Chrysin Modulates Genes Related to Inflammation, Tissue Remodeling, and Cell Proliferation in the Gastric Ulcer Healing
Abstract
1. Introduction
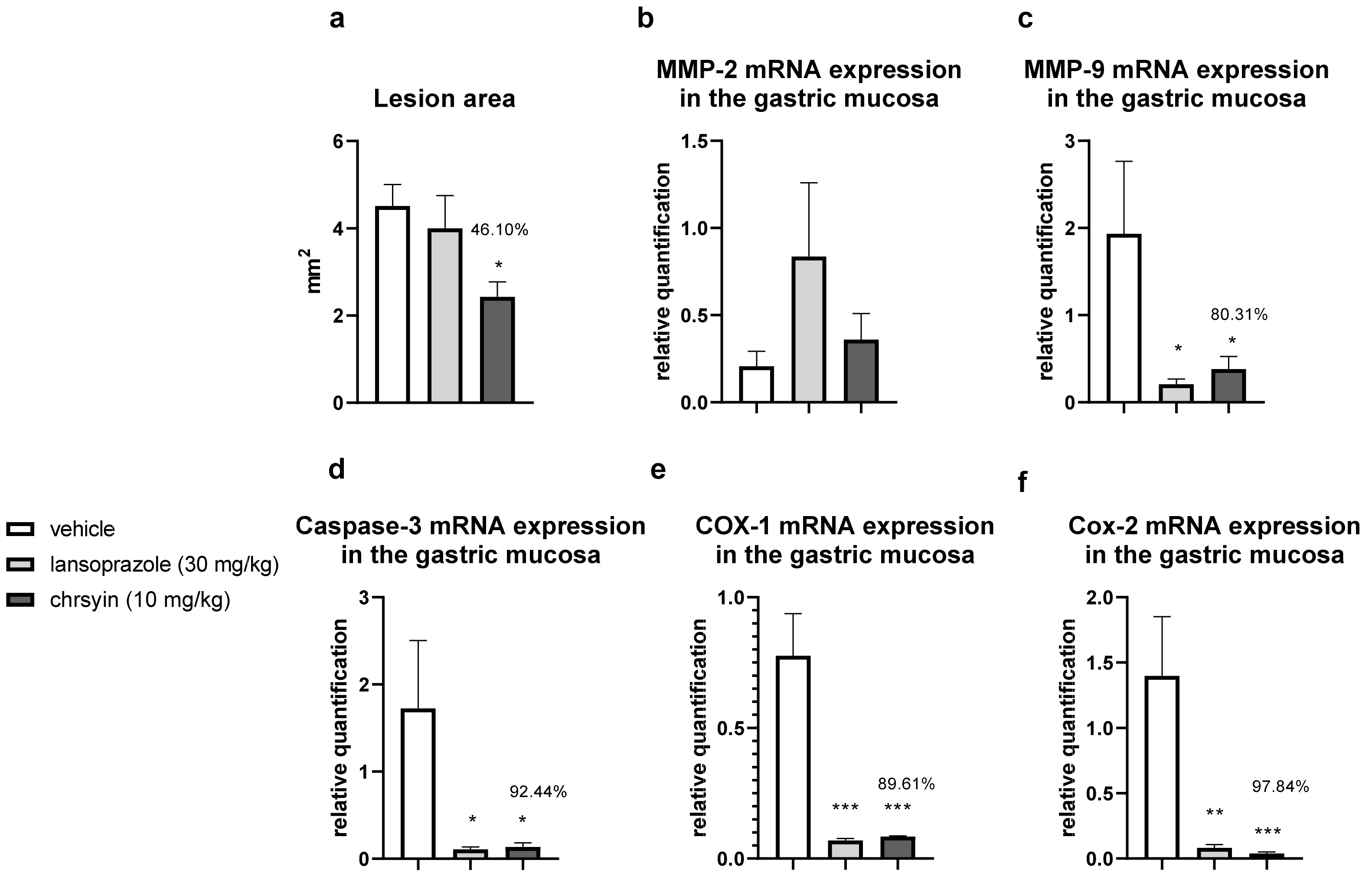
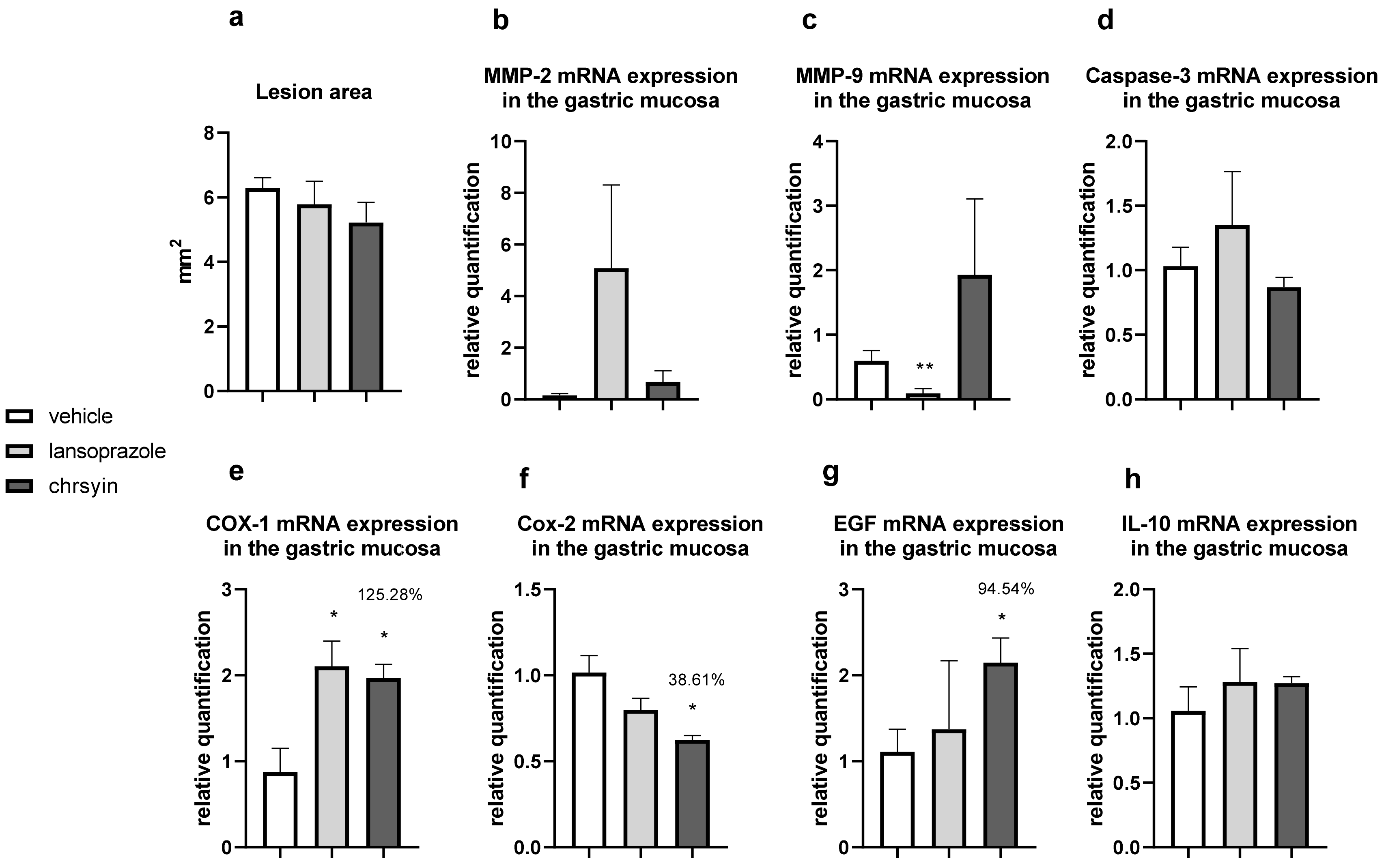
2. Results and Discussion
3. Materials and Methods
3.1. Animals
3.2. Evaluation of Gastric Protective and Healing Effects of Chrysin
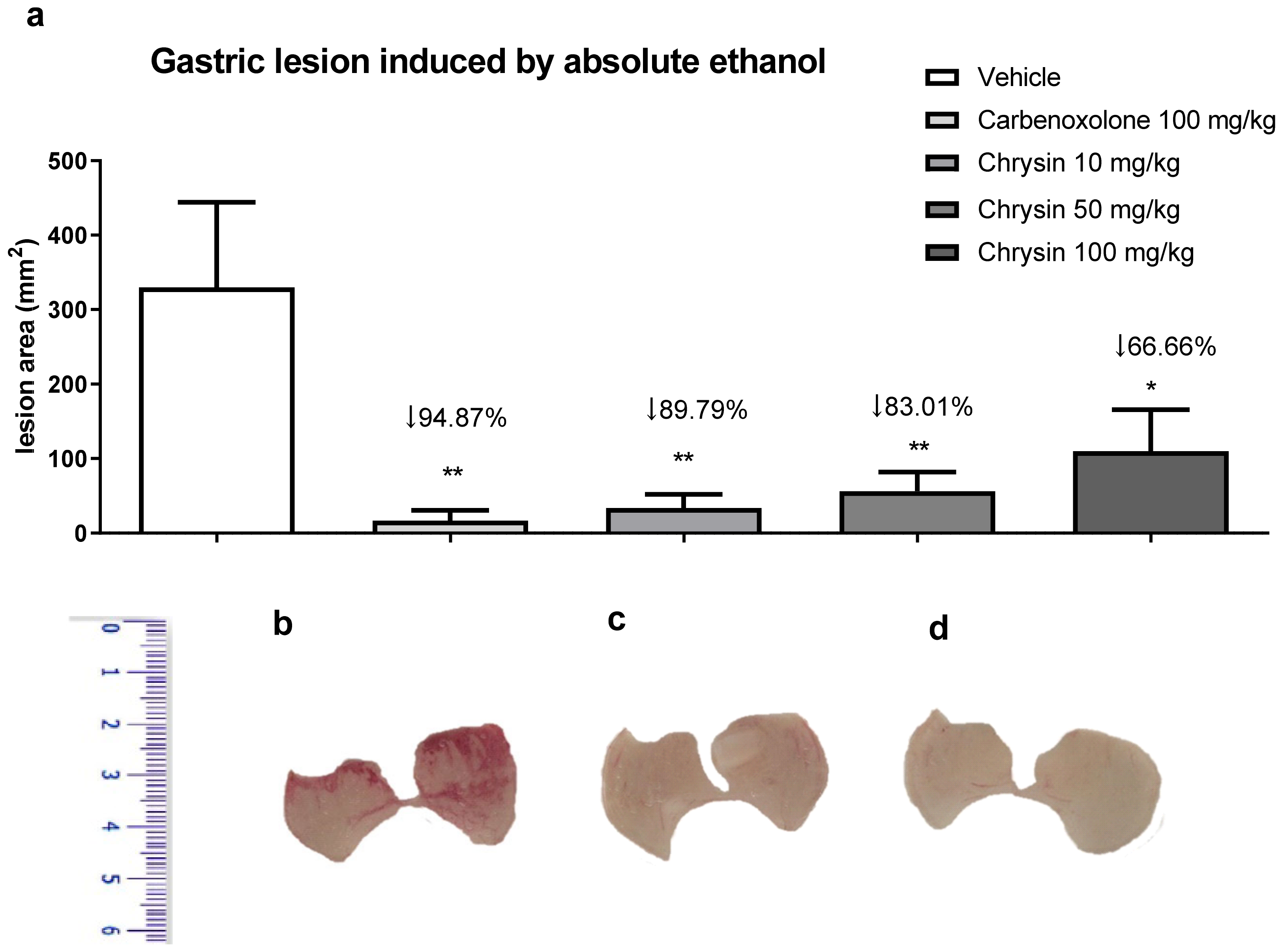
3.2.1. Gastric Lesions Induced Absolute Ethanol [34]
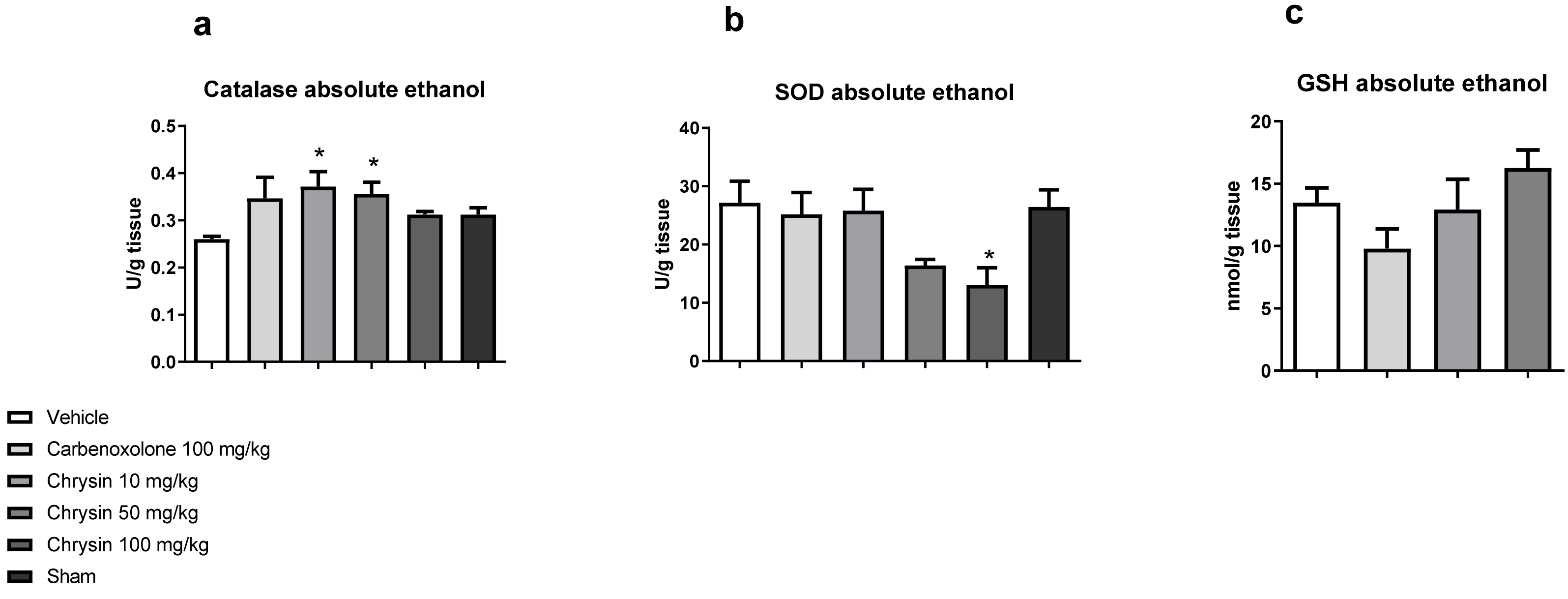
3.2.2. Quantification of Catalase Activity [35]
3.2.3. Quantification of Superoxide Dismutase Activity [36]
3.2.4. Quantification of Reduced Glutathione Levels [37]
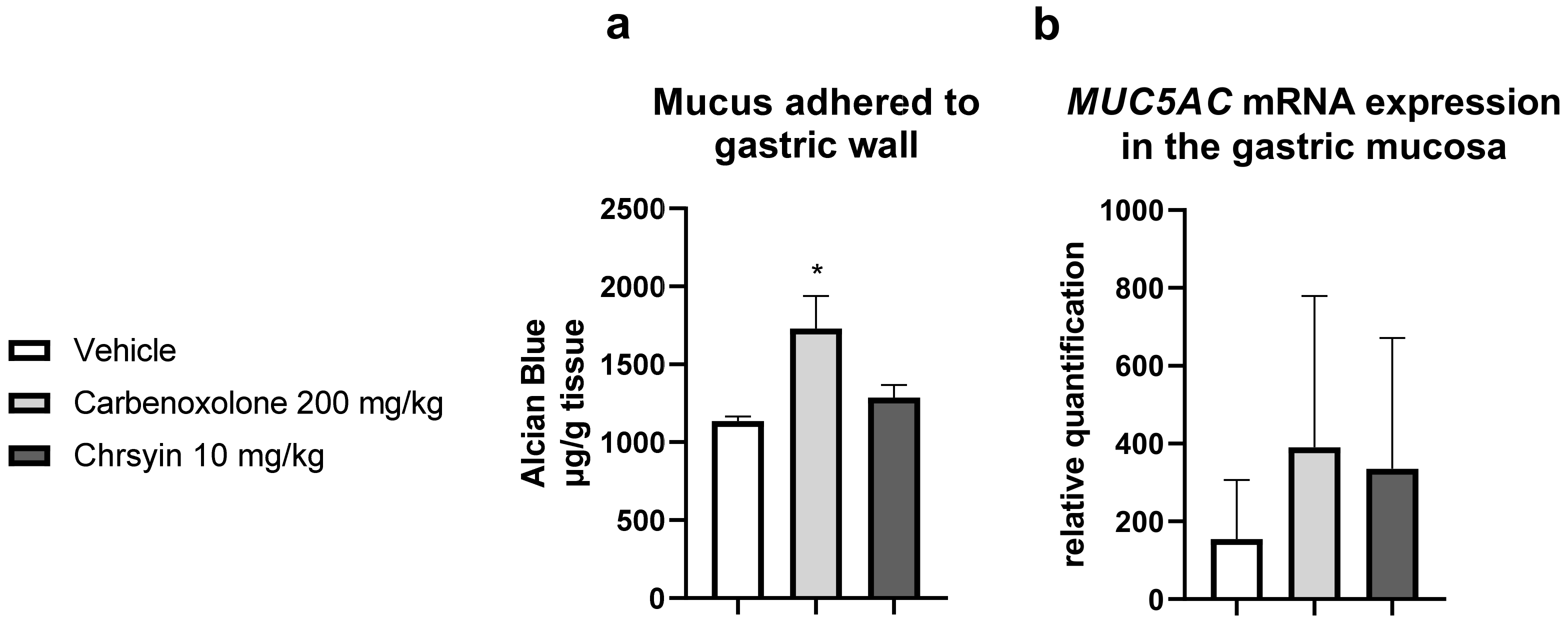
3.2.5. Quantification of Mucus Adhered to Gastric Wall [38]
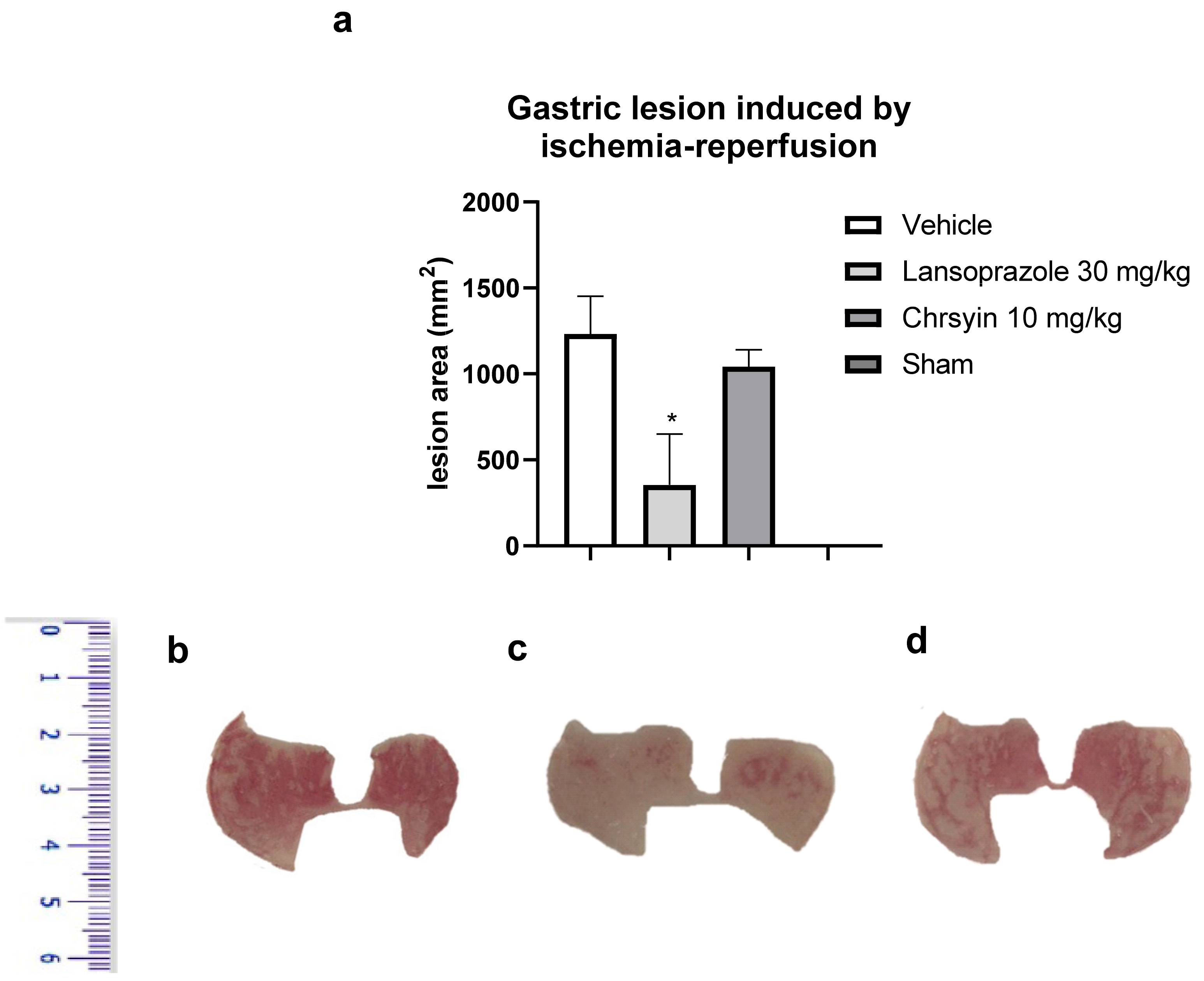
3.2.6. Gastric Ulcer Induced by Ischemia-Reperfusion [39]
3.2.7. Gastric Ulcer Induced by Acetic Acid [40,41,42]
3.2.8. Gastric Mucosa RNA Extraction and mRNA Studies [43,44]
3.3. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| CAT | catalase |
| COX-1 | cyclooxygenase 1 |
| COX-2 | cyclooxygenase 2 |
| EGF | epidermal growth factor |
| GSH | reduced glutathione |
| IL-10 | interleukin 10 |
| MMP-2 | metalloproteinase 2 |
| MMP-9 | metalloproteinase 9 |
| MUC5AC | mucin 5ac |
| NSAID | non-steroidal anti-inflammatory drug |
| PPI | proton pump inhibitor |
| SOD | super oxide dismutase |
References
- Kirsch, J.M.; Hirsch-Reilly, C. Peptic ulcer disease. Acute Care Gen. Surg. Work. Manag. 2017, 390, 159–164. [Google Scholar]
