High Pretransplant BAFF Levels and B-cell Subset Polarized towards a Memory Phenotype as Predictive Biomarkers for Antibody-Mediated Rejection
Abstract
1. Introduction
2. Results
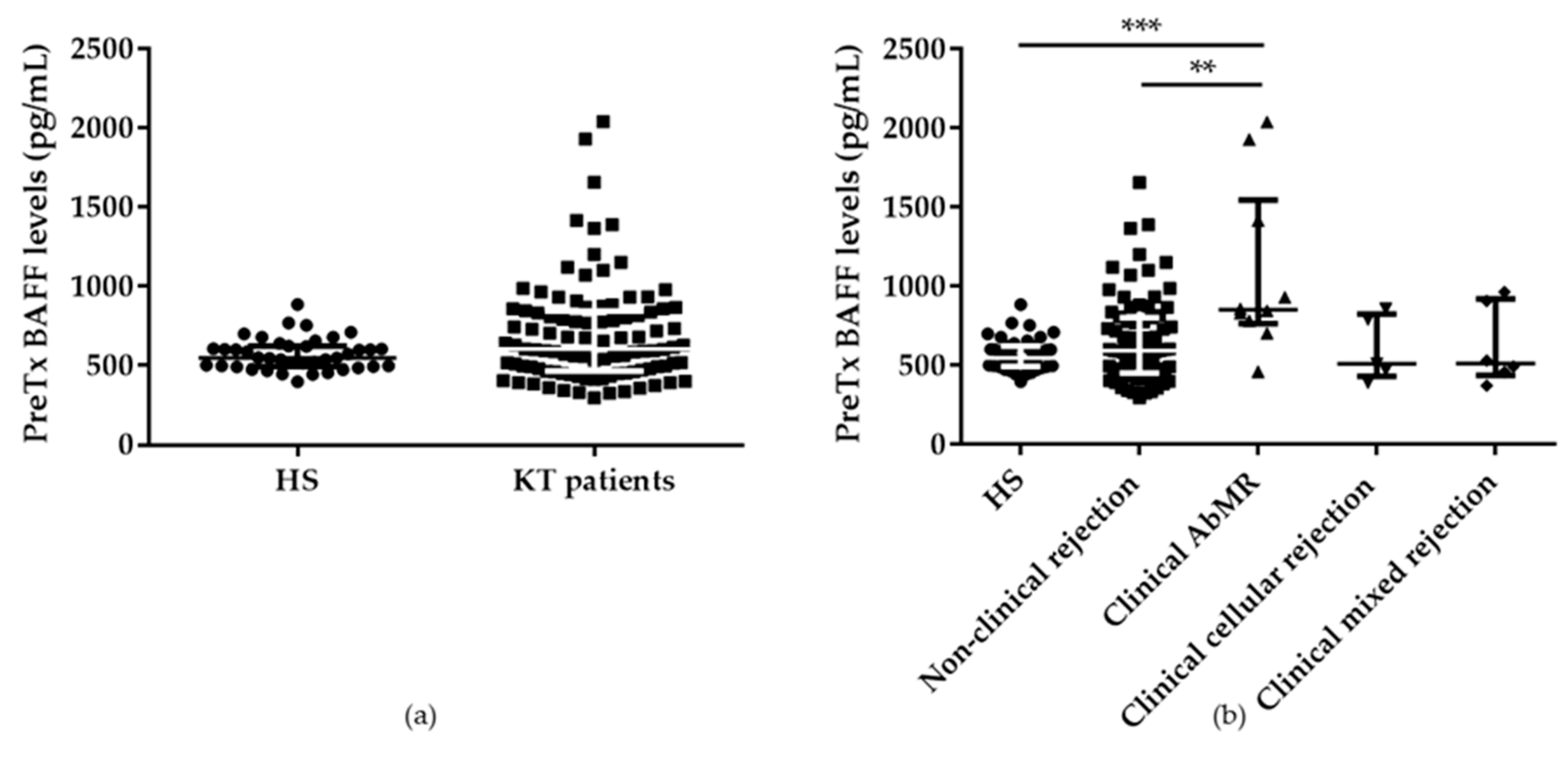
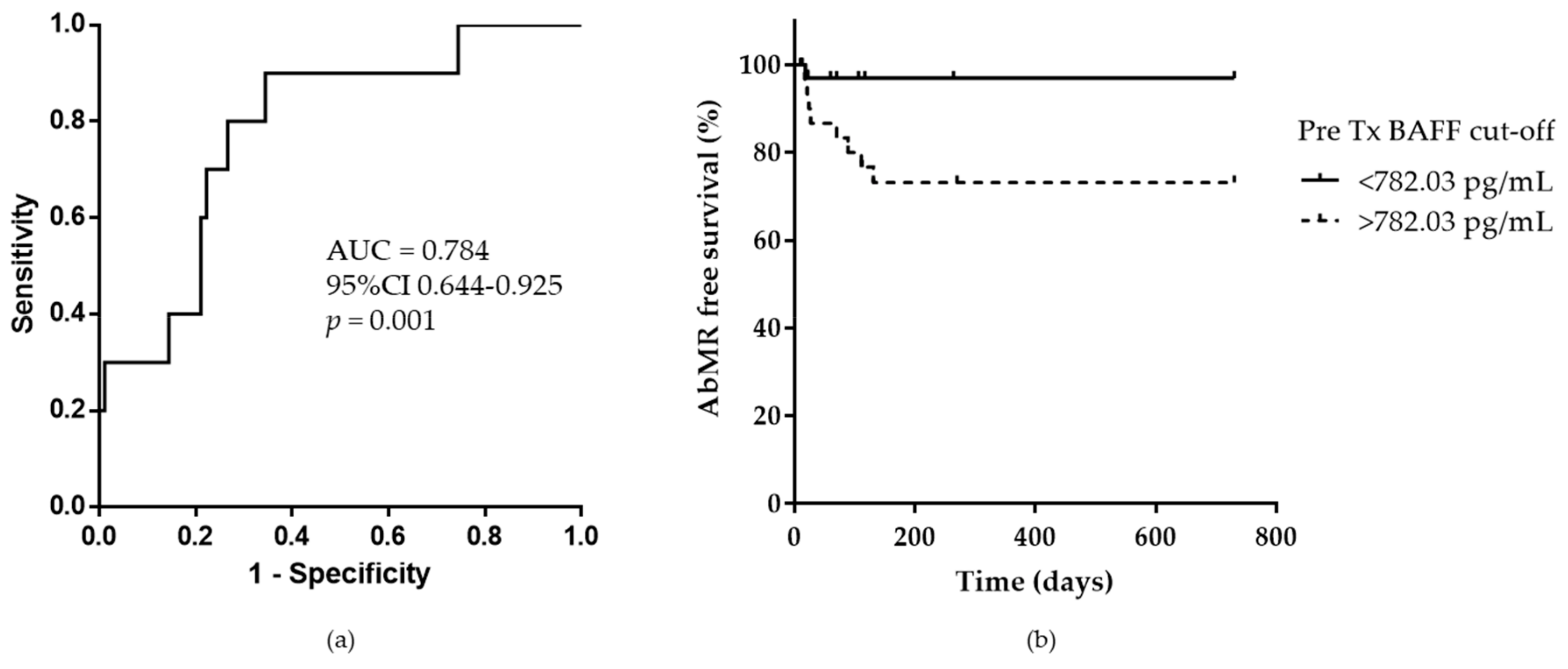
2.1. Pretransplant Serum BAFF Levels in Kidney Transplant Patients
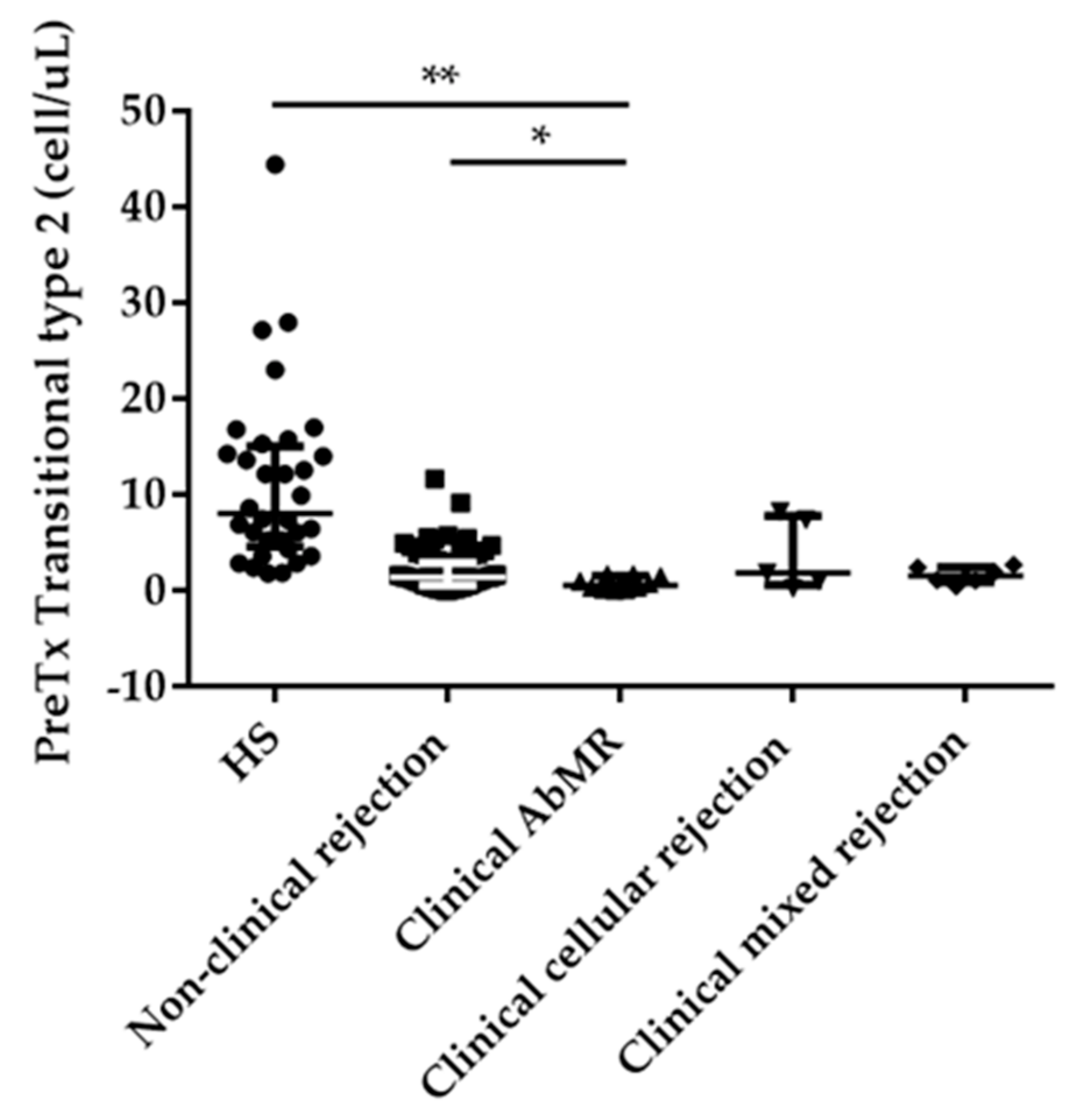
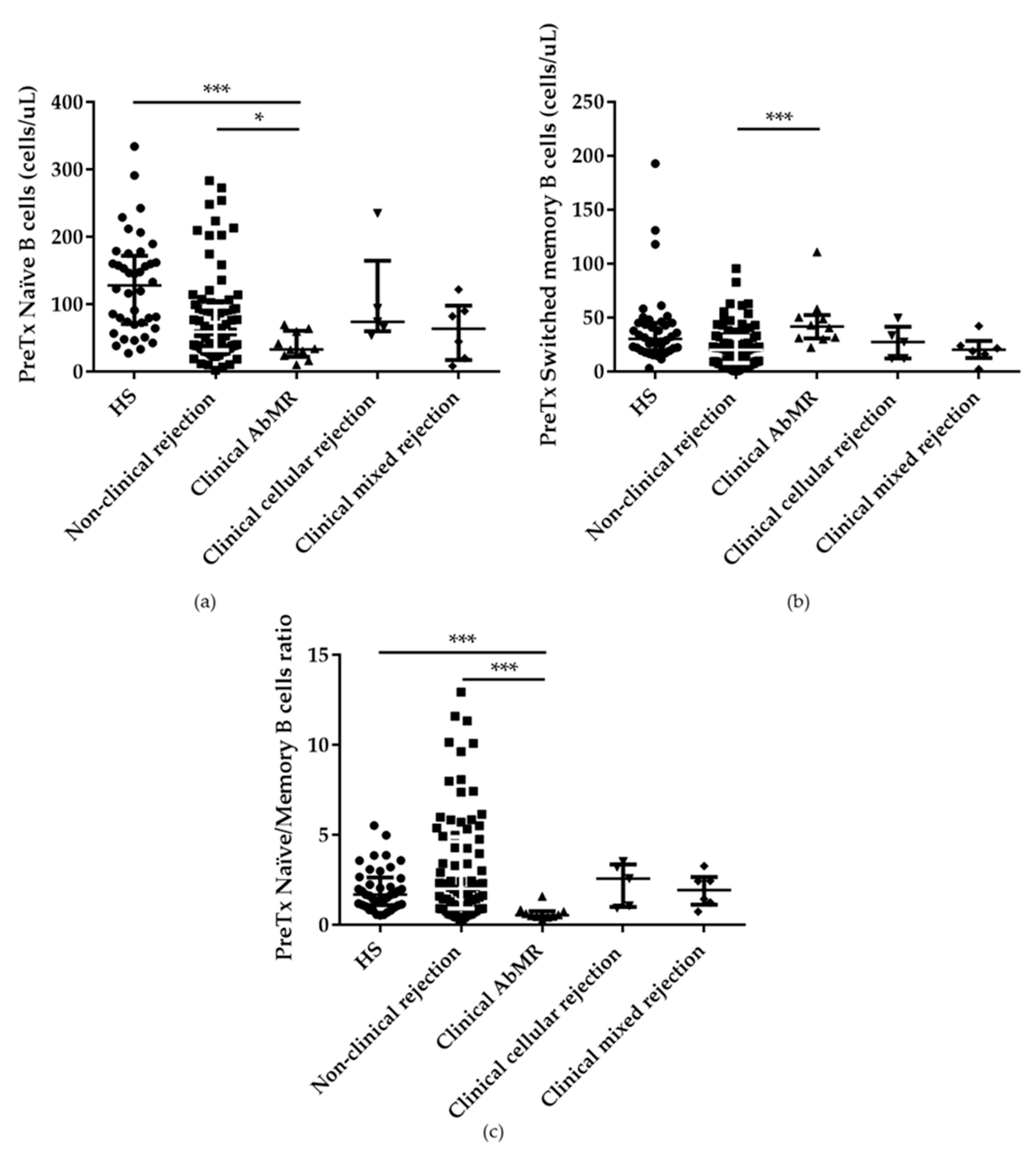
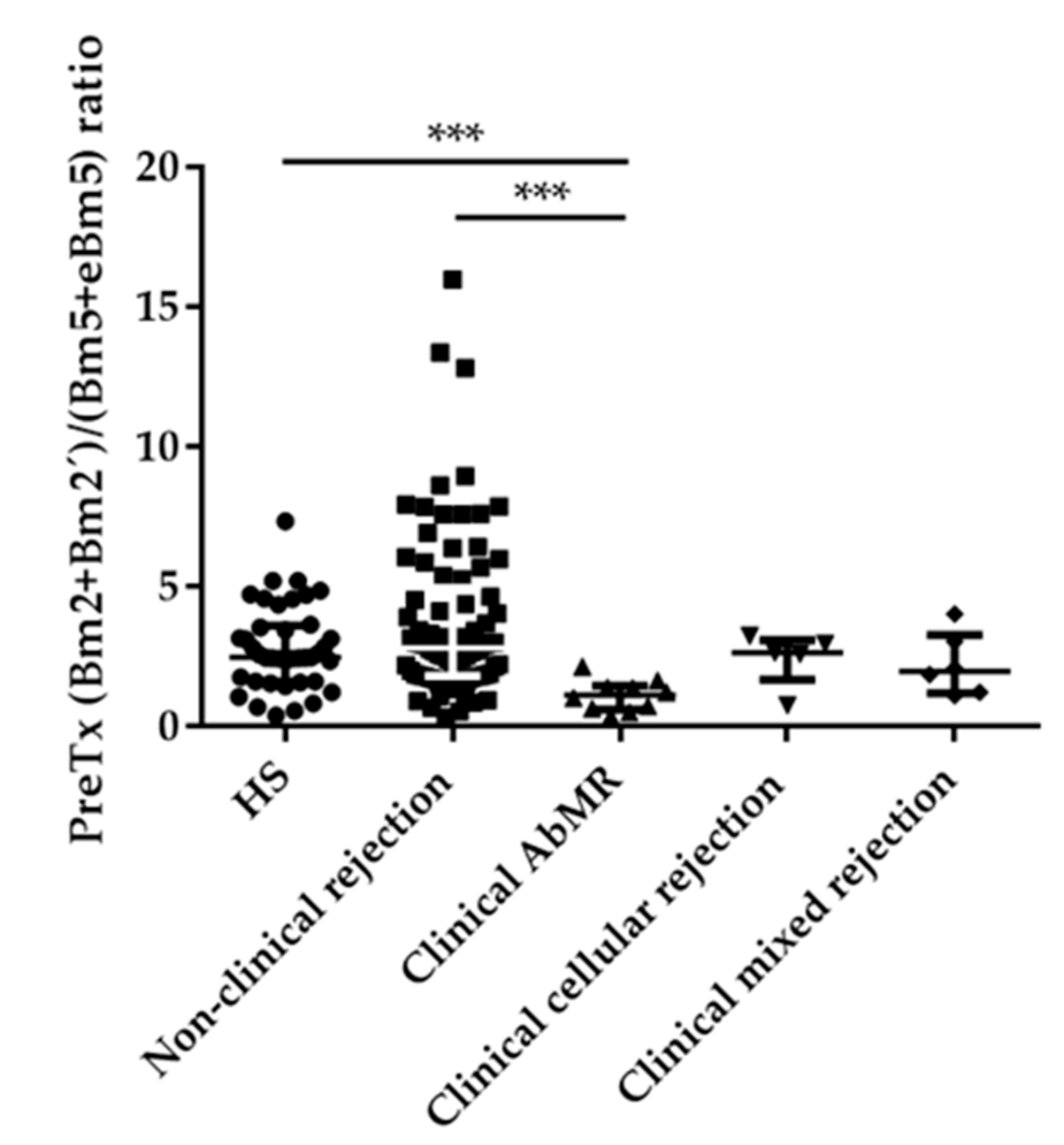
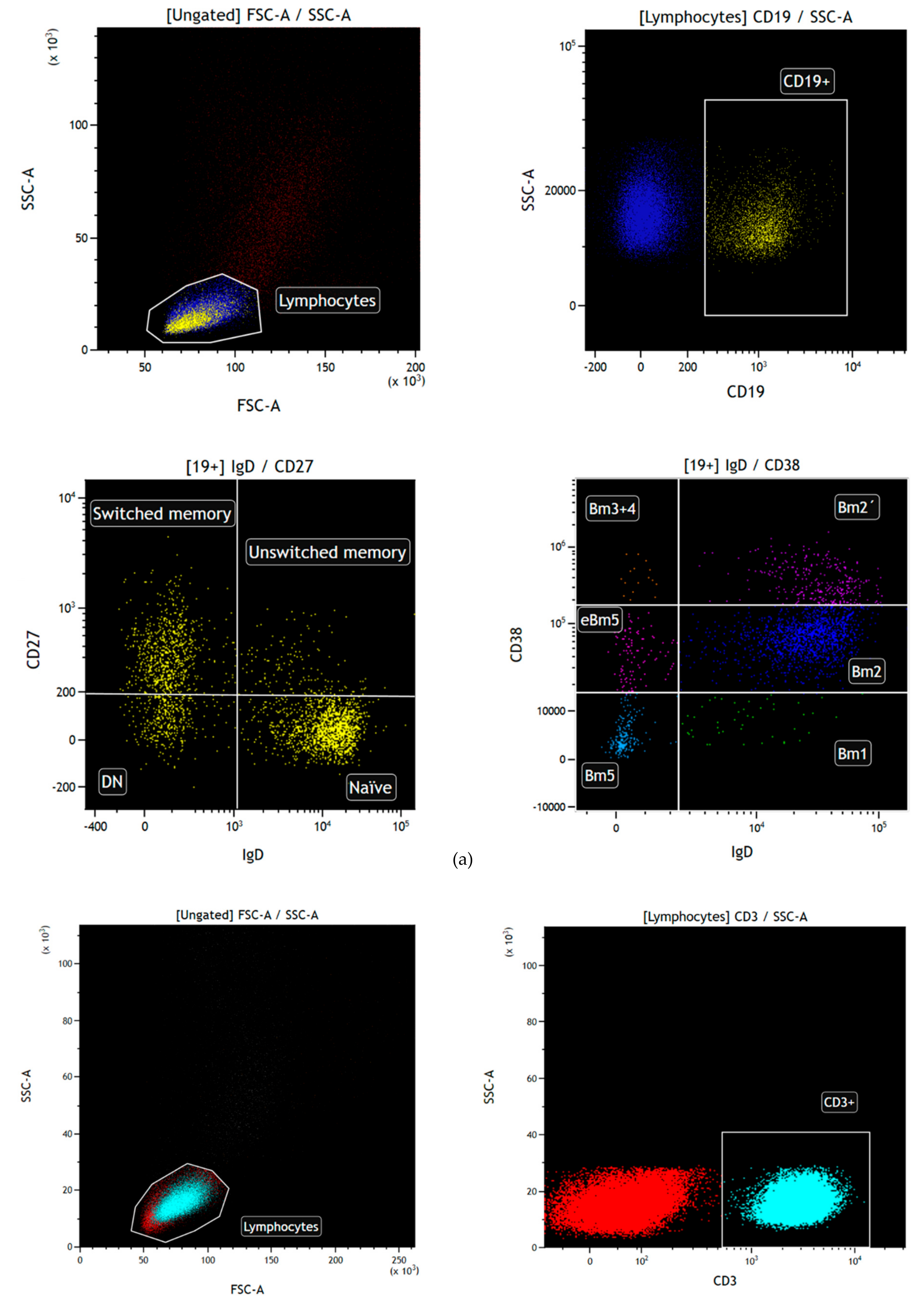
2.2. Pretransplant B Cell Subpopulations Distribution in AbMR Patients
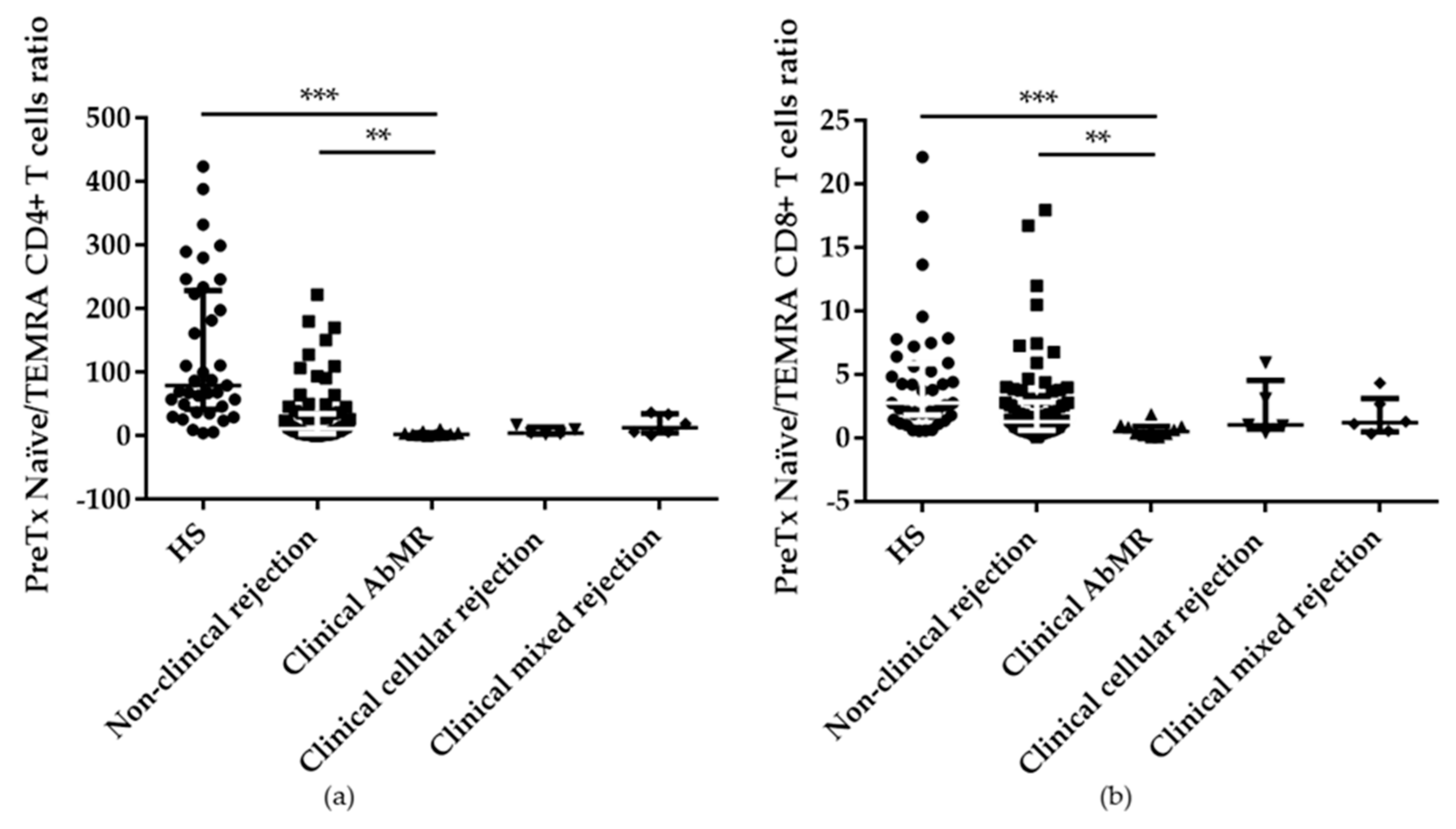
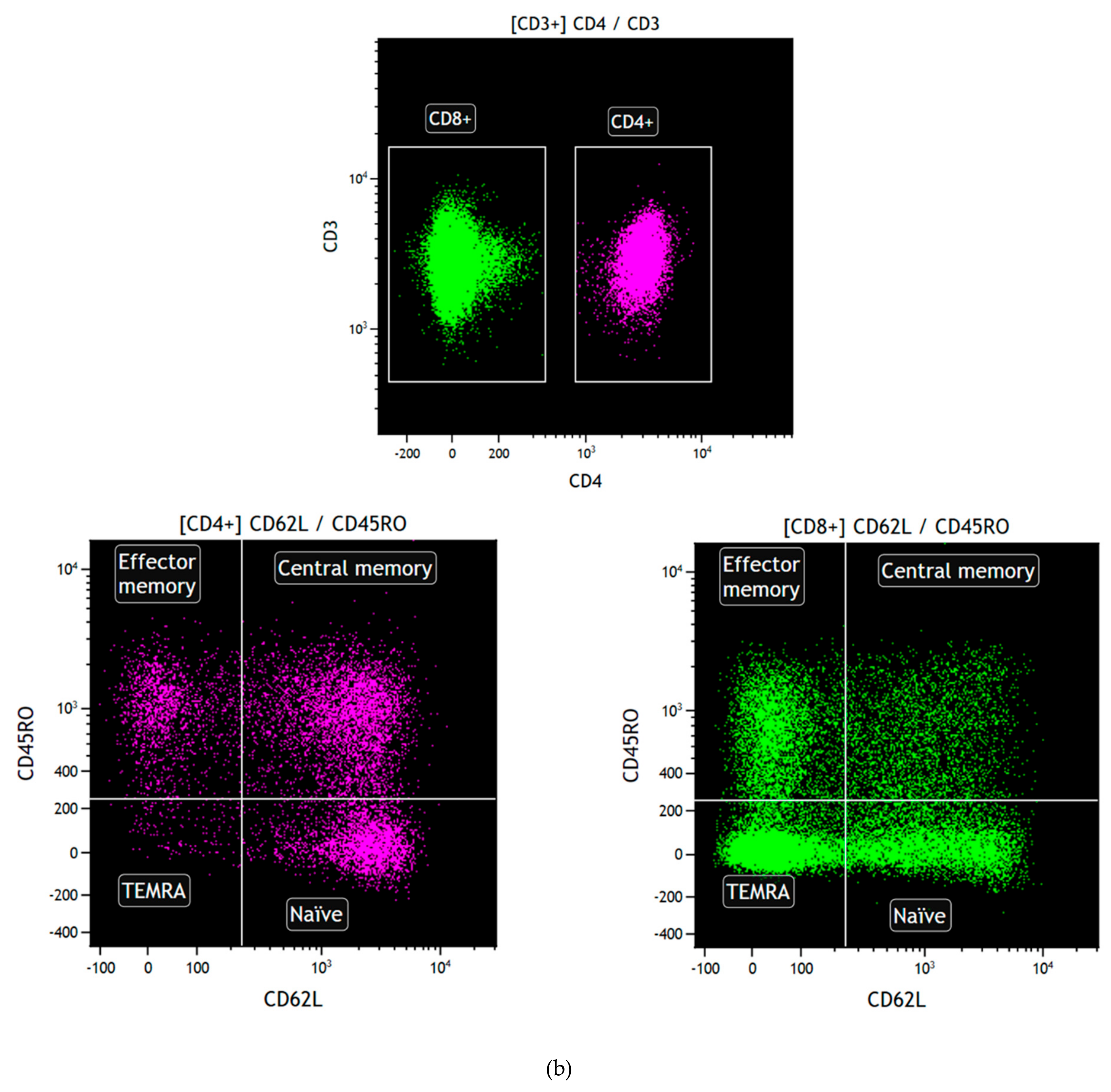
2.3. Pretransplant T cell Subpopulations Distribution in AbMR Patients
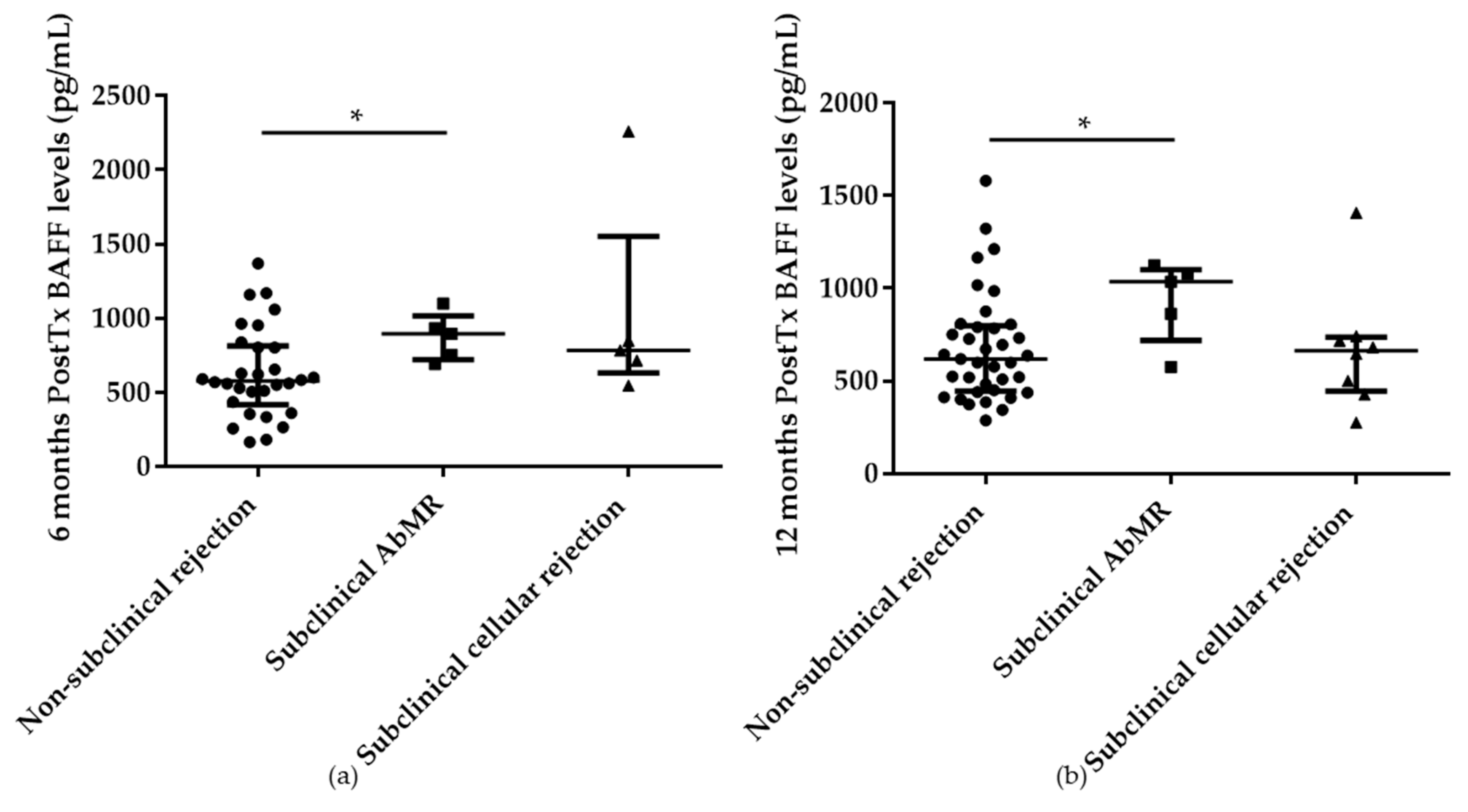
2.4. Serum BAFF Levels at 6 and 12 Months Posttransplantation in Subclinical AbMR Patients
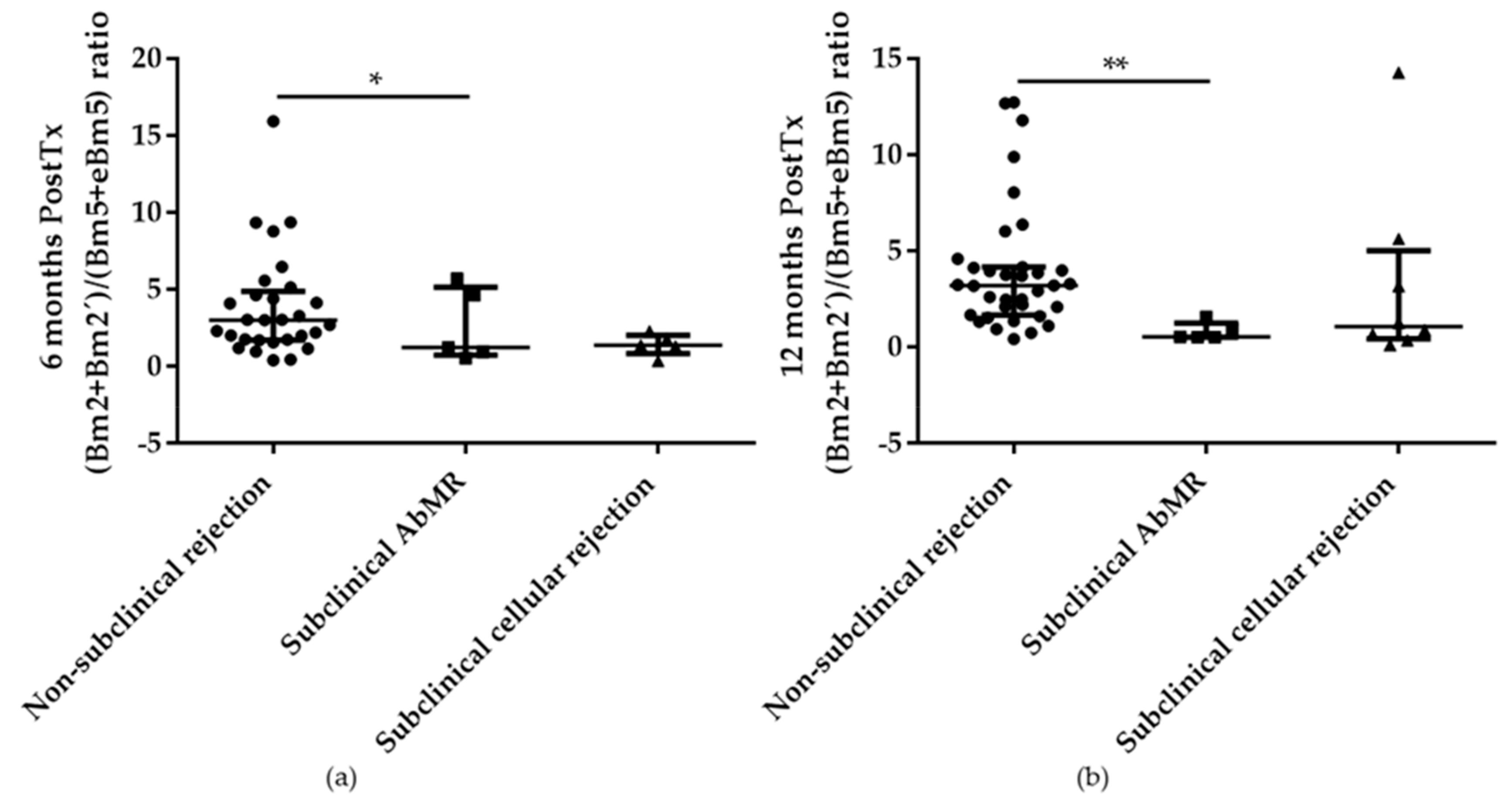
2.5. B and T cell Subpopulations Distribution at 6 and 12 Months after Kidney Transplantation in Subclinical AbMR Patients
3. Discussion
4. Materials and Methods
4.1. Patients
4.2. ELISA serum BAFF levels
4.3. Anti-HLA Antibodies and DSA
4.4. Flow Cytometry for B Cell Subsets
4.5. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AbMR | Antibody-mediated rejection |
| DSA | Donor-specific antibodies |
| BAFF | B-cell activating factor |
| BAFFR | BAFF receptor |
| KT | Kidney transplantation |
| PRA | Panel-reactive antibodies |
| IQR | Interquartile range |
| SD | Standard deviation |
| HS | Healthy subjects |
| cPRA | Calculated-panel reactive antibodies |
| ROC | Receiver operational curve |
| AUC | Area under curve |
| CI | Confidence interval |
| Tx | Transplantation |
| CIT | Cold ischemia time |
| DGF | Delayed graft function |
| HR | Hazard ratio |
| ESRD | End stage renal disease |
| PKD | Polycystic kidney disease |
| PreTx | Pre-transplantation |
| PostTx | Post-transplantation |
| SSOP | Sequence-specific oligonucleotide probes |
| SSP | Sequence-specific primers |
| FITC | Fluorescein isothiocyanate |
| PE | Phycoerythrin |
| APC | Allophycocyanin |
| Cy5.5 | Cyanine 5.5 |
| Cy7 | Cyanine 7 |
References
- Clatworthy, M.R. Targeting B Cells and Antibody in Transplantation. Am. J. Transplant. 2011, 11, 1359–1367. [Google Scholar] [CrossRef]
