Osteogenic Potential of Bovine Bone Graft in Combination with Laser Photobiomodulation: An Ex Vivo Demonstrative Study in Wistar Rats by Cross-Linked Studies Based on Synchrotron Microtomography and Histology
Abstract
1. Introduction
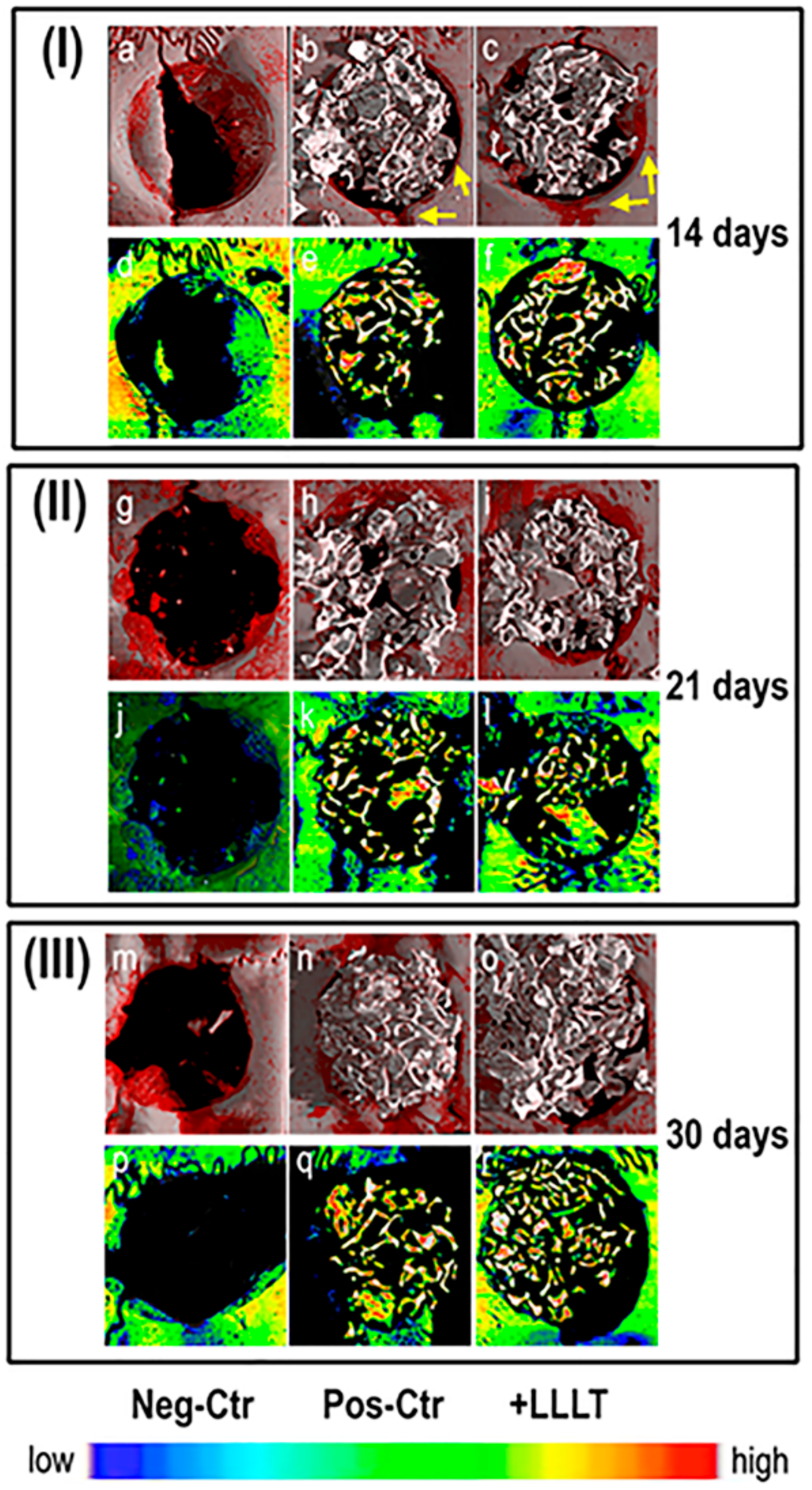
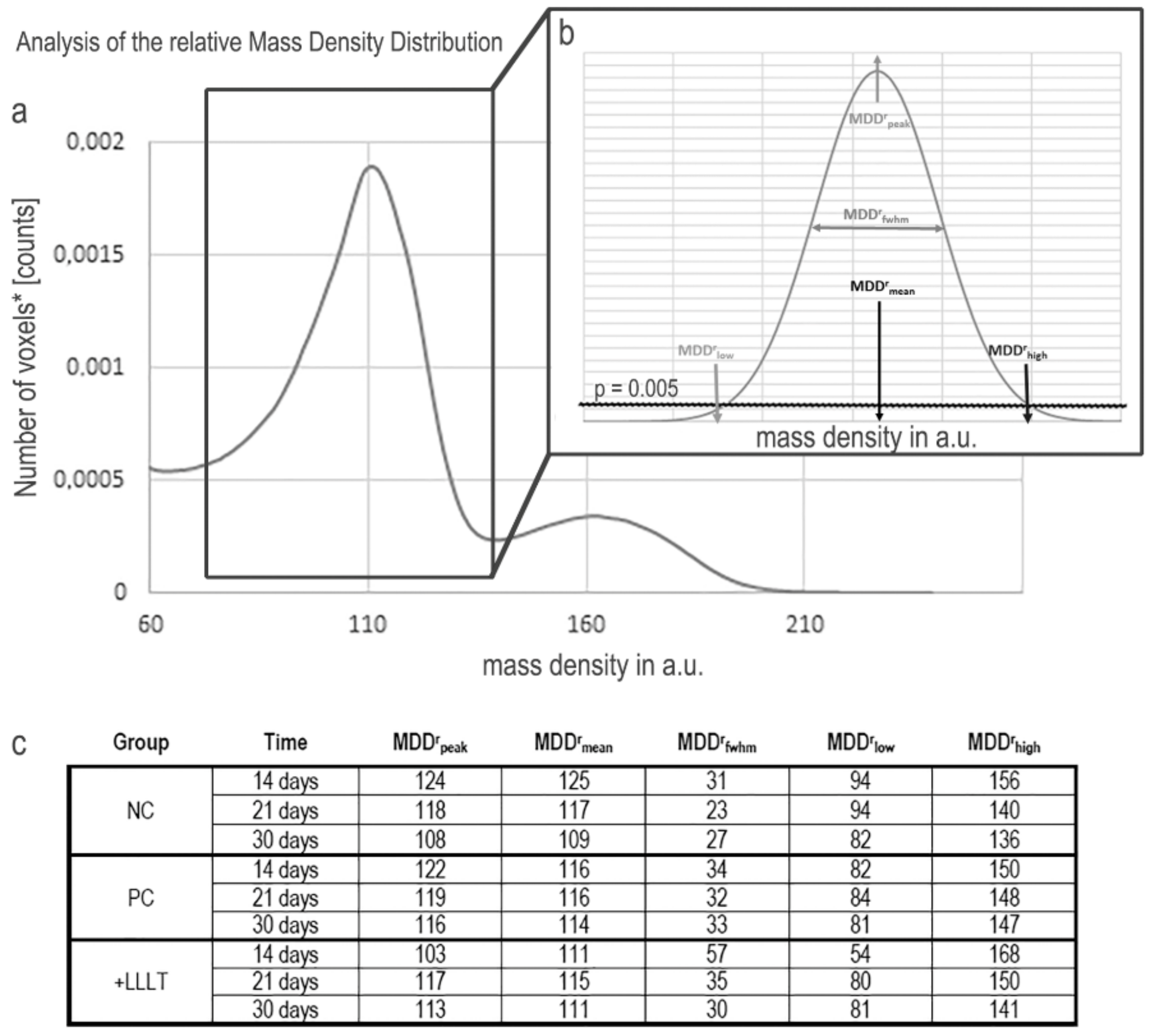
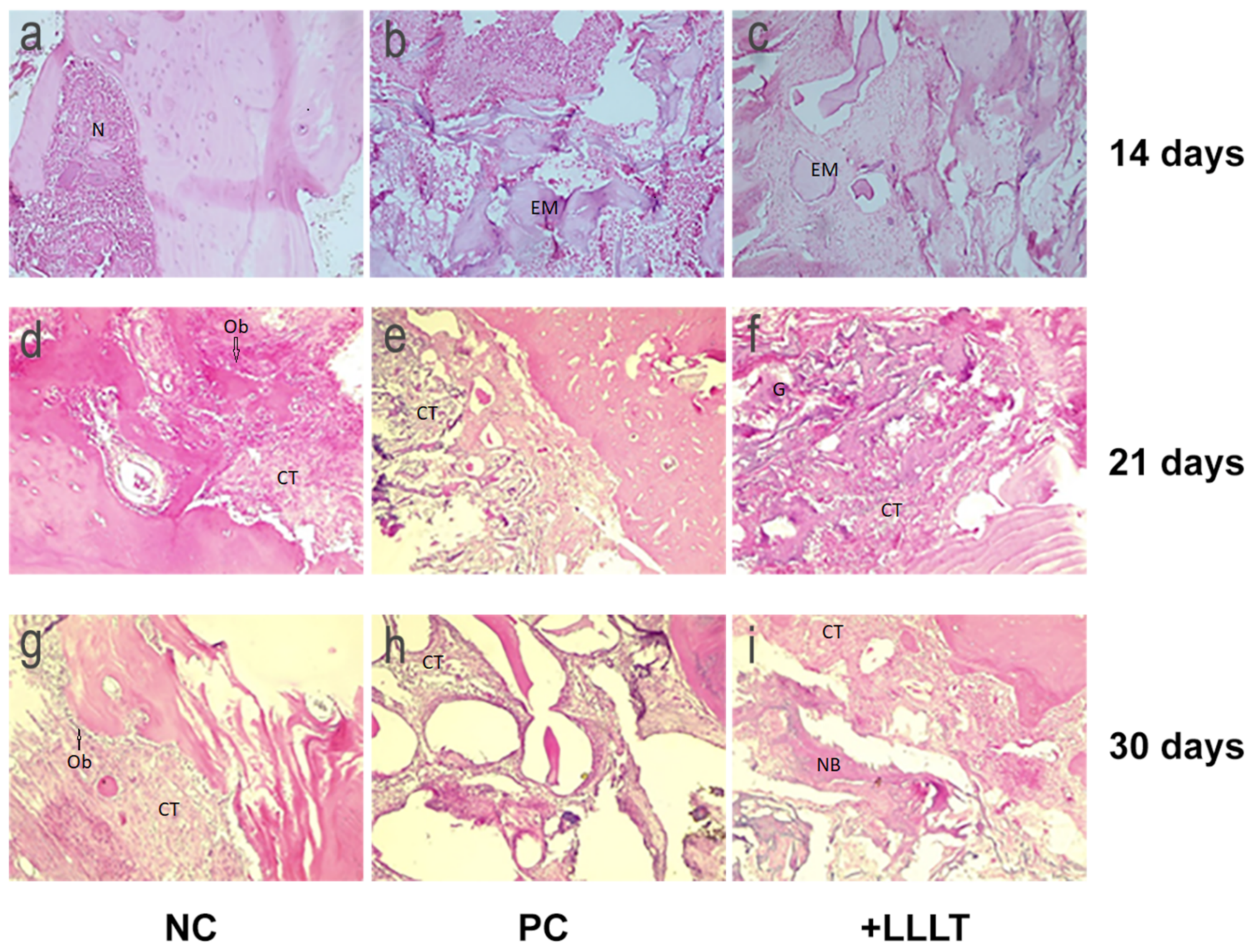
2. Results
2.1. Synchrotron Radiation-Based Micro-Tomography
2.2. Histology
3. Discussion
4. Materials and Methods
4.1. Animal Model and Groups of Study
4.2. Surgical Procedure
4.3. Photobiomodulation Protocol
4.4. Samples Collection
4.5. Synchrotron Radiation-Based Micro-Tomography
4.6. Histology
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| LLLT | Low level laser therapy |
| 3D | Three-dimensional |
| MDDr | Relative bone mineral density distribution |
| Micro-CT | Microtomography |
References
- Paknejad, M.; Rokn, A.; Rouzmeh, N.; Heidari, M.; Titidej, A.; Kharazifard, M.J.; Mehrfard, A. Histologic evaluation of bone healing capacity following application of inorganic bovine bone and a new allograft material in rabbit calvaria. J. Dent. (Tehran) 2015, 12, 31–38. [Google Scholar]
- Clokie, C.M.; Moghadam, H.; Jackson, M.T.; Sandor, G.K. Closure of critical sized defects with allogenic and alloplastic bone substitutes. J. Craniofac. Surg. 2002, 13, 111–121, discussion 122–123. [Google Scholar] [CrossRef] [PubMed]
- Barboza, E.P.; Duarte, M.E.; Geolas, L.; Sorensen, R.G.; Riedel, G.E.; Wikesjo, U.M. Ridge augmentation following implantation of recombinant human bone morphogenetic protein-2 in the dog. J. Periodontol. 2000, 71, 488–496. [Google Scholar] [CrossRef]
- Wang, W.; Yeung, K.W.K. Bone grafts and biomaterials substitutes for bone defect repair: A review. Bioact. Mater. 2017, 2, 224–247. [Google Scholar] [CrossRef] [PubMed]
- Yamada, M.; Egusa, H. Current bone substitutes for implant dentistry. J. Prosthodont. Res. 2018, 62, 152–161. [Google Scholar] [CrossRef]
- Aghaloo, T.L.; Moy, P.K. Which hard tissue augmentation techniques are the most successful in furnishing bony support for implant placement? Int. J. Oral. Maxillofac. Implant. 2007, 22, 49–70. [Google Scholar]
- Rakhmatia, Y.D.; Ayukawa, Y.; Furuhashi, A.; Koyano, K. Current barrier membranes: Titanium mesh and other membranes for guided bone regeneration in dental applications. J. Prosthodont. Res. 2013, 57, 3–14. [Google Scholar] [CrossRef]
