Exploring Frequencies of Circulating Specific Th17 Cells against Myeloperoxidase and Proteinase 3 in ANCA Associated Vasculitis
Abstract
1. Introduction
2. Results
2.1. Baseline Characteristics of the Population
2.2. IL-17 Concentration in Serum and Urine Samples
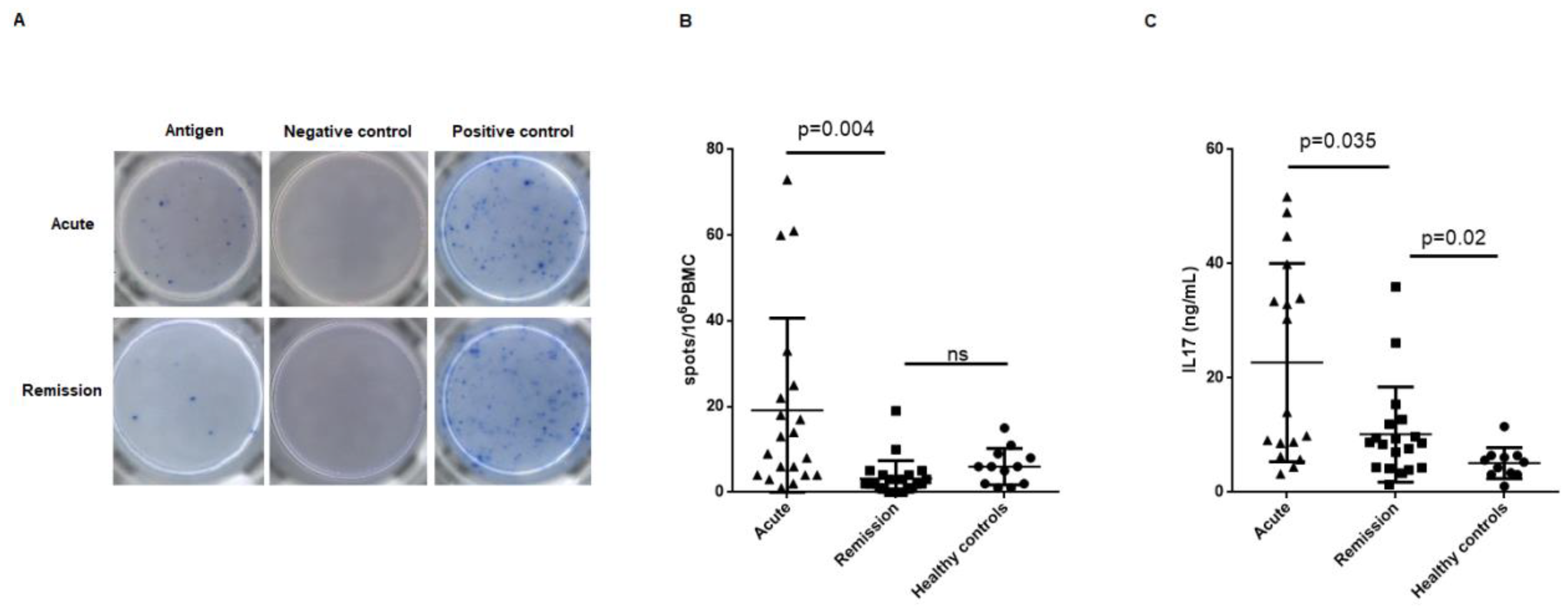
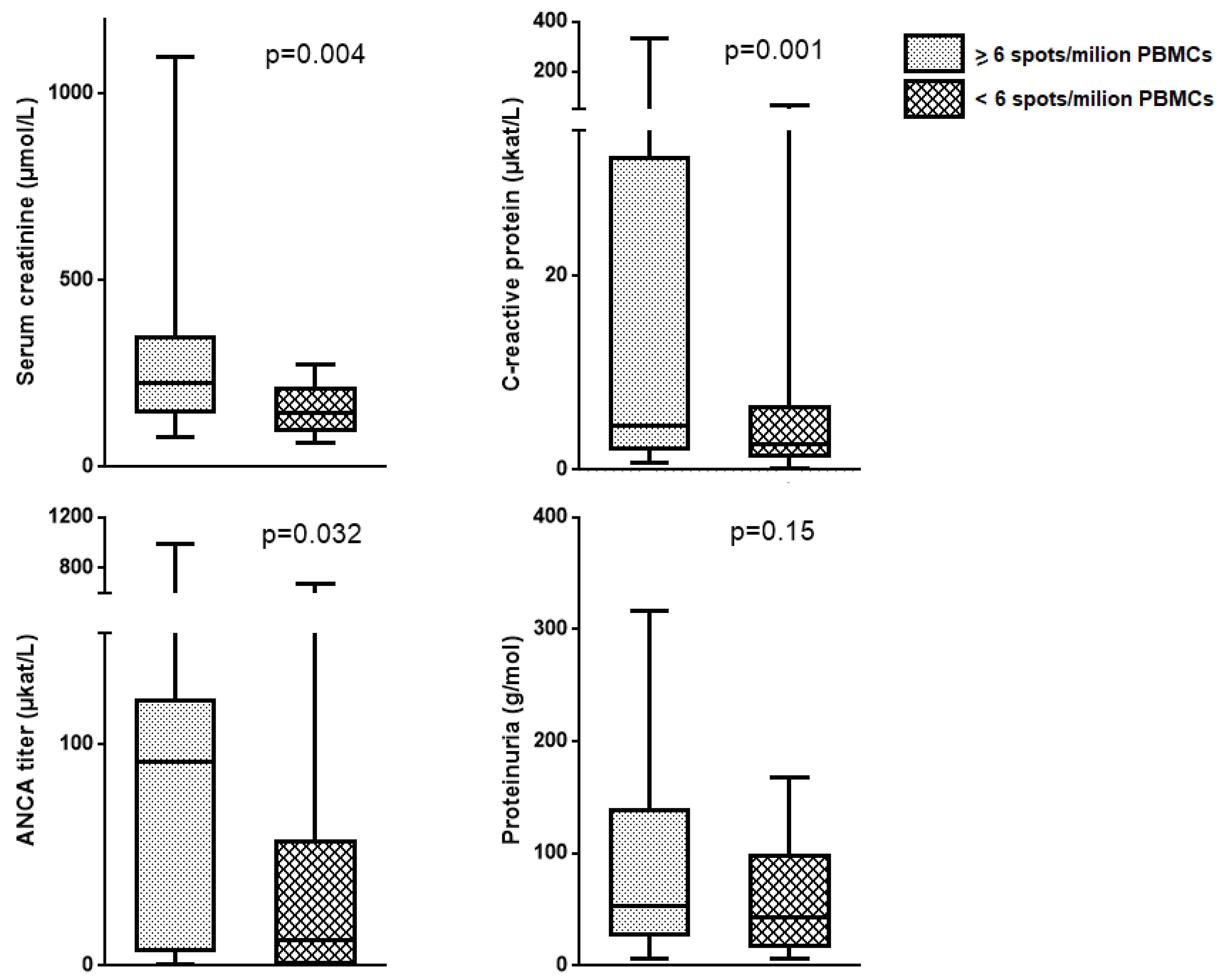
2.3. Circulating MPO-Specific and PR3-Specific Th17 Frequencies, Supernatant IL-17 Concentration and AAV Disease Phase
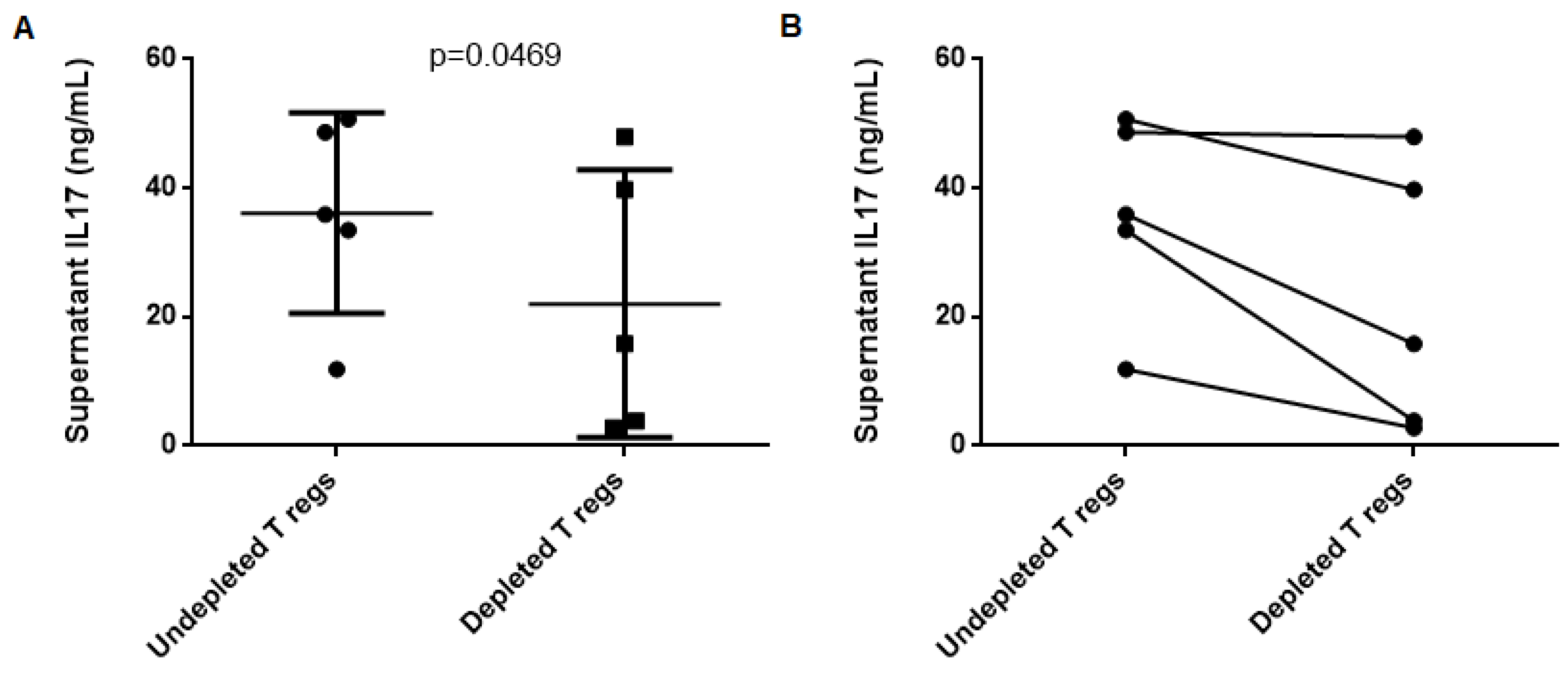
2.4. T Regulatory Lymphocytes do not Suppress Th17 Response to MPO in AAV Patients
3. Discussion
4. Methods
4.1. Experimental Design and Study Population
4.2. Clinical Variables
4.3. Sampling and Laboratory Procedures
4.4. IL-17 Levels in Urine, Serum, and Cell Culture Supernatant
4.5. Circulating Th17 Cell Frequencies
4.6. MPO-Specific Functional Suppressive Activity of Regulatory T cells
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Savage, C.O.S. Pathogenesis of anti-neutrophil cytoplasmic autoantibody (ANCA)-associated vasculitis. Clin. Exp. Immunol. 2011, 164 (Suppl. 1), 23–26. [Google Scholar] [CrossRef] [PubMed]
- Salvadori, M.; Tsalouchos, A. Antineutrophil cytoplasmic antibody associated vasculitides with renal involvement: Open challenges in the remission induction therapy. World J. Nephrol. 2018, 7, 71–83. [Google Scholar] [CrossRef] [PubMed]
- Wilde, B.; Thewissen, M.; Damoiseaux, J.; van Paassen, P.; Witzke, O.; Tervaert, J.W.C. T cells in ANCA-associated vasculitis: What can we learn from lesional versus circulating T cells? Arthritis Res. Ther. 2010, 12, 204. [Google Scholar] [CrossRef]
- Tabarkiewicz, J.; Pogoda, K.; Karczmarczyk, A.; Pozarowski, P.; Giannopoulos, K. The Role of IL-17 and Th17 Lymphocytes in Autoimmune Diseases. Arch. Immunol. Ther. Exp. 2015, 63, 435–449. [Google Scholar] [CrossRef] [PubMed]
- Azizi, G.; Jadidi-Niaragh, F.; Mirshafiey, A. Th17 Cells in Immunopathogenesis and treatment of rheumatoid arthritis. Int. J. Rheum. Dis. 2013, 16, 243–253. [Google Scholar] [CrossRef] [PubMed]
