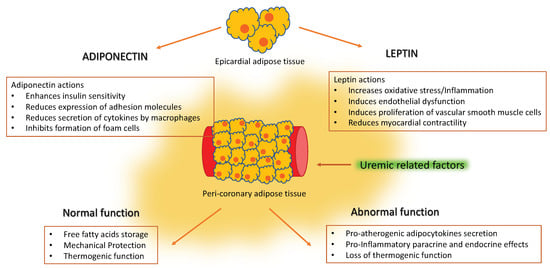
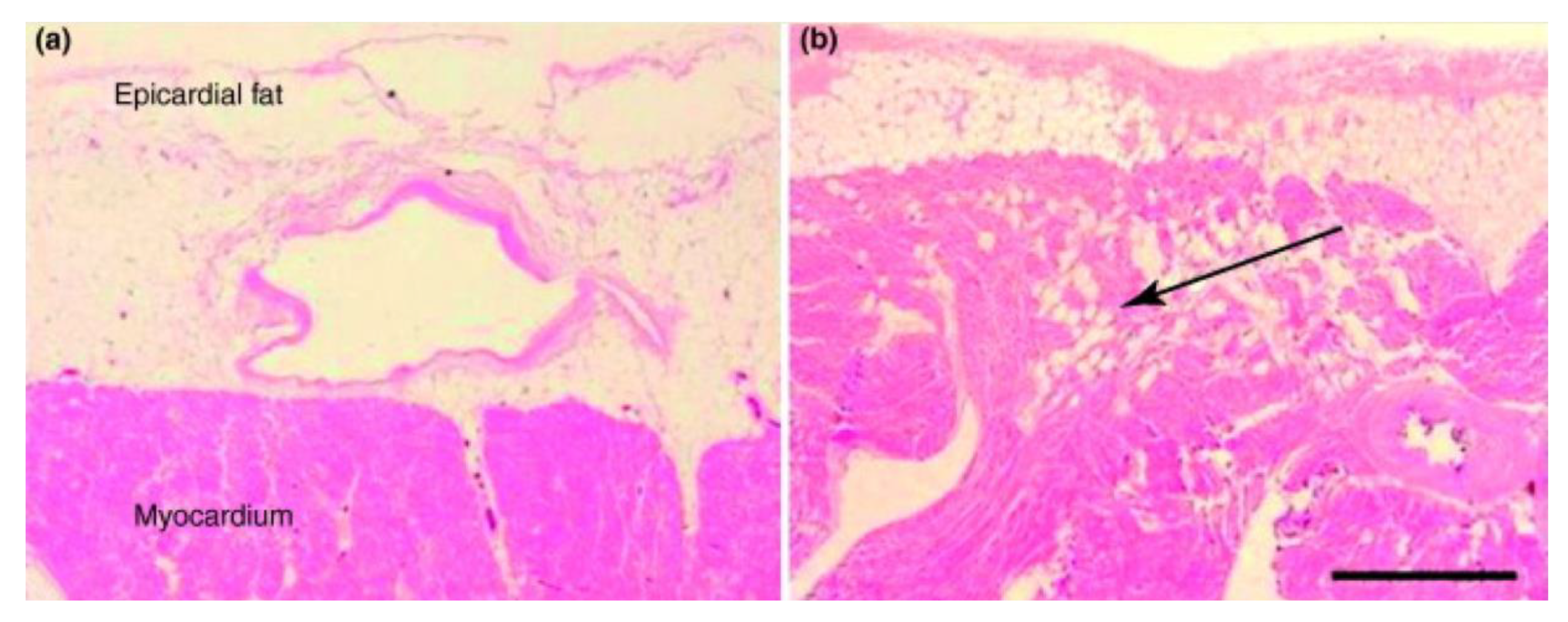
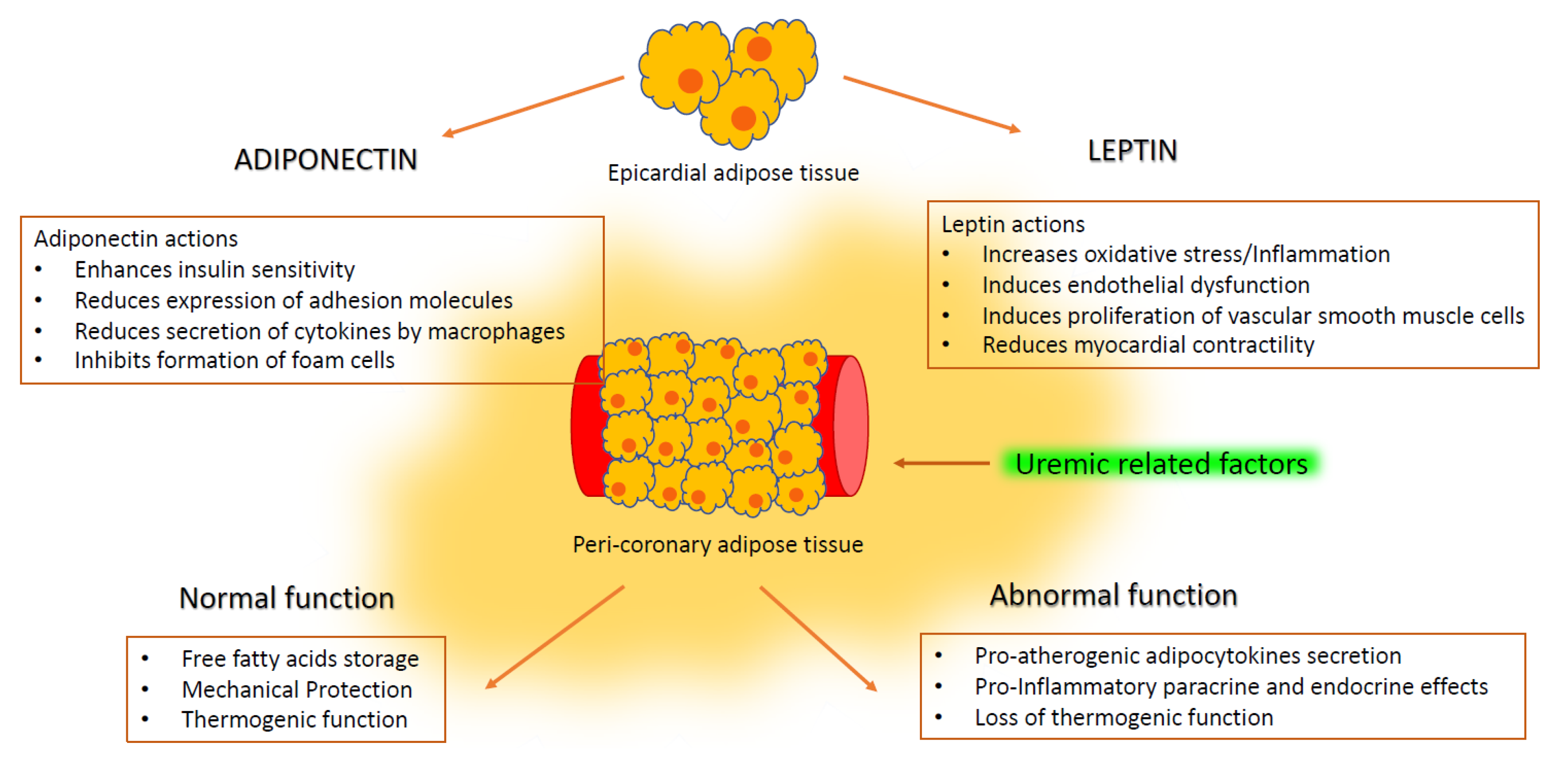
3. Epicardial Adipose Tissue
4. Epicardial Adipose Tissue in Renal Disease
5. Adipocytokines and Vascular Disease
6. Adiponectin in Renal Disease
7. Leptin in Renal Disease
8. Therapeutic Approaches
9. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Russo, R.; Di Iorio, B.; Di Lullo, L.; Russo, D. Epicardial adipose tissue: New parameter for cardiovascular risk assessment in high risk populations. J. Nephrol. 2018, 31, 847–853. [Google Scholar] [CrossRef] [PubMed]
- Bornachea, O.; Vea, A.; Llorente-Cortes, V. Interplay between epicardial adipose tissue, metabolic and cardiovascular diseases. Clin. Investig. Arterioscler. 2018, 30, 230–239. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Singh, H.; Khanijoun, H.K.; Iacobellis, G. Echocardiographic assessment of epicardial adipose tissue—A marker of visceral adiposity. Mcgill. J. Med. 2007, 10, 26–30. [Google Scholar] [PubMed]
- Janik, M.; Hartlage, G.; Alexopoulos, N.; Mirzoyev, Z.; McLean, D.S.; Arepalli, C.D.; Chen, Z.; Stillman, A.E.; Raggi, P. Epicardial adipose tissue volume and coronary artery calcium to predict myocardial ischemia on positron emission tomography-computed tomography studies. J. Nucl. Cardiol. 2010, 17, 841–847. [Google Scholar] [CrossRef]
- Nelson, A.J.; Worthley, M.I.; Psaltis, P.J.; Carbone, A.; Dundon, B.K.; Duncan, R.F.; Piantadosi, C.; Lau, D.H.; Sanders, P.; Wittert, G.A.; et al. Validation of cardiovascular magnetic resonance assessment of pericardial adipose tissue volume. J. Cardiovasc. Magn. Reson. 2009, 11, 15. [Google Scholar] [CrossRef]
- Alexopoulos, N.; McLean, D.S.; Janik, M.; Arepalli, C.D.; Stillman, A.E.; Raggi, P. Epicardial adipose tissue and coronary artery plaque characteristics. Atherosclerosis 2010, 210, 150–154. [Google Scholar] [CrossRef]
- Nerlekar, N.; Brown, A.J.; Muthalaly, R.G.; Talman, A.; Hettige, T.; Cameron, J.D.; Wong, D.T.L. Association of epicardial adipose tissue and high-risk plaque characteristics: A systematic review and meta-analysis. J. Am. Heart Assoc. 2017, 6, e006379. [Google Scholar] [CrossRef]
- Bachar, G.N.; Dicker, D.; Kornowski, R.; Atar, E. Epicardial adipose tissue as a predictor of coronary artery disease in asymptomatic subjects. Am. J. Cardiol. 2012, 110, 534–538. [Google Scholar] [CrossRef]
- Ding, J.; Hsu, F.-C.; Harris, T.B.; Liu, Y.; Kritchevsky, S.B.; Szklo, M.; Ouyang, P.; Espeland, M.A.; Lohman, K.K.; Criqui, M.H.; et al. The association of pericardial fat with incident coronary heart disease: The Multi-Ethnic Study of Atherosclerosis (MESA). Am. J. Clin. Nutr. 2009, 90, 499–504. [Google Scholar] [CrossRef]
- Turkmen, K.; Kayikcioglu, H.; Ozbek, O.; Solak, Y.; Kayrak, M.; Samur, C.; Anil, M.; Zeki Tonbul, H. The relationship between epicardial adipose tissue and malnutrition, inflammation, atherosclerosis/calcification syndrome in ESRD patients. Clin. J. Am. Soc. Nephrol. 2011, 6, 1920–1925. [Google Scholar] [CrossRef]
