AMP-Activated Protein Kinase (AMPK) at the Crossroads Between CO2 Retention and Skeletal Muscle Dysfunction in Chronic Obstructive Pulmonary Disease (COPD)
Abstract
1. Introduction
1.1. AMPK and Pulmonary Disease
1.2. General Concepts about Skeletal Muscle Dysfunction in COPD
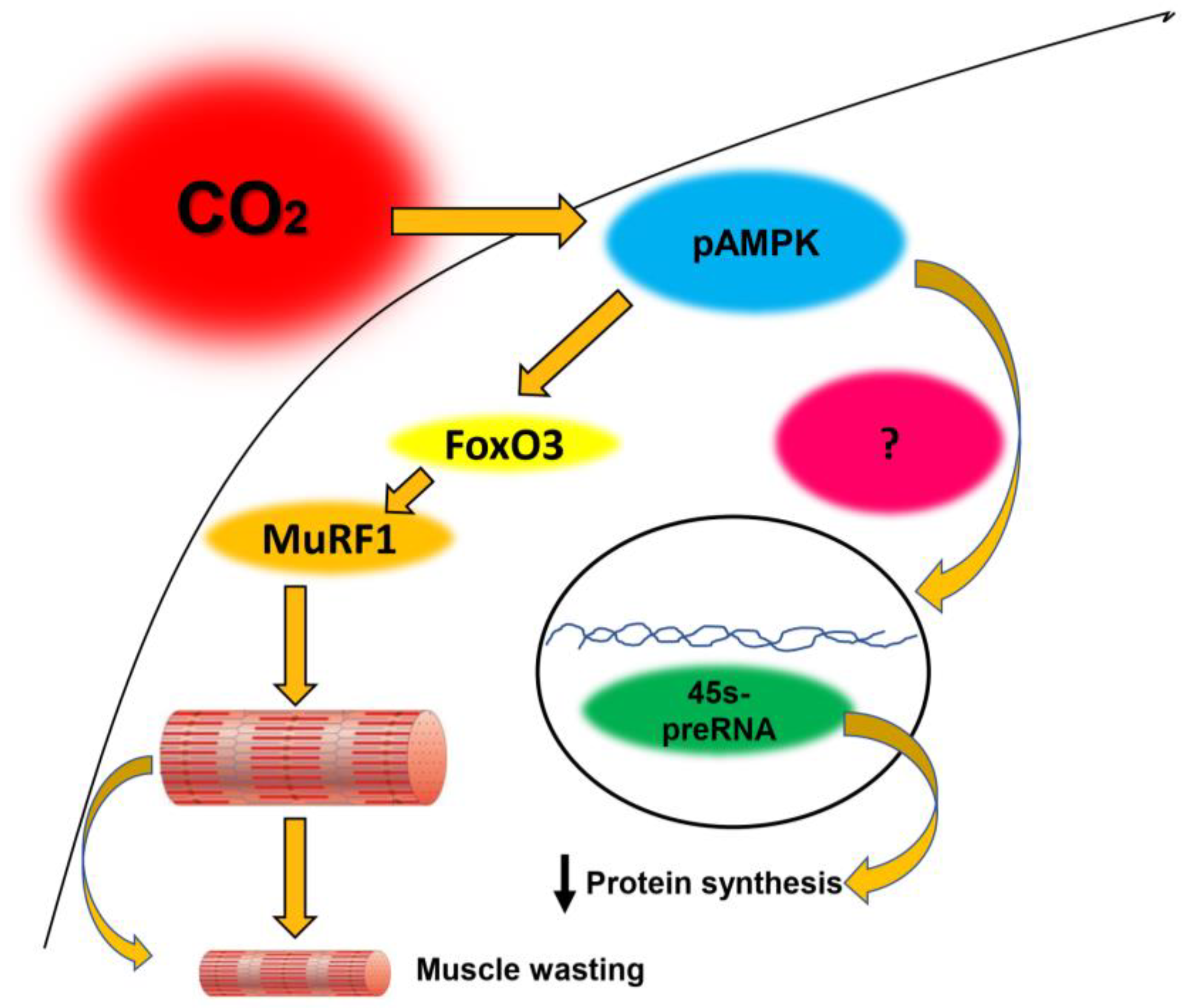
1.3. CO2-Mediated AMPK Activation Accelerates Protein Muscle Degradation
1.4. CO2-Mediataed AMPK Activation Attenuates Muscle Protein Synthesis
1.5. Other Effects of CO2 on Skeletal Muscle Potentially Regulated by AMPK
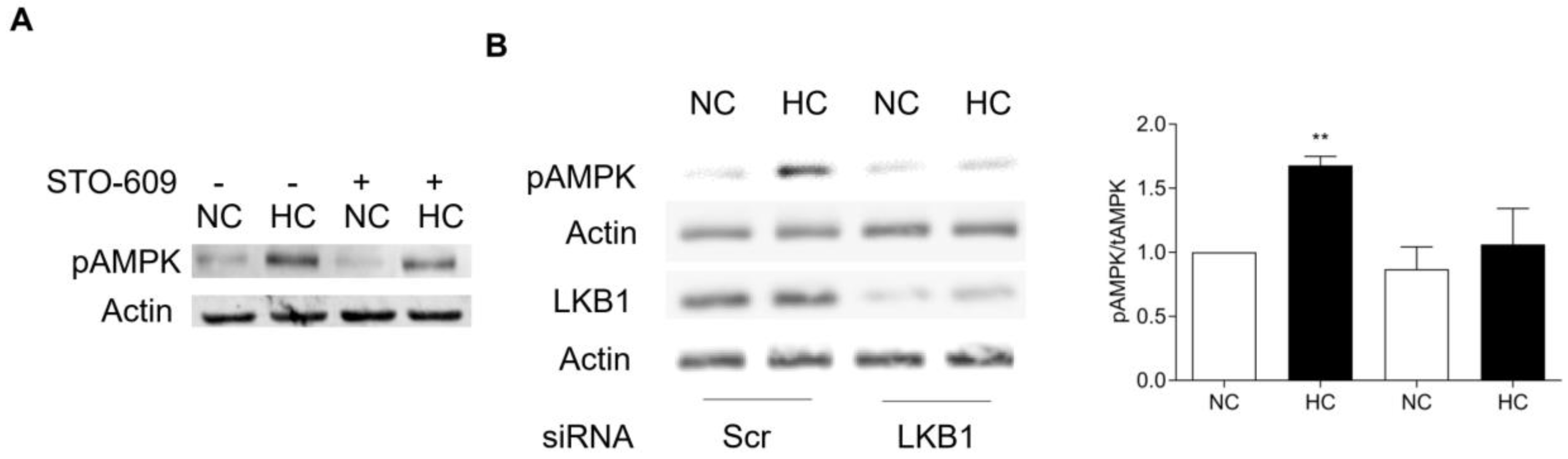
2. How is AMPK Regulated by CO2?
2.1. Interactions Between CO2 Retention, AMPK, and Autophagy
2.2. Limitations and Future Avenues to Investigate the Process of CO2-Induced Muscle Dysfunction
3. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Belkin, R.A.; Henig, N.R.; Singer, L.G.; Chaparro, C.; Rubenstein, R.C.; Xie, S.X.; Yee, J.Y.; Kotloff, R.M.; Lipson, D.A.; Bunin, G.R. Risk factors for death of patients with cystic fibrosis awaiting lung transplantation. Am. J. Respir. Crit. Care Med. 2006, 173, 659–666. [Google Scholar] [CrossRef]
- Vadasz, I.; Hubmayr, R.D.; Nin, N.; Sporn, P.H.; Sznajder, J.I. Hypercapnia: A nonpermissive environment for the lung. Am. J. Respir Cell Mol. Biol. 2012, 46, 417–421. [Google Scholar] [CrossRef]
- Weinberger, S.E.; Schwartzstein, R.M.; Weiss, J.W. Hypercapnia. N. Engl. J. Med. 1989, 321, 1223–1231. [Google Scholar] [CrossRef]
- Rodriguez-Roisin, R.; Drakulovic, M.; Rodriguez, D.A.; Roca, J.; Barbera, J.A.; Wagner, P.D. Ventilation-perfusion imbalance and chronic obstructive pulmonary disease staging severity. J. Appl. Physiol. 2009, 106, 1902–1908. [Google Scholar] [CrossRef] [PubMed]
- Tobin, M.J.; Laghi, F.; Brochard, L. Role of the respiratory muscles in acute respiratory failure of COPD: Lessons from weaning failure. J. Appl. Physiol. 2009, 107, 962–970. [Google Scholar] [CrossRef] [PubMed]
- Brat, K.; Plutinsky, M.; Hejduk, K.; Svoboda, M.; Popelkova, P.; Zatloukal, J.; Volakova, E.; Fecaninova, M.; Heribanova, L.; Koblizek, V. Respiratory parameters predict poor outcome in COPD patients, category GOLD 2017 B. Int. J. Chron. Obstruct. Pulmon. Dis. 2018, 13, 1037–1052. [Google Scholar] [CrossRef]
- Nin, N.; Muriel, A.; Penuelas, O.; Brochard, L.; Lorente, J.A.; Ferguson, N.D.; Raymondos, K.; Rios, F.; Violi, D.A.; Thille, A.W.; et al. Severe hypercapnia and outcome of mechanically ventilated patients with moderate or severe acute respiratory distress syndrome. Intensive Care Med. 2017, 43, 200–208. [Google Scholar] [CrossRef] [PubMed]
- Andersen, O.S. Blood acid-base alignment nomogram. Scales for pH, pCO2 base excess of whole blood of different hemoglobin concentrations, plasma bicarbonate, and plasma total-CO2. Scand. J. Clin. Lab Investig. 1963, 15, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Missner, A.; Kugler, P.; Saparov, S.M.; Sommer, K.; Mathai, J.C.; Zeidel, M.L.; Pohl, P. Carbon dioxide transport through membranes. J. Biol. Chem. 2008, 283, 25340–25347. [Google Scholar] [CrossRef]
- Lu, Z.; Casalino-Matsuda, S.M.; Nair, A.; Buchbinder, A.; Budinger, G.R.S.; Sporn, P.H.S.; Gates, K.L. A role for heat shock factor 1 in hypercapnia-induced inhibition of inflammatory cytokine expression. FASEB J. 2018, 32, 3614–3622. [Google Scholar] [CrossRef]
- Vohwinkel, C.U.; Lecuona, E.; Sun, H.; Sommer, N.; Vadasz, I.; Chandel, N.S.; Sznajder, J.I. Elevated CO2 levels cause mitochondrial dysfunction and impair cell proliferation. J. Biol. Chem. 2011, 286, 37067–37076. [Google Scholar] [CrossRef] [PubMed]
