Increased Urinary 3-Mercaptolactate Excretion and Enhanced Passive Systemic Anaphylaxis in Mice Lacking Mercaptopyruvate Sulfurtransferase, a Model of Mercaptolactate-Cysteine Disulfiduria
Abstract
1. Introduction
2. Results
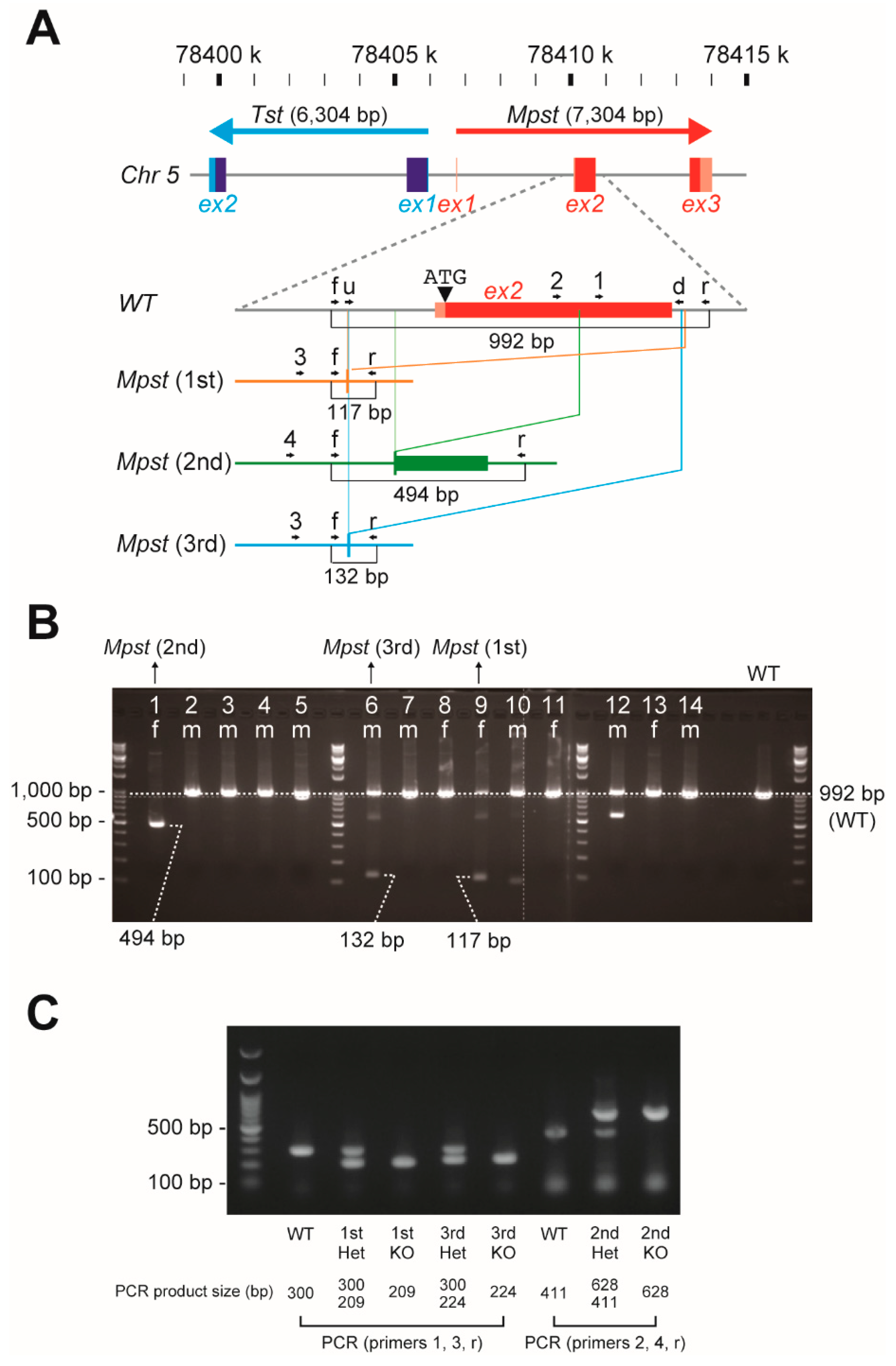
2.1. Establishment of Three Independent Mpst Mutant Lines
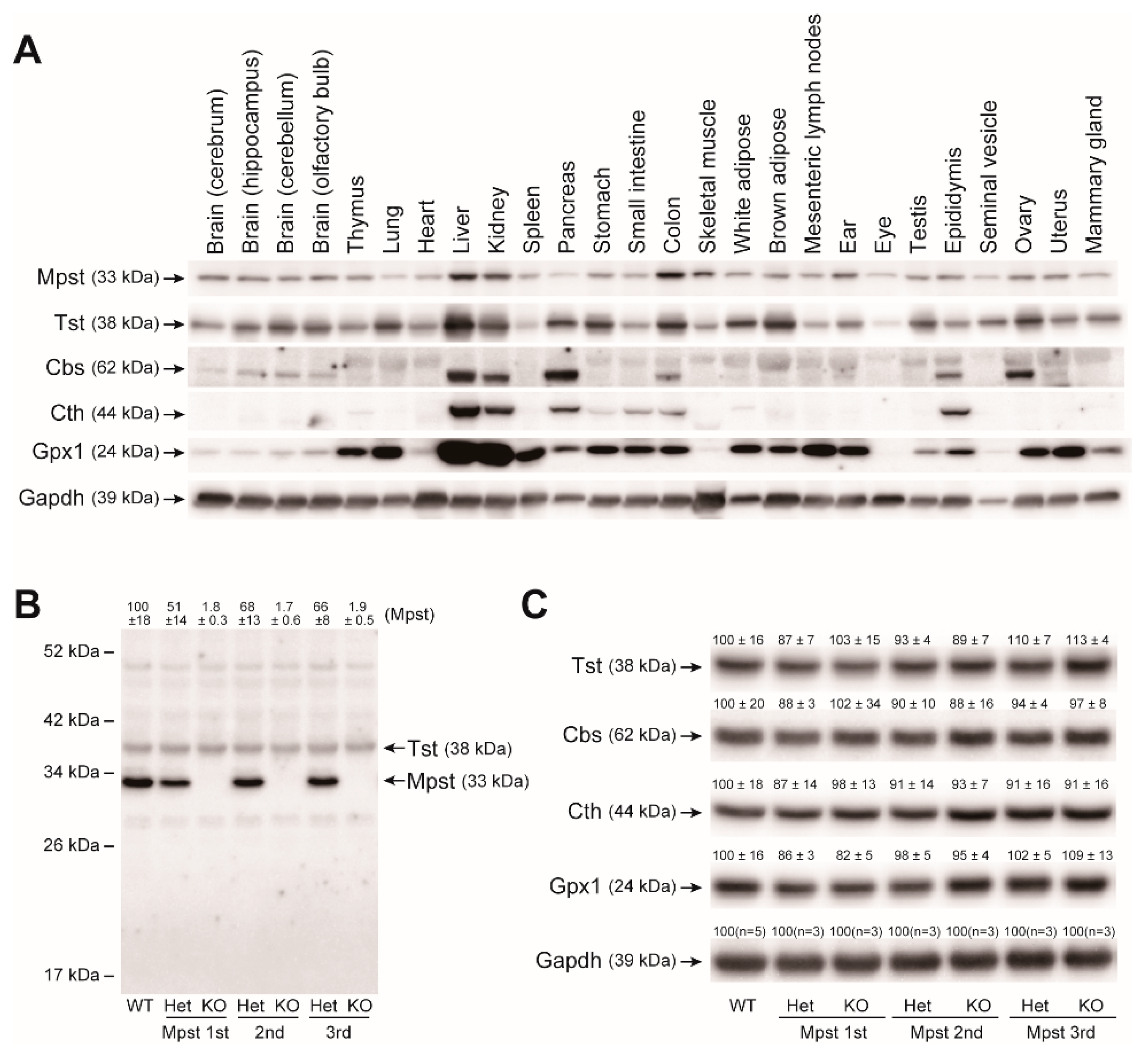
2.2. Mpst, Tst, Cbs, and Cth Expression in Mpst Mutant Livers
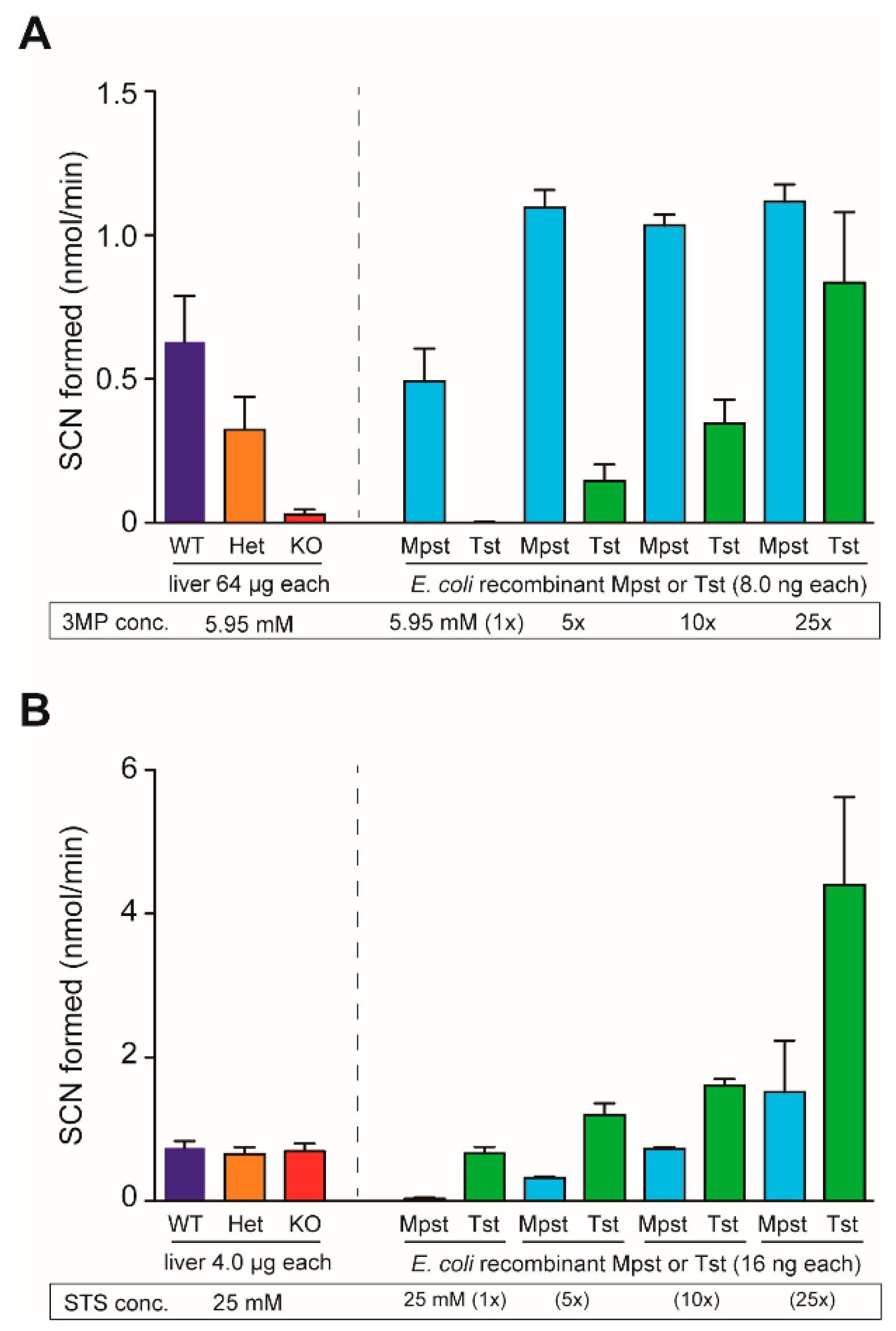
2.3. Increased Urinary Excretion of 3-Mercaptolactate in Mpst-KO Mice
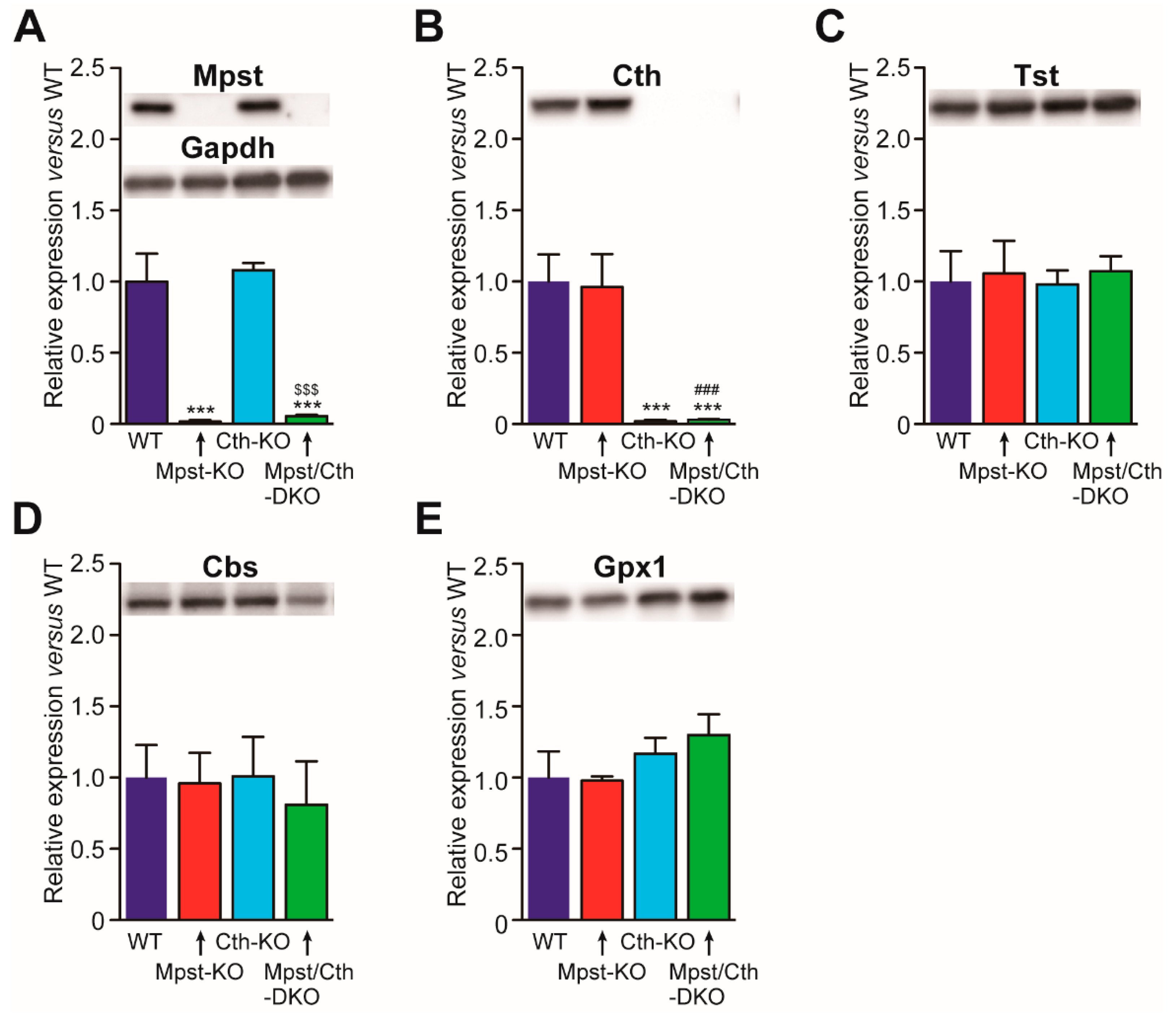
2.4. Generation of Mice Lacking Both Mpst and Cth
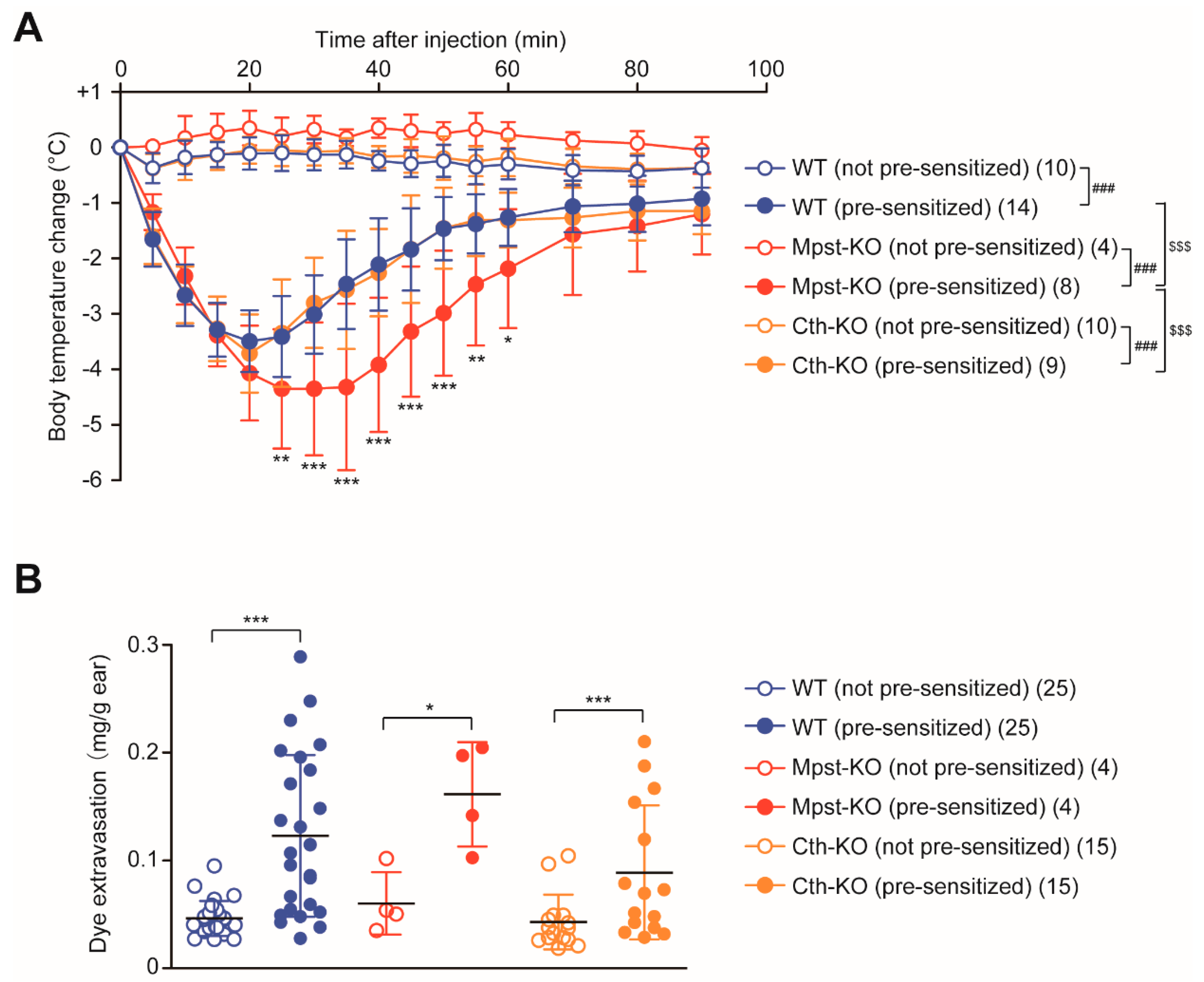
2.5. Enhanced PSA Response in Mpst-KO Mice
3. Discussion
4. Materials and Methods
4.1. Generation of Mpst (1st–3rd)-KO Mice
4.2. Preparation of Mpst and Tst Recombinant Proteins
4.3. Rabbit Polyclonal Mpst Antibody Production
4.4. Western Blot Analyses
4.5. Mpst and Tst Activity Assays
4.6. Measurements of Amino Acid/Thiol Compound Levels and Biochemical Parameters
4.7. PSA and PCA Assays
4.8. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ALT | Alanine aminotransferase |
| AST | Aspartate aminotransferase |
| BUN | Blood urea nitrogen |
| Cars2 | Cysteinyl-tRNA synthetase 2 |
| Cbs | Cystathionine β-synthase |
| CPK | Creatine phosphokinase |
| CRE | Creatinine |
| Cth | Cystathionine γ-lyas |
| DKO | Double knockout |
| DNP | 2,4-dinitrophenyl |
| Gpx1 | Glutathione peroxidase 1 |
| GSH | Glutathione |
| GSSG | Glutathione (oxidized form) |
| Hcy | Homocysteine |
| H2S | Hydrogen sulfide |
| LDH | Lactate dehydrogenase |
| MCDU | Mercaptolactate-cysteine disulfiduria |
| 3-ML | 3-Mercaptolactate |
| 3-MP | 3-Mercaptopyruvate |
| Mpst | Mercaptopyruvate sulfurtransferase |
| PCA | Passive cutaneous anaphylaxis |
| PSA | Passive systemic anaphylaxis |
| RSS | Reactive sulfur species |
| TBARS | Thiobarbituric acid reactive substances |
| Tst | Thiosulfate sulfurtransferase |
| UA | Uric acid |
| WT | Wild-type |
References
- Ampola, M.G.; Efron, M.L.; Bixby, E.M.; Meshorer, E. Mental deficiency and a new aminoaciduria. Am. J. Dis. Child 1969, 117, 66–70. [Google Scholar] [CrossRef] [PubMed]
- Crawhall, J.C.; Parker, R.; Sneddon, W.; Young, E.P.; Ampola, M.G.; Efron, M.L.; Bixby, E.M. Beta mercaptolactate-cysteine disulfide: Analog of cystine in the urine of a mentally retarded patient. Science 1968, 160, 419–420. [Google Scholar] [CrossRef] [PubMed]
- Crawhall, J.C.; Parker, R.; Sneddon, W.; Young, E.P. Beta-mercaptolactate-cysteine disulfide in the urine of a mentally retarded patient. Am. J. Dis. Child 1969, 117, 71–82. [Google Scholar] [PubMed]
- Crawhall, J.C.; Bir, K.; Purkiss, P.; Stanbury, J.B. Sulfur amino acids as precursors of beta-mercaptolactate-cysteine disulfide in human subjects. Biochem. Med. 1971, 5, 109–115. [Google Scholar] [CrossRef]