- Cordeiro, A.C.; Amparo, F.C.; Oliveira, M.A.C.; Amodeo, C.; Smanio, P.; Pinto, I.M.; Lindholm, B.; Stenvinkel, P.; Carrero, J.J. Epicardial fat accumulation, cardiometabolic profile and cardiovascular events in patients with stages 3-5 chronic kidney disease. J. Intern. Med. 2015, 278, 77–87. [Google Scholar] [CrossRef]
- Gansevoort, R.T.; Correa-Rotter, R.; Hemmelgarn, B.R.; Jafar, T.H.; Heerspink, H.J.L.; Mann, J.F.; Matsushita, K.; Wen, C.P. Chronic kidney disease and cardiovascular risk: Epidemiology, mechanisms, and prevention. Lancet 2013, 382, 339–352. [Google Scholar] [CrossRef]
- Sarnak, M.J.; Levey, A.S.; Schoolwerth, A.C.; Coresh, J.; Culleton, B.; Hamm, L.L.; McCullough, P.A.; Kasiske, B.L.; Kelepouris, E.; Klag, M.J.; et al. Kidney disease as a risk factor for development of cardiovascular disease: A statement from the American Heart Association councils on kidney in cardiovascular disease, high blood pressure research, clinical cardiology, and epidemiology and prevention. Hypertension 2003, 42, 1050–1065. [Google Scholar] [CrossRef]
- Levin, A.; Rigatto, C.; Brendan, B.; Madore, F.; Muirhead, N.; Holmes, D. Cohort profile: Canadian study of prediction of death, dialysis and interim cardiovascular events (CanPREDDICT ). BMC Nephrol. 2013, 14, 121. [Google Scholar] [CrossRef]
- Berl, T.; Henrich, W. Kidney-heart interactions: Epidemiology, pathogenesis, and treatment. Clin. J. Am. Soc. Nephrol. 2006, 1, 8–18. [Google Scholar] [CrossRef]
- D’Marco, L.; Bellasi, A.; Raggi, P. Cardiovascular biomarkers in chronic kidney disease: State of current research and clinical applicability. Dis. Markers 2015, 2015, 1–16. [Google Scholar] [CrossRef]
- Vickery, S.; Webb, M.C.; Price, C.P.; John, R.I.; Abbas, N.A.; Lamb, E.J. Prognostic value of cardiac biomarkers for death in a non-dialysis chronic kidney disease population. Nephrol. Dial. Transplant. 2008, 11, 3546–3553. [Google Scholar] [CrossRef]
- Rabkin, S.W. Epicardial fat: Properties, function and relationship to obesity. Obes. Rev. 2007, 8, 253–261. [Google Scholar] [CrossRef]
- Iacobellis, G.; Bianco, A.C. Epicardial adipose tissue: Emerging physiological, pathophysiological and clinical features. Trends Endocrinol. Metab. 2011, 22, 450–457. [Google Scholar] [CrossRef]
- Marchington, J.M.; Mattacks, C.A.; Pond, C.M. Adipose tissue in the mammalian heart and pericardium: Structure, foetal development and biochemical properties. Comp. Biochem. Physiol. B. 1989, 94, 225–232. [Google Scholar] [CrossRef]
- Corradi, D.; Maestri, R.; Callegari, S.; Pastori, P.; Goldoni, M.; Luong, T.V.; Bordi, C. The ventricular epicardial fat is related to the myocardial mass in normal, ischemic and hypertrophic hearts. Cardiovasc. Pathol. 2004, 13, 313–316. [Google Scholar] [CrossRef] [PubMed]
- Pezeshkian, M.; Noori, M.; Najjarpour-Jabbari, H.; Abolfathi, A.; Darabi, M.; Darabi, M.; Darabi, M.; Shaaker, M.; Shahmohammadi, G. Fatty acid composition of epicardial and subcutaneous human adipose tissue. Metab. Syndr. Relat. Disord. 2009, 7, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Sacks, H.S.; Fain, J.N.; Holman, B.; Cheema, P.; Chary, A.; Parks, F.; Karas, J.; Optican, R.; Bahouth, S.W.; Garrett, E.; et al. Uncoupling protein-1 and related messenger ribonucleic acids in human epicardial and other adipose tissues: Epicardial fat functioning as brown fat. J. Clin. Endocrinol. Metab. 2009, 94, 3611–3615. [Google Scholar] [CrossRef] [PubMed]