- Redfield, R.R.; Rodriguez, E.; Parsons, R.; Vivek, K.; Mustafa, M.M.; Noorchashm, H.; Naji, A. Essential Role for B Cells in Transplantation Tolerance. Curr. Opin. Immunol. 2011, 23, 685–691. [Google Scholar] [CrossRef]
- Kirk, A.D.; Turgeon, N.A.; Iwakoshi, N.N. B Cells and Transplantation Tolerance. Nat. Rev. Nephrol. 2010, 6, 584–593. [Google Scholar] [CrossRef]
- Lund, F.E.; Randall, T.D. Effector and Regulatory B Cells: Modulators of CD4+ T Cell Immunity. Nat. Rev. Immunol. 2010, 10, 236–247. [Google Scholar] [CrossRef] [PubMed]
- Gaston, R.S.; Cecka, J.M.; Kasiske, B.L.; Fieberg, A.M.; Leduc, R.; Cosio, F.C.; Gourishankar, S.; Grande, J.; Halloran, P.; Hunsicker, L.; et al. Evidence for Antibody-Mediated Injury as a Major Determinant of Late Kidney Allograft Failure. Transplantation 2010, 90, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, W.M.; Valujskikh, A.; Fairchild, R.L. Mechanisms of Antibody-Mediated Acute and Chronic Rejection of Kidney Allografts. Curr. Opin. Organ Transplant. 2016, 21, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Sellarés, J.; de Freitas, D.G.; Mengel, M.; Reeve, J.; Einecke, G.; Sis, B.; Hidalgo, L.G.; Famulski, K.; Matas, A.; Halloran, P.F. Understanding the Causes of Kidney Transplant Failure: The Dominant Role of Antibody-Mediated Rejection and Nonadherence. Am. J. Transplant 2012, 12, 388–399. [Google Scholar] [CrossRef]
- Roufosse, C.; Simmonds, N.; Clahsen-van Groningen, M.; Haas, M.; Henriksen, K.J.; Horsfield, C.; Loupy, A.; Mengel, M.; Perkowska-Ptasińska, A.; Rabant, M.; et al. A 2018 Reference Guide to the Banff Classification of Renal Allograft Pathology. Transplantation 2018, 102, 1795–1814. [Google Scholar] [CrossRef]
- Mackay, F.; Schneider, P.; Rennert, P.; Browning, J. BAFF AND APRIL: A Tutorial on B Cell Survival. Annu. Rev. Immunol. 2003, 21, 231–264. [Google Scholar] [CrossRef]
- Hengeveld, P.J.; Kersten, M.J. B-Cell Activating Factor in the Pathophysiology of Multiple Myeloma: A Target for Therapy? Blood Cancer J. 2015, 5, e282. [Google Scholar] [CrossRef]
- Vigolo, M.; Chambers, M.G.; Willen, L.; Chevalley, D.; Maskos, K.; Lammens, A.; Tardivel, A.; Das, D.; Kowalczyk-Quintas, C.; Schuepbach-Mallepell, S.; et al. A Loop Region of BAFF Controls B Cell Survival and Regulates Recognition by Different Inhibitors. Nat. Commun. 2018, 9, 1199. [Google Scholar] [CrossRef] [PubMed]