- Hannestad, U.; Martensson, J.; Sjodahl, R.; Sorbo, B. 3-mercaptolactate cysteine disulfiduria: Biochemical studies on affected and unaffected members of a family. Biochem. Med. 1981, 26, 106–114. [Google Scholar] [CrossRef]
- Niederwiesler, A.; Giliberti, P.; Baerlocher, K. Beta-mercaptolactate cysteine disulfiduria in two normal sisters. Isolation and characterization of beta-mercaptolactate cysteine disulfide. Clin. Chim. Acta 1973, 43, 405–416. [Google Scholar] [CrossRef]
- Nagahara, N.; Nagano, M.; Ito, T.; Shimamura, K.; Akimoto, T.; Suzuki, H. Antioxidant enzyme, 3-mercaptopyruvate sulfurtransferase-knockout mice exhibit increased anxiety-like behaviors: A model for human mercaptolactate-cysteine disulfiduria. Sci. Rep. 2013, 3, 1986. [Google Scholar] [CrossRef]
- Nagahara, N. Catalytic site cysteines of thiol enzyme: Sulfurtransferases. J. Amino Acids 2011, 2011, 709404. [Google Scholar] [CrossRef]
- Nagahara, N.; Okazaki, T.; Nishino, T. Cytosolic mercaptopyruvate sulfurtransferase is evolutionarily related to mitochondrial rhodanese. Striking similarity in active site amino acid sequence and the increase in the mercaptopyruvate sulfurtransferase activity of rhodanese by site-directed mutagenesis. J. Biol. Chem. 1995, 270, 16230–16235. [Google Scholar]
- Nagahara, N.; Tanaka, M.; Tanaka, Y.; Ito, T. Novel Characterization of antioxidant enzyme, 3-mercaptopyruvate sulfurtransferase-knockout mice: Overexpression of the evolutionarily-related enzyme rhodanese. Antioxidants 2019, 8, 116. [Google Scholar] [CrossRef]
- Ubuka, T.; Ohta, J.; Akagi, R.; Hosaki, Y.; Ishimoto, Y.; Kiguchi, S.; Ikeda, T.; Ishino, K. Metabolism ofL-cysteine via transamination pathway (3-mercaptopyruvate pathway). Amino Acids 1992, 3, 243–252. [Google Scholar] [CrossRef] [PubMed]
- Nagahara, N.; Ito, T.; Minami, M. Mercaptopyruvate sulfurtransferase as a defense against cyanide toxication: Molecular properties and mode of detoxification. Histol. Histopathol. 1999, 14, 1277–1286. [Google Scholar] [PubMed]
- Wrobel, M.; Jurkowska, H.; Sliwa, L.; Srebro, Z. Sulfurtransferases and cyanide detoxification in mouse liver, kidney, and brain. Toxicol. Mech. Methods 2004, 14, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Nagahara, N. Multiple role of 3-mercaptopyruvate sulfurtransferase: Antioxidative function, H2S and polysulfide production and possible SOx production. Br. J. Pharmacol. 2018, 175, 577–589. [Google Scholar] [CrossRef]
- Williams, R.A.; Kelly, S.M.; Mottram, J.C.; Coombs, G.H. 3-Mercaptopyruvate sulfurtransferase of Leishmania contains an unusual C-terminal extension and is involved in thioredoxin and antioxidant metabolism. J. Biol. Chem. 2003, 278, 1480–1486. [Google Scholar] [CrossRef]
- Kimura, H. Signaling molecules: Hydrogen sulfide and polysulfide. Antioxid. Redox Signal. 2015, 22, 362–376. [Google Scholar] [CrossRef]
