Differential Effects of 2-Hydroxypropyl-Cyclodextrins on Lipid Accumulation in Npc1-Null Cells
Abstract
1. Introduction
2. Results
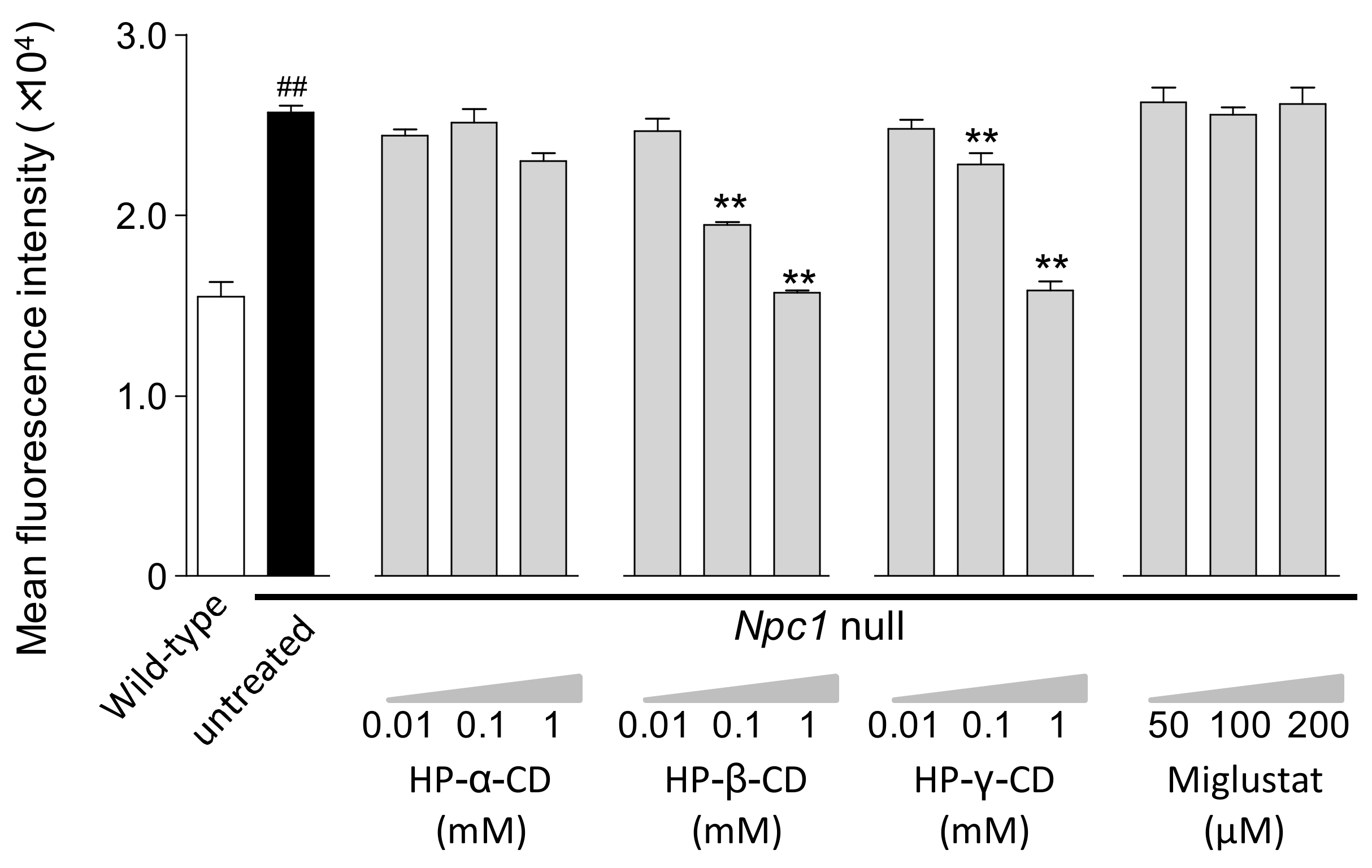
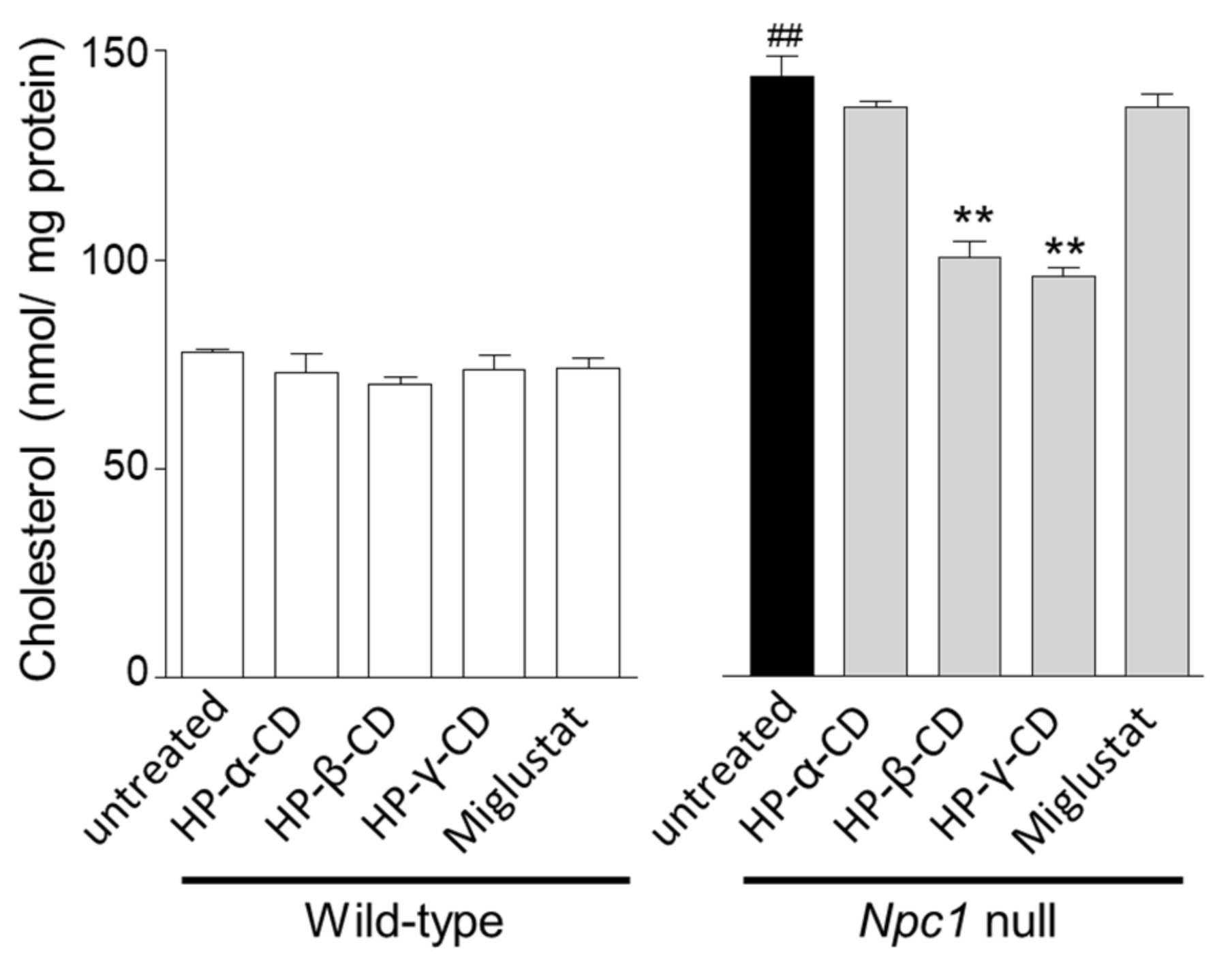
2.1. Effects of Cyclodextrins and Miglustat on LysoTracker® Fluorescence Intensity and Intracellular Cholesterol Level in Npc1-Null CHO Cells
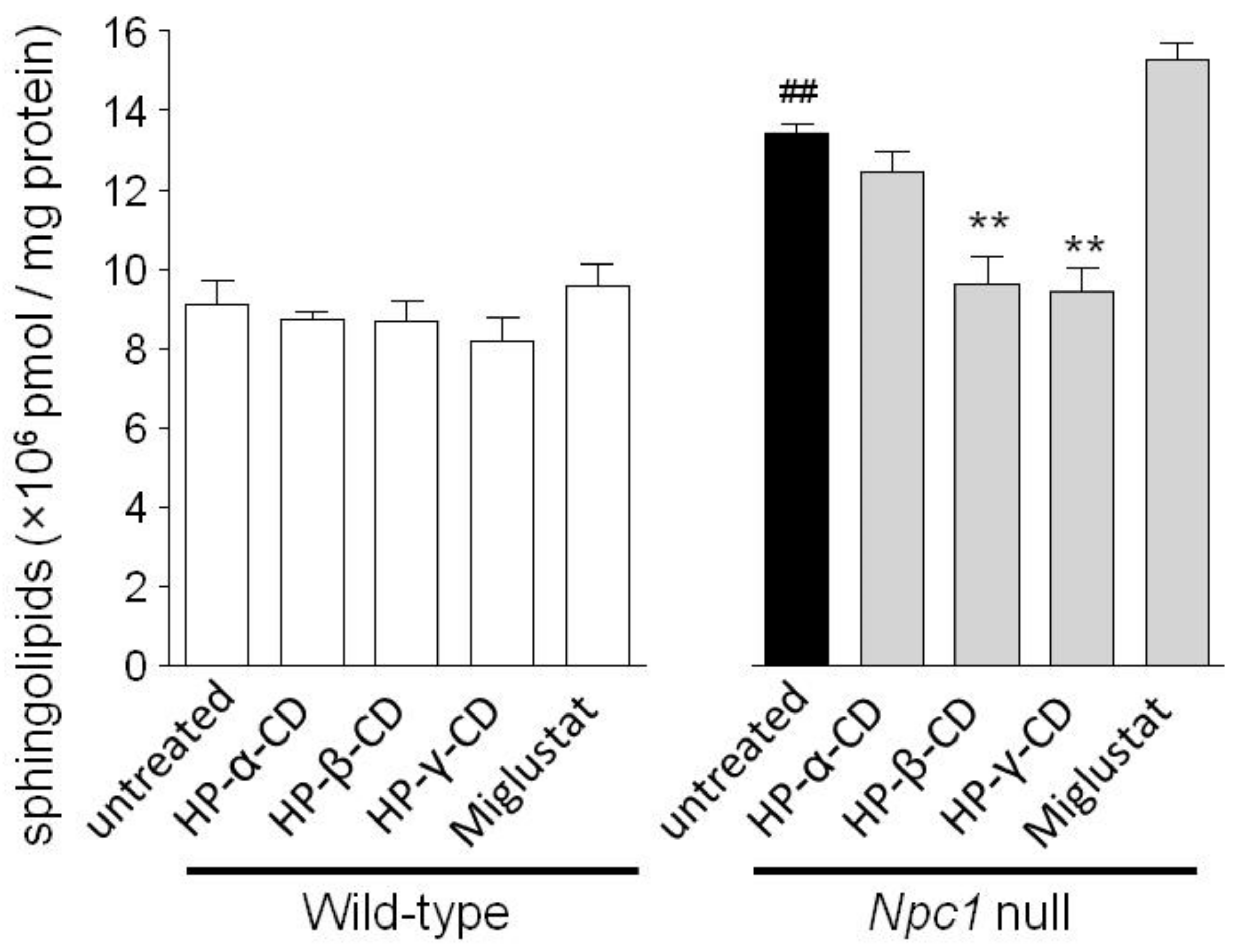
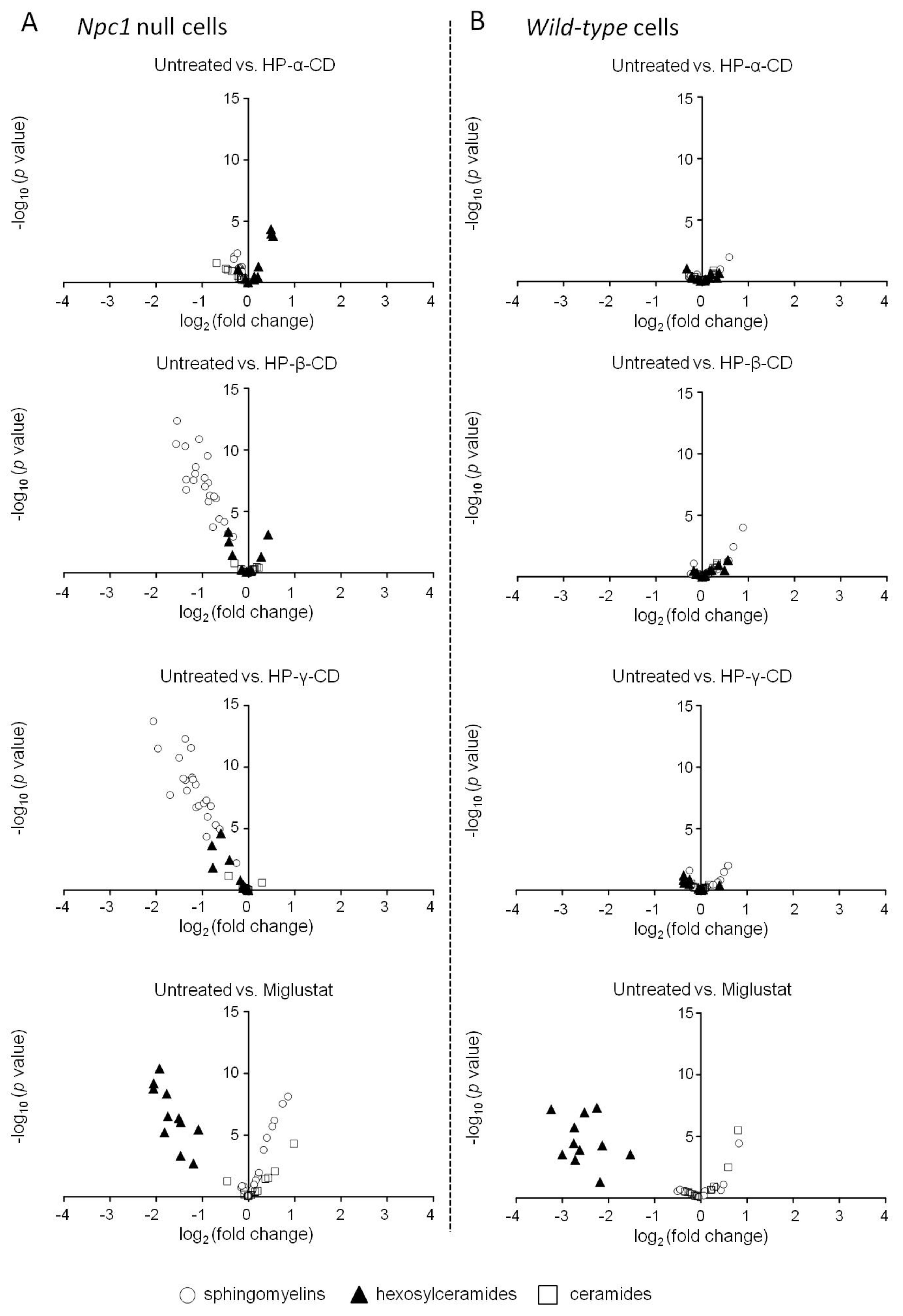
2.2. Effects of Cyclodextrins and Miglustat on Intracellular Sphingolipid Levels
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Cell Culture
4.2.1. Measurement of the LysoTracker® Fluorescence Intensity
4.2.2. Measurement of Intracellular Cholesterol Levels
4.2.3. Measurement of the intracellular sphingolipid level by High-Performance Liquid Chromatography–Tandem Mass Spectrometry (LC–MS/MS)
4.2.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| NPC | Niemann–Pick disease type C |
| CD | Cyclodextrin |
| HP-β-CD | 2-Hydroxypropyl-β-cyclodextrin |
| HP-γ-CD | 2-Hydroxypropyl-γ-cyclodextrin |
| HP-α-CD | 2-Hydroxypropyl-α-cyclodextrin |
| CHO | Chinese hamster ovary |
| WT | Wild-type |
| S.E.M. | Standard error of the mean |
| DMEM | Dulbecco’s modified Eagle’s medium |
| FBS | Fetal bovine serum |
| BCA | Bicinchoninic acid |
| LC/MS/MS | High-performance liquid chromatography–tandem mass spectrometry |
| iPS | Induced pluripotent stem |
| LAMP | Lysosomal-associated membrane protein |
References