- Iacobellis, G. Epicardial and pericardial fat: Close, but very different. Obesity 2009, 17, 625–627. [Google Scholar] [CrossRef]
- Akoumianakis, I.; Antoniades, C. The interplay between adipose tissue and the cardiovascular system: Is fat always bad? Cardiovasc. Res. 2017, 113, 999–1008. [Google Scholar] [CrossRef]
- Turer, A.T.; Scherer, P.E. Adiponectin: Mechanistic insights and clinical implications. Diabetologia 2012, 55, 2319–2326. [Google Scholar] [CrossRef]
- Salazar, J.; Luzardo, E.; Mejías, J.C.; Rojas, J.; Ferreira, A.; Rivas-Ríos, J.R.; Bermúdez, V. Epicardial Fat: Physiological, Pathological, and Therapeutic Implications. Cardiol. Res. Pract. 2016, 2016, 1291537. [Google Scholar] [CrossRef]
- Ouchi, N.; Parker, J.L.; Lugus, J.J.; Walsh, K. Adipokines in inflammation and metabolic disease. Nat. Rev. Immunol. 2011, 11, 85–97. [Google Scholar] [CrossRef]
- Kaisar, O.M.; Johnson, D.W.; Prins, J.B.; Isbel, N. The role of novel biomarkers of cardiovascular disease in chronic kidney disease: Focus on adiponectin and leptin. Curr. Cardiol. Rev. 2008, 287–292. [Google Scholar] [CrossRef]
- Wong, H.K.; Cheung, T.T.; Cheung, B.M.Y. Adrenomedullin and cardiovascular diseases. JRSM Cardiovasc. Dis. 2012, 1, 1–7. [Google Scholar] [CrossRef]
- Scholze, A.; Tepel, M. Role of leptin in reverse epidemiology in chronic kidney disease. Semin. Dial. 2007, 20, 534–538. [Google Scholar] [CrossRef]
- Ueno, K.; Anzai, T.; Jinzaki, M.; Yamada, M.; Jo, Y.; Maekawa, Y.; Kawamura, A.; Yoshikawa, T.; Tanami, Y.; Sato, K.; et al. Increased Epicardial Fat Volume Quantified by 64-Multidetector Computed Tomography is Associated With Coronary Atherosclerosis and Totally Occlusive Lesions. Circ. J. 2009, 73, 1927–1933. [Google Scholar] [CrossRef] [PubMed]
- Reinhardt, M.; Cushman, T.R.; Thearle, M.S.; Krakoff, J. Epicardial adipose tissue is a predictor of decreased kidney function and coronary artery calcification in youth- and early adult onset type 2 diabetes mellitus. J. Endocrinol. Investig. 2019, 42, 979–986. [Google Scholar] [CrossRef]
- Nakanishi, K.; Fukuda, S.; Tanaka, A.; Otsuka, K.; Taguchi, H.; Yoshikawa, J.; Shimada, K. Epicardial adipose tissue accumulation is associated with renal dysfunction and coronary plaque morphology on multidetector computed tomography. Circ. J. 2015, 80, 196–201. [Google Scholar] [CrossRef] [PubMed]
- Karohl, C.; D’Marco, L.; Bellasi, A.; Raggi, P. Hybrid myocardial imaging for risk stratification prior to kidney transplantation: Added value of coronary calcium and epicardial adipose tissue. J. Nucl. Cardiol. 2013, 20, 1013–1020. [Google Scholar] [CrossRef] [PubMed]
- D’Marco, L.G.; Bellasi, A.; Kim, S.; Chen, Z.; Block, G.A.; Raggi, P. Epicardial adipose tissue predicts mortality in incident hemodialysis patients: A substudy of the Renagel in New Dialysis trial. Nephrol. Dial. Transplant. 2013, 28, 2586–2595. [Google Scholar] [CrossRef] [PubMed]