- Shibuya, N.; Mikami, Y.; Kimura, Y.; Nagahara, N.; Kimura, H. Vascular endothelium expresses 3-mercaptopyruvate sulfurtransferase and produces hydrogen sulfide. J. Biochem. 2009, 146, 623–626. [Google Scholar] [CrossRef]
- Shibuya, N.; Koike, S.; Tanaka, M.; Ishigami-Yuasa, M.; Kimura, Y.; Ogasawara, Y.; Fukui, K.; Nagahara, N.; Kimura, H. A novel pathway for the production of hydrogen sulfide from D-cysteine in mammalian cells. Nat. Commun. 2013, 4, 1366. [Google Scholar] [CrossRef]
- Nagahara, N.; Koike, S.; Nirasawa, T.; Kimura, H.; Ogasawara, Y. Alternative pathway of H2S and polysulfides production from sulfurated catalytic-cysteine of reaction intermediates of 3-mercaptopyruvate sulfurtransferase. Biochem. Biophys. Res. Commun. 2018, 496, 648–653. [Google Scholar] [CrossRef]
- Kimura, Y.; Toyofuku, Y.; Koike, S.; Shibuya, N.; Nagahara, N.; Lefer, D.; Ogasawara, Y.; Kimura, H. Identification of H2S3 and H2S produced by 3-mercaptopyruvate sulfurtransferase in the brain. Sci. Rep. 2015, 5, 14774. [Google Scholar] [CrossRef]
- Yadav, P.K.; Yamada, K.; Chiku, T.; Koutmos, M.; Banerjee, R. Structure and kinetic analysis of H2S production by human mercaptopyruvate sulfurtransferase. J. Biol. Chem. 2013, 288, 20002–20013. [Google Scholar] [CrossRef] [PubMed]
- Akaike, T.; Ida, T.; Wei, F.Y.; Nishida, M.; Kumagai, Y.; Alam, M.M.; Ihara, H.; Sawa, T.; Matsunaga, T.; Kasamatsu, S.; et al. Cysteinyl-tRNA synthetase governs cysteine polysulfidation and mitochondrial bioenergetics. Nat. Commun. 2017, 8, 1177. [Google Scholar] [CrossRef] [PubMed]
- Ishii, I.; Akahoshi, N.; Yamada, H.; Nakano, S.; Izumi, T.; Suematsu, M. Cystathionine gamma-lyase-deficient mice require dietary cysteine to protect against acute lethal myopathy and oxidative injury. J. Biol. Chem. 2010, 285, 26358–26368. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, M.; Unoki, T.; Shinkai, Y.; Ishii, I.; Ida, T.; Akaike, T.; Yamamoto, M.; Kumagai, Y. Environmental electrophile-mediated toxicity in mice lacking Nrf2, CSE, or both. Environ. Health Perspect. 2019, 127, 67002. [Google Scholar] [CrossRef] [PubMed]
- Hagiya, Y.; Kamata, S.; Mitsuoka, S.; Okada, N.; Yoshida, S.; Yamamoto, J.; Ohkubo, R.; Abiko, Y.; Yamada, H.; Akahoshi, N.; et al. Hemizygosity of transsulfuration genes confers increased vulnerability against acetaminophen-induced hepatotoxicity in mice. Toxicol. Appl. Pharmacol. 2015, 282, 195–206. [Google Scholar] [CrossRef]
- Yamada, H.; Akahoshi, N.; Kamata, S.; Hagiya, Y.; Hishiki, T.; Nagahata, Y.; Matsuura, T.; Takano, N.; Mori, M.; Ishizaki, Y.; et al. Methionine excess in diet induces acute lethal hepatitis in mice lacking cystathionine gamma-lyase, an animal model of cystathioninuria. Free Radic. Biol. Med. 2012, 52, 1716–1726. [Google Scholar] [CrossRef]
