A Pentapeptide with Tyrosine Moiety as Fluorescent Chemosensor for Selective Nanomolar-Level Detection of Copper(II) Ions
Abstract
1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. Materials
3.2. Methods
3.2.1. UV Absorption Spectroscopy
3.2.2. Steady-State and Time-Resolved Fluorescence Spectroscopy
3.2.3. Analytical Procedure
3.2.4. NMR Spectroscopy
3.2.5. Liquid Chromatography Coupled with Mass Spectrometry (LC-MS)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Pesavento, M.; Alberti, G.; Biesuz, R. Analytical methods for determination of free metal ion concentration, labile species fraction and metal complexation capacity of environmental waters: A review. Anal. Chim. Acta 2009, 631, 129–141. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Hao, D.; Shen, W.; Qian, Z.; Zhu, C. Highly sensitive fluorescent sensor for copper (II) based on amplified fluorescence quenching of a water-soluble NIR emitting conjugated polymer. Microchim. Acta 2012, 177, 357–364. [Google Scholar] [CrossRef]
- Cowan, J.A. Inorganic Biochemistry: An Introduction, 2nd ed.; John Wiley & Sons: Hoboken, NJ, USA, 1997. [Google Scholar]
- Lazarchick, J. Update on anemia and neutropenia in copper deficiency. Curr. Opin. Hematol. 2012, 19, 58–60. [Google Scholar] [CrossRef] [PubMed]
- Bayindir, S. A simple rhodanine-based fluorescent sensor for mercury and copper: The recognition of Hg2+ in aqueous solution, and Hg2+/Cu2+ in organic solvent. J. Photochem. Photobiol. A Chem. 2019, 372, 235–244. [Google Scholar] [CrossRef]
- Liu, S.R.; Wu, S.P. An NBD-based sensitive and selective fluorescent sensor for copper (II) ion. J. Fluoresc. 2011, 21, 1599–1605. [Google Scholar] [CrossRef]
- Gaggelli, E.; Kozlowski, H.; Valensin, D.; Valensin, G. Copper homeostasis and neurodegenerative disorders (Alzheimer’s, prion, and Parkinson’s diseases and amyotrophic lateral sclerosis). Chem. Rev. 2006, 106, 1995–2044. [Google Scholar] [CrossRef]
- Yin, B.C.; Ye, B.C.; Tan, W.; Wang, H.; Xie, C.C. An allosteric dual-DNAzyme unimolecular probe for colorimetric detection of copper (II). J. Am. Chem. Soc. 2009, 131, 14624–14625. [Google Scholar] [CrossRef]
- Chaiyo, S.; Siangproh, W.; Apilux, A.; Chailapakul, O. Highly selective and sensitive paper-based colorimetric sensor using thiosulfate catalytic etching of silver nanoplates for trace determination of copper ions. Anal. Chim. Acta 2015, 866, 75–83. [Google Scholar] [CrossRef]
- Kaur, N.; Kumar, S. A diamide–diamine based Cu2+ chromogenic sensor for highly selective visual and spectrophotometric detection. Tetrahedron Lett. 2006, 47, 4109–4112. [Google Scholar] [CrossRef]
- Becker, J.S.; Matusch, A.; Depboylu, C.; Dobrowolska, J.; Zoriy, M.V. Quantitative imaging of selenium, copper, and zinc in thin sections of biological tissues (Slugs−Genus Arion) measured by laser ablation inductively coupled plasma mass spectrometry. Anal. Chem. 2007, 79, 6074–6080. [Google Scholar] [CrossRef]
- Doner, G.; Ege, A. Determination of copper, cadmium and lead in seawater and mineral water by flame atomic absorption spectrometry after coprecipitation with aluminum hydroxide. Anal. Chim. Acta 2005, 547, 14–17. [Google Scholar] [CrossRef]
- Herzog, G.; Arrigan, D.W. Application of disorganized monolayer films on gold electrodes to the prevention of surfactant inhibition of the voltammetric detection of trace metals via anodic stripping of underpotential deposits: Detection of copper. Anal. Chem. 2003, 75, 319–323. [Google Scholar] [CrossRef] [PubMed]