- Yang, Y.; Wang, S.; Bao, Y.R.; Li, T.J.; Yang, G.L.; Chang, X.; Meng, X.S. Anti-ulcer effect and potential mechanism of licoflavone by regulating inflammation mediators and amino acid metabolism. J. Ethnopharm. 2017, 199, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Minozzo, B.R.; Lemes, B.M.; Justo, A.D.S.; Lara, J.E.; Petry, V.E.K.; Fernandes, D.; Belló, C.; Vellosa, J.C.R.; Campagnoli, E.B.; Nunes, O.C.; et al. Anti-ulcer mechanisms of polyphenols extract of Euphorbia umbellata (Pax) Bruyns (Euphorbiaceae). J. Ethnopharm. 2016, 191, 29–40. [Google Scholar] [CrossRef] [PubMed]
- De Souza Almeida, E.S.; Filho, V.C.; Niero, R.; Clasen, B.K.; Balogun, S.O.; De Oliveira Martins, D.T. Pharmacological mechanisms underlying the anti-ulcer activity of methanol extract and canthin-6-one of Simaba ferruginea A. St-Hil. in animal models. J. Ethnopharm. 2011, 134, 630–636. [Google Scholar] [CrossRef] [PubMed]
- Cheung, K.S.; Chan, E.W.; Wong, A.Y.S.; Chen, L.; Wong, I.C.K.; Leung, W.K. Long-term proton pump inhibitors and risk of gastric cancer development after treatment for Helicobacter pylori: A population-based study. Gut 2018, 67, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Medic-Saric, M.; Rastija, V.; Bojic, M. Recent advances in the application of high performance liquid chromatography in the analysis of polyphenols in wine and propolis. J. AOAC Int. 2011, 94, 32–42. [Google Scholar] [PubMed]
- Pichichero, E.; Cicconi, R.; Mattei, M.; Canini, A. Chrysin-induced apoptosis is mediated through p38 and Bax activation in B16-F1 and A375 melanoma cells. Int. J. Oncol. 2011, 38, 473–483. [Google Scholar]
- Mani, R.; Natesan, V. Chrysin: Sources, beneficial pharmacological activities, and molecular mechanism of action. Phytochemistry 2018, 145, 187–196. [Google Scholar] [CrossRef]