- Nakano, S.; Ishii, I.; Shinmura, K.; Tamaki, K.; Hishiki, T.; Akahoshi, N.; Ida, T.; Nakanishi, T.; Kamata, S.; Kumagai, Y.; et al. Hyperhomocysteinemia abrogates fasting-induced cardioprotection against ischemia/reperfusion by limiting bioavailability of hydrogen sulfide anions. J. Mol. Med. 2015, 93, 879–889. [Google Scholar] [CrossRef]
- Han, S.J.; Noh, M.R.; Jung, J.M.; Ishii, I.; Yoo, J.; Kim, J.I.; Park, K.M. Hydrogen sulfide-producing cystathionine gamma-lyase is critical in the progression of kidney fibrosis. Free Radic. Biol. Med. 2017, 112, 423–432. [Google Scholar] [CrossRef]
- Akahoshi, N.; Handa, H.; Takemoto, R.; Kamata, S.; Yoshida, M.; Onaka, T.; Ishii, I. Preeclampsia-like features and partial lactation failure in mice lacking cystathionine gamma-lyase-an animal model of cystathioninuria. Int. J. Mol. Sci. 2019, 20, 3507. [Google Scholar] [CrossRef]
- Bhatia, M. H2S and Inflammation: An Overview. Handb. Exp. Pharmacol. 2015, 230, 165–180. [Google Scholar]
- Flannigan, K.L.; Wallace, J.L. Hydrogen sulfide-based anti-inflammatory and chemopreventive therapies: An experimental approach. Curr. Pharm. Des. 2015, 21, 3012–3022. [Google Scholar] [CrossRef] [PubMed]
- Simons, F.E.; Ardusso, L.R.; Bilo, M.B.; Cardona, V.; Ebisawa, M.; El-Gamal, Y.M.; Lieberman, P.; Lockey, R.F.; Muraro, A.; Roberts, G.; et al. International consensus on (ICON) anaphylaxis. World Allergy Organ. J. 2014, 7, 9. [Google Scholar] [CrossRef] [PubMed]
- Reber, L.L.; Hernandez, J.D.; Galli, S.J. The pathophysiology of anaphylaxis. J. Allergy Clin. Immunol. 2017, 140, 335–348. [Google Scholar] [CrossRef] [PubMed]
- Ogasawara, Y.; Lacourciere, G.M.; Ishii, K.; Stadtman, T.C. Characterization of potential selenium-binding proteins in the selenophosphate synthetase system. Proc. Natl. Acad. Sci. USA 2005, 102, 1012–1016. [Google Scholar] [CrossRef]
- Balbino, B.; Sibilano, R.; Starkl, P.; Marichal, T.; Gaudenzio, N.; Karasuyama, H.; Bruhns, P.; Tsai, M.; Reber, L.L.; Galli, S.J. Pathways of immediate hypothermia and leukocyte infiltration in an adjuvant-free mouse model of anaphylaxis. J. Allergy Clin. Immunol. 2017, 139, 584–596. [Google Scholar] [CrossRef]
- Makabe-Kobayashi, Y.; Hori, Y.; Adachi, T.; Ishigaki-Suzuki, S.; Kikuchi, Y.; Kagaya, Y.; Shirato, K.; Nagy, A.; Ujike, A.; Takai, T.; et al. The control effect of histamine on body temperature and respiratory function in IgE-dependent systemic anaphylaxis. J. Allergy Clin. Immunol. 2002, 110, 298–303. [Google Scholar] [CrossRef]
- Soriano, R.N.; Braga, S.P.; Breder, J.S.C.; Batalhao, M.E.; Oliveira-Pelegrin, G.R.; Ferreira, L.F.R.; Rocha, M.J.A.; Carnio, E.C.; Branco, L.G.S. Endogenous peripheral hydrogen sulfide is propyretic: Its permissive role in brown adipose tissue thermogenesis in rats. Exp. Physiol. 2018, 103, 397–407. [Google Scholar] [CrossRef]