- Vanier, M.T. Niemann-Pick disease type C. Orphanet J. Rare Dis. 2010, 5, 16. [Google Scholar] [CrossRef] [PubMed]
- Carstea, E.D.; Morris, J.A.; Coleman, K.G.; Loftus, S.K.; Zhang, D.; Cummings, C.; Gu, J.; Rosenfeld, M.A.; Pavan, W.J.; Krizman, D.B.; et al. Niemann-Pick C1 disease gene: homology to mediators of cholesterol homeostasis. Science 1997, 277, 228–231. [Google Scholar] [CrossRef] [PubMed]
- Naureckiene, S.; Sleat, D.E.; Lackland, H.; Fensom, A.; Vanier, M.T.; Wattiaux, R.; Jadot, M.; Lobel, P. Identification of HE1 as the second gene of Niemann-Pick C disease. Science 2000, 290, 2298–2301. [Google Scholar] [CrossRef] [PubMed]
- Ikonen, E. Cellular cholesterol trafficking and compartmentalization. Nat. Rev. Mol. Cell Biol. 2008, 9, 125–138. [Google Scholar] [CrossRef]
- Brown, M.S.; Goldstein, J.L. A receptor-mediated pathway for cholesterol homeostasis. Science 1986, 232, 34–47. [Google Scholar] [CrossRef]
- Vanier, M.T.; Millat, G. Niemann-Pick disease type C. Clin. Genet. 2003, 64, 269–281. [Google Scholar] [CrossRef]
- Zervas, M.; Somers, K.L.; Thrall, M.A.; Walkley, S.U. Critical role for glycosphingolipids in Niemann-Pick disease type C. Curr. Biol. CB 2001, 11, 1283–1287. [Google Scholar] [CrossRef]
- Zervas, M.; Dobrenis, K.; Walkley, S.U. Neurons in Niemann-Pick disease type C accumulate gangliosides as well as unesterified cholesterol and undergo dendritic and axonal alterations. J. Neuropathol. Exp. Neurol. 2001, 60, 49–64. [Google Scholar] [CrossRef]