- Rivera-Yañez, N.; Rodriguez-Canales, M.; Nieto-Yañez, O.; Jimenez-Estrada, M.; Ibarra-Barajas, M.; Canales-Martinez, M.M.; Rodriguez-Monroy, M.A. Hypoglycaemic and Antioxidant Effects of Propolis of Chihuahua in a Model of Experimental Diabetes. Evid. Based Complement. Altern. Med. 2018, 2018. [Google Scholar] [CrossRef]
- Lin, Y.M.; Chen, C.I.; Hsiang, Y.P.; Hsu, Y.C.; Cheng, K.C.; Chien, P.H.; Pan, H.L.; Lu, C.C.; Chen, Y.J. Chrysin Attenuates Cell Viability of Human Colorectal Cancer Cells through Autophagy Induction Unlike 5-Fluorouracil/Oxaliplatin. Int. J. Mol. Sci. 2018, 19, 1763. [Google Scholar] [CrossRef]
- Yu, C.H.; Suh, B.; Shin, I.; Kim, E.H.; Kim, D.; Shin, Y.J.; Chang, S.Y.; Baek, S.H.; Kim, H.; Bae, O.N. Inhibitory effects of a novel chrysin-derivative, CPD 6, on acute and chronic skin inflammation. Int. J. Mol. Sci. 2019, 20, 2607. [Google Scholar] [CrossRef] [PubMed]
- Dos Reis Lívero, F.A.; da Silva, L.M.; Ferreira, D.M.; Galuppo, L.F.; Borato, D.G.; Prando, T.B.L.; Lourenço, E.L.B.; Strapasson, R.L.B.; Stefanello, M.É.A.; de Paula Werner, M.F.; et al. Hydroethanolic extract of Baccharis trimera promotes gastroprotection and healing of acute and chronic gastric ulcers induced by ethanol and acetic acid. Naunyn. Schmiedebergs. Arch. Pharm. 2016, 389, 985–998. [Google Scholar] [CrossRef] [PubMed]
- Kwiecien, S.; Brzozowski, T.; Konturek, S.J. Effects of reactive oxygen species action on gastric mucosa in various models of mucosal injury. J. Physiol. Pharm. 2002, 53, 39–50. [Google Scholar]
- Rajasekaran, A.; Sivakumar, V.; Darlinquine, S. Role of Blepharis maderaspatensis and Ammannia baccifera plant extracts on in vitro oxygen radical scavenging, secretion of gastric fluid and gastroprotection on ulcer induced rats. Pharm. Biol. 2012, 50, 1085–1095. [Google Scholar] [CrossRef]