- Rauch, M.; Tussiwand, R.; Bosco, N.; Rolink, A.G. Crucial Role for BAFF-BAFF-R Signaling in the Survival and Maintenance of Mature B Cells. PLoS ONE 2009, 4, e5456. [Google Scholar] [CrossRef] [PubMed]
- Schweighoffer, E.; Tybulewicz, V.L. Signalling for B Cell Survival. Curr. Opin. Cell Biol. 2018, 51, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Hoek, K.L.; Carlesso, G.; Clark, E.S.; Khan, W.N. Absence of Mature Peripheral B Cell Populations in Mice with Concomitant Defects in B Cell Receptor and BAFF-R Signaling. J. Immunol. 2009, 183, 5630–5643. [Google Scholar] [CrossRef] [PubMed]
- Thaunat, O.; Patey, N.; Gautreau, C.; Lechaton, S.; Fremeaux-Bacchi, V.; Dieu-Nosjean, M.-C.; Cassuto-Viguier, E.; Legendre, C.; Delahousse, M.; Lang, P.; et al. B Cell Survival in Intragraft Tertiary Lymphoid Organs after Rituximab Therapy. Transplantation 2008, 85, 1648–1653. [Google Scholar] [CrossRef] [PubMed]
- Tangye, S.G.; Bryant, V.L.; Cuss, A.K.; Good, K.L. BAFF, APRIL and Human B Cell Disorders. Semin. Immunol. 2006, 18, 305–317. [Google Scholar] [CrossRef] [PubMed]
- Mackay, F.; Schneider, P. Cracking the BAFF Code. Nat. Publ. Group 2009, 9, 491–502. [Google Scholar] [CrossRef]
- Vincent, F.B.; Morand, E.F.; Schneider, P.; Mackay, F. The BAFF/APRIL System in SLE Pathogenesis. Nat. Rev. Rheumatol. 2014, 10, 365–373. [Google Scholar] [CrossRef]
- Thompson, N.; Isenberg, D.A.; Jury, E.C.; Ciurtin, C. Exploring BAFF: Its Expression, Receptors and Contribution to the Immunopathogenesis of Sjögren’s Syndrome. Rheumatology 2016, 55, 1548–1555. [Google Scholar] [CrossRef]
- Wei, F.; Chang, Y.; Wei, W. The Role of BAFF in the Progression of Rheumatoid Arthritis. Cytokine 2015, 76, 537–544. [Google Scholar] [CrossRef]
- Matsushita, T.; Hasegawa, M.; Yanaba, K.; Kodera, M.; Takehara, K.; Sato, S. Elevated Serum BAFF Levels in Patients with Systemic Sclerosis: Enhanced BAFF Signaling in Systemic Sclerosis B Lymphocytes. Arthritis Rheum. 2006, 54, 192–201. [Google Scholar] [CrossRef] [PubMed]
- Kannel, K.; Alnek, K.; Vahter, L.; Gross-Paju, K.; Uibo, R.; Kisand, K.V. Changes in Blood B Cell-Activating Factor (BAFF) Levels in Multiple Sclerosis: A Sign of Treatment Outcome. PLoS ONE 2015, 10, e0143393. [Google Scholar] [CrossRef] [PubMed]