- Simion, M.; Trisi, P.; Piattelli, A. Vertical ridge augmentation using a membrane technique associated with osseointegrated implants. Int. J. Periodontics. Restor. Dent. 1994, 14, 496–511. [Google Scholar]
- Tinti, C.; Parma-Benfenati, S.; Polizzi, G. Vertical ridge augmentation: What is the limit? Int. J. Periodontics. Restor. Dent. 1996, 16, 220–229. [Google Scholar]
- Simion, M.; Jovanovic, S.A.; Trisi, P.; Scarano, A.; Piattelli, A. Vertical ridge augmentation around dental implants using a membrane technique and autogenous bone or allografts in humans. Int. J. Periodontics. Restor. Dent. 1998, 18, 8–23. [Google Scholar]
- Buser, D.; Bragger, U.; Lang, N.P.; Nyman, S. Regeneration and enlargement of jaw bone using guided tissue regeneration. Clin. Oral. Implant. Res. 1990, 1, 22–32. [Google Scholar] [CrossRef] [PubMed]
- Buser, D.; Dula, K.; Hirt, H.P.; Schenk, R.K. Lateral ridge augmentation using autografts and barrier membranes: A clinical study with 40 partially edentulous patients. J. Oral. Maxillofac. Surg. 1996, 54, 420–432. [Google Scholar] [CrossRef]
- Funato, A.; Ishikawa, T.; Kitajima, H.; Yamada, M.; Moroi, H. A novel combined surgical approach to vertical alveolar ridge augmentation with titanium mesh, resorbable membrane, and rhPDGF-BB: A retrospective consecutive case series. Int. J. Periodontics. Restor. Dent. 2013, 33, 437–445. [Google Scholar] [CrossRef] [PubMed]
- Jo, S.H.; Kim, Y.K.; Choi, Y.H. Histological Evaluation of the Healing Process of Various Bone Graft Materials after Engraftment into the Human Body. Materials 2018, 11, 714. [Google Scholar] [CrossRef]
- Iezzi, G.; Piattelli, A.; Giuliani, A.; Mangano, C.; Barone, A.; Manzon, L.; Degidi, M.; Scarano, A.; Filippone, A.; Perrotti, V. Molecular, Cellular and Pharmaceutical Aspects of Bone Grafting Materials and Membranes During Maxillary Sinus-lift Procedures. Part 2: Detailed Characteristics of the Materials. Curr. Pharm. Biotechnol. 2017, 18, 33–44. [Google Scholar] [CrossRef]
- Behnia, H.; Khoshzaban, A.; Zarinfar, M.; Mashhadi Abbas, F.; Bahraminasab, H.; Khojasteh, A. Histological Evaluation of regeneration in rabbit calvarial bone defects using demineralized bone matrix, mesenchymal stem cells and platelet rich in growth factors. J. Dent. Sch. 2012, 30, 143–154. [Google Scholar]