- Al-Quraishy, S.; Othman, M.S.; Dkhil, M.A.; Abdel Moneim, A.E. Olive (Olea europaea) leaf methanolic extract prevents HCl/ethanol-induced gastritis in rats by attenuating inflammation and augmenting antioxidant enzyme activities. Biomed. Pharm. 2017, 91, 338–349. [Google Scholar] [CrossRef]
- Naz, S.; Imran, M.; Rauf, A.; Orhan, I.E.; Shariati, M.A.; Iahtisham Ul, H.; IqraYasmin; Shahbaz, M.; Qaisrani, T.B.; Shah, Z.A.; et al. Chrysin: Pharmacological and therapeutic properties. Life Sci. 2019, 235, 116797. [Google Scholar] [CrossRef]
- Laine, L.; Takeuchi, K.; Tarnawski, A. Gastric Mucosal Defense and Cytoprotection: Bench to Bedside. Gastroenterology 2008, 135, 41–60. [Google Scholar] [CrossRef]
- Liu, Z.; Luo, Y.; Cheng, Y.; Zou, D.; Zeng, A.; Yang, C.; Xu, J.; Zhan, H. Gastrin attenuates ischemia-reperfusion-induced intestinal injury in rats. Exp. Biol. Med. 2016, 241, 873–881. [Google Scholar] [CrossRef]
- Magierowski, M.; Magierowska, K.; Hubalewska-Mazgaj, M.; Sliwowski, Z.; Pajdo, R.; Ginter, G.; Kwiecien, S.; Brzozowski, T.; Nagahara, N.; Wrobel, M. Exogenous and endogenous hydrogen sulfide protects gastric mucosa against the formation and time-dependent development of ischemia/reperfusion-induced acute lesions progressing into deeper ulcerations. Molecules 2017, 22, 295. [Google Scholar] [CrossRef]
- Mózsik, G. Gastric cytoprotection 30 years after its discovery by André Robert: A personal perspective. Inflammopharmacology 2010, 18, 209–221. [Google Scholar] [CrossRef]