- Schuster, A.; Jung, B.; Hofbauer, J.; Kühne, L.; Zecher, D.; Banas, B.; Bergler, T. B-Cell Activating Factor BAFF Reflects Patients’ Immunological Risk Profile after Kidney Transplantation. Transpl. Immunol. 2017, 45, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Friebus-Kardash, J.; Wilde, B.; Keles, D.; Heinold, A.; Kribben, A.; Witzke, O.; Heinemann, F.M.; Eisenberger, U. Pretransplant Serum BAFF Levels Are Associated with Pretransplant HLA Immunization and Renal Allograft Survival. Transpl. Immunol. 2018, 47, 10–17. [Google Scholar] [CrossRef]
- Min, J.W.; Kim, K.W.; Kim, B.-M.; Doh, K.C.; Choi, M.S.; Choi, B.S.; Park, C.W.; Yang, C.W.; Kim, Y.-S.; Oh, E.-J.; et al. Clinical Significance of Pre- and Post-Transplant BAFF Levels in Kidney Transplant Recipients. PLoS ONE 2016, 11, e0162964. [Google Scholar] [CrossRef]
- Xu, H.; He, X.; Liu, Q.; Shi, D.; Chen, Y.; Zhu, Y.; Zhang, X. Abnormal High Expression of B-Cell Activating Factor Belonging to the TNF Superfamily (BAFF) Associated with Long-Term Outcome In. TPS 2009, 41, 1552–1556. [Google Scholar] [CrossRef]
- Sango, C.; Merino, D.; San Segundo, D.; Rodrigo, E.; Lopez-Hoyos, M.; Benito, A.; Ángeles Ramos, M.; Gómez-Román, J.; Arias, M. B-Cell-Activating Factor Levels Are Associated with Antibody-Mediated Histological Damage in Kidney Transplantation. Transplant. Proc. 2016, 48, 2910–2912. [Google Scholar] [CrossRef]
- Banham, G.; Prezzi, D.; Harford, S.; Taylor, C.J.; Hamer, R.; Higgins, R.; Bradley, J.A.; Clatworthy, M.R. Elevated Pretransplantation Soluble BAFF Is Associated with an Increased Risk of Acute Antibody-Mediated Rejection. Transplantation 2013, 96, 413–420. [Google Scholar] [CrossRef]
- Wang, X.-Z.; Wan, Z.; Xue, W.-J.; Zheng, J.; Li, Y.; Ding, C.G. B-Cell Activating Factor Predicts Acute Rejection Risk in Kidney Transplant Recipients: A 6-Month Follow-Up Study. Front. Immunol. 2019, 10, 1046. [Google Scholar] [CrossRef]
- Pongpirul, W.; Chancharoenthana, W.; Pongpirul, K.; Leelahavanichkul, A.; Kittikowit, W.; Jutivorakool, K.; Nonthasoot, B.; Avihingsanon, Y.; Eiam-Ong, S.; Praditpornsilpa, K.; et al. B-Cell Activating Factor, a Predictor of Antibody Mediated Rejection in Kidney Transplantation Recipients. Nephrology 2018, 23, 169–174. [Google Scholar] [CrossRef]