- Harvey, R.A.; Ferrier, D.A. Lippincott’s Illustrated Reviews: Biochemistry, 5th ed.; Woulters Kluwer–Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2011; pp. 261–276. [Google Scholar]
- Watanabe, M.; Osada, J.; Aratani, Y.; Kluckman, K.; Reddick, R.; Malinow, M.R.; Maeda, N. Mice deficient in cystathionine beta-synthase: Animal models for mild and severe homocyst(e)inemia. Proc. Natl. Acad. Sci. USA 1995, 92, 1585–1589. [Google Scholar] [CrossRef]
- Akahoshi, N.; Kobayashi, C.; Ishizaki, Y.; Izumi, T.; Himi, T.; Suematsu, M.; Ishii, I. Genetic background conversion ameliorates semi-lethality and permits behavioral analyses in cystathionine beta-synthase-deficient mice, an animal model for hyperhomocysteinemia. Hum. Mol. Genet. 2008, 17, 1994–2005. [Google Scholar] [CrossRef]
- Hashimoto, M.; Yamashita, Y.; Takemoto, T. Electroporation of Cas9 protein/sgRNA into early pronuclear zygotes generates non-mosaic mutants in the mouse. Dev. Biol. 2016, 418, 1–9. [Google Scholar] [CrossRef]
- Namekata, K.; Enokido, Y.; Ishii, I.; Nagai, Y.; Harada, T.; Kimura, H. Abnormal lipid metabolism in cystathionine beta-synthase-deficient mice, an animal model for hyperhomocysteinemia. J. Biol. Chem. 2004, 279, 52961–52969. [Google Scholar] [CrossRef] [PubMed]
- Jarabak, R.; Westley, J. 3-Mercaptopyruvate sulfurtransferase: Rapid equilibrium-ordered mechanism with cyanide as the acceptor substrate. Biochemistry 1980, 19, 900–904. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Rao, P.; Bhattacharya, R. Dose and time-dependent effects of cyanide on thiosulfate sulfurtransferase, 3-mercaptopyruvate sulfurtransferase, and cystathionine lambda-lyase activities. J. Biochem. Mol. Toxicol. 2013, 27, 499–507. [Google Scholar] [CrossRef] [PubMed]
- Iciek, M.; Kotanska, M.; Knutelska, J.; Bednarski, M.; Zygmunt, M.; Kowalczyk-Pachel, D.; Bilska-Wilkosz, A.; Gorny, M.; Sokolowska-Jezewicz, M. The effect of NaCl on the level of reduced sulfur compounds in rat liver. Implications for blood pressure increase. Postepy Hig. Med. Dosw. 2017, 71, 564–576. [Google Scholar] [CrossRef]
- Yamamoto, J.; Kamata, S.; Miura, A.; Nagata, T.; Kainuma, R.; Ishii, I. Differential adaptive responses to 1- or 2-day fasting in various mouse tissues revealed by quantitative PCR analysis. FEBS Open Bio 2015, 5, 357–368. [Google Scholar] [CrossRef]
- Isokawa, M.; Funatsu, T.; Tsunoda, M. Fast and simultaneous analysis of biothiols by high-performance liquid chromatography with fluorescence detection under hydrophilic interaction chromatography conditions. Analyst 2013, 138, 3802–3808. [Google Scholar] [CrossRef]
- Isokawa, M.; Shimosawa, T.; Funatsu, T.; Tsunoda, M. Determination and characterization of total thiols in mouse serum samples using hydrophilic interaction liquid chromatography with fluorescence detection and mass spectrometry. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2016, 1019, 59–65. [Google Scholar] [CrossRef]