- Adinortey, M.B.; Ansah, C.; Galyuon, I.; Nyarko, A. In Vivo Models Used for Evaluation of Potential Antigastroduodenal Ulcer Agents. Ulcers 2013, 2013, 295. [Google Scholar] [CrossRef]
- Takagi, K.; Okabe, S.; Saziki, R. A new method for the production of chronic gastric ulcer in rats and the effect of several drugs on its healing. Jpn. J. Pharm. 1969, 19, 418–426. [Google Scholar] [CrossRef] [PubMed]
- Périco, L.L.; Heredia-Vieira, S.C.; Beserra, F.P.; De Cássia Dos Santos, R.; Weiss, M.B.; Resende, F.A.; Dos Santos Ramos, M.A.; Bonifácio, B.V.; Bauab, T.M.; Varanda, E.A.; et al. Does the gastroprotective action of a medicinal plant ensure healing effects? An integrative study of the biological effects of Serjania marginata Casar. (Sapindaceae) in rats. J. Ethnopharm. 2015, 172, 312–324. [Google Scholar] [CrossRef] [PubMed]
- Schmassmann, A. Mechanisms of ulcer healing and effects of nonsteroidal anti-inflammatory drugs. Am. J. Med. 1998, 104, 43S–51S; discussion 79S–80S. [Google Scholar] [CrossRef]
- Tombulturk, F.K.; Soydas, T.; Sarac, E.Y.; Tuncdemir, M.; Coskunpinar, E.; Polat, E.; Sirekbasan, S.; Kanigur-Sultuybek, G. Regulation of MMP 2 and MMP 9 expressions modulated by AP-1 (c-jun) in wound healing: Improving role of Lucilia sericata in diabetic rats. Acta Diabetol. 2019, 56, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Gyenge, M.; Amagase, K.; Kunimi, S.; Matsuoka, R.; Takeuchi, K. Roles of pro-angiogenic and anti-angiogenic factors as well as matrix metalloproteinases in healing of NSAID-induced small intestinal ulcers in rats. Life Sci. 2013, 93, 441–447. [Google Scholar] [CrossRef]
- Lempinen, M.; Inkinen, K.; Wolff, H.; Ahonen, J. Matrix metalloproteinases 2 and 9 in indomethacin-induced rat gastric ulcer. Eur. Surg. Res. 2000, 32, 169–176. [Google Scholar] [CrossRef]