- Schroeder, J.E.; Mosheiff, R. Tissue engineering approaches for bone repair: Concepts and evidence. Injury 2011, 42, 609–613. [Google Scholar] [CrossRef]
- Lysiak-Drwal, K.; Dominiak, M.; Solski, L.; Zywicka, B.; Pielka, S.; Konopka, T.; Gerber, H. Early histological evaluation of bone defect healing with and without guided bone regeneration techniques: Experimental animal studies. Postepy. Hig. Med. Dosw. 2008, 62, 282–288. [Google Scholar]
- Hitti, R.A.; Kerns, D.G. Guided Bone Regeneration in the Oral Cavity: A Review. Open Pathol. J. 2011, 511, 33–45. [Google Scholar] [CrossRef]
- Urist, M.R.; McLean, F.C. Recent advances in physiology of bone. I. J. Bone Jt. Surg. Am. 1963, 45, 1305–1313. [Google Scholar] [CrossRef]
- Deniz, E.; Arslan, A.; Diker, N.; Olgac, V.; Kilic, E. Evaluation of light-emitting diode photobiomodulation on bone healing of rat calvarial defects. Biotechnol. Biotechnol. Equip. 2015, 29, 1–8. [Google Scholar] [CrossRef]
- Soares, L.G.; Marques, A.M.; Barbosa, A.F.; Santos, N.R.; Aciole, J.M.; Souza, C.M.; Pinheiro, A.L.; Silveira, L., Jr. Raman study of the repair of surgical bone defects grafted with biphasic synthetic microgranular HA + β-calcium triphosphate and irradiated or not with λ780 nm laser. Lasers Med. Sci. 2014, 29, 1575–1584. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, A.L.; Gerbi, M.E. Photoengineering of bone repair processes. Photomed. Laser Surg. 2006, 24, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Karu, T.I.; Pyatibrat, L.V.; Afanasyeva, N.I. A novel mitochondrial signaling pathway activated by visibleto-near infrared radiation. Photochem. Photobiol. 2004, 80, 366–372. [Google Scholar] [CrossRef]
- Ahmed, S.; Bewsh, G.; Bhat, S.; Babu, R. Low level laser therapy: Healing at the speed of light. J. Evol. Med. Dent. Sci. 2013, 2, 7441–7463. [Google Scholar] [CrossRef]
- Barber, A.; Luger, J.E.; Karpf, A.; Salame, K.; Shlomi, B.; Kogan, G.; Nissan, M.; Alon, M.; Rochkind, S. Advances in Laser Therapy for Bone Repair. Laser Ther. 2001, 13, 80–85. [Google Scholar] [CrossRef]
- Dörtbudak, O.; Haas, R.; Mallath-Pokorny, G. Biostimulation of bone marrow cells with a diode soft laser. Clin. Oral. Implants Res. 2000, 11, 540–545. [Google Scholar] [CrossRef]