- Iacobellis, G.; Pistilli, D.; Gucciardo, M.; Leonetti, F.; Miraldi, F.; Brancaccio, G.; Gallo, P.; di Gioia, C.R. Adiponectin expression in human epicardial adipose tissue in vivo is lower in patients with coronary artery disease. Cytokine 2005, 29, 251–255. [Google Scholar] [CrossRef]
- Cheng, K.H.; Chu, C.S.; Lee, K.T.; Lin, T.H.; Hsieh, C.C.; Chiu, C.C.; Voon, W.C.; Sheu, S.H.; Lai, W.T. Adipocytokines and proinflammatory mediators from abdominal and epicardial adipose tissue in patients with coronary artery disease. Int. J. Obes. 2008, 32, 268–274. [Google Scholar] [CrossRef]
- Mazurek, T.; Zhang, L.F.; Zalewski, A.; Mannion, J.D.; Diehl, J.T.; Arafat, H.; Sarov-Blat, L.; O’Brien, S.; Keiper, E.A.; Johnson, A.G.; et al. Human epicardial adipose tissue is a source of inflammatory mediators. Circulation 2003, 108, 2460–2466. [Google Scholar] [CrossRef]
- Ouchi, N.; Kihara, S.; Arita, Y.; Okamoto, Y.; Maeda, K.; Kuriyama, H.; Hotta, K.; Nishida, M.; Takahashi, M.; Muraguchi, M.; et al. Adiponectin, an adipocyte-derived plasma protein, inhibits endothelial NF-kappaB signaling through a cAMP-dependent pathway. Circulation 2000, 102, 1296–1301. [Google Scholar] [CrossRef]
- Baker, A.R.; Silva, N.F.; da Quinn, D.W.; Harte, A.L.; Pagano, D.; Bonser, R.S.; Kumar, S.; McTernan, P.G. Human epicardial adipose tissue expresses a pathogenic profile of adipocytokines in patients with cardiovascular disease. Cardiovasc. Diabetol. 2006, 5, 1. [Google Scholar] [CrossRef] [PubMed]
- Ghantous, C.M.; Azrak, Z.; Hanache, S.; Abou-Kheir, W.; Zeidan, A. Differential role of leptin and adiponectin in cardiovascular system. Int. J. Endocrinol. 2015, 2015, 534320. [Google Scholar] [CrossRef] [PubMed]
- Kershaw, E.E.; Flier, J.S. Adipose tissue as an endocrine organ. J. Clin. Endocrinol. Metab. 2004, 89, 2548–2556. [Google Scholar] [CrossRef] [PubMed]
- Trujillo, M.E.; Sullivan, S.; Harten, I.; Schneider, S.H.; Greenberg, A.S.; Fried, S.K. Interleukin-6 regulates human adipose tissue lipid metabolism and leptin production in vitro. J. Clin. Endocrinol. Metab. 2004, 89, 5577–5582. [Google Scholar] [CrossRef]
- Fisher, F.F.M.; Trujillo, M.E.; Hanif, W.; Barnett, A.H.; McTernan, P.G.; Scherer, P.E.; Kumar, S. Serum high molecular weight complex of adiponectin correlates better with glucose tolerance than total serum adiponectin in Indo-Asian males. Diabetologia 2005, 48, 1084–1087. [Google Scholar] [CrossRef]
- Bouskila, M.; Pajvani, U.B.; Scherer, P.E. Adiponectin: A relevant player in PPARgamma-agonist-mediated improvements in hepatic insulin sensitivity? Int. J. Obes. 2005, 29, S17–S23. [Google Scholar] [CrossRef]
- Whitehead, J.P.; Richards, A.A.; Hickman, I.J.; Macdonald, G.A.; Prins, J.B. Adiponectin—A key adipokine in the metabolic syndrome. Diabetes Obes. Metab. 2006, 8, 264–280. [Google Scholar] [CrossRef]
- Komura, N.; Kihara, S.; Sonoda, M.; Maeda, N.; Tochino, Y.; Funahashi, T.; Shimomura, I. Increment and impairment of adiponectin in renal failure. Cardiovasc. Res. 2010, 86, 471–477. [Google Scholar] [CrossRef]