- Vadasz, I.; Dada, L.A.; Briva, A.; Trejo, H.E.; Welch, L.C.; Chen, J.; Toth, P.T.; Lecuona, E.; Witters, L.A.; Schumacker, P.T.; et al. AMP-activated protein kinase regulates CO2-induced alveolar epithelial dysfunction in rats and human cells by promoting Na,K-ATPase endocytosis. J. Clin. Investig. 2008, 118, 752–762. [Google Scholar] [CrossRef] [PubMed]
- Jaitovich, A.; Barreiro, E. Skeletal Muscle Dysfunction in Chronic Obstructive Pulmonary Disease. What We Know and Can Do for Our Patients. Am. J. Respir. Crit. Care Med. 2018, 198, 175–186. [Google Scholar] [CrossRef] [PubMed]
- Jaitovich, A.; Khan, M.; Itty, R.; Chieng, H.C.; Dumas, C.L.; Nadendla, P.; Fantauzzi, J.P.; Yucel, R.M.; Feustel, P.J.; Judson, M.A. ICU Admission Muscle and Fat Mass, Survival, and Disability at Discharge: A Prospective Cohort Study. Chest 2019, 155, 322–330. [Google Scholar] [CrossRef] [PubMed]
- Schols, A.M.; Broekhuizen, R.; Weling-Scheepers, C.A.; Wouters, E.F. Body composition and mortality in chronic obstructive pulmonary disease. Am. J. Clin. Nutr. 2005, 82, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Shrikrishna, D.; Patel, M.; Tanner, R.J.; Seymour, J.M.; Connolly, B.A.; Puthucheary, Z.A.; Walsh, S.L.; Bloch, S.A.; Sidhu, P.S.; Hart, N.; et al. Quadriceps wasting and physical inactivity in patients with COPD. Eur. Respir. J. 2012, 40, 1115–1122. [Google Scholar] [CrossRef]
- Swallow, E.B.; Reyes, D.; Hopkinson, N.S.; Man, W.D.; Porcher, R.; Cetti, E.J.; Moore, A.J.; Moxham, J.; Polkey, M.I. Quadriceps strength predicts mortality in patients with moderate to severe chronic obstructive pulmonary disease. Thorax 2007, 62, 115–120. [Google Scholar] [CrossRef]
- Sharma, R.; Florea, V.G.; Bolger, A.P.; Doehner, W.; Florea, N.D.; Coats, A.J.; Hodson, M.E.; Anker, S.D.; Henein, M.Y. Wasting as an independent predictor of mortality in patients with cystic fibrosis. Thorax 2001, 56, 746–750. [Google Scholar] [CrossRef][Green Version]
- Marquis, K.; Debigare, R.; Lacasse, Y.; LeBlanc, P.; Jobin, J.; Carrier, G.; Maltais, F. Midthigh muscle cross-sectional area is a better predictor of mortality than body mass index in patients with chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2002, 166, 809–813. [Google Scholar] [CrossRef]
- Puthucheary, Z.A.; Rawal, J.; McPhail, M.; Connolly, B.; Ratnayake, G.; Chan, P.; Hopkinson, N.S.; Phadke, R.; Dew, T.; Sidhu, P.S.; et al. Acute skeletal muscle wasting in critical illness. JAMA 2013, 310, 1591–1600. [Google Scholar] [CrossRef]
- Barreiro, E.; Jaitovich, A. Skeletal muscle dysfunction in COPD: Relevance of nutritional support and pulmonary rehabilitation. J. Thorac. Dis. 2018, 10, S1330–S1331. [Google Scholar] [CrossRef]
- Bodine, S.C.; Baehr, L.M. Skeletal muscle atrophy and the E3 ubiquitin ligases MuRF1 and MAFbx/atrogin-1. Am. J. Physiol. Endocrinol. Metab. 2014, 307, E469–E484. [Google Scholar] [CrossRef]
- Hardie, D.G.; Ross, F.A.; Hawley, S.A. AMPK: A nutrient and energy sensor that maintains energy homeostasis. Nat. Rev. Mol. Cell Biol. 2012, 13, 251–262. [Google Scholar] [CrossRef] [PubMed]
- Carling, D.; Viollet, B. Beyond energy homeostasis: The expanding role of AMP-activated protein kinase in regulating metabolism. Cell Metab. 2015, 21, 799–804. [Google Scholar] [CrossRef] [PubMed]
- Viollet, B. The Energy Sensor AMPK: Adaptations to Exercise, Nutritional and Hormonal Signals. In Hormones, Metabolism and the Benefits of Exercise; Spiegelman, B., Ed.; Springer: Cham, Switzerland, 2017; pp. 13–24. [Google Scholar] [CrossRef]
- Hurley, R.L.; Anderson, K.A.; Franzone, J.M.; Kemp, B.E.; Means, A.R.; Witters, L.A. The Ca2+/calmodulin-dependent protein kinase kinases are AMP-activated protein kinase kinases. J. Biol. Chem. 2005, 280, 29060–29066. [Google Scholar] [CrossRef] [PubMed]