- Taketomi, Y.; Ueno, N.; Kojima, T.; Sato, H.; Murase, R.; Yamamoto, K.; Tanaka, S.; Sakanaka, M.; Nakamura, M.; Nishito, Y.; et al. Mast cell maturation is driven via a group III phospholipase A2-prostaglandin D2-DP1 receptor paracrine axis. Nat. Immunol. 2013, 14, 554–563. [Google Scholar] [CrossRef]
| Parental Genotypes | Genotypes of Offsprings (≥3-week-old) | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Male | × | Female | Total Litter No. | Average Litter Size | Mpst-WT | Mpst-Het | Mpst-KO | Sex Ratio Male:Female | ||||||||||
| Mpst | Cth | Mpst | Cth | Cth-WT | Cth-Het | Cth-KO | Cth-WT | Cth-Het | Cth-KO | Cth-WT | Cth-Het | Cth-KO | ||||||
| Mpst single mutant | ||||||||||||||||||
| Het (1st) | WT | 27 | 5.1 ± 2.6 | 59 | 78 | 52:85 | ||||||||||||
| Het (1st) | Het (1st) | 81 | 5.7 ± 2.2 | 114 | 234 | 112 | 244:216 | |||||||||||
| KO (1st) | KO (1st) | 5 | 5.2 ± 1.8 | 26 | 16:10 | |||||||||||||
| Het (2nd) | WT | 14 | 5.5 ± 2.0 | 37 | 40 | 41:36 | ||||||||||||
| Het (2nd) | Het (2nd) | 17 | 5.0 ± 2.3 | 28 | 36 | 21 | 35:50 | |||||||||||
| KO (2nd) | KO (2nd) | 10 | 4.0 ± 2.0 | 40 | 21:19 | |||||||||||||
| Het (3rd) | WT | 9 | 3.6 ± 2.4 | 16 | 16 | 16:16 | ||||||||||||
| Het (3rd) | Het (3rd) | 30 | 5.1 ± 2.0 | 30 | 73 | 49 | 83:69 | |||||||||||
| KO (3rd) | KO (3rd) | 15 | 5.4 ± 2.4 | 81 | 39:42 | |||||||||||||
| Mpst (1st)/Cth double mutant | ||||||||||||||||||
| Het | KO | Het | KO | 11 | 4.6 ± 1.6 | 12 | 25 | 14 | 27:24 | |||||||||
| KO | Het | KO | Het | 29 | 5.8 ± 2.4 | 50 | 87 | 32 | 88:81 | |||||||||
| KO | KO | KO | KO | 8 | 3.6 ± 1.9 | 29 | 18:11 | |||||||||||
| Mpst | Mpst (1st)/Cth | |||||
|---|---|---|---|---|---|---|
| Mice | WT (a) | 1st KO (b) | 2nd KO | 3rd KO | Cth-KO (c) | -DKO (d) |
| Serum (µM) | (n = 10) | (n = 7) | (n = 7) | (n = 7) | (n = 5) | (n = 10) |
| Ala | 479 ± 128 | 377 ± 56 | 439 ± 79 | 430 ± 88 | 574 ± 239 | 407 ± 88 |
| Arg | 120 ± 19 c | 104 ± 23 c | 120 ± 14 | 113 ± 24 | 168 ± 37 abd | 146 ± 26 c |
| Asn/Asp | 6.28 ± 2.66 c | 9.33 ± 6.68 c | 17.4 ± 9.4 | 6.01 ± 4.41 | 28.1 ± 17.8 abd | 14.3 ± 6.4 c |
| Gln | 807 ± 135 | 752 ± 133 | 762 ± 74 | 801 ± 35 | 935 ± 172 d | 654 ± 147 c |
| Glu | 27.4 ± 21.4 c | 26.1 ± 17.9 c | 36.7 ± 15.7 | 19.9 ± 8.1 | 54.5 ± 17.9 ab | 39.2 ± 10.7 |
| Gly | 328 ± 75 | 291 ± 38 | 352 ± 28 | 306 ± 64 | 368 ± 109 | 345 ± 49 |
| His | 42.0 ± 24.5 cd | 44.2 ± 23.3 cd | 85.1 ± 19.1 | 39.3 ± 20 | 131 ± 58 ab | 90.4 ± 10.4 ab |
| Ile | 109 ± 18 | 106 ± 18 | 113 ± 21 | 105 ± 14 | 123 ± 20 | 106 ± 20 |
| Leu | 159 ± 21 | 155 ± 22 | 170 ± 26 | 169 ± 15 | 196 ± 11 d | 156 ± 32 c |
| Lys | 217 ± 37 | 226 ± 42 | 211 ± 48 | 255 ± 33 | 298 ± 24 | 184 ± 44 |
| Met | 75.3 ± 19.4 | 69.9 ± 16.7 c | 86.9 ± 8.0 | 67.6 ± 11.0 | 104 ± 25 b | 96.6 ± 22 |