- Talaat, R.M.; Mohamed, S.F.; Bassyouni, I.H.; Raouf, A.A. Th1/Th2/Th17/Treg cytokine imbalance in systemic lupus erythematosus (SLE) patients: Correlation with disease activity. Cytokine 2015, 72, 146–153. [Google Scholar] [CrossRef]
- Saadoun, D.; Garrido, M.; Comarmond, C.; Desbois, A.C.; Domont, F.; Savey, L.; Terrier, B.; Geri, G.; Rosenzwajg, M.; Klatzmann, D.; et al. Th1 and Th17 Cytokines Drive Inflammation in Takayasu Arteritis. Arthritis Rheumatol. 2015, 67, 1353–1360. [Google Scholar] [CrossRef]
- Nanke, Y.; Yago, T.; Kotake, S. The Role of Th17 Cells in the Pathogenesis of Behcet’s Disease. J. Clin. Med. 2017, 6, 74. [Google Scholar] [CrossRef]
- Kuwabara, T.; Ishikawa, F.; Kondo, M.; Kakiuchi, T. The Role of IL-17 and Related Cytokines in Inflammatory Autoimmune Diseases. Mediat. Inflamm. 2017, 2017, 3908061. [Google Scholar] [CrossRef]
- Pelletier, M.; Maggi, L.; Micheletti, A.; Lazzeri, E.; Tamassia, N.; Costantini, C.; Cosmi, L.; Lunardi, C.; Annunziato, F.; Romagnani, S.; et al. Evidence for a cross-talk between human neutrophils and Th17 cells Evidence for a cross-talk between human neutrophils and Th17 cells. Blood 2014, 115, 335–343. [Google Scholar] [CrossRef]
- Nogueira, E.; Hamour, S.; Sawant, D.; Henderson, S.; Mansfield, N.; Chavele, K.M.; Pusey, C.D.; Salama, A.D. Serum IL-17 and IL-23 levels and autoantigen-specific Th17 cells are elevated in patients with ANCA-associated vasculitis. Nephrol. Dial. Transplant. 2010, 25, 2209–2217. [Google Scholar] [CrossRef] [PubMed]
- Szczeklik, W.; Jakieła, B.; Wawrzycka-Adamczyk, K.; Sanak, M.; Hubalewska-Mazgaj, M.; Padjas, A.; Surmiak, M.; Szczeklik, K.; Sznajd, J.; Musiał, J. Skewing toward Treg and Th2 responses is a characteristic feature of sustained remission in ANCA-positive granulomatosis with polyangiitis. Eur. J. Immunol. 2017, 47, 724–733. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, P.; Andersson, C.; Cassel, P.; Nyström, S.; Ernerudh, J. Increase in Th17-associated CCL20 and decrease in Th2-associated CCL22 plasma chemokines in active ANCA-associated vasculitis. Scand. J. Rheumatol. 2015, 44, 80–83. [Google Scholar] [CrossRef] [PubMed]