- Moerner, W.E.; Fromm, D.P. Methods of single-molecule fluorescence spectroscopy and microscopy. Rev. Scient. Instrum. 2003, 74, 3597–3619. [Google Scholar] [CrossRef]
- Chen, W.; Liu, X.; Chen, S.; Song, X.; Kang, J. A real-time colorimetric and ratiometric fluorescent probe for rapid detection of SO2 derivatives in living cells based on a near-infrared benzopyrylium dye. RSC Adv. 2015, 5, 25409–25415. [Google Scholar] [CrossRef]
- Carter, K.P.; Young, A.M.; Palmer, A.E. Fluorescent sensors for measuring metal ions in living systems. Chem. Rev. 2014, 114, 4564–4601. [Google Scholar] [CrossRef] [PubMed]
- Dai, Q.; Liu, H.; Gao, C.; Li, W.; Zhu, C.; Lin, C.; Tan, Y.; Yuan, Z.; Jiang, Y. A one-step synthesized acridine-based fluorescent chemosensor for selective detection of copper(II) ions and living cell imaging. New J. Chem. 2018, 42, 613–618. [Google Scholar] [CrossRef]
- Han, J.; Tang, X.; Wang, Y.; Liu, R.; Wang, L.; Ni, L. A quinoline-based fluorescence “on-off-on” probe for relay identification of Cu2+ and Cd2+ ions. Spectrochim. Acta A 2018, 205, 597–602. [Google Scholar] [CrossRef]
- Puangsamlee, T.; Tachapermpon, Y.; Kammalun, P.; Sukrat, K.; Wainiphithapong, C.; Sirirak, J.; Wanichacheva, N. Solvent control bifunctional fluorescence probe for selective detection of Cu2+ and Hg2+ via the excimer of pyrenylacetamide subunits. J. Luminesc. 2018, 196, 227–235. [Google Scholar] [CrossRef]
- Raju, M.; Nair, R.R.; Raval, I.H.; Haldar, S.; Chatterjee, P.B. A water soluble Cu2+-specific colorimetric probe can also detect Zn2+ in live shrimp and aqueous environmental samples by fluorescence channel. Sens. Act. B Chem. 2018, 260, 364–370. [Google Scholar] [CrossRef]
- Makowska, J.; Żamojć, K.; Wyrzykowski, D.; Wiczk, W.; Chmurzyński, L. Copper(II) complexation by fragment of central part of FBP28 protein from Mus musculus. Biophys. Chem. 2018, 241, 55–60. [Google Scholar] [CrossRef]
- Tominaga, T.T.; Imasato, H.; Nascimento, O.R.; Tabak, M. Interaction of tyrosine and tyrosine dipeptides with Cu2+ ions: A fluorescence study. Anal. Chim. Acta 1995, 315, 217–224. [Google Scholar] [CrossRef]
- Makowska, J.; Żamojć, K.; Wyrzykowski, D.; Żmudzińska, W.; Uber, D.; Wierzbicka, M.; Wiczk, W.; Chmurzyński, L. Probing the binding of Cu2+ ions to a fragment of the Aβ(1–42) polypeptide using fluorescence spectroscopy, isothermal titration calorimetry and molecular dynamics simulations. Biophys. Chem. 2016, 216, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Kozłowski, H.; Kowalik-Jankowska, T.; Jeżowska-Bojczuk, M. Chemical and biological aspects of Cu2+ interactions with peptides and aminoglycosides. Coord. Chem. Rev. 2005, 249, 2323–2334. [Google Scholar] [CrossRef]
- Hoernke, M.; Koksch, B.; Brezesinski, G. Amyloidogenic peptides at hydrophobic–hydrophilic interfaces: Coordination affinities and the chelate effect dictate the competitive binding of Cu2+ and Zn2+. ChemPhysChem 2011, 12, 2225–2229. [Google Scholar] [CrossRef]
- Yip, T.T.; Nakagawa, Y.; Porath, J. Evaluation of the interaction of peptides with Cu(II), Ni(II), and Zn(II) by high-performance immobilized metal ion affinity chromatography. Anal. Biochem. 1989, 183, 159–171. [Google Scholar] [CrossRef]
- Sigel, H.; Martin, R.B. Coordinating properties of the amide bond. Stability and structure of metal ion complexes of peptides and related ligands. Chem. Rev. 1982, 82, 385–426. [Google Scholar] [CrossRef]