| Phe | 95.6 ± 26.9 | 79.5 ± 13.9 | 86.0 ± 7.0 | 89.8 ± 6.2 | 106 ± 23 | 83.3 ± 13.4 |
| Pro | 131 ± 68 | 91.2± 26.7 | 120 ± 30 | 94.5 ± 28.4 | 177 ± 89 | 135 ± 36 |
| Ser | 145 ± 58 | 120 ± 18 | 143 ± 18 | 125 ± 29 | 175 ± 62 | 141 ± 30 |
| Thr | 175 ± 42 | 151 ± 35 | 180 ± 21 | 163 ± 45 | 199 ± 35 | 194 ± 43 |
| Trp | 71.2 ± 17.1 | 73.9 ± 11.1 | 61.5 ± 16.5 | 62.9 ± 12.0 | 69.9 ± 9.6 | 82.9 ± 22.3 |
| Tyr | 94.8 ± 32.0 | 87.6 ± 25.0 | 110 ± 18 | 101 ± 26 | 99.2 ± 58.9 | 80.3 ± 30.4 |
| Val | 242 ± 58 | 225 ± 40 c | 253 ± 29 | 226 ± 27 | 304 ± 43 b | 251 ± 41 |
| Cystathionine | 44.1 ± 19.7 cd | 39.5 ± 12.8 cd | 56.2 ± 12.6 | 37.6 ± 8.1 | 124 ± 15 ab | 133 ± 32 ab |
| Citrulline | 79.2 ± 19.3 cd | 63.0 ± 15.2 cd | 68.6 ± 14.1 | 67.2 ± 12.7 | 169 ± 24 ab | 135 ± 34 ab |
| Ornithine | 42.1 ± 10.9 c | 35.1 ± 10.2 c | 35.7 ± 6.6 | 43.0 ± 16.2 | 66.4 ± 19.1 ab | 49.5 ± 13.8 |
| Taurine | 675 ± 349 | 491 ± 136 | 642 ± 181 | 514 ± 88 | 601 ± 240 | 664 ± 235 |
| Total Cys | 319 ± 40 d | 322 ± 52 d | 341 ± 78 | 315 ± 32 | 274 ± 11 | 261 ± 51 ab |
| Total Hcy | 5.78 ± 1.22 cd | 5.90 ± 2.05 cd | 5.58 ± 0.77 | 6.23 ± 1.11 | 83.6 ± 18.6 abd | 121 ± 15 abc |
| Total GSH | 252 ± 71 cd | 230 ± 141 d | 221 ± 42 | 224 ± 37 | 124 ± 59 a | 107 ± 42 ab |
| Total Cys-Gly | 3.23 ± 0.39 b | 4.43 ± 0.59 ac | 4.79 ± 0.55 | 3.93 ± 0.39 | 3.15 ± 0.93 b | 3.87 ± 0.86 |
| Total γ Glu-Cys | 10.4 ± 2.8 | 13.2 ± 4.0 cd | 12.1 ± 2.4 | 8.37 ± 1.94 | 8.17 ± 0.45 b | 8.32 ± 2.16 b |
| Urine (mmol/mol creatinine) | ||||||
| (n = 10) | (n = 9) | (n = 7) | (n = 7) | (n = 6) | (n = 9) | |
| 3-ML | 10.4 ± 2.0 bd | 61.3 ± 9.9 acd | 57.4 ± 29.4 | 75.9 ± 20.7 | 16.9 ± 3.2 bd | 91.2 ± 21.0 abc |
| Total Cys | 156 ± 28 cd | 187 ± 24 cd | 222 ± 50 | 211 ± 84 | 624 ± 272 ab | 601 ± 115 ab |
| Total Hcy | 7.32 ± 2.66 cd | 6.24 ± 2.81 cd | 6.15 ± 1.11 | 5.44 ± 1.24 | 169 ± 51 ab | 195 ± 69 ab |
| Total GSH | 2.00 ± 1.28 | 1.40 ± 0.81 | 2.31 ± 1.09 | 1.74 ± 0.44 | 2.67 ± 1.84 | 2.15 ± 1.09 |
| Total Cys-Gly | 26.6 ± 10.0 cd | 25.4 ± 7.1 cd | 41.3 ± 9.6 | 32.4 ± 12.0 | 85.8 ± 37.7 ab | 82.4 ± 33.9 ab |
| Total γ Glu-Cys | 10.2 ± 4.9 cd | 9.45 ± 3.70 cd | 21.9 ± 7.0 | 14.3 ± 3.5 | 24.3 ± 13.0 ab | 26.8 ± 7.7 ab |
| Liver | ||||||
| Total GSH (nmol/mg protein; n = 5 each) | ||||||
| 10.6 ± 1.5 cd | 10.9 ± 1.7 cd | N.T. | N.T. | 6.51 ± 0.75 ab | 6.25 ± 1.25 ab | |
| GSSG/tGSH (%; n = 5 each) | ||||||
| 4.04 ± 0.48 cd | 3.82 ± 0.48 cd | N.T. | N.T. | 5.42 ± 0.62 ab | 5.69 ± 0.89 ab | |
| Mpst | Mpst (1st)/Cth | |||||
|---|---|---|---|---|---|---|
| Mice | WT (a) | 1st KO (b) | 2nd KO | 3rd KO | Cth-KO (c) | -DKO (d) |
| Serum | (8) | (7) | (7) | (7) | (5) | (10) |
| Albumin (g/dL) | 2.10 ± 0.12 | 2.21 ± 0.18 d | 2.14 ± 0.10 | 2.19 ± 0.20 | 2.00 ± 0.19 | 1.97 ± 0.16 b |
| ALT (IU/L) | 17.5 ± 10.8 | 12.1 ± 2.2 d | 16.3 ± 5.0 | 16.0 ± 5.3 | 22.0 ± 8.1 | 29.2 ± 12.6 b |