- Rimbert, M.; Hamidou, M.; Braudeau, C.; Puéchal, X.; Teixeira, L.; Caillon, H.; Néel, A.; Audrain, M.; Guillevin, L.; Josien, R. Decreased Numbers of Blood Dendritic Cells and Defective Function of Regulatory T Cells in Antineutrophil Cytoplasmic Antibody-Associated Vasculitis. Rieux-Laucat F, editor. PLoS ONE 2011, 6, e18734. [Google Scholar] [CrossRef]
- Abdulahad, W.H.; Stegeman, C.A.; Limburg, P.C.; Kallenberg, C.G.M. Skewed distribution of Th17 lymphocytes in patients with Wegener’s granulomatosis in remission. Arthritis Rheum. 2008, 58, 2196–2205. [Google Scholar] [CrossRef]
- Lee, R.G. The Balance of Th17 Versus Treg Cells in Autoimmunity. Int. J. Mol. Sci. 2018, 19, 730. [Google Scholar]
- Abdulahad, W.H.; van der Geld, Y.M.; Stegeman, C.A.; Kallenberg, C.G.M. Persistent expansion of CD4+ effector memory T cells in Wegener’s granulomatosis. Kidney Int. 2006, 70, 938–947. [Google Scholar] [CrossRef]
- Abdulahad, W.H.; Stegeman, C.A.; van der Geld, Y.M.; Doornbos-van der Meer, B.; Limburg, P.C.; Kallenberg, C.G.M. Functional defect of circulating regulatory CD4+ T cells in patients with Wegener’s granulomatosis in remission. Arthritis Rheum. 2007, 56, 2080–2091. [Google Scholar] [CrossRef]
- Kälsch, A.I.; Csernok, E.; Münch, D.; Birck, R.; Yard, B.A.; Gross, W.; Kälsch, T.; Schmitt, W.H. Use of Highly Sensitive C-Reactive Protein for Followup of Wegener’s Granulomatosis. J. Rheumatol. 2010, 37, 2319–2325. [Google Scholar] [CrossRef]
- Tomasson, G.; Grayson, P.C.; Mahr, A.D.; Lavalley, M.; Merkel, P.A. Value of ANCA measurements during remission to predict a relapse of ANCA-associated vasculitis—A meta-analysis. Rheumatology 2012, 51, 100–109. [Google Scholar] [CrossRef]