- Kumada, M.; Kihara, S.; Sumitsuji, S.; Kawamoto, T.; Matsumoto, S.; Ouchi, N.; Arita, Y.; Okamoto, Y.; Shimomura, I.; Hiraoka, H.; et al. Association of hypoadiponectinemia with coronary artery disease in men. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 85–89. [Google Scholar] [CrossRef]
- Zoccali, C.; Mallamaci, F. Adiponectin and leptin in chronic kidney disease: Causal factors or mere risk markers? J. Ren. Nutr. 2011, 21, 87–91. [Google Scholar] [CrossRef]
- Zoccali, C.; Mallamaci, F.; Tripepi, G.; Benedetto, F.A.; Cutrupi, S.; Parlongo, S.; Malatino, L.S.; Bonanno, G.; Seminara, G.; Rapisarda, F.; et al. Adiponectin, metabolic risk factors, and cardiovascular events among patients with end-stage renal disease. J. Am. Soc. Nephrol. 2002, 13, 134–141. [Google Scholar] [PubMed]
- Becker, B.; Kronenberg, F.; Kielstein, J.T.; Haller, H.; Morath, C. Renal insulin resistance syndrome, adiponectin and cardiovascular events in patients with kidney disease: The Mild and Moderate Kidney Disease Study. J. Am. Soc. Nephrol. 2005, 16, 1091–1098. [Google Scholar] [CrossRef] [PubMed]
- Menon, V.; Li, L.; Wang, X.; Greene, T.; Balakrishnan, V.; Madero, M.; Pereira, A.A.; Beck, G.J.; Kusek, J.W.; Collins, A.J.; et al. Adiponectin and mortality in patients with chronic kidney disease. J. Am. Soc. Nephrol. 2006, 17, 2599–2606. [Google Scholar] [CrossRef] [PubMed]
- Nisoli, E.; Tonello, C.; Briscini, L.; Flaim, R.; Carruba, M.O. Leptin and nerve growth factor regulate adipose tissue. Nat. Med. 1996, 2, 130. [Google Scholar] [CrossRef] [PubMed]
- Stenvinkel, P. Leptin—A new hormone of definite interest for the nephrologist. Nephrol. Dial. Transplant. 1998, 13, 1099–1101. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Fried, S.K.; Ricci, M.R.; Russell, C.D.; Laferrère, B. Regulation of leptin production in humans. J. Nutr. 2000, 130, 3127S–3131S. [Google Scholar] [CrossRef]
- Considine, R.V.; Sinha, M.K.; Heiman, M.L.; Kriauciunas, A.; Stephens, T.W.; Nyce, M.R.; Ohannesian, J.P.; Marco, C.C.; McKee, L.J.; Bauer, T.L.; et al. Serum immunoreactive-leptin concentrations in normal-weight and obese humans. N. Engl. J. Med. 1996, 334, 292–295. [Google Scholar] [CrossRef]
- Maffei, M.; Halaas, J.; Ravussin, E.; Pratley, R.E.; Lee, G.H.; Zhang, Y.; Fei, H.; Kim, S.; Lallone, R.; Ranganathan, S.; et al. Leptin levels in human and rodent: Measurement of plasma leptin and ob RNA in obese and weight-reduced subjects. Nat. Med. 1995, 1, 1155–1161. [Google Scholar] [CrossRef]
- Boden, G.; Sargrad, K.; Homko, C.; Mozzoli, M.; Stein, T.P. Effect of a low-carbohydrate diet on appetite, blood glucose levels, and insulin resistance in obese patients with type 2 diabetes. Ann. Intern. Med. 2005, 142, 403–411. [Google Scholar] [CrossRef]
- Merabet, E.; Dagogo-Jack, S.; Coyne, D.W.; Klein, S.; Santiago, J.V.; Hmiel, S.P.; Landt, M. Increased plasma leptin concentration in end-stage renal disease. J. Clin. Endocrinol. Metab. 1997, 82, 847–850. [Google Scholar] [CrossRef]