- Peskin, A.V.; Pace, P.E.; Winterbourn, C.C. Enhanced hyperoxidation of peroxiredoxin 2 and peroxiredoxin 3 in the presence of bicarbonate/CO2. Free Radic. Biol. Med. 2019, 145, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Truzzi, D.R.; Coelho, F.R.; Paviani, V.; Alves, S.V.; Netto, L.E.S.; Augusto, O. The bicarbonate/carbon dioxide pair increases hydrogen peroxide-mediated hyperoxidation of human peroxiredoxin 1. J. Biol. Chem. 2019, 294, 14055–14067. [Google Scholar] [CrossRef]
- Maltais, F.; Decramer, M.; Casaburi, R.; Barreiro, E.; Burelle, Y.; Debigare, R.; Dekhuijzen, P.N.; Franssen, F.; Gayan-Ramirez, G.; Gea, J.; et al. An official American Thoracic Society/European Respiratory Society statement: Update on limb muscle dysfunction in chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2014, 189, e15–e62. [Google Scholar] [CrossRef]
- Allaire, J.; Maltais, F.; Doyon, J.F.; Noel, M.; LeBlanc, P.; Carrier, G.; Simard, C.; Jobin, J. Peripheral muscle endurance and the oxidative profile of the quadriceps in patients with COPD. Thorax 2004, 59, 673–678. [Google Scholar] [CrossRef]
- Casaburi, R.; Patessio, A.; Ioli, F.; Zanaboni, S.; Donner, C.F.; Wasserman, K. Reductions in exercise lactic acidosis and ventilation as a result of exercise training in patients with obstructive lung disease. Am. Rev. Respir. Dis. 1991, 143, 9–18. [Google Scholar] [CrossRef]
- Stephenson, D.G.; Williams, D.A. Calcium-activated force responses in fast- and slow-twitch skinned muscle fibres of the rat at different temperatures. J. Physiol. 1981, 317, 281–302. [Google Scholar] [CrossRef] [PubMed]
- Schiaffino, S.; Reggiani, C. Fiber types in mammalian skeletal muscles. Physiol. Rev. 2011, 91, 1447–1531. [Google Scholar] [CrossRef] [PubMed]
- Ciciliot, S.; Rossi, A.C.; Dyar, K.A.; Blaauw, B.; Schiaffino, S. Muscle type and fiber type specificity in muscle wasting. Int. J. Biochem. Cell. Biol. 2013, 45, 2191–2199. [Google Scholar] [CrossRef] [PubMed]
- Maltais, F.; LeBlanc, P.; Whittom, F.; Simard, C.; Marquis, K.; Belanger, M.; Breton, M.J.; Jobin, J. Oxidative enzyme activities of the vastus lateralis muscle and the functional status in patients with COPD. Thorax 2000, 55, 848–853. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, H.M.; Maarbjerg, S.J.; Crane, J.D.; Jeppesen, J.; Jorgensen, S.B.; Schertzer, J.D.; Shyroka, O.; Kiens, B.; van Denderen, B.J.; Tarnopolsky, M.A.; et al. AMP-activated protein kinase (AMPK) beta1beta2 muscle null mice reveal an essential role for AMPK in maintaining mitochondrial content and glucose uptake during exercise. Proc. Natl. Acad. Sci. USA 2011, 108, 16092–16097. [Google Scholar] [CrossRef] [PubMed]
- Korponay, T.C.; Balnis, J.; Vincent, C.E.; Singer, D.V.; Chopra, A.; Adam, A.P.; Ginnan, R.; Singer, H.A.; Jaitovich, A. High CO2 Downregulates Skeletal Muscle Protein Anabolism via AMP-activated Protein Kinase alpha2-mediated Depressed Ribosomal Biogenesis. Am. J. Respir. Cell. Mol. Biol. 2020, 62, 74–86. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wang, Y.; Hou, L.; Xiong, Y.; Zhao, S. Fibroblast Growth Factor 21 (FGF21) Promotes Formation of Aerobic Myofibers via the FGF21-SIRT1-AMPK-PGC1alpha Pathway. J. Cell Physiol. 2017, 232, 1893–1906. [Google Scholar] [CrossRef]
- Nin, N.; Angulo, M.; Briva, A. Effects of hypercapnia in acute respiratory distress syndrome. Ann. Transl. Med. 2018, 6, 37. [Google Scholar] [CrossRef]
- Jaitovich, A.; Angulo, M.; Lecuona, E.; Dada, L.A.; Welch, L.C.; Cheng, Y.; Gusarova, G.; Ceco, E.; Liu, C.; Shigemura, M.; et al. High CO2 levels cause skeletal muscle atrophy via AMP-activated kinase (AMPK), FoxO3a protein, and muscle-specific Ring finger protein 1 (MuRF1). J. Biol. Chem. 2015, 290, 9183–9194. [Google Scholar] [CrossRef]