- Yang, H.; Liu, Z.Q.; Zhou, Z.G.; Shi, E.X.; Li, F.Y.; Du, Y.K.; Yi, Y.; Huang, C.H. Highly selective ratiometric fluorescent sensor for Cu(II) with two urea groups. Tetrahedr. Lett. 2006, 47, 2911–2914. [Google Scholar] [CrossRef]
- Ma, D.L.; He, H.Z.; Chan, D.S.H.; Wong, C.Y.; Leung, C.H. A colorimetric and luminescent dual-modal assay for Cu(II) ion detection using an iridium(III) complex. PLoS ONE 2014, 9, e99930. [Google Scholar] [CrossRef]
- Arabahmadi, R. A reversible fluorescence “ON–OFF–ON” sensor for sequential detection of F− and Cu2+ ions and its application as a molecular-scale logic device and security keypad lock. J. Coord. Chem. 2019, 72, 1187–1202. [Google Scholar] [CrossRef]
- Fu, Y.; Feng, Q.C.; Jiang, X.J.; Xu, H.; Li, M.; Zang, S.Q. New fluorescent sensor for Cu2+ and S2− in 100% aqueous solution based on displacement approach. Dalton Trans. 2014, 43, 5815–5822. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, L.; Wang, L.; Guo, Y.; Cai, L.; Yu, M.; Wei, L. Highly sensitive and selective fluorescent sensor for Zn2+/Cu2+ and new approach for sensing Cu2+ by central metal displacement. Chem. Commun. 2011, 47, 5798–5800. [Google Scholar] [CrossRef] [PubMed]
- Formica, M.; Fusi, V.; Giorgi, L.; Micheloni, M. New fluorescent chemosensors for metal ions in solution. Coord. Chem. Rev. 2012, 256, 170–192. [Google Scholar] [CrossRef]
- Praikaew, P.; Maniam, S.; Charoenpanich, A.; Sirirak, J.; Promarak, V.; Langford, S.J.; Wanichacheva, N. Water-soluble Cu2+-fluorescent sensor based on core-substituted naphthalene diimide and its application in drinking water analysis and live cell imaging. J. Photochem. Photobiol. A Chem. 2019, 382, 111852. [Google Scholar] [CrossRef]
- Yang, W.; Chen, X.; Su, H.; Fang, W.; Zhang, Y. The fluorescence regulation mechanism of the paramagnetic metal in a biological HNO sensor. Chem. Commun. 2015, 51, 9616–9619. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.S.; Kwon, P.S.; Lee, J.W.; Kim, J.I.; Hong, C.S.; Kim, J.W.; Yan, S.; Lee, J.Y.; Lee, J.H.; Joo, T.; et al. Coumarin-derived Cu2+-selective fluorescence sensor: Synthesis, mechanisms, and applications in living cells. J. Am. Chem. Soc. 2009, 131, 2008–2012. [Google Scholar] [CrossRef] [PubMed]
- Bosque-Sendra, J.M.; Almansa-López, E.; García-Campaña, M.; Cuadros-Rodríguez, L. Data analysis in the determination of stoichiometries and stability constants of complexes. Anal. Sci. 2003, 19, 1431–1439. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gans, P.; Sabatini, A.; Vacca, A. HypSpec. 2008. Available online: http://www.hyperquad.co.uk (accessed on 16 August 2019).
- Abbas, I.; Vranic, M.; Hoffmann, H.; El-Khatib, A.; Montes-Bayón, M.; Möller, H.; Weller, M. Investigations of the copper peptide hepcidin-25 by LC-MS/MS and NMR. Int. J. Mol. Sci. 2018, 19, 2271. [Google Scholar] [CrossRef]
- Gaggelli, E.; D’Amelio, N.; Valensin, D.; Valensin, G. 1H NMR studies of copper binding by histidine-containing peptides. Magn. Reson. Chem. 2003, 41, 877–883. [Google Scholar] [CrossRef]
- Zoroddu, M.A.; Serenella, M.; Peana, M. Copper and nickel binding in multi-histidinic peptide fragments. J. Inorg. Biochem. 2009, 103, 1214–1220. [Google Scholar] [CrossRef]
- De Ricco, R.; Potocki, S.; Kozłowski, H.; Valensin, D. NMR investigations of metal interactions with unstructured soluble protein domains. Coord. Chem. Rev. 2014, 269, 1–12. [Google Scholar] [CrossRef]