- Ueda, Y.; Shimizu, N. Effects of pulse frequency of low-level laser therapy (LLLT) on bone nodule formation in rat calvarial cells. J. Clin. Laser Med. Surg. 2003, 21, 271–277. [Google Scholar] [CrossRef]
- Stein, A.; Benayahu, D.; Maltz, L.; Oron, U. Low-level laser irradiation promotes proliferation and differentiation of human osteoblasts in vitro. Photomed. Laser Surg. 2005, 23, 161–166. [Google Scholar] [CrossRef]
- Kandra, M.; Lyngstadaas, S.P.; Haanaes, H.R.; Mustafa, K. Effect of laser therapy on attachment, proliferation and differentiation on human osteoblast-like cells cultured on titanium implant material. Biomaterials 2005, 26, 3503–3509. [Google Scholar]
- Ozawa, Y.; Shimizu, N.; Mishima, H.; Yamaguchi, M.; Takiguchi, H.; Iwasawa, T.; Abiko, Y. Stimulatory effects of low-power laser irradiation on bone formation in vitro. SPIE 1984, 1995, 281–288. [Google Scholar]
- Guzzardella, G.A.; Fini, M.; Torricelli, P.; Giavaresi, G.; Giardino, R. Laser stimulation on bone defect healing: An in vitro study. Lasers Med. Sci. 2002, 17, 216–220. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, A.L.B.; Limeira Júnior, F.A.; Gerbi, M.E.M.M.; Ramalho, L.M.; Marzola, C.; Ponzi, E.A.; Soares, A.O.; De Carvalho, L.C.; Lima, H.C.; Gonçalves, T.O. Effect of 830-nm laser light on the repair of bone defects grafted with inorganic bovine bone and decalcified cortical oss osseous membrane. J. Clin. Laser Med. Surg. 2003, 21, 383–388. [Google Scholar] [CrossRef] [PubMed]
- Rochkind, S.; Kogan, G.; Luger, E.G.; Salame, K.; Karp, E.; Graif, M.; Weiss, J. Molecular structure of the bony tissue after experimental trauma to the mandibular region followed by laser therapy. Photomed. Laser Surg. 2004, 22, 249–253. [Google Scholar] [CrossRef] [PubMed]
- Silva Júnior, A.N.; Pinheiro, A.L.B.; Oliveira, M.G.; Weismann, R.; Ramalho, L.M.; Nicolau, R.A. Computerized morphometric assessment of the effect of low-level laser therapy on bone repair. J. Clin. Laser Med. Surg. 2002, 20, 83–87. [Google Scholar] [CrossRef]
- Pinheiro, A.L.B.; Oliveira, M.A.M.; Martins, P.P.M. Biomodulação da cicatrização óssea pós-implantar com o uso da laserterapia não-cirúrgica: Estudo por microscopia eletrônica de varredura. Rev. Foufba 2001, 22, 12–19. [Google Scholar]
- Lopes, C.B.; Pinheiro, A.L.B.; Sathaiah, S.; Duarte, J.; Cristinamartins, M. Infrared laser light reduces loading time of dental implants: A Raman sapectroscopic study. Photomed. Laser Surg. 2005, 23, 27–31. [Google Scholar] [CrossRef]