| AST (IU/L) | 45.3 ± 9.7 | 39.6 ± 12.3 d | 42.6 ± 16.6 | 42.1 ± 14.0 | 51.0 ± 20.4 | 75.2 ± 40.4 b |
| BUN (mg/dL) | 25.2 ± 3.3 | 25.4 ± 3.4 | 25.8 ± 1.9 | 26.8 ± 5.3 | 26.5 ± 2.5 | 26.5 ± 3.1 |
| CPK (IU/L) | 394 ± 110 | 306 ± 96 d | 448 ± 222 | 271 ± 62 | 351 ± 84 | 470 ± 154 b |
| CRE (mg/dL) | < 0.2 | < 0.2 | < 0.2 | < 0.2 | < 0.2 | < 0.2 |
| LDH (IU/L) | 173 ± 48 d | 157 ± 16 d | 282 ± 93 | 184 ± 28 | 225 ± 54 | 308 ± 80 ab |
| T-bilirubin (mg/dL) | 0.29 ± 0.11 | 0.30 ± 0.12 | 0.37 ± 0.11 | 0.27 ± 0.08 | 0.36 ± 0.11 | 0.35 ± 0.07 |
| T-protein (g/dL) | 4.59 ± 0.22 d | 4.71 ± 0.44 d | 4.39 ± 0.23 | 4.63 ± 0.49 | 4.26 ± 0.27 | 4.13 ± 0.23 ab |
| UA (mg/dL) | 2.81 ± 0.79 cd | 1.97 ± 1.12 | 1.74 ± 0.60 | 2.17 ± 0.79 | 1.60 ± 0.39 a | 1.63 ± 0.40 a |
| (6) | (5) | (6) | (8) | |||
| TBARS (nmol/mL) | 1.68 ± 0.22 | 1.68 ± 0.23 | N.T. | N.T. | 1.87 ± 0.41 | 1.50 ± 0.17 |
| Liver | ||||||
| TBARS | (6) | (5) | (6) | (8) | ||
| (nmol/µg protein) | 522 ± 29 d | 546 ± 77 | N.T. | N.T. | 659 ± 78 | 702 ± 169 a |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akahoshi, N.; Minakawa, T.; Miyashita, M.; Sugiyama, U.; Saito, C.; Takemoto, R.; Honda, A.; Kamichatani, W.; Kamata, S.; Anan, Y.; et al. Increased Urinary 3-Mercaptolactate Excretion and Enhanced Passive Systemic Anaphylaxis in Mice Lacking Mercaptopyruvate Sulfurtransferase, a Model of Mercaptolactate-Cysteine Disulfiduria. Int. J. Mol. Sci. 2020, 21, 818. https://doi.org/10.3390/ijms21030818
Akahoshi N, Minakawa T, Miyashita M, Sugiyama U, Saito C, Takemoto R, Honda A, Kamichatani W, Kamata S, Anan Y, et al. Increased Urinary 3-Mercaptolactate Excretion and Enhanced Passive Systemic Anaphylaxis in Mice Lacking Mercaptopyruvate Sulfurtransferase, a Model of Mercaptolactate-Cysteine Disulfiduria. International Journal of Molecular Sciences. 2020; 21(3):818. https://doi.org/10.3390/ijms21030818
Chicago/Turabian StyleAkahoshi, Noriyuki, Tatsuro Minakawa, Masashi Miyashita, Uran Sugiyama, Chihiro Saito, Rintaro Takemoto, Akihiro Honda, Waka Kamichatani, Shotaro Kamata, Yasumi Anan, and et al. 2020. "Increased Urinary 3-Mercaptolactate Excretion and Enhanced Passive Systemic Anaphylaxis in Mice Lacking Mercaptopyruvate Sulfurtransferase, a Model of Mercaptolactate-Cysteine Disulfiduria" International Journal of Molecular Sciences 21, no. 3: 818. https://doi.org/10.3390/ijms21030818
APA StyleAkahoshi, N., Minakawa, T., Miyashita, M., Sugiyama, U., Saito, C., Takemoto, R., Honda, A., Kamichatani, W., Kamata, S., Anan, Y., & Ishii, I. (2020). Increased Urinary 3-Mercaptolactate Excretion and Enhanced Passive Systemic Anaphylaxis in Mice Lacking Mercaptopyruvate Sulfurtransferase, a Model of Mercaptolactate-Cysteine Disulfiduria. International Journal of Molecular Sciences, 21(3), 818. https://doi.org/10.3390/ijms21030818