- Shiota, S.; Okada, T.; Naitoh, H.; Ochi, R.; Fukuchi, Y. Hypoxia and hypercapnia affect contractile and histological properties of rat diaphragm and hind limb muscles. Pathophysiology 2004, 11, 23–30. [Google Scholar] [CrossRef]
- Sharabi, K.; Hurwitz, A.; Simon, A.J.; Beitel, G.J.; Morimoto, R.I.; Rechavi, G.; Sznajder, J.I.; Gruenbaum, Y. Elevated CO2 levels affect development, motility, and fertility and extend life span in Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA 2009, 106, 4024–4029. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, K.; Yakabe, Y. AMPK activation stimulates myofibrillar protein degradation and expression of atrophy-related ubiquitin ligases by increasing FOXO transcription factors in C2C12 myotubes. Biosci. Biotechnol. Biochem. 2007, 71, 1650–1656. [Google Scholar] [CrossRef] [PubMed]
- Silberstein, L.; Webster, S.G.; Travis, M.; Blau, H.M. Developmental progression of myosin gene expression in cultured muscle cells. Cell 1986, 46, 1075–1081. [Google Scholar] [CrossRef]
- Bodine, S.C.; Latres, E.; Baumhueter, S.; Lai, V.K.; Nunez, L.; Clarke, B.A.; Poueymirou, W.T.; Panaro, F.J.; Na, E.; Dharmarajan, K.; et al. Identification of ubiquitin ligases required for skeletal muscle atrophy. Science 2001, 294, 1704–1708. [Google Scholar] [CrossRef]
- Greer, E.L.; Oskoui, P.R.; Banko, M.R.; Maniar, J.M.; Gygi, M.P.; Gygi, S.P.; Brunet, A. The energy sensor AMP-activated protein kinase directly regulates the mammalian FOXO3 transcription factor. J. Biol. Chem. 2007, 282, 30107–30119. [Google Scholar] [CrossRef]
- Goodman, C.A.; Mabrey, D.M.; Frey, J.W.; Miu, M.H.; Schmidt, E.K.; Pierre, P.; Hornberger, T.A. Novel insights into the regulation of skeletal muscle protein synthesis as revealed by a new nonradioactive in vivo technique. FASEB J. 2011, 25, 1028–1039. [Google Scholar] [CrossRef]
- Chaillou, T.; Kirby, T.J.; McCarthy, J.J. Ribosome biogenesis: Emerging evidence for a central role in the regulation of skeletal muscle mass. J. Cell Physiol. 2014, 229, 1584–1594. [Google Scholar] [CrossRef]
- Mayer, C.; Grummt, I. Ribosome biogenesis and cell growth: mTOR coordinates transcription by all three classes of nuclear RNA polymerases. Oncogene 2006, 25, 6384–6391. [Google Scholar] [CrossRef]
- Stec, M.J.; Mayhew, D.L.; Bamman, M.M. The effects of age and resistance loading on skeletal muscle ribosome biogenesis. J. Appl. Physiol. 2015, 119, 851–857. [Google Scholar] [CrossRef]
- Drygin, D.; Rice, W.G.; Grummt, I. The RNA polymerase I transcription machinery: An emerging target for the treatment of cancer. Annu. Rev. Pharmacol. Toxicol. 2010, 50, 131–156. [Google Scholar] [CrossRef]
- Hoppe, S.; Bierhoff, H.; Cado, I.; Weber, A.; Tiebe, M.; Grummt, I.; Voit, R. AMP-activated protein kinase adapts rRNA synthesis to cellular energy supply. Proc. Natl. Acad. Sci. USA 2009, 106, 17781–17786. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.; Yano, H.; Ogasawara, S.; Yoshioka, S.; Imamura, H.; Okamoto, K.; Tsuneoka, M. Mild Glucose Starvation Induces KDM2A-Mediated H3K36me2 Demethylation through AMPK To Reduce rRNA Transcription and Cell Proliferation. Mol. Cell Biol. 2015, 35, 4170–4184. [Google Scholar] [CrossRef] [PubMed]
- Barreiro, E.; Gea, J. Molecular and biological pathways of skeletal muscle dysfunction in chronic obstructive pulmonary disease. Chron Respir. Dis. 2016, 13, 297–311. [Google Scholar] [CrossRef] [PubMed]
- Mauro, A. Satellite cell of skeletal muscle fibers. J. Biophys. Biochem. Cytol. 1961, 9, 493–495. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Price, F.; Rudnicki, M.A. Satellite cells and the muscle stem cell niche. Physiol. Rev. 2013, 93, 23–67. [Google Scholar] [CrossRef] [PubMed]