- Deschamps, P.; Zerrouk, N.; Martens, T.; Charlot, M.F.; Girerd, J.J.; Chaumeil, J.C.; Tomas, A. Copper complexation by amino acid: L-glutamine–copper(II)–L-histidine ternary system. J. Trace Microprobe Tech. 2003, 21, 729–741. [Google Scholar] [CrossRef]
- Gianelli, L.; Amendola, V.; Fabbrizzi, L.; Pallavicini, P.; Mellerio, G.G. Investigation of reduction of Cu(II) complexes in positive-ion mode electrospray mass spectrometry. Rapid Commun. Mass Spectrom. 2001, 15, 2347–2353. [Google Scholar] [CrossRef]
- Lavanant, H.; Virelizier, H.; Hoppilliard, Y. Reduction of copper(II) complexes by electron capture in an electrospray ionization source. J. Am. Soc. Mass Spectrom. 1998, 9, 1217–1221. [Google Scholar] [CrossRef]
- An, Y.; Wang, P.; Yue, Z. A sequential and reversibility fluorescent pentapeptide probe for Cu(II) ions and hydrogen sulfide detections and its application in two different living cells imaging. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2019, 216, 319–327. [Google Scholar] [CrossRef]
- Kaewnok, N.; Petdum, A.; Sirirak, J.; Charoenpanich, A.; Panchan, W.; Sahasithiwat, S.; Sooksimuang, T.; Wanichacheva, N. Novel Cu2+-specific “Turn-ON” fluorescent probe based on [5]helicene with very large Stokes shift and its potential application in living cells. New J. Chem. 2008, 42, 5540–5547. [Google Scholar] [CrossRef]
- Wang, P.; Wu, J. Highly selective and sensitive detection of Zn(II) and Cu(II) ions using a novel peptide fluorescent probe by two different mechanisms and its application in live cell imaging. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2019, 208, 140–149. [Google Scholar] [CrossRef]
- Seo, H.; An, M.; Kim, B.Y.; Choi, J.H.; Helal, A.; Kim, H.S. Highly selective fluorescent probe for sequential recognition of copper (II) and iodide ions. Tetrahedron 2017, 73, 4684–4691. [Google Scholar] [CrossRef]
- Lu, M.C.; Chiu, L.Y.; Chiu, L.Y.; Lin, C.Y.; Horng, J.C. Highly selective and water-soluble peptidyl chemosensors for copper(ii) and mercury(ii) ions based on a β-hairpin structure. Anal. Meth. 2013, 5, 1702–1707. [Google Scholar] [CrossRef]
- Uber, D.; Wyrzykowski, D.; Tiberi, C.; Sabatino, G.; Żmudzińska, G.; Chmurzyński, L.; Papini, A.M.; Makowska, J. Conformation-dependent affinity of Cu(II) ions peptide complexes derived from the human Pin1 protein. J. Therm. Anal. Calorim. 2017, 127, 1431–1443. [Google Scholar] [CrossRef]
- Pelton, J.T.; McLean, L.R. Spectroscopic methods for analysis of protein secondary structure. Anal. Biochem. 2000, 277, 167–176. [Google Scholar] [CrossRef]
- Lakowicz, J.R. Principles of Fluorescence Spectroscopy, 3rd ed.; Springer Science & Business Media: Berlin, Germany, 2013. [Google Scholar]
- Żamojć, K.; Wiczk, W.; Chmurzyński, L. The influence of the type of substituents and the solvent on the interactions between different coumarins and selected TEMPO analogues–Fluorescence quenching studies. Chem. Phys. 2018, 513, 188–194. [Google Scholar] [CrossRef]
- Żamojć, K.; Zdrowowicz, M.; Hać, A.; Witwicki, M.; Rudnicki-Velasquez, P.B.; Wyrzykowski, D.; Wiczk, W.; Chmurzyński, L. Dihydroxy-substituted coumarins as fluorescent probes for nanomolar-level detection of the 4-amino-TEMPO spin label. Int. J. Mol. Struct. 2019, 20, 3802. [Google Scholar] [CrossRef] [PubMed]
- Goddard, T.D.; Kneller, D.G. SPARKY3. 2002. Available online: https://omictools.com/sparky-tool (accessed on 5 November 2019).