- Neßlauer, A.-M.; Gläser, A.; Gräler, M.; Engelmann, R.; Müller-Hilke, B.; Frank, M.; Burstein, C.; Rolfs, A.; Neidhardt, J.; Wree, A.; et al. A therapy with miglustat, 2-hydroxypropyl-ß-cyclodextrin and allopregnanolone restores splenic cholesterol homeostasis in Niemann-pick disease type C1. Lipids Health Dis. 2019, 18. [Google Scholar] [CrossRef]
- Pineda, M.; Wraith, J.E.; Mengel, E.; Sedel, F.; Hwu, W.-L.; Rohrbach, M.; Bembi, B.; Walterfang, M.; Korenke, G.C.; Marquardt, T.; et al. Miglustat in patients with Niemann-Pick disease Type C (NP-C): a multicenter observational retrospective cohort study. Mol. Genet. Metab. 2009, 98, 243–249. [Google Scholar] [CrossRef]
- Patterson, M.C.; Vecchio, D.; Jacklin, E.; Abel, L.; Chadha-Boreham, H.; Luzy, C.; Giorgino, R.; Wraith, J.E. Long-term miglustat therapy in children with Niemann-Pick disease type C. J. Child Neurol. 2010, 25, 300–305. [Google Scholar] [CrossRef] [PubMed]
- Szakszon, K.; Szegedi, I.; Magyar, Á.; Oláh, É.; Andrejkovics, M.; Balla, P.; Lengyel, A.; Berényi, E.; Balogh, I. Complete recovery from psychosis upon miglustat treatment in a juvenile Niemann–Pick C patient. Eur. J. Paediatr. Neurol. 2014, 18, 75–78. [Google Scholar] [CrossRef] [PubMed]
- Zarowski, M.; Steinborn, B.; Gurda, B.; Dvorakova, L.; Vlaskova, H.; Kothare, S.V. Treatment of cataplexy in Niemann-Pick disease type C with the use of miglustat. Eur. J. Paediatr. Neurol. EJPN Off. J. Eur. Paediatr. Neurol. Soc. 2011, 15, 84–87. [Google Scholar] [CrossRef] [PubMed]