- Díez, J.J.; Iglesias, P.; Fernández-Reyes, M.J.; Aguilera, A.; Bajo, M.A.; Alvarez-Fidalgo, P.; Codoceo, R.; Selgas, R. Serum concentrations of leptin, adiponectin and resistin, and their relationship with cardiovascular disease in patients with end-stage renal disease. Clin. Endocrinol. 2005, 62, 242–249. [Google Scholar] [CrossRef] [PubMed]
- Teta, D.; Bevington, A.; Brown, J.; Pawluczyk, I.; Harris, K.; Walls, J. Acidosis downregulates leptin production from cultured adipocytes through a glucose transport-dependent post-transcriptional mechanism. J. Am. Soc. Nephrol. 2003, 14, 2248–2254. [Google Scholar] [CrossRef] [PubMed]
- Ciccone, M.; Vettor, R.; Pannacciulli, N.; Minenna, A.; Bellacicco, M.; Rizzon, P.; Giorgino, R.; De Pergola, G. Plasma leptin is independently associated with the intima-media thickness of the common carotid artery. Int. J. Obes. Relat. Metab. Disord. 2001, 25, 805–810. [Google Scholar] [CrossRef] [PubMed]
- Singhal, A.; Farooqi, I.S.; Cole, T.J.; Rahilly, S.O.; Fewtrell, M.; Kattenhorn, M.; Lucas, A.; Deanfield, J. Influence of leptin on arterial distensibility. Circulation 2002, 106, 1919–1924. [Google Scholar] [CrossRef]
- Lee, M.-C.; Chen, Y.-C.; Ho, G.-J.; Shih, M.-H.; Chou, K.-C.; Hsu, B.-G. Serum leptin levels positively correlate with peripheral arterial stiffness in kidney transplantation patients. Transplant. Proc. 2014, 46, 353–358. [Google Scholar] [CrossRef]
- Aguilera, A.; Bajo, M.A.; Rebollo, F.; Díez, J.J.; Díaz, C.; Paiva, A.; Codoceo, R.; Selgas, R. Leptin as a marker of nutrition and cardiovascular risk in peritoneal dialysis patients. Adv. Perit. Dial. 2002, 18, 212–217. [Google Scholar]
- Noor, S.; Alam, F.; Fatima, S.S.; Khan, M.; Rehman, R. Role of Leptin and dyslipidemia in chronic kidney disease. Pak. J. Pharm. Sci. 2018, 31, 893–897. [Google Scholar]
- Kastarinen, H.; Kesäniemi, Y.A.; Ukkola, O. Leptin and lipid metabolism in chronic kidney failure. Scand. J. Clin. Lab. Investig. 2009, 69, 401–408. [Google Scholar] [CrossRef]
- Scholze, A.; Rattensperger, D.; Zidek, W.; Tepel, M. Low serum leptin predicts mortality in patients with chronic kidney disease stage 5. Obesity 2007, 15, 1617–1622. [Google Scholar] [CrossRef]
- Beberashvili, I.; Sinuani, I.; Azar, A.; Yasur, H.; Feldman, L.; Averbukh, Z.; Weissgarten, J. Longitudinal study of leptin levels in chronic hemodialysis patients. Nutr. J. 2011, 10, 68. [Google Scholar] [CrossRef]
- Tsai, Y.-C.; Lee, C.-T.; Huang, T.-L.; Cheng, B.-C.; Kuo, C.-C.; Su, Y.; Ng, H.Y.; Yang, C.C.; Chuang, F.R.; Liao, S.C. Inflammatory marker but not adipokine predicts mortality among long-term hemodialysis patients. Mediators. Inflamm. 2007, 2007, 19891. [Google Scholar] [CrossRef][Green Version]
- Nakazato, R.; Rajani, R.; Cheng, V.Y.; Shmilovich, H.; Nakanishi, R.; Otaki, Y.; Gransar, H.; Slomka, P.J.; Hayes, S.W.; Thomson, L.E.; et al. Weight change modulates epicardial fat burden: A 4-year serial study with non-contrast computed tomography. Atherosclerosis 2012, 220, 139–144. [Google Scholar] [CrossRef]
- Parisi, V.; Petraglia, L.; D’Esposito, V.; Cabaro, S.; Rengo, G.; Caruso, A.; Grimaldi, M.G.; Baldascino, F.; De Bellis, A.; Vitale, D.; et al. Statin therapy modulates thickness and inflammatory profile of human epicardial adipose tissue. Int. J. Cardiol. 2019, 274, 326–330. [Google Scholar] [CrossRef]