- Sethi, S.; Murphy, T.F. Infection in the pathogenesis and course of chronic obstructive pulmonary disease. N. Engl. J. Med. 2008, 359, 2355–2365. [Google Scholar] [CrossRef]
- Puthucheary, Z.A.; Hart, N. Skeletal muscle mass and mortality―but what about functional outcome? Crit. Care 2014, 18, 110. [Google Scholar] [CrossRef]
- Abdulai, R.M.; Jensen, T.J.; Patel, N.R.; Polkey, M.I.; Jansson, P.; Celli, B.R.; Rennard, S.I. Deterioration of Limb Muscle Function during Acute Exacerbation of Chronic Obstructive Pulmonary Disease. Am. J. Respir. Crit. Care Med. 2018, 197, 433–449. [Google Scholar] [CrossRef]
- Donaldson, G.C.; Seemungal, T.A.; Bhowmik, A.; Wedzicha, J.A. Relationship between exacerbation frequency and lung function decline in chronic obstructive pulmonary disease. Thorax 2002, 57, 847–852. [Google Scholar] [CrossRef]
- Tanabe, N.; Muro, S.; Hirai, T.; Oguma, T.; Terada, K.; Marumo, S.; Kinose, D.; Ogawa, E.; Hoshino, Y.; Mishima, M. Impact of exacerbations on emphysema progression in chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2011, 183, 1653–1659. [Google Scholar] [CrossRef]
- Fenn, W.O.; Rahn, H.; Otis, A.B. A theoretical study of the composition of the alveolar air at altitude. Am. J. 1946, 146, 637–653. [Google Scholar] [CrossRef] [PubMed]
- Tang, A.H.; Rando, T.A. Induction of autophagy supports the bioenergetic demands of quiescent muscle stem cell activation. EMBO J. 2014, 33, 2782–2797. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.G.; Plas, D.R.; Kubek, S.; Buzzai, M.; Mu, J.; Xu, Y.; Birnbaum, M.J.; Thompson, C.B. AMP-activated protein kinase induces a p53-dependent metabolic checkpoint. Mol. Cell 2005, 18, 283–293. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Zhu, M.J.; Dodson, M.V.; Du, M. AMP-activated protein kinase stimulates Warburg-like glycolysis and activation of satellite cells during muscle regeneration. J. Biol. Chem. 2015, 290, 26445–26456. [Google Scholar] [CrossRef]
- Shan, T.; Zhang, P.; Liang, X.; Bi, P.; Yue, F.; Kuang, S. Lkb1 is indispensable for skeletal muscle development, regeneration, and satellite cell homeostasis. Stem Cells 2014, 32, 2893–2907. [Google Scholar] [CrossRef]
- Helenius, I.T.; Haake, R.J.; Kwon, Y.J.; Hu, J.A.; Krupinski, T.; Casalino-Matsuda, S.M.; Sporn, P.H.S.; Sznajder, J.I.; Beitel, G.J. Identification of Drosophila Zfh2 as a Mediator of Hypercapnic Immune Regulation by a Genome-Wide RNA Interference Screen. J. Immunol. 2016, 196, 655–667. [Google Scholar] [CrossRef]
- Helenius, I.T.; Krupinski, T.; Turnbull, D.W.; Gruenbaum, Y.; Silverman, N.; Johnson, E.A.; Sporn, P.H.; Sznajder, J.I.; Beitel, G.J. Elevated CO2 suppresses specific Drosophila innate immune responses and resistance to bacterial infection. Proc. Natl. Acad. Sci. USA 2009, 106, 18710–18715. [Google Scholar] [CrossRef]
- Smith, C.D.; Compton, R.A.; Bowler, J.S.; Kemp, J.T.; Sudweeks, S.N.; Thomson, D.M.; Winder, W.W. Characterization of the liver kinase B1-mouse protein-25 -Ste-20-related adaptor protein complex in adult mouse skeletal muscle. J. Appl. Physiol. 2011, 111, 1622–1628. [Google Scholar] [CrossRef][Green Version]
- Thomson, D.M.; Hancock, C.R.; Evanson, B.G.; Kenney, S.G.; Malan, B.B.; Mongillo, A.D.; Brown, J.D.; Hepworth, S.; Fillmore, N.; Parcell, A.C.; et al. Skeletal muscle dysfunction in muscle-specific LKB1 knockout mice. J. Appl. Physiol. 2010, 108, 1775–1785. [Google Scholar] [CrossRef]
- Miura, S.; Kai, Y.; Tadaishi, M.; Tokutake, Y.; Sakamoto, K.; Bruce, C.R.; Febbraio, M.A.; Kita, K.; Chohnan, S.; Ezaki, O. Marked phenotypic differences of endurance performance and exercise-induced oxygen consumption between AMPK and LKB1 deficiency in mouse skeletal muscle: Changes occurring in the diaphragm. Am. J. Physiol. Endocrinol. Metab. 2013, 305, E213–E229. [Google Scholar] [CrossRef]