- Rhee, R.L.; Davis, J.C.; Ding, L.; Fervenza, F.C.; Hoffman, G.S.; Kallenberg, C.G.; Langford, C.A.; McCune, W.J.; Monach, P.A.; Seo, P.; et al. The Utility of Urinalysis in Determining the Risk of Renal Relapse in ANCA-Associated Vasculitis. Clin. J. Am. Soc. Nephrol. 2018, 13, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Wilde, B.; Thewissen, M.; Damoiseaux, J.; Hilhorst, M.; van Paassen, P.; Witzke, O.; Tervaert, J.W. Th17 expansion in granulomatosis with polyangiitis (Wegener’s): The role of disease activity, immune regulation and therapy. Arthritis Res. Ther. 2012, 14, R227. [Google Scholar] [CrossRef] [PubMed]
- Krohn, S.; Nies, J.F.; Kapffer, S.; Schmidt, T.; Riedel, J.H.; Kaffke, A.; Peters, A.; Borchers, A.; Steinmetz, O.M.; Krebs, C.F.; et al. IL-17C/IL-17 Receptor E Signaling in CD4+ T Cells Promotes TH17 Cell-Driven Glomerular Inflammation. J. Am. Soc. Nephrol. 2018, 29, 1210–1222. [Google Scholar] [CrossRef]
- Crome, S.Q.; Wang, A.Y.; Levings, M.K. Translational mini-review series on Th17 cells: Function and regulation of human T helper 17 cells in health and disease. Clin. Exp. Immunol. 2010, 159, 109–119. [Google Scholar] [CrossRef]
- Matsumoto, K.; Kanmatsuse, K. Increased Urinary Excretion of Interleukin-17 in Nephrotic Patients. Nephron 2002, 91, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Velden, J.; Paust, H.J.; Hoxha, E.; Turner, J.E.; Steinmetz, O.M.; Wolf, G.; Jabs, W.J.; Özcan, F.; Beige, J.; Heering, P.J.; et al. Renal IL-17 expression in human ANCA-associated glomerulonephritis. Am. J. Physiol. Ren. Physiol. 2012, 302, F1663–F1673. [Google Scholar] [CrossRef]
- Reijnders, T.D.Y.; Stegeman, C.A.; Huitema, M.G.; Rutgers, A.; Heeringa, P.; Abdulahad, W.H. Unraveling the identity of FoxP3+ regulatory T cells in Granulomatosis with Polyangiitis patients. Sci. Rep. 2019, 9, 8273. [Google Scholar] [CrossRef]
- Langley, R.G.; Elewski, B.E.; Lebwohl, M.; Reich, K.; Griffiths, C.E.; Papp, K.; Puig, L.; Nakagawa, H.; Spelman, L.; Sigurgeirsson, B.; et al. Secukinumab in Plaque Psoriasis—Results of Two Phase 3 Trials. N. Engl. J. Med. 2014, 371, 326–338. [Google Scholar] [CrossRef]