- Kościelska-Kasprzak, K.; Bartoszek, D.; Myszka, M.; Kamińska, D.; Boratyńska, M.; Krajewska, M.; Klinger, M. B Cell Activating Factor (BAFF) in Long-Term Kidney Transplant Recipients Is Not a Prognostic Marker for Allograft Dysfunction or Survival. Transplant. Proc. 2018, 50, 1750–1754. [Google Scholar] [CrossRef] [PubMed]
- Slavcev, A.; Brozova, J.; Slatinska, J.; Sekerkova, Z.; Honsova, E.; Skibova, J.; Striz, I.; Viklicky, O. Soluble BAFF Cytokine Levels and Antibody-Mediated Rejection of the Kidney Allograft. Arch. Immunol. Ther. Exp. 2016, 64 (Suppl. 1), 47–53. [Google Scholar] [CrossRef] [PubMed]
- Lehnhardt, A.M.; Strecker, M.; Eiermann, T.; Marget, M.; Thaiss, F.; Nashan, B.; Koch, M. High B-Cell Activating Factor Is Not Associated with Worse 3-Year Graft Outcome in Blood Group-Incompatible Kidney Transplantation with Rituximab Induction. Clin. Transplant. 2015, 29, 359–364. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wang, S.; Su, X.; Fu, Q.; Li, J.; Wang, J.; Deng, R.; Wu, C.; Huang, Q.; Liu, L.; et al. The Role of Soluble B Cell-Activating Factor in Further Stratifying the Risk of Antibody-Mediated Rejection Post-Renal Transplant: A Meta-Analysis. Int. Immunopharmacol. 2020, 79, 106059. [Google Scholar] [CrossRef]
- MacLeod, M.K.L.; Kappler, J.W.; Marrack, P. Memory CD4 T Cells: Generation, Reactivation and Re-Assignment. Immunology 2010, 130, 10–15. [Google Scholar] [CrossRef]
- Bohnhorst, J.Ø.; Bjørgan, M.B.; Thoen, J.E.; Natvig, J.B.; Thompson, K.M. Bm1–Bm5 Classification of Peripheral Blood B Cells Reveals Circulating Germinal Center Founder Cells in Healthy Individuals and Disturbance in the B Cell Subpopulations in Patients with Primary Sjögren’s Syndrome. J. Immunol. 2001, 167, 3610–3618. [Google Scholar] [CrossRef]
- Thibault-Espitia, A.; Foucher, Y.; Danger, R.; Migone, T.; Pallier, A.; Castagnet, S.; G-Gueguen, C.; Devys, A.; C-Gautier, A.; Giral, M.; et al. BAFF and BAFF-R Levels Are Associated with Risk of Long-Term Kidney Graft Dysfunction and Development of Donor-Specific Antibodies. Am. J. Transpl. 2012, 12, 2754–2762. [Google Scholar] [CrossRef]
- Xu, H.; He, X.; Xu, R. B Cell Activating Factor, Renal Allograft Antibody-Mediated Rejection, and Long-Term Outcome. J. Immunol. Res. 2018, 2018, 5251801. [Google Scholar] [CrossRef]