- Salt, I.; Celler, J.W.; Hawley, S.A.; Prescott, A.; Woods, A.; Carling, D.; Hardie, D.G. AMP-activated protein kinase: Greater AMP dependence, and preferential nuclear localization, of complexes containing the alpha2 isoform. Biochem. J. 1998, 334, 177–187. [Google Scholar] [CrossRef] [PubMed]
- Yamauchi, T.; Kamon, J.; Minokoshi, Y.; Ito, Y.; Waki, H.; Uchida, S.; Yamashita, S.; Noda, M.; Kita, S.; Ueki, K.; et al. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat. Med. 2002, 8, 1288–1295. [Google Scholar] [CrossRef] [PubMed]
- Berger, K.I.; Ayappa, I.; Chatr-Amontri, B.; Marfatia, A.; Sorkin, I.B.; Rapoport, D.M.; Goldring, R.M. Obesity hypoventilation syndrome as a spectrum of respiratory disturbances during sleep. Chest 2001, 120, 1231–1238. [Google Scholar] [CrossRef] [PubMed]
- Berger, K.I.; Ayappa, I.; Sorkin, I.B.; Norman, R.G.; Rapoport, D.M.; Goldring, R.M. CO2 homeostasis during periodic breathing in obstructive sleep apnea. J. Appl. Physiol. 2000, 88, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Coffey, V.G.; Zhong, Z.; Shield, A.; Canny, B.J.; Chibalin, A.V.; Zierath, J.R.; Hawley, J.A. Early signaling responses to divergent exercise stimuli in skeletal muscle from well-trained humans. FASEB J. 2006, 20, 190–192. [Google Scholar] [CrossRef] [PubMed]
- Langen, R.C.; Schols, A.M.; Kelders, M.C.; van der Velden, J.L.; Wouters, E.F.; Janssen-Heininger, Y.M. Muscle wasting and impaired muscle regeneration in a murine model of chronic pulmonary inflammation. Am. J. Respir. Cell Mol. Biol. 2006, 35, 689–696. [Google Scholar] [CrossRef] [PubMed]
- Kneppers, A.E.M.; Haast, R.A.M.; Langen, R.C.J.; Verdijk, L.B.; Leermakers, P.A.; Gosker, H.R.; van Loon, L.J.C.; Lainscak, M.; Schols, A. Distinct skeletal muscle molecular responses to pulmonary rehabilitation in chronic obstructive pulmonary disease: A cluster analysis. J. Cachexia Sarcopenia Muscle 2019, 10, 311–322. [Google Scholar] [CrossRef]
- Klionsky, D.J.; Abdelmohsen, K.; Abe, A.; Abedin, M.J.; Abeliovich, H.; Acevedo Arozena, A.; Adachi, H.; Adams, C.M.; Adams, P.D.; Adeli, K.; et al. Guidelines for the use and interpretation of assays for monitoring autophagy (3rd edition). Autophagy 2016, 12, 1–222. [Google Scholar] [CrossRef]
- Guo, Y.; Gosker, H.R.; Schols, A.M.; Kapchinsky, S.; Bourbeau, J.; Sandri, M.; Jagoe, R.T.; Debigare, R.; Maltais, F.; Taivassalo, T.; et al. Autophagy in locomotor muscles of patients with chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2013, 188, 1313–1320. [Google Scholar] [CrossRef]
- Hussain, S.N.; Sandri, M. Role of autophagy in COPD skeletal muscle dysfunction. J. Appl. Physiol. 2013, 114, 1273–1281. [Google Scholar] [CrossRef]
- Masiero, E.; Agatea, L.; Mammucari, C.; Blaauw, B.; Loro, E.; Komatsu, M.; Metzger, D.; Reggiani, C.; Schiaffino, S.; Sandri, M. Autophagy is required to maintain muscle mass. Cell Metab. 2009, 10, 507–515. [Google Scholar] [CrossRef] [PubMed]
- Gouzi, F.; Blaquiere, M.; Catteau, M.; Bughin, F.; Maury, J.; Passerieux, E.; Ayoub, B.; Mercier, J.; Hayot, M.; Pomies, P. Oxidative stress regulates autophagy in cultured muscle cells of patients with chronic obstructive pulmonary disease. J. Cell. Physiol. 2018, 233, 9629–9639. [Google Scholar] [CrossRef] [PubMed]
- Plant, P.J.; Brooks, D.; Faughnan, M.; Bayley, T.; Bain, J.; Singer, L.; Correa, J.; Pearce, D.; Binnie, M.; Batt, J. Cellular markers of muscle atrophy in chronic obstructive pulmonary disease. Am. J. Respir. Cell Mol. Biol. 2010, 42, 461–471. [Google Scholar] [CrossRef] [PubMed]