- Roschger, P.; Paschalis, E.P.; Fratzl, P.; Klaushofer, K. Bone mineralization density distribution in health and disease. Bone 2008, 42, 456–466. [Google Scholar] [CrossRef]
- Oliveira, P.; Sperandio, E.; Fernandes, K.R.; Pastor, F.A.; Nonaka, K.O.; Renno, A.C. Comparison of the effects of low-level laser therapy and low-intensity pulsed ultrasound on the process of bone repair in the rat tibia. Rev. Bras. Fisioter. 2011, 15, 200–205. [Google Scholar] [CrossRef]
- Renno, A.C.M.; McDonnell, P.A.; Parizotto, N.A.; Laakso, E.L. The effects of laser irradiation on osteoblast and osteosarcoma cell proliferation and differentiation in vitro. Photomed. Laser Surg. 2007, 25, 275–280. [Google Scholar] [CrossRef]
- Takauti, C.A.; Futema, F.; Brito Junior, R.B.; Abrahão, A.C.; Costa, C.; Queiroz, C.S. Assessment of bone healing in rabbit calvaria grafted with three different biomaterials. Braz. Dent. J. 2014, 25, 379–384. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rokn, A.R.; Khodadoostan, M.A.; Ghahroudi, A.A.R.R.; Aar, R.; Motahhary, P.; Javad, M.; Fard, K.; Bruyn, D.; Afzalifar, R.; Soolari, E.; et al. Bone formation with two types of grafting materials: A histologic and histomorphometric study. Open Dent. J. 2011, 5, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Turri, A.; Dahlin, C. Comparative maxillary bone-defect healing by calcium-sulphate or deproteinized bovine bone particles and extra cellular matrix membranes in a guided bone regeneration setting: An experimental study in rabbits. Clin. Oral. Implant. Res. 2015, 26, 501–506. [Google Scholar] [CrossRef] [PubMed]
- Carter, D.R.; Loboa, E.G.; Polefka, E.G.; Beaupre, G.S. Mechanical Influences on Skeletal Regeneration. In Human Biomechanics and Injury Prevention; Kajzer, J., Tanaka, E., Yamada, H., Eds.; Springer: Tokyo, Japan, 2000; pp. 129–136. [Google Scholar]
- Lakey, L.A.; Akella, R.; Ranieru, J.P. Angiogenesis: Implications for tissue repair. In Bone Engineering; Davies, J.E., Ed.; EM Squared Incorporated: Toronto, ON, Canada, 2000; pp. 137–142. [Google Scholar]