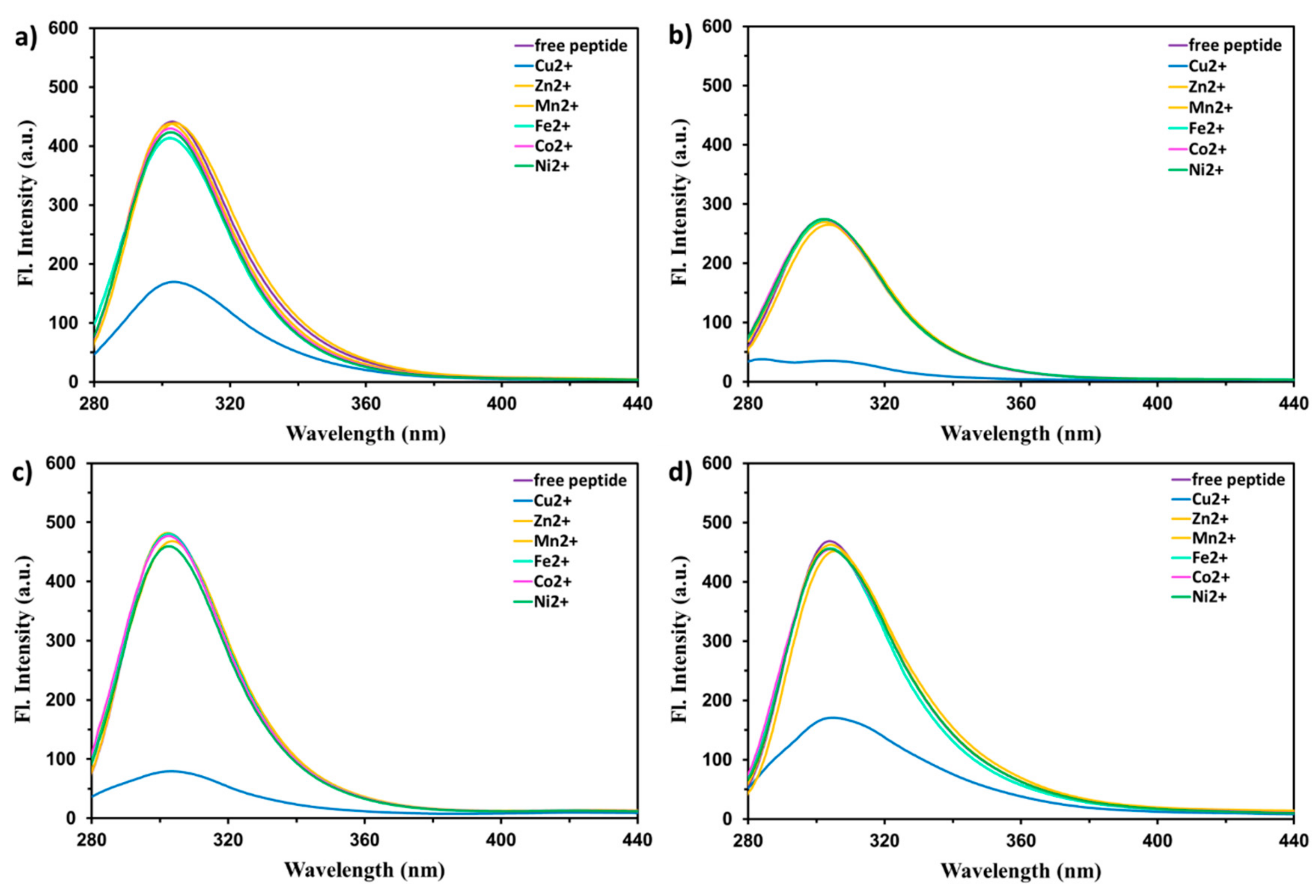

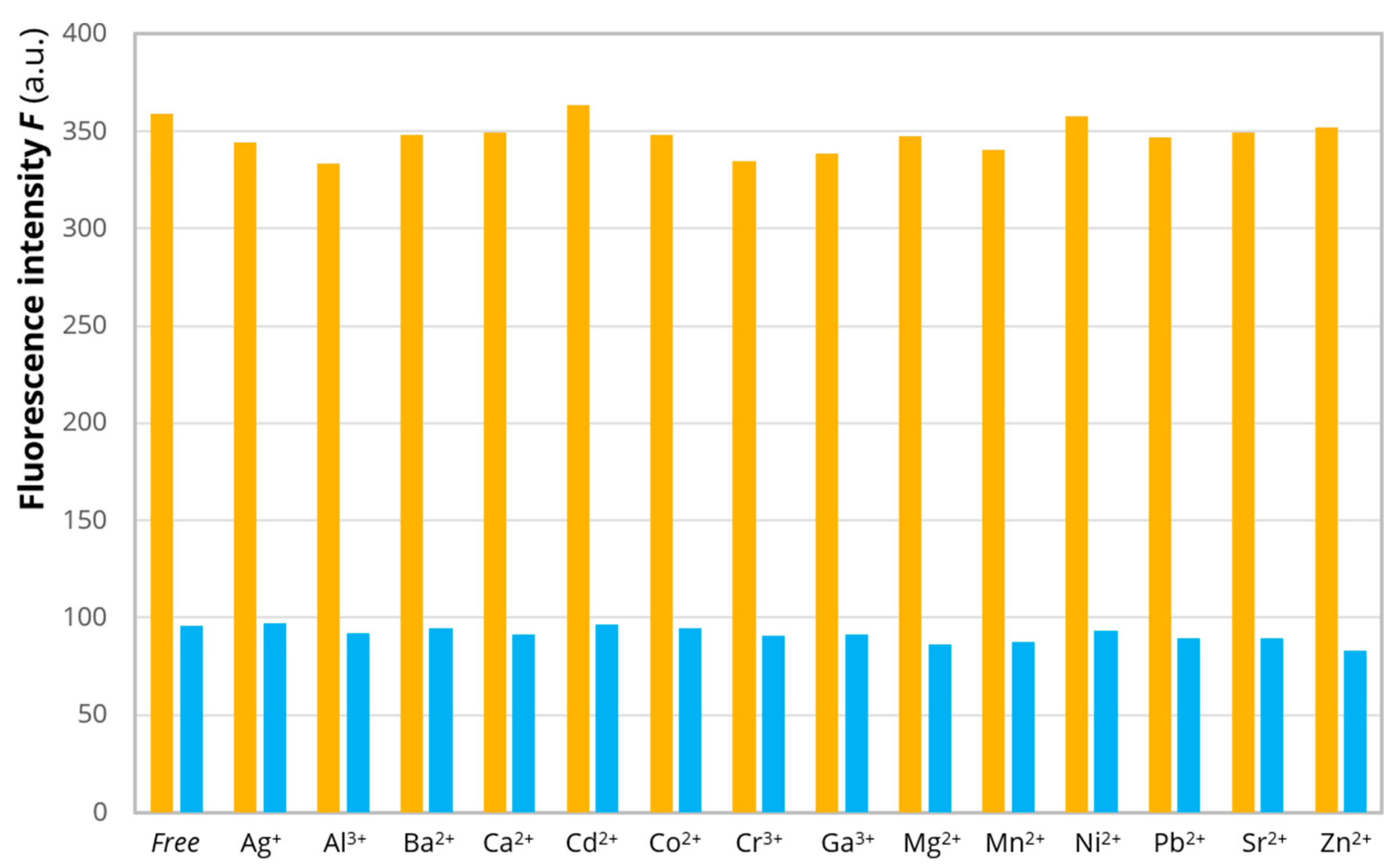
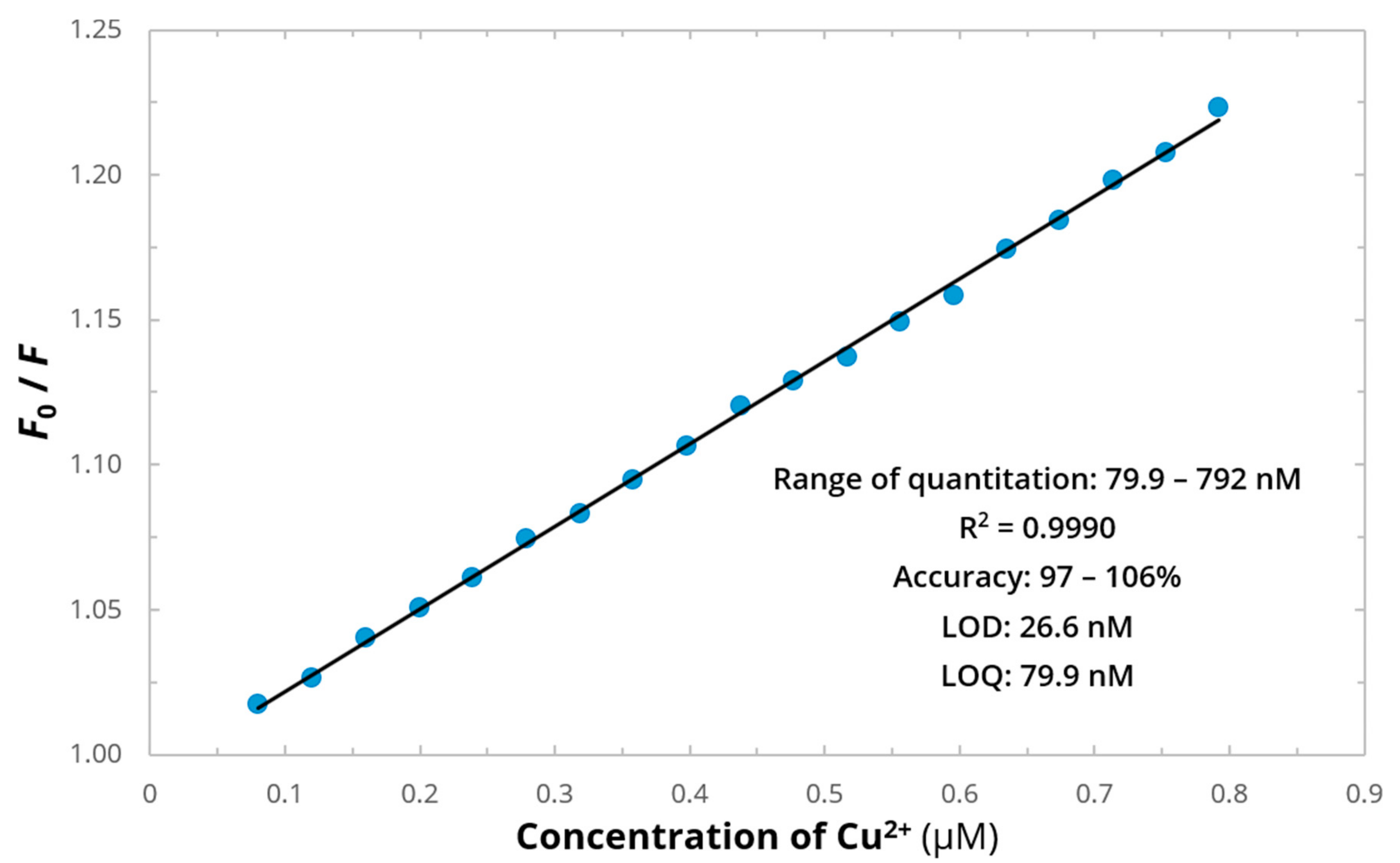

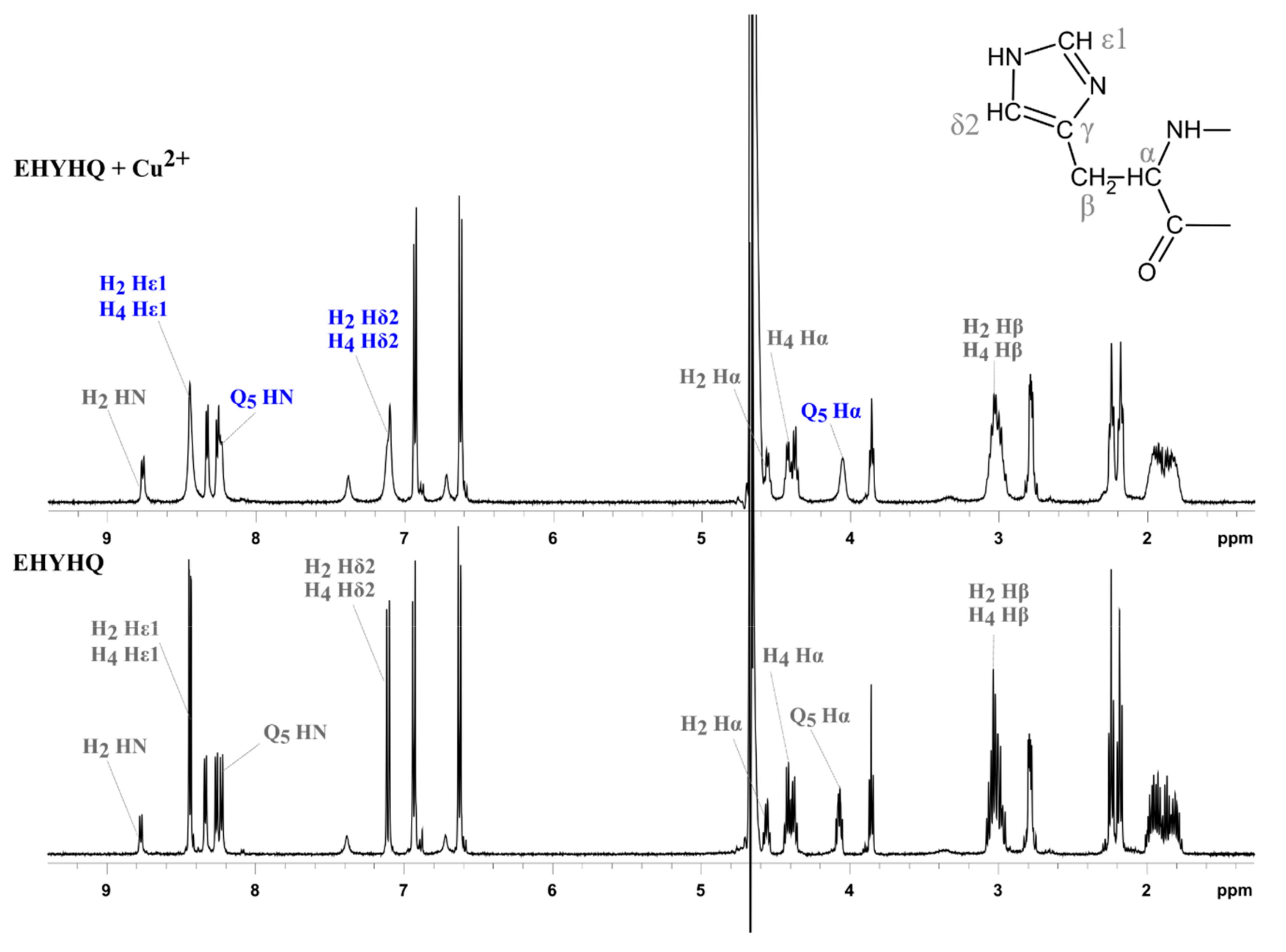

| Reaction | logKn | Cu2+-EYHHQ | Cu2+-EHYHQ | Cu2+-EHHQY | Cu2+-KYHHE |
|---|---|---|---|---|---|
| Cu + P = CuP | logK1 | 1.48 (±0.03) | 1.25 (±0.01) | 1.55 (±0.01) | 1.46 (±0.01) |
| CuP + P = CuP2 | logK2 | 3.34 (±0.08) | 3.25 (±0.02) | 3.34 (±0.02) | 2.55 (±0.05) |
| Cu + 2P = CuP2 | logK | 4.82 (±0.04) | 4.50 (±0.01) | 4.89 (±0.01) | (±0.01) |
| Reference | LOD | Operation System | Reversibility | pH |
|---|---|---|---|---|