- Li, S.L.; Zhao, J.R.; Ren, X.Y.; Xie, J.P.; Ma, Q.Z.; Rong, Q.H. Increased expression of matrix metalloproteinase-9 associated with gastric ulcer recurrence. World J. Gastroenterol. 2013, 19, 4590–4595. [Google Scholar] [CrossRef]
- Yang, M.; Xiong, J.; Zou, Q.; Wang, D.D.; Huang, C.X. Chrysin attenuates interstitial fibrosis and improves cardiac function in a rat model of acute myocardial infarction. J. Mol. Histol. 2018, 49, 555–565. [Google Scholar] [CrossRef]
- Shalini, S.; Dorstyn, L.; Dawar, S.; Kumar, S. Old, new and emerging functions of caspases. Cell Death Differ. 2015, 22, 526–539. [Google Scholar] [CrossRef]
- Long, X.; Zhao, X.; Wang, W.; Zhang, Y.; Wang, H.; Liu, X.; Suo, H. Protective effect of silkworm pupa oil on hydrochloric acid/ethanol-induced gastric ulcers. J. Sci. Food Agric. 2019, 99, 2974–2986. [Google Scholar] [CrossRef] [PubMed]
- Milani, S.; Calabro, A. Role of growth factors and their receptors in gastric ulcer healing. Microsc. Res. Tech. 2001, 53, 360–371. [Google Scholar] [CrossRef] [PubMed]
- Arab, H.H.; Salama, S.A.; Omar, H.A.; Arafa, E.S.A.; Maghrabi, I.A. Diosmin protects against ethanol-induced gastric injury in rats: Novel anti-ulcer actions. PLoS ONE 2015, 10, e0122417. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Chen, Y.; Zhang, J.; Wang, L.; Jin, Z.; Huang, H.; Man, S.; Gao, W. Evaluation of protective effects of costunolide and dehydrocostuslactone on ethanol-induced gastric ulcer in mice based on multi-pathway regulation. Chem. Biol. Interact. 2016, 250, 68–77. [Google Scholar] [CrossRef]
- Aebi, H. Catalase in vitro. Methods Enzym. 1984, 105, 121–126. [Google Scholar]