- Garavello-Freitas, I.; Baranauskas, V.; Joazeiro, P.P.; Padovani, C.R.; Dal Pai-Silva, M.; da Cruz-Hofling, M.A. Low-power laser irradiation improves histomorphometrical parameters and bone matrix organization during tibia wound healing in rats. J. Photochem. Photobiol. B. 2003, 70, 81–89. [Google Scholar] [CrossRef]
- Campana, V.; Moya, M.; Gavotto, A.; Juri, H.; Palma, J.A. Effects of diclofenac sodium and He:Ne laser irradiation on plasmatic fibrinogen levels in inflammatory processes. J. Clin. Laser Med. Surg. 1998, 16, 317–320. [Google Scholar] [CrossRef]
- Hall, G.; Anneroth, G.; Schennings, T.; Zetterqvist, L.; Ryden, H. Effect of low level energy laser irradiation on wound healing. An experimental study in rats. Swed. Dent. J. 1994, 18, 29–34. [Google Scholar]
- Luca, R.; Todea, C.D.; Duma, V.-F.; Bradu, A.; Podoleanu, A. Quantitative assessment of rat bone regeneration using complex master–slave optical coherence tomography. Quant. Imaging Med. Surg. 2019, 9, 782–798. [Google Scholar] [CrossRef] [PubMed]
- Soleimani, M.; Abbasnia, E.; Fathi, M.; Sahraei, H.; Fathi, Y.; Kaka, G. The effects of low-level laser irradiation on differentiation and proliferation of human bone marrow mesenchymal stem cells into neurons and osteoblasts--an in vitro study. Lasers Med. Sci. 2012, 27, 423–430. [Google Scholar] [CrossRef]
- Saygun, I.; Nizam, N.; Ural, A.U.; Serdar, M.A.; Avcu, F.; Tozum, T.F. Low-level laser irradiation affects the release of basic fibroblast growth factor (bFGF), insulin-like growth factor-I (IGF-I), and receptor of IGF-I (IGFBP3) from osteoblasts. Photomed. Laser Surg. 2012, 30, 149–154. [Google Scholar] [CrossRef]
- Weber, J.B.B. Avaliação do Efeito da Laserterapia (GaAlAs) Nos Enxertos ósseos Autógenos em Ratos: Estudo Morfológico [Doctoral dissertation]. Ph.D. Thesis, Universidade Católica do Rio Grande do Sul, Porto Alegre, Brazil, 2003. Available online: http://tede2.pucrs.br/tede2/bitstream/tede/1118/1/431499.pdf.
- Rechenberg B, Animal models in bone repair. Drug Discov. Today 2014, 13, 23–27.
- Lopes-Martins, R. Low level laser therapy [LLLT] in inflammatory and rheumatic diseases: A review of therapeutic mechanisms. Curr. Rheumatol. Rev. 2007, 3, 147–154. [Google Scholar] [CrossRef]
- Hamblin, M.R.; Ferraresi, C.; Huang, Y.Y.M.D.; Freitas, L.F.; Carroll, J.D. Low-Level Light Therapy: Photobiomodulation; SPIE Press Book: Bellingham, WA, USA, 2018; Volume TT115, Available online: https://spie.org/Publications/Book/2295637?SSO=1.