- Pineda, M.; Juríčková, K.; Karimzadeh, P.; Kolnikova, M.; Malinova, V.; Insua, J.L.; Velten, C.; Kolb, S.A. Disease characteristics, prognosis and miglustat treatment effects on disease progression in patients with Niemann-Pick disease Type C: an international, multicenter, retrospective chart review. Orphanet J. Rare Dis. 2019, 14, 32. [Google Scholar] [CrossRef]
- Colaco, A.; Kaya, E.; Adriaenssens, E.; Davis, L.C.; Zampieri, S.; Fernández-Suárez, M.E.; Tan, C.Y.; Deegan, P.B.; Porter, F.D.; Galione, A.; et al. Mechanistic convergence and shared therapeutic targets in Niemann-Pick disease. J. Inherit. Metab. Dis. 2019. [Google Scholar] [CrossRef]
- Liu, B.; Turley, S.D.; Burns, D.K.; Miller, A.M.; Repa, J.J.; Dietschy, J.M. Reversal of defective lysosomal transport in NPC disease ameliorates liver dysfunction and neurodegeneration in the npc1−/− mouse. Proc. Natl. Acad. Sci. USA 2009, 106, 2377–2382. [Google Scholar] [CrossRef]
- Davidson, C.D.; Ali, N.F.; Micsenyi, M.C.; Stephney, G.; Renault, S.; Dobrenis, K.; Ory, D.S.; Vanier, M.T.; Walkley, S.U. Chronic Cyclodextrin Treatment of Murine Niemann-Pick C Disease Ameliorates Neuronal Cholesterol and Glycosphingolipid Storage and Disease Progression. PLoS ONE 2009, 4, e6951. [Google Scholar] [CrossRef]
- Rosenbaum, A.I.; Zhang, G.; Warren, J.D.; Maxfield, F.R. Endocytosis of beta-cyclodextrins is responsible for cholesterol reduction in Niemann-Pick type C mutant cells. Proc. Natl. Acad. Sci. USA 2010, 107, 5477–5482. [Google Scholar] [CrossRef]