- Li, W.W.; Peng, X.; Liu, Y.; Liu, H.; Liu, F.; He, L.; Liu, Y.; Zhang, F.; Guo, C.; Chen, G.; et al. TLR9 and BAFF: Their Expression in Patients with IgA Nephropathy. Mol. Med. Rep. 2014, 10, 1469–1474. [Google Scholar] [CrossRef]
- McCarthy, E.M.; Lee, R.Z.; Ní Gabhann, J.; Smith, S.; Cunnane, G.; Doran, M.F.; Howard, D.; O’Connell, P.; Kearns, G.; Jefferies, C.A. Elevated B Lymphocyte Stimulator Levels Are Associated with Increased Damage in an Irish Systemic Lupus Erythematosus Cohort. Rheumatology 2013, 52, 1279–1284. [Google Scholar] [CrossRef]
- Jonsson, M.V.; Szodoray, P.; Jellestad, S.; Jonsson, R.; Skarstein, K. Association between Circulating Levels of the Novel TNF Family Members APRIL and BAFF and Lymphoid Organization in Primary Sjögren’s Syndrome. J. Clin. Immunol. 2005, 25, 189–201. [Google Scholar] [CrossRef] [PubMed]
- Salazar-Camarena, D.C.; Ortiz-Lazareno, P.C.; Cruz, A.; Oregon-Romero, E.; Machado-Contreras, J.R.; Muñoz-Valle, J.F.; Orozco-López, M.; Marín-Rosales, M.; Palafox-Sánchez, C.A. Association of BAFF, APRIL Serum Levels, BAFF-R, TACI and BCMA Expression on Peripheral B-Cell Subsets with Clinical Manifestations in Systemic Lupus Erythematosus. Lupus 2016, 25, 582–592. [Google Scholar] [CrossRef] [PubMed]
- Bosello, S.; Youinou, P.; Daridon, C.; Tolusso, B.; Bendaoud, B.; Pietrapertosa, D.; Morelli, A.; Ferraccioli, G.F. Concentrations of BAFF Correlate with Autoantibody Levels, Clinical Disease Activity, and Response to Treatment in Early Rheumatoid Arthritis. J. Rheumatol. 2008, 35, 1256–1264. [Google Scholar] [PubMed]
- Mariette, X.; Roux, S.; Zhang, J.; Bengoufa, D.; Lavie, F.; Zhou, T.; Kimberly, R. The Level of BLyS (BAFF) Correlates with the Titre of Autoantibodies in Human Sjögren’s Syndrome. Ann. Rheum. Dis. 2003, 62, 168–171. [Google Scholar] [CrossRef]
- Newell, K.A.; Asare, A.; Kirk, A.D.; Gisler, T.D.; Bourcier, K.; Suthanthiran, M.; Burlingham, W.J.; Marks, W.H.; Sanz, I.; Lechler, R.I.; et al. Identification of a B Cell Signature Associated with Renal Transplant Tolerance in Humans. J. Clin. Investig. 2010, 120, 1836–1847. [Google Scholar] [CrossRef]
- Sagoo, P.; Perucha, E.; Sawitzki, B.; Tomiuk, S.; Stephens, D.A.; Miqueu, P.; Chapman, S.; Craciun, L.; Sergeant, R.; Brouard, S.; et al. Development of a Cross-Platform Biomarker Signature to Detect Renal Transplant Tolerance in Humans. J. Clin. Investig. 2010, 120, 1848–1861. [Google Scholar] [CrossRef]