- Hamblin, M. Mechanisms and applications of the anti-inflammatory effects of photobiomodulation. AIMS Biophys. 2017, 4, 337–361. [Google Scholar] [CrossRef] [PubMed]
- Nicolau, R.A.; Martinez, M.S.; Rigau, J.; Tomas, J. Effect of low power 655 nm diode laser irradiation on the neuromuscular junctions of the mouse diaphragm. Lasers Surg. Med. 2004, 34, 277–284. [Google Scholar] [CrossRef]
- Khadra, M.; Kasem, N.; Haanaes, H.R.; Ellingsen, J.E.; Lyngstadaas, S.P. Enhancement of bone formation in rat calvarial bone defects using low-level laser therapy. Oral. Surg. Oral. Med. Oral. Pathol. Oral. Radiol. Endod. 2004, 97, 693–700. [Google Scholar] [CrossRef]
- Weber, J.B.; Pinheiro, A.L.; de Oliveira, M.G.; Oliveira, F.A.; Ramalho, L.M. Laser therapy improves healing of bone defects submitted to autologous bone graft. Photomed. Laser Surg. 2006, 24, 38–44. [Google Scholar] [CrossRef]
- Giuliani, A.; Moroncini, F.; Mazzoni, S.; Belicchi, M.L.; Villa, C.; Erratico, S.; Colombo, E.; Calcaterra, F.; Brambilla, L.; Torrente, Y.; et al. Polyglycolic Acid–Polylactic Acid scaffold response to different progenitor cell in vitro cultures: A demonstrative and comparative X-Ray Synchrotron Radiation Phase-Contrast Microtomography study. Tissue Eng. Part C Methods 2014, 20, 308–316. [Google Scholar] [CrossRef] [PubMed]
- Manescu, A.; Giuliani, A.; Mazzoni, S.; Mohammadi, S.; Tromba, G.; Diomede, F.; Zini, N.; Piattelli, A.; Trubiani, O. Osteogenic potential of Dual-blocks cultured with periodontal ligament stem cells: In-vitro and synchrotron microtomography study. J. Periodontal Res. 2016, 51, 112–124. [Google Scholar] [CrossRef] [PubMed]
- Mazzoni, S.; Mohammadi, S.; Tromba, G.; Diomede, F.; Piattelli, A.; Trubiani, O.; Giuliani, A. Role of cortico-cancellous heterologous bone in human periodontal ligament stem cell xeno-free culture studied by Synchrotron radiation phase-contrast microtomography. Int. J. Mol. Sci. 2017, 18, 364. [Google Scholar] [CrossRef] [PubMed]
- Andreollo, N.A.; Santos, E.F.; Araújo, M.R.; Lopes, L.R. Rat’s age versus human’s age: What is the relationship. Arq. Bras. Cir. Dig. 2012, 25, 49–51. [Google Scholar] [CrossRef]
- Stavropoulos, A.; Sculean, A.; Bosshardt, D.D.; Buser, D.; Klinge, B. Pre-clinical in vivo models for the screening of bone biomaterials for oral/craniofacial indications: Focus on small-animal models. Periodontol. 2000 2015, 68, 55–65. [Google Scholar] [CrossRef]
- Silva, Y.S.; Deboni, M.C.Z.; Arana-Chaves, V.E.; Naclério-Homem, M.G. Novel Model of Mono Cortical Bone Defect in Rat Mandible: An Interesting Tool for Osseous Investigations. IJHS 2016, 4, 47–54. [Google Scholar]
- Paganin, D.; Mayo, S.C.; Gureyev, T.E.; Miller, P.R.; Wilkins, S.W. Simultaneous phase and amplitude extraction from a single defocused image of a homogeneous object. J. Microsc. 2002, 206, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Brun, F.; Massimi, L.; Fratini, M.; Dreossi, D.; Billé, F.; Accardo, A.; Pugliese, R.; Cedola, A. SYRMEP Tomo Project: A graphical user interface for customizing CT reconstruction workflows. Adv. Struct. Chem. Imaging 2017, 3, 4. [Google Scholar] [CrossRef] [PubMed]
- Giuliani, A.; Iezzi, G.; Mozzati, M.; Gallesio, G.; Mazzoni, S.; Tromba, G.; Zanini, F.; Piattelli, A.; Mortellaro, C. Bisphosphonate-related Osteonecrosis of the Human Jaw: A combined 3D assessment of Bone Descriptors by Histology and Synchrotron Radiation-based Microtomography. Oral. Oncol. 2018, 82, 200–202. [Google Scholar] [CrossRef] [PubMed]
- Weitkamp, T.; Haas, D.; Wegrzynek, D.; Rack, A. ANKAphase: Software for single-distance phase retrieval from inline X-ray phase-contrast radiographs. J. Synchrotron. Radiat. 2011, 18, 617–629. [Google Scholar] [CrossRef] [PubMed]
- Torres, C.S.; dos Santos, J.N.; Monteiro, J.S.; Amorim, P.G.; Pinheiro, A.L. Does the Use of Laser Photobiomodulation, Bone Morphogenetic Proteins, and Guided Bone Regeneration Improve the Outcome of Autologous Bone Grafts? An in Vivo Study in a Rodent Model. Photomed. Laser Surg. 2008, 26, 371–377. [Google Scholar] [CrossRef] [PubMed]
- Manescu, A.; Oancea, R.; Todea, C.; Rusu, L.C.; Mazzoni, S.; Negrutiu, M.L.; Sinescu, C.; Giuliani, A. On Long Term Effects of Low Power Laser Therapy on Bone Repair: A Demonstrative Study by Synchrotron Radiation-based Phase-Contrast Microtomography. Int. J. Radiol. Imaging Technol. 2016, 2, 10. [Google Scholar] [CrossRef]