- Liu, B.; Ramirez, C.M.; Miller, A.M.; Repa, J.J.; Turley, S.D.; Dietschy, J.M. Cyclodextrin overcomes the transport defect in nearly every organ of NPC1 mice leading to excretion of sequestered cholesterol as bile acid. J. Lipid Res. 2010, 51, 933–944. [Google Scholar] [CrossRef]
- Ramirez, C.M.; Liu, B.; Taylor, A.M.; Repa, J.J.; Burns, D.K.; Weinberg, A.G.; Turley, S.D.; Dietschy, J.M. Weekly Cyclodextrin Administration Normalizes Cholesterol Metabolism in Nearly Every Organ of the Niemann-Pick Type C1 Mouse and Markedly Prolongs Life. Pediatr. Res. 2010, 68, 309–315. [Google Scholar] [CrossRef]
- Vite, C.H.; Bagel, J.H.; Swain, G.P.; Prociuk, M.; Sikora, T.U.; Stein, V.M.; O’Donnell, P.; Ruane, T.; Ward, S.; Crooks, A.; et al. Intracisternal cyclodextrin prevents cerebellar dysfunction and Purkinje cell death in feline Niemann-Pick type C1 disease. Sci. Transl. Med. 2015, 7, 276ra26. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.; Yamada, Y.; Ishitsuka, Y.; Matsuo, M.; Shiraishi, K.; Wada, K.; Uchio, Y.; Kondo, Y.; Takeo, T.; Nakagata, N.; et al. Efficacy of 2-Hydroxypropyl-β-cyclodextrin in Niemann–Pick Disease Type C Model Mice and Its Pharmacokinetic Analysis in a Patient with the Disease. Biol. Pharm. Bull. 2015, 38, 844–851. [Google Scholar] [CrossRef] [PubMed]