- Alexopoulos, N.; Melek, B.H.; Arepalli, C.D.; Hartlage, G.-R.; Chen, Z.; Kim, S.; Stillman, A.E.; Raggi, P. Effect of intensive versus moderate lipid-lowering therapy on epicardial adipose tissue in hyperlipidemic post-menopausal women: A substudy of the BELLES trial (Beyond Endorsed Lipid Lowering with EBT Scanning). J. Am. Coll. Cardiol. 2013, 61, 1956–1961. [Google Scholar] [CrossRef]
- Subbotin, V.M. Neovascularization of coronary tunica intima (DIT) is the cause of coronary atherosclerosis. Lipoproteins invade coronary intima via neovascularization from adventitial vasa vasorum, but not from the arterial lumen: A hypothesis. Theor. Biol. Med. Model. 2012, 9, 11. [Google Scholar] [CrossRef]
- Lima-Martínez, M.M.; Paoli, M.; Rodney, M.; Balladares, N.; Contreras, M.; D’Marco, L.; Iacobellis, G. Effect of sitagliptin on epicardial fat thickness in subjects with type 2 diabetes and obesity: A pilot study. Endocrine 2016, 51, 448–455. [Google Scholar] [CrossRef]
- Sacks, H.S.; Fain, J.N.; Cheema, P.; Bahouth, S.W.; Garrett, E.; Wolf, R.Y.; Wolford, D.; Samaha, J. Inflammatory genes in epicardial fat contiguous with coronary atherosclerosis in the metabolic syndrome and type 2 diabetes: Changes associated with pioglitazone. Diabetes Care 2011, 34, 730–733. [Google Scholar] [CrossRef]
- Ko, S.M.; Zhang, C.; Chen, Z.; D’Marco, L.; Bellasi, A.; Stillman, A.E.; Block, G.; Raggi, P. Epicardial adipose tissue volume increase in hemodialysis patients treated with sevelamer or calcium-based phosphate binders: A substudy of the Renagel in new dialysis trial. J. Nephrol. 2016, 29, 683–690. [Google Scholar] [CrossRef]
- Marchington, J.M.; Pond, C.M. Site-specific properties of pericardial and epicardial adipose tissue: The effects of insulin and high-fat feeding on lipogenesis and the incorporation of fatty acids in vitro. Int. J. Obes. 1990, 14, 1013–1022. [Google Scholar]
- Ishikawa, Y.; Ishii, T.; Asuwa, N.; Masuda, S. Absence of atherosclerosis evolution in the coronary arterial segment covered by myocardial tissue in cholesterol-fed rabbits. Virchows Arch. 1997, 430, 163–171. [Google Scholar] [CrossRef]
- Wang, J.; Chen, D.; Cheng, X.M.; Zhang, Q.G.; Peng, Y.P.; Wang, L.J.; He, S.Q.; Gong, J.B. Influence of phenotype conversion of epicardial adipocytes on the coronary atherosclerosis and its potential molecular mechanism. Am. J. Transl. Res. 2015, 7, 1712–1723. [Google Scholar]
- Bale, L.K.; West, S.A.; Conover, C.A. Characterization of mouse pericardial fat: Regulation by PAPP-A. Growth. Horm. IGF Res. 2018, 42, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Dalal, R.; Cao, C.D.; Postoak, J.L.; Yang, G.; Zhang, Q.; Wang, Z.; Lal, H.; Van Kaer, L. IL-10-producing B cells are enriched in murine pericardial adipose tissues and ameliorate the outcome of acute myocardial infarction. Proc. Natl. Acad. Sci. USA 2019, 116, 21673–21684. [Google Scholar] [CrossRef] [PubMed]
| Adipokine | Metabolism in CKD | Cardiovascular Effects | |||||