- Heidt, S.; Vergunst, M.; Anholts, J.D.H.; Reinders, M.E.J.; De Fijter, J.W.; Eikmans, M.; Claas, F.H.J. B Cell Markers of Operational Tolerance Can Discriminate Acute Kidney Allograft Rejection from Stable Graft Function. Transplantation 2015, 99, 1058–1064. [Google Scholar] [CrossRef]
- Nouël, A.; Ségalen, I.; Jamin, C.; Doucet, L.; Caillard, S.; Renaudineau, Y.; Pers, J.-O.; Le Meur, Y.; Hillion, S. B Cells Display an Abnormal Distribution and an Impaired Suppressive Function in Patients with Chronic Antibody-Mediated Rejection. Kidney Int. 2014, 85, 590–599. [Google Scholar] [CrossRef]
- Blair, P.A.; Noreña, L.Y.; Flores-Borja, F.; Rawlings, D.J.; Isenberg, D.A.; Ehrenstein, M.R.; Mauri, C. CD19+CD24hiCD38hi B Cells Exhibit Regulatory Capacity in Healthy Individuals but Are Functionally Impaired in Systemic Lupus Erythematosus Patients. Immunity 2010, 32, 129–140. [Google Scholar] [CrossRef]
- Haas, M.; Loupy, A.; Lefaucheur, C.; Roufosse, C.; Glotz, D.; Seron, D.; Nankivell, B.J.; Halloran, P.F.; Colvin, R.B.; Akalin, E.; et al. The Banff 2017 Kidney Meeting Report: Revised Diagnostic Criteria for Chronic Active T Cell-Mediated Rejection, Antibody-Mediated Rejection, and Prospects for Integrative Endpoints for next-Generation Clinical Trials. Am. J. Transpl. 2018, 18, 293–307. [Google Scholar] [CrossRef]
- San Segundo, D.; Millán, O.; Muñoz-Cacho, P.; Boix, F.; Paz-Artal, E.; Talayero, P.; Morales, J.M.; Muro, M.; De Cos, M.Á.; Guirado, L.; et al. High Proportion of Pretransplantation Activated Regulatory T Cells (CD4+CD25highCD62L+CD45RO+) Predicts Acute Rejection in Kidney Transplantation: Results of a Multicenter Study. Transplantation 2014, 98, 1213–1218. [Google Scholar] [CrossRef] [PubMed]
- San Segundo, D.; López-Hoyos, M.; Arias, M. Regulatory B-Cells in Transplantation. Antibodies 2013, 2, 587–597. [Google Scholar] [CrossRef]
| ESRD (n = 109) | ||||
|---|---|---|---|---|
| n | Mean / Median | SD / IQR | % | |
| Recipient age (years) | 109 | 55 | 43–62 | |
| Recipient gender (male %) | 57.8 | |||
| Etiology of ESRD1: | ||||
| Glomerular | 37 | 33.9 | ||
| Diabetes mellitus | 28 | 25.7 | ||
| PKD | 16 | 14.7 | ||
| Interstitial | 9 | 8.3 | ||
| Vascular | 8 | 7.3 | ||
| Non-filiated | 7 | 6.4 | ||