- Luqmani, R.A.; Bacon, P.A.; Moots, R.J.; Janssen, B.A.; Pall, A.; Emery, P.; Savage, C.; Adu, D. Birmingham Vasculitis Activity Score (BVAS) in systemic necrotizing vasculitis. QJM 1994, 87, 671–678. [Google Scholar]
- Yates, M.; Watts, R.; Bajema, I.; Cid, M.; Crestani, B.; Hauser, T.; Hellmich, B.; Holle, J.; Laudien, M.; Little, M.A.; et al. Validation of the EULAR/ERA-EDTA recommendations for the management of ANCA-associated vasculitis by disease content experts. RMD Open 2017, 3, e000449. [Google Scholar] [CrossRef]
- Berden, A.E.; Ferrario, F.; Hagen, E.C.; Jayne, D.R.; Jennette, J.C.; Joh, K.; Neumann, I.; Noël, L.H.; Pusey, C.D.; Waldherr, R.; et al. Histopathologic Classification of ANCA-Associated Glomerulonephritis. J. Am. Soc. Nephrol. 2010, 21, 1628–1636. [Google Scholar] [CrossRef] [PubMed]

| Acute (n = 21) | Remission (n = 22) | p-Value | |
|---|---|---|---|
| Male/Female Sex (%) | 47.6/52.3 | 40.1/59.9 | 0.658 |
| Age (years) | 61.52 ± 20.23 | 65.81 ± 13.60 | 0.417 |
| BMI | 30.79 ± 6.85 | 27.17 ± 6.43 | 0.18 |
| Time since diagnostic (months) | 62.86 ± 48.43 | ||
| Recently diagnosed patients Patients in flare | 0 ± 0 100.60 ± 90.84 | 0.001 0.20 | |
| Creatinine (µmol/L) | 280.19 ± 248.99 | 158.95 ± 60.13 | 0.041 |
| Proteinuria (g/mol) | 97.13 ± 79.36 | 46.29 ± 39.55 | 0.015 |
| ANCA titer (karb.u./L) | 228.4 ± 382.28 | 76.66 ± 161.84 | 0.104 |
| ANCA specificity (%) | 1 | ||
| MPO | 76.2 | 72.7 | |
| PR3 | 23.8 | 27.3 | |
| CRP (µkat/L) | 42.90 ± 79.95 | 6.09 ± 14.94 | 0.056 |
| Hematuria (%) | 76.2 | 59 | 0.232 |
| History of prior relapse | - | 40.9 | |
| Lung involvement (%) | 28.6 | 31.8 | 0.817 |
| Healthy Controls (n = 12) | |
|---|---|
| Male/Female Sex (%) | 41.66/58.33 |
| Age (years) | 36 ± 11.28 |
| Body Mass Index | 26.58 ± 2.24 |
| Univariate Analysys | |||
| Variable | OR | CI (95%) | p-Value |
| Specific Th17 frequency | 1.233 | 1.048–1.452 | 0.012 * |