|---|---|---|---|---|---|---|---|
| Accumulation | Outcome | Oxidative Stress | Ischemia/Reperfusion | LV Hypertrophy | Remodeling | Inflammation | |
| Adiponectin | Yes | Inflammation/CVD | ⇩ | ⇩ | ⇩ | ⇩ | ⇩ |
| Leptin | Yes | Inflammation/CVD | ⇧ | ⇩ | ⇧ | ⇧ | ⇧ |
| Visfatin | Yes | endothelial damage/lipid dysregulation/CVD | ⇧ | ⇧ | ⇩ | ⇩ | ⇧ |
| Apelin | Yes | Inflammation/CVD | ⇩ | ⇩ | U | U | ⇩ |
| Resistin | Yes | endothelial damage/inflammation/CVD | ⇧ | ⇧ | ⇧ | ⇧ | ⇧ |
| Omentin | Yes | endothelial damage/inflammation/CVD | ⇩ | ⇩ | U | U | ⇩ |
| Publication | Experimental Animal | Outcome |
|---|---|---|
| Marchington et al. [79] | Guinea pigs | Epicardial adipose tissue stores energy and protects the coronary circulation from elevated fatty acid levels |
| Ishikawa et al. [80] | Rabbits | In cholesterol-fed animals, atherosclerosis does not develop in coronary artery segments embedded in myocardial bridges, but only in segments surrounded by epicardial adipose tissue |
| Wang et al. [81] | New Zealand white rabbits | A high-fat diet induces a phenotype conversion in the epicardial adipose tissue from brown to white adipose tissue with focal development of atherosclerosis and progressive increase of leptin mRNA and IL-6 expression. |
| Bale et al. [82] | Mice | Mouse pericardial fat has the characteristics of visceral fat and is regulated by pregnancy-associated plasma protein-A (PAPP-A) that affects insulin sensitivity. |
| Wu et al. [83] | Mice | The pericardial adipose tissue has a higher concentration of IL-10-producing B cells than other adipose tissues, and these cells have anti-inflammatory activity following myocardial infarction. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
D’Marco, L.; Puchades, M.J.; Gorriz, J.L.; Romero-Parra, M.; Lima-Martínez, M.; Soto, C.; Bermúdez, V.; Raggi, P. Epicardial Adipose Tissue, Adiponectin and Leptin: A Potential Source of Cardiovascular Risk in Chronic Kidney Disease. Int. J. Mol. Sci. 2020, 21, 978. https://doi.org/10.3390/ijms21030978
D’Marco L, Puchades MJ, Gorriz JL, Romero-Parra M, Lima-Martínez M, Soto C, Bermúdez V, Raggi P. Epicardial Adipose Tissue, Adiponectin and Leptin: A Potential Source of Cardiovascular Risk in Chronic Kidney Disease. International Journal of Molecular Sciences. 2020; 21(3):978. https://doi.org/10.3390/ijms21030978
Chicago/Turabian StyleD’Marco, Luis, Maria Jesús Puchades, Jose Luis Gorriz, Maria Romero-Parra, Marcos Lima-Martínez, Carlos Soto, Valmore Bermúdez, and Paolo Raggi. 2020. "Epicardial Adipose Tissue, Adiponectin and Leptin: A Potential Source of Cardiovascular Risk in Chronic Kidney Disease" International Journal of Molecular Sciences 21, no. 3: 978. https://doi.org/10.3390/ijms21030978
APA StyleD’Marco, L., Puchades, M. J., Gorriz, J. L., Romero-Parra, M., Lima-Martínez, M., Soto, C., Bermúdez, V., & Raggi, P. (2020). Epicardial Adipose Tissue, Adiponectin and Leptin: A Potential Source of Cardiovascular Risk in Chronic Kidney Disease. International Journal of Molecular Sciences, 21(3), 978. https://doi.org/10.3390/ijms21030978