- Winterbourn, C.C.; Hawkins, R.E.; Brian, M.; Carrell, R.W. The estimation of red cell superoxide dismutase activity. J. Lab. Clin. Med. 1975, 85, 337–341. [Google Scholar]
- Faure, P.; Lafond, J.-L. Measurement of plasma sulfhydryl and carbonyl groups as a possible indicator of protein oxidation. In Analysis of Free Radicals in Biological Systems; Birkhäuser Basel: Basel, Switzerland, 1995; pp. 237–248. [Google Scholar]
- Rafatullah, S.; Tariq, M.; Al-Yahya, M.A.; Mossa, J.S.; Ageel, A.M. Evaluation of turmeric (Curcuma longa) for gastric and duodenal antiulcer activity in rats. J. Ethnopharm. 1990, 29, 25–34. [Google Scholar] [CrossRef]
- Ueda, S.; Yoshikawa, T.; Takahashi, S.; Ichikawa, H.; Yasuda, M.; Oyamada, H.; Tanigawa, T.; Sugino, S.; Kondo, M. Role of free radicals and lipid peroxidation in gastric mucosal injury induced by ischemia-reperfusion in rats. Scand. J. Gastroenterol. Suppl. 1989, 162, 55–58. [Google Scholar] [CrossRef]
- Morimoto, Y.; Shimohara, K.; Oshima, S.; Sukamoto, T. Effects of the new anti-ulcer agent KB-5492 on experimental gastric mucosal lesions and gastric mucosal defensive factors, as compared to those of teprenone and cimetidine. Jpn. J. Pharmacol. 1991, 57, 495–505. [Google Scholar] [CrossRef]
- Okabe, S.; Roth, J.L.; Pfeiffer, C.J. A method for experimental, penetrating gastric and duodenal ulcers in rats. Observations on normal healing. Am. J. Dig. Dis. 1971, 16, 277–284. [Google Scholar] [CrossRef]
- Konturek, S.J.; Dembinski, A.; Warzecha, Z.; Brzozowski, T.; Gregory, H. Role of epidermal growth factor in healing of chronic gastroduodenal ulcers in rats. Gastroenterology 1988, 94, 1300–1307. [Google Scholar] [CrossRef]