| Other causes | 4 | 3.7 | ||
| Retransplantation | 23 | 21.1 | ||
| Hypersensitized (>90%) | 7 | 6.4 | ||
| PreTx Anti-HLA Ab | 33 | 30.3 | ||
| PreTx DSA | 3 | 2.8 | ||
| Donor age (years) | 52 | 44–62 | ||
| DGF | 26 | 23.9 | ||
| CIT (hours) | 17 | 9–21 | ||
| Induction therapy: | 78 | 71.6 | ||
| Thymoglobulin | 53 | 67.9 | ||
| Basiliximab | 25 | 32.1 | ||
| Biopsy C4d+ | 13 | 59.1 | ||
| Biopsy g+ptc ≥ 2 | 18 | 81.8 | ||
| Clinical rejection | 22 | 20.2 | ||
| Clinical AbMR | 11 | 10.1 | ||
| HLA-A Mismatches | 1.21 | 0.63 | ||
| HLA-B Mismatches | 1.50 | 0.62 | ||
| HLA-C Mismatches | 1.37 | 0.62 | ||
| HLA-DRB1 Mismatches | 1.32 | 0.67 | ||
| HLA-DQB1 Mismatches | 1.04 | 0.67 | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Irure-Ventura, J.; San Segundo, D.; Rodrigo, E.; Merino, D.; Belmar-Vega, L.; Ruiz San Millán, J.C.; Valero, R.; Benito, A.; López-Hoyos, M. High Pretransplant BAFF Levels and B-cell Subset Polarized towards a Memory Phenotype as Predictive Biomarkers for Antibody-Mediated Rejection. Int. J. Mol. Sci. 2020, 21, 779. https://doi.org/10.3390/ijms21030779
Irure-Ventura J, San Segundo D, Rodrigo E, Merino D, Belmar-Vega L, Ruiz San Millán JC, Valero R, Benito A, López-Hoyos M. High Pretransplant BAFF Levels and B-cell Subset Polarized towards a Memory Phenotype as Predictive Biomarkers for Antibody-Mediated Rejection. International Journal of Molecular Sciences. 2020; 21(3):779. https://doi.org/10.3390/ijms21030779
Chicago/Turabian StyleIrure-Ventura, Juan, David San Segundo, Emilio Rodrigo, David Merino, Lara Belmar-Vega, Juan Carlos Ruiz San Millán, Rosalía Valero, Adalberto Benito, and Marcos López-Hoyos. 2020. "High Pretransplant BAFF Levels and B-cell Subset Polarized towards a Memory Phenotype as Predictive Biomarkers for Antibody-Mediated Rejection" International Journal of Molecular Sciences 21, no. 3: 779. https://doi.org/10.3390/ijms21030779
APA StyleIrure-Ventura, J., San Segundo, D., Rodrigo, E., Merino, D., Belmar-Vega, L., Ruiz San Millán, J. C., Valero, R., Benito, A., & López-Hoyos, M. (2020). High Pretransplant BAFF Levels and B-cell Subset Polarized towards a Memory Phenotype as Predictive Biomarkers for Antibody-Mediated Rejection. International Journal of Molecular Sciences, 21(3), 779. https://doi.org/10.3390/ijms21030779