- Kondo, Y.; Tokumaru, H.; Ishitsuka, Y.; Matsumoto, T.; Taguchi, M.; Motoyama, K.; Higashi, T.; Arima, H.; Matsuo, M.; Higaki, K.; et al. In vitro evaluation of 2-hydroxyalkylated β-cyclodextrins as potential therapeutic agents for Niemann-Pick Type C disease. Mol. Genet. Metab. 2016, 118, 214–219. [Google Scholar] [CrossRef]
- Abi-Mosleh, L.; Infante, R.E.; Radhakrishnan, A.; Goldstein, J.L.; Brown, M.S. Cyclodextrin overcomes deficient lysosome-to-endoplasmic reticulum transport of cholesterol in Niemann-Pick type C cells. Proc. Natl. Acad. Sci. USA 2009, 106, 19316–19321. [Google Scholar] [CrossRef]
- Cartledge, J.D.; Midgley, J.; Gazzard, B.G. Itraconazole cyclodextrin solution: the role of in vitro susceptibility testing in predicting successful treatment of HIV-related fluconazole-resistant and fluconazole-susceptible oral candidosis. AIDS Lond. Engl. 1997, 11, 163–168. [Google Scholar] [CrossRef]
- Ory, D.S.; Ottinger, E.A.; Farhat, N.Y.; King, K.A.; Jiang, X.; Weissfeld, L.; Berry-Kravis, E.; Davidson, C.D.; Bianconi, S.; Keener, L.A.; et al. Intrathecal 2-hydroxypropyl-β-cyclodextrin decreases neurological disease progression in Niemann-Pick disease, type C1: a non-randomised, open-label, phase 1-2 trial. Lancet Lond. Engl. 2017, 390, 1758–1768. [Google Scholar] [CrossRef]
- Safety and Efficacy of Intravenous Trappsol Cyclo (HPBCD) in Niemann-Pick Type C Patients - Full Text View - ClinicalTrials. gov Available online: https://clinicaltrials.gov/ct2/show/NCT02912793 (accessed on 24 January 2020).