- Leermakers, P.A.; Schols, A.; Kneppers, A.E.M.; Kelders, M.; de Theije, C.C.; Lainscak, M.; Gosker, H.R. Molecular signalling towards mitochondrial breakdown is enhanced in skeletal muscle of patients with chronic obstructive pulmonary disease (COPD). Sci. Rep. 2018, 8, 15007. [Google Scholar] [CrossRef]
- Russell, R.C.; Tian, Y.; Yuan, H.; Park, H.W.; Chang, Y.Y.; Kim, J.; Kim, H.; Neufeld, T.P.; Dillin, A.; Guan, K.L. ULK1 induces autophagy by phosphorylating Beclin-1 and activating VPS34 lipid kinase. Nat. Cell Biol. 2013, 15, 741–750. [Google Scholar] [CrossRef]
- Otomo, C.; Metlagel, Z.; Takaesu, G.; Otomo, T. Structure of the human ATG12~ATG5 conjugate required for LC3 lipidation in autophagy. Nat. Struct. Mol. Biol. 2013, 20, 59–66. [Google Scholar] [CrossRef]
- Kneppers, A.E.M.; Langen, R.C.J.; Gosker, H.R.; Verdijk, L.B.; Cebron Lipovec, N.; Leermakers, P.A.; Kelders, M.; de Theije, C.C.; Omersa, D.; Lainscak, M.; et al. Increased Myogenic and Protein Turnover Signaling in Skeletal Muscle of Chronic Obstructive Pulmonary Disease Patients with Sarcopenia. J. Am. Med. Dir. Assoc. 2017, 18, 637.e1–637.e11. [Google Scholar] [CrossRef]
- Puig-Vilanova, E.; Rodriguez, D.A.; Lloreta, J.; Ausin, P.; Pascual-Guardia, S.; Broquetas, J.; Roca, J.; Gea, J.; Barreiro, E. Oxidative stress, redox signaling pathways, and autophagy in cachectic muscles of male patients with advanced COPD and lung cancer. Free Radic. Biol. Med. 2015, 79, 91–108. [Google Scholar] [CrossRef]
- Frudd, K.; Burgoyne, T.; Burgoyne, J.R. Oxidation of Atg3 and Atg7 mediates inhibition of autophagy. Nat. Commun. 2018, 9, 95. [Google Scholar] [CrossRef]
- Chiang, G.G.; Abraham, R.T. Phosphorylation of mammalian target of rapamycin (mTOR) at Ser-2448 is mediated by p70S6 kinase. J. Biol. Chem. 2005, 280, 25485–25490. [Google Scholar] [CrossRef]
- Kim, J.; Kundu, M.; Viollet, B.; Guan, K.L. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat. Cell Biol. 2011, 13, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Millet, G.P.; Debevec, T.; Brocherie, F.; Girard, O.; Pialoux, V.; Wust, R.C.; Degens, H.; Zuo, L.; He, F.; Chaillou, T.; et al. Commentaries on Viewpoint: Human skeletal muscle wasting in hypoxia: A matter of hypoxic dose? J. Appl. Physiol. 2017, 122, 409–411. [Google Scholar] [CrossRef] [PubMed]
- Larsson, L.; Ansved, T. Effects of long-term physical training and detraining on enzyme histochemical and functional skeletal muscle characteristic in man. Muscle Nerve 1985, 8, 714–722. [Google Scholar] [CrossRef] [PubMed]
- Jaitovich, A. Hypercapnic respiratory failure-driven skeletal muscle dysfunction: It is time for animal models-based mechanistic research. Adv. Exp. Med. Biol. 2020, in press. [Google Scholar]
- Langen, R.C.; Haegens, A.; Vernooy, J.H.; Wouters, E.F.; de Winther, M.P.; Carlsen, H.; Steele, C.; Shoelson, S.E.; Schols, A.M. NF-kappaB activation is required for the transition of pulmonary inflammation to muscle atrophy. Am. J. Respir. Cell. Mol. Biol. 2012, 47, 288–297. [Google Scholar] [CrossRef]
- Toledo, A.C.; Magalhaes, R.M.; Hizume, D.C.; Vieira, R.P.; Biselli, P.J.; Moriya, H.T.; Mauad, T.; Lopes, F.D.; Martins, M.A. Aerobic exercise attenuates pulmonary injury induced by exposure to cigarette smoke. Eur. Respir. J. 2012, 39, 254–264. [Google Scholar] [CrossRef]
- Evans, W.J. What is sarcopenia? J. Gerontol A Biol. Sci. Med. Sci 1995, 50 Spec. No, 5–8. [Google Scholar] [CrossRef]
- Jones, S.E.; Maddocks, M.; Kon, S.S.; Canavan, J.L.; Nolan, C.M.; Clark, A.L.; Polkey, M.I.; Man, W.D. Sarcopenia in COPD: Prevalence, clinical correlates and response to pulmonary rehabilitation. Thorax 2015, 70, 213–218. [Google Scholar] [CrossRef]