- Dos Santos, T.W.; Miranda, J.; Teixeira, L.; Aiastui, A.; Matheu, A.; Gambero, A.; Portillo, M.P.; Ribeiro, M.L. Yerba Mate Stimulates Mitochondrial Biogenesis and Thermogenesis in High-Fat-Diet-Induced Obese Mice. Mol. Nutr. Food Res. 2018, 62, 1800142. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fagundes, F.L.; Piffer, G.d.M.; Périco, L.L.; Rodrigues, V.P.; Hiruma-Lima, C.A.; dos Santos, R.d.C. Chrysin Modulates Genes Related to Inflammation, Tissue Remodeling, and Cell Proliferation in the Gastric Ulcer Healing. Int. J. Mol. Sci. 2020, 21, 760. https://doi.org/10.3390/ijms21030760
Fagundes FL, Piffer GdM, Périco LL, Rodrigues VP, Hiruma-Lima CA, dos Santos RdC. Chrysin Modulates Genes Related to Inflammation, Tissue Remodeling, and Cell Proliferation in the Gastric Ulcer Healing. International Journal of Molecular Sciences. 2020; 21(3):760. https://doi.org/10.3390/ijms21030760
Chicago/Turabian StyleFagundes, Felipe Leonardo, Graziele de Morais Piffer, Larissa Lucena Périco, Vinicius Peixoto Rodrigues, Clélia Akiko Hiruma-Lima, and Raquel de Cássia dos Santos. 2020. "Chrysin Modulates Genes Related to Inflammation, Tissue Remodeling, and Cell Proliferation in the Gastric Ulcer Healing" International Journal of Molecular Sciences 21, no. 3: 760. https://doi.org/10.3390/ijms21030760
APA StyleFagundes, F. L., Piffer, G. d. M., Périco, L. L., Rodrigues, V. P., Hiruma-Lima, C. A., & dos Santos, R. d. C. (2020). Chrysin Modulates Genes Related to Inflammation, Tissue Remodeling, and Cell Proliferation in the Gastric Ulcer Healing. International Journal of Molecular Sciences, 21(3), 760. https://doi.org/10.3390/ijms21030760