| [17] | 120 nM | ON-OFF (HEPES buffer) | - | 5.0–8.0 |
| [46] | 23.5 nM | ON-OFF (HEPES buffer) | yes | 6.0–12.0 |
| [34] | 10.9 nM | ON-OFF (PBS buffer) | - | 5.0–8.0 |
| [47] | 40.4 nM | OFF-ON (H2O:CH3CN, 1:9 v/v) | - | 5.0–7.0 |
| [48] | 15 nM | ON-OFF (HEPES buffer) | yes | 7.0–12.0 |
| [49] | 1 μM | ON-OFF (EtOH) | - | - |
| [50] | 0.55–0.62 μM | ON-OFF (phosphate buffer) | - | 7.0–11.0 |
| This work | 26.6 nM | ON-OFF (MES buffer) | yes | 4.0–11.0 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Żamojć, K.; Kamrowski, D.; Zdrowowicz, M.; Wyrzykowski, D.; Wiczk, W.; Chmurzyński, L.; Makowska, J. A Pentapeptide with Tyrosine Moiety as Fluorescent Chemosensor for Selective Nanomolar-Level Detection of Copper(II) Ions. Int. J. Mol. Sci. 2020, 21, 743. https://doi.org/10.3390/ijms21030743
Żamojć K, Kamrowski D, Zdrowowicz M, Wyrzykowski D, Wiczk W, Chmurzyński L, Makowska J. A Pentapeptide with Tyrosine Moiety as Fluorescent Chemosensor for Selective Nanomolar-Level Detection of Copper(II) Ions. International Journal of Molecular Sciences. 2020; 21(3):743. https://doi.org/10.3390/ijms21030743
Chicago/Turabian StyleŻamojć, Krzysztof, Dominik Kamrowski, Magdalena Zdrowowicz, Dariusz Wyrzykowski, Wiesław Wiczk, Lech Chmurzyński, and Joanna Makowska. 2020. "A Pentapeptide with Tyrosine Moiety as Fluorescent Chemosensor for Selective Nanomolar-Level Detection of Copper(II) Ions" International Journal of Molecular Sciences 21, no. 3: 743. https://doi.org/10.3390/ijms21030743
APA StyleŻamojć, K., Kamrowski, D., Zdrowowicz, M., Wyrzykowski, D., Wiczk, W., Chmurzyński, L., & Makowska, J. (2020). A Pentapeptide with Tyrosine Moiety as Fluorescent Chemosensor for Selective Nanomolar-Level Detection of Copper(II) Ions. International Journal of Molecular Sciences, 21(3), 743. https://doi.org/10.3390/ijms21030743