- Huang, D.; Swanson, E.A.; Lin, C.P.; Schuman, J.S.; Stinson, W.G.; Chang, W.; Hee, M.R.; Flotte, T.; Gregory, K.; Puliafito, C.A.; et al. Optical coherence tomography. Science 1991, 254, 1178–1181. [Google Scholar] [CrossRef]
- Drexler, W.; Liu, M.; Kumar, A.; Kamali, T.; Unterhuber, A.; Leitgeb, R.A. Optical coherence tomography today: Speed, contrast, and multimodality. J. Biomed. Opt. 2014, 19, 071412. [Google Scholar] [CrossRef]
- Podoleanu, A.; Bradu, A. Master–slave interferometry for parallel spectral domain interferometry sensing and versatile 3D optical coherence tomography. Opt. Express. 2013, 21, 19324–19338. [Google Scholar] [CrossRef]
- Monroy, G.L.; Won, J.; Spillman, D.R.; Dsouza, R.; Boppart, S.A. Clinical translation of handheld optical coherence tomography: Practical considerations and recent advancements. J. Biomed. Opt. 2017, 22, 121715. [Google Scholar] [CrossRef] [PubMed]
- Duma, V.F.; Dobre, G.; Demian, D.; Cernat, R.; Dobre, G.; Negrutiu, M.L.; Topala, F.I.; Hutiu, G.; Bradu, A.; Podoleanu, A.G. Handheld scanning probes for optical coherence tomography. Rom. Rep. Phys. 2015, 67, 1346–1358. [Google Scholar]
- Rominu, M.; Manescu, A.; Sinescu, C.; Negrutiu, M.L.; Topala, F.; Rominu, R.O.; Bradu, A.; Jackson, D.A.; Giuliani, A.; Podoleanu, A.G. Zirconia enriched dental adhesive: A solution for OCT contrast enhancement. Demonstrative study by synchrotron radiation microtomography. Dent. Mater. 2014, 30, 417–423. [Google Scholar] [CrossRef] [PubMed]


| Manufacturer | IRRADIA Mid-Laser® Stockholm, Sweden |
|---|---|
| Model Identifier | MID-laser; Serial no 8110131-4 |
| Year Produced | 2007 |
| Number and type of emitters | Gallium-Aluminum–Arsenide laser (GaAlAs) laser |
| Wavelength and bandwidth | 808 nm |
| Pulse mode | CW |
| Beam spot size at target | 1 cm2 |
| Irradiance at target | 450 mW/cm2 |
| If pulsed peak irradiance | 450 mW/cm2 |
| Exposure duration | 17 s per point, 85 s per session |
| Radiant exposure | 24.075 J/cm2 |
| Radiant energy | 18.9 J |
| Number of points irradiated | 5 |
| Area irradiated | 1 cm2 |
| Application technique | Photobiomodulation was applied to the skin covering the surgical defect in four peripheral opposite points and in one central point of the defect (the size of the defect was 5 mm in diameter), using a plastic surgical guide |
| Number and frequency of treatment sessions | Surgery day and every 48 h after the surgery, for 14 days, 21 days, and 30 days respectively |
| Total radiant energy over entire treatment course | 151.2 J for the 14 days group; 226.8 J for the 21 days group; 302.4 J for the 30 days group |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luca, R.E.; Giuliani, A.; Mănescu, A.; Heredea, R.; Hoinoiu, B.; Constantin, G.D.; Duma, V.-F.; Todea, C.D. Osteogenic Potential of Bovine Bone Graft in Combination with Laser Photobiomodulation: An Ex Vivo Demonstrative Study in Wistar Rats by Cross-Linked Studies Based on Synchrotron Microtomography and Histology. Int. J. Mol. Sci. 2020, 21, 778. https://doi.org/10.3390/ijms21030778
Luca RE, Giuliani A, Mănescu A, Heredea R, Hoinoiu B, Constantin GD, Duma V-F, Todea CD. Osteogenic Potential of Bovine Bone Graft in Combination with Laser Photobiomodulation: An Ex Vivo Demonstrative Study in Wistar Rats by Cross-Linked Studies Based on Synchrotron Microtomography and Histology. International Journal of Molecular Sciences. 2020; 21(3):778. https://doi.org/10.3390/ijms21030778
Chicago/Turabian StyleLuca, Ruxandra Elena, Alessandra Giuliani, Adrian Mănescu, Rodica Heredea, Bogdan Hoinoiu, George Dumitru Constantin, Virgil-Florin Duma, and Carmen Darinca Todea. 2020. "Osteogenic Potential of Bovine Bone Graft in Combination with Laser Photobiomodulation: An Ex Vivo Demonstrative Study in Wistar Rats by Cross-Linked Studies Based on Synchrotron Microtomography and Histology" International Journal of Molecular Sciences 21, no. 3: 778. https://doi.org/10.3390/ijms21030778
APA StyleLuca, R. E., Giuliani, A., Mănescu, A., Heredea, R., Hoinoiu, B., Constantin, G. D., Duma, V.-F., & Todea, C. D. (2020). Osteogenic Potential of Bovine Bone Graft in Combination with Laser Photobiomodulation: An Ex Vivo Demonstrative Study in Wistar Rats by Cross-Linked Studies Based on Synchrotron Microtomography and Histology. International Journal of Molecular Sciences, 21(3), 778. https://doi.org/10.3390/ijms21030778