| Serum IL-17 | 0.993 | 0.979–1.008 | 0.377 |
| Urinary IL-17 | 0.732 | 0.488–1.097 | 0.13 |
| Supernatant IL-17 | 1.076 | 1.012–1.144 | 0.02 * |
| Serum creatinine | 1.007 | 1–1.014 | 0.065 |
| GFR | 1.002 | 0.99–1.013 | 0.785 |
| ANCA titer | 1.002 | 0.999–1.006 | 0.14 |
| CRP | 1.003 | 0.999–1.68 | 0.09 |
| Multivariate Analysis | |||
| Variable | OR | CI (95%) | p-Value |
| Specific Th17 frequency | 1.183 | 1.006–1.390 | 0.042 * |
| Supernatant IL-17 | 1.079 | 1.004–1.160 | 0.04 * |
| Serum Creatinine | 0.999 | 0.986–1.013 | 0.939 |
| CRP | 1.021 | 0.980–1.064 | 0.313 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martinez Valenzuela, L.; Draibe, J.; Quero, M.; Fulladosa, X.; Cruzado, J.M.; Bestard, O.; Torras, J. Exploring Frequencies of Circulating Specific Th17 Cells against Myeloperoxidase and Proteinase 3 in ANCA Associated Vasculitis. Int. J. Mol. Sci. 2019, 20, 5820. https://doi.org/10.3390/ijms20235820
Martinez Valenzuela L, Draibe J, Quero M, Fulladosa X, Cruzado JM, Bestard O, Torras J. Exploring Frequencies of Circulating Specific Th17 Cells against Myeloperoxidase and Proteinase 3 in ANCA Associated Vasculitis. International Journal of Molecular Sciences. 2019; 20(23):5820. https://doi.org/10.3390/ijms20235820
Chicago/Turabian StyleMartinez Valenzuela, Laura, Juliana Draibe, Maria Quero, Xavier Fulladosa, Josep Maria Cruzado, Oriol Bestard, and Juan Torras. 2019. "Exploring Frequencies of Circulating Specific Th17 Cells against Myeloperoxidase and Proteinase 3 in ANCA Associated Vasculitis" International Journal of Molecular Sciences 20, no. 23: 5820. https://doi.org/10.3390/ijms20235820
APA StyleMartinez Valenzuela, L., Draibe, J., Quero, M., Fulladosa, X., Cruzado, J. M., Bestard, O., & Torras, J. (2019). Exploring Frequencies of Circulating Specific Th17 Cells against Myeloperoxidase and Proteinase 3 in ANCA Associated Vasculitis. International Journal of Molecular Sciences, 20(23), 5820. https://doi.org/10.3390/ijms20235820