- Soga, M.; Ishitsuka, Y.; Hamasaki, M.; Yoneda, K.; Furuya, H.; Matsuo, M.; Ihn, H.; Fusaki, N.; Nakamura, K.; Nakagata, N.; et al. HPGCD Outperforms HPBCD as A Potential Treatment for Niemann-Pick Disease Type C During Disease Modeling with iPS Cells. STEM CELLS 2014, 33, 1075–1088. [Google Scholar] [CrossRef]
- Davidson, C.D.; Fishman, Y.I.; Puskás, I.; Szemán, J.; Sohajda, T.; McCauliff, L.A.; Sikora, J.; Storch, J.; Vanier, M.T.; Szente, L.; et al. Efficacy and ototoxicity of different cyclodextrins in Niemann–Pick C disease. Ann. Clin. Transl. Neurol. 2016, 3, 366–380. [Google Scholar] [CrossRef]
- Ito, J.; Nagayasu, Y.; Yokoyama, S. Cholesterol–sphingomyelin interaction in membrane and apolipoprotein-mediated cellular cholesterol efflux. J. Lipid Res. 2000, 41, 894–904. [Google Scholar]
- Wanikawa, M.; Nakamura, H.; Emori, S.; Hashimoto, N.; Murayama, T. Accumulation of sphingomyelin in Niemann-Pick disease type C cells disrupts Rab9-dependent vesicular trafficking of cholesterol. J. Cell. Physiol. 2020, 235, 2300–2309. [Google Scholar] [CrossRef]
- Long, Y.; Xu, M.; Li, R.; Dai, S.; Beers, J.; Chen, G.; Soheilian, F.; Baxa, U.; Wang, M.; Marugan, J.J.; et al. Induced Pluripotent Stem Cells for Disease Modeling and Evaluation of Therapeutics for Niemann-Pick Disease Type A. Stem Cells Transl. Med. 2016, 5, 1644–1655. [Google Scholar] [CrossRef]
- Irie, T.; Fukunaga, K.; Pitha, J. Hydroxypropylcyclodextrins in Parenteral use. I: Lipid Dissolution and Effects on Lipid Transfers in Vitro. J. Pharm. Sci. 1992, 81, 521–523. [Google Scholar] [CrossRef]
- Regier, D.S.; Proia, R.L.; D’Azzo, A.; Tifft, C.J. The GM1 and GM2 Gangliosidoses: Natural History and Progress toward Therapy. Pediatr. Endocrinol. Rev. PER 2016, 13 Suppl. 1, 663–673. [Google Scholar]
- Bembi, B.; Marchetti, F.; Guerci, V.I.; Ciana, G.; Addobbati, R.; Grasso, D.; Barone, R.; Cariati, R.; Fernandez-Guillen, L.; Butters, T.; et al. Substrate reduction therapy in the infantile form of Tay-Sachs disease. Neurology 2006, 66, 278–280. [Google Scholar] [CrossRef]
- Maegawa, G.H.B.; van Giersbergen, P.L.M.; Yang, S.; Banwell, B.; Morgan, C.P.; Dingemanse, J.; Tifft, C.J.; Clarke, J.T.R. Pharmacokinetics, safety and tolerability of miglustat in the treatment of pediatric patients with GM2 gangliosidosis. Mol. Genet. Metab. 2009, 97, 284–291. [Google Scholar] [CrossRef]
- Shapiro, B.E.; Pastores, G.M.; Gianutsos, J.; Luzy, C.; Kolodny, E.H. Miglustat in late-onset Tay-Sachs disease: a 12-month, randomized, controlled clinical study with 24 months of extended treatment. Genet. Med. Off. J. Am. Coll. Med. Genet. 2009, 11, 425–433. [Google Scholar] [CrossRef]
- Patterson, M.C.; Vecchio, D.; Prady, H.; Abel, L.; Wraith, J.E. Miglustat for treatment of Niemann-Pick C disease: a randomised controlled study. Lancet Neurol. 2007, 6, 765–772. [Google Scholar] [CrossRef]
- Yu, D.; Swaroop, M.; Wang, M.; Baxa, U.; Yang, R.; Yan, Y.; Coksaygan, T.; DeTolla, L.; Marugan, J.J.; Austin, C.P.; et al. Niemann-Pick Disease Type C: Induced Pluripotent Stem Cell-Derived Neuronal Cells for Modeling Neural Disease and Evaluating Drug Efficacy. J. Biomol. Screen. 2014, 19, 1164–1173. [Google Scholar] [CrossRef]
- Schlegel, V.; Thieme, M.; Holzmann, C.; Witt, M.; Grittner, U.; Rolfs, A.; Wree, A. Pharmacologic Treatment Assigned for Niemann Pick Type C1 Disease Partly Changes Behavioral Traits in Wild-Type Mice. Int. J. Mol. Sci. 2016, 17. [Google Scholar] [CrossRef]