- McDonald, M.L.; Diaz, A.A.; Ross, J.C.; San Jose Estepar, R.; Zhou, L.; Regan, E.A.; Eckbo, E.; Muralidhar, N.; Come, C.E.; Cho, M.H.; et al. Quantitative computed tomography measures of pectoralis muscle area and disease severity in chronic obstructive pulmonary disease. A cross-sectional study. Ann. Am. Thorac. Soc. 2014, 11, 326–334. [Google Scholar] [CrossRef]
- Hopkinson, N.S.; Tennant, R.C.; Dayer, M.J.; Swallow, E.B.; Hansel, T.T.; Moxham, J.; Polkey, M.I. A prospective study of decline in fat free mass and skeletal muscle strength in chronic obstructive pulmonary disease. Respir. Res. 2007, 8, 25. [Google Scholar] [CrossRef]
- Van den Borst, B.; Koster, A.; Yu, B.; Gosker, H.R.; Meibohm, B.; Bauer, D.C.; Kritchevsky, S.B.; Liu, Y.; Newman, A.B.; Harris, T.B.; et al. Is age-related decline in lean mass and physical function accelerated by obstructive lung disease or smoking? Thorax 2011, 66, 961–969. [Google Scholar] [CrossRef]
- Campbell, E.J. Animal models of emphysema: The next generations. J. Clin. Investig. 2000, 106, 1445–1446. [Google Scholar] [CrossRef] [PubMed]
- Zheng, T.; Zhu, Z.; Wang, Z.; Homer, R.J.; Ma, B.; Riese, R.J., Jr.; Chapman, H.A., Jr.; Shapiro, S.D.; Elias, J.A. Inducible targeting of IL-13 to the adult lung causes matrix metalloproteinase- and cathepsin-dependent emphysema. J. Clin. Investig. 2000, 106, 1081–1093. [Google Scholar] [CrossRef] [PubMed]
- Balnis, J.; Korponay, T.C.; Vincent, C.E.; Singer, D.V.; Adam, A.P.; Lacomis, D.; Lee, C.G.; Elias, J.A.; Singer, H.A.; Jaitovich, A. IL-13-driven pulmonary emphysema leads to skeletal muscle dysfunction attenuated by endurance exercise. J. Appl. Physiol. 2020, 128, 134–148. [Google Scholar] [CrossRef] [PubMed]
- Degens, H.; Gayan-Ramirez, G.; van Hees, H.W. Smoking-induced skeletal muscle dysfunction: From evidence to mechanisms. Am. J. Respir. Crit. Care Med. 2015, 191, 620–625. [Google Scholar] [CrossRef]
- Chan, S.M.; Cerni, C.; Passey, S.; Seow, H.J.; Bernardo, I.; Poel, C.V.; Dobric, A.; Brassington, K.; Selemidis, S.; Bozinovski, S.; et al. Cigarette Smoking Exacerbates Skeletal Muscle Injury Without Compromising its Regenerative Capacity. Am. J. Respir. Cell Mol. Biol. 2019. [Google Scholar] [CrossRef]
- Basic, V.T.; Tadele, E.; Elmabsout, A.A.; Yao, H.; Rahman, I.; Sirsjo, A.; Abdel-Halim, S.M. Exposure to cigarette smoke induces overexpression of von Hippel-Lindau tumor suppressor in mouse skeletal muscle. Am. J. Physiol. Lung Cell. Mol. Physiol. 2012, 303, L519–L527. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balnis, J.; Korponay, T.C.; Jaitovich, A. AMP-Activated Protein Kinase (AMPK) at the Crossroads Between CO2 Retention and Skeletal Muscle Dysfunction in Chronic Obstructive Pulmonary Disease (COPD). Int. J. Mol. Sci. 2020, 21, 955. https://doi.org/10.3390/ijms21030955
Balnis J, Korponay TC, Jaitovich A. AMP-Activated Protein Kinase (AMPK) at the Crossroads Between CO2 Retention and Skeletal Muscle Dysfunction in Chronic Obstructive Pulmonary Disease (COPD). International Journal of Molecular Sciences. 2020; 21(3):955. https://doi.org/10.3390/ijms21030955
Chicago/Turabian StyleBalnis, Joseph, Tanner C. Korponay, and Ariel Jaitovich. 2020. "AMP-Activated Protein Kinase (AMPK) at the Crossroads Between CO2 Retention and Skeletal Muscle Dysfunction in Chronic Obstructive Pulmonary Disease (COPD)" International Journal of Molecular Sciences 21, no. 3: 955. https://doi.org/10.3390/ijms21030955
APA StyleBalnis, J., Korponay, T. C., & Jaitovich, A. (2020). AMP-Activated Protein Kinase (AMPK) at the Crossroads Between CO2 Retention and Skeletal Muscle Dysfunction in Chronic Obstructive Pulmonary Disease (COPD). International Journal of Molecular Sciences, 21(3), 955. https://doi.org/10.3390/ijms21030955