- Higaki, K.; Ninomiya, H.; Sugimoto, Y.; Suzuki, T.; Taniguchi, M.; Niwa, H.; Pentchev, P.G.; Vanier, M.T.; Ohno, K. Isolation of NPC1-Deficient Chinese Hamster Ovary Cell Mutants by Gene Trap Mutagenesis. J. Biochem. (Tokyo) 2001, 129, 875–880. [Google Scholar] [CrossRef]
- Furukawa, J.-I.; Soga, M.; Okada, K.; Yokota, I.; Piao, J.; Irie, T.; Era, T.; Shinohara, Y. Impact of the Niemann-Pick c1 Gene Mutation on the Total Cellular Glycomics of CHO Cells. J. Proteome Res. 2017, 16, 2802–2810. [Google Scholar] [CrossRef]
- Li, J.; Deffieu, M.S.; Lee, P.L.; Saha, P.; Pfeffer, S.R. Glycosylation inhibition reduces cholesterol accumulation in NPC1 protein-deficient cells. Proc. Natl. Acad. Sci. USA 2015, 112, 14876–14881. [Google Scholar] [CrossRef]
- Kennedy, B.E.; Madreiter, C.T.; Vishnu, N.; Malli, R.; Graier, W.F.; Karten, B. Adaptations of energy metabolism associated with increased levels of mitochondrial cholesterol in Niemann-Pick type C1-deficient cells. J. Biol. Chem. 2014, 289, 16278–16289. [Google Scholar] [CrossRef]
- Te Vruchte, D.; Speak, A.O.; Wallom, K.L.; Al Eisa, N.; Smith, D.A.; Hendriksz, C.J.; Simmons, L.; Lachmann, R.H.; Cousins, A.; Hartung, R.; et al. Relative acidic compartment volume as a lysosomal storage disorder–associated biomarker. J. Clin. Invest. 2014, 124, 1320–1328. [Google Scholar] [CrossRef]
- Te Vruchte, D.; Galione, A.; Strupp, M.; Mann, M. Effects of N-Acetyl-Leucine and its enantiomers in Niemann-Pick disease type C cells. bioRxiv 2019, 826222. [Google Scholar]
- Tanaka, Y.; Ishitsuka, Y.; Yamada, Y.; Kondo, Y.; Takeo, T.; Nakagata, N.; Higashi, T.; Motoyama, K.; Arima, H.; Matsuo, M.; et al. Influence of Npc1 genotype on the toxicity of hydroxypropyl-β-cyclodextrin, a potentially therapeutic agent, in Niemann–Pick Type C disease models. Mol. Genet. Metab. Rep. 2014, 1, 19–30. [Google Scholar] [CrossRef]
- Muranaka, H.; Hayashi, A.; Minami, K.; Kitajima, S.; Kohno, S.; Nishimoto, Y.; Nagatani, N.; Suzuki, M.; Kulathunga, L.a.N.; Sasaki, N.; et al. A distinct function of the retinoblastoma protein in the control of lipid composition identified by lipidomic profiling. Oncogenesis 2017, 6, e350. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hoque, S.; Kondo, Y.; Sakata, N.; Yamada, Y.; Fukaura, M.; Higashi, T.; Motoyama, K.; Arima, H.; Higaki, K.; Hayashi, A.; et al. Differential Effects of 2-Hydroxypropyl-Cyclodextrins on Lipid Accumulation in Npc1-Null Cells. Int. J. Mol. Sci. 2020, 21, 898. https://doi.org/10.3390/ijms21030898
Hoque S, Kondo Y, Sakata N, Yamada Y, Fukaura M, Higashi T, Motoyama K, Arima H, Higaki K, Hayashi A, et al. Differential Effects of 2-Hydroxypropyl-Cyclodextrins on Lipid Accumulation in Npc1-Null Cells. International Journal of Molecular Sciences. 2020; 21(3):898. https://doi.org/10.3390/ijms21030898
Chicago/Turabian StyleHoque, Sanzana, Yuki Kondo, Nodoka Sakata, Yusei Yamada, Madoka Fukaura, Taishi Higashi, Keiichi Motoyama, Hidetoshi Arima, Katsumi Higaki, Akio Hayashi, and et al. 2020. "Differential Effects of 2-Hydroxypropyl-Cyclodextrins on Lipid Accumulation in Npc1-Null Cells" International Journal of Molecular Sciences 21, no. 3: 898. https://doi.org/10.3390/ijms21030898
APA StyleHoque, S., Kondo, Y., Sakata, N., Yamada, Y., Fukaura, M., Higashi, T., Motoyama, K., Arima, H., Higaki, K., Hayashi, A., Komiya, T., Ishitsuka, Y., & Irie, T. (2020). Differential Effects of 2-Hydroxypropyl-Cyclodextrins on Lipid Accumulation in Npc1-Null Cells. International Journal of Molecular Sciences, 21(3), 898. https://doi.org/10.3390/ijms21030